Open Access Article
Open Access ArticleCore–shell defective TiO2 nanoparticles by femtosecond laser irradiation with enhanced photocatalytic performance†
Bersu
Bastug Azer
 *a,
Ahmet
Gulsaran
a,
Joel R.
Pennings
a,
Reza
Karimi
b,
Aydin
Ashrafi Belgabad
bc,
Alexander H.
Xu
ad,
Liena
Zaidan
e,
Samed
Kocer
af,
Joseph
Sanderson
b,
Michal
Bajcsy
eg,
Michael A.
Pope
*a,
Ahmet
Gulsaran
a,
Joel R.
Pennings
a,
Reza
Karimi
b,
Aydin
Ashrafi Belgabad
bc,
Alexander H.
Xu
ad,
Liena
Zaidan
e,
Samed
Kocer
af,
Joseph
Sanderson
b,
Michal
Bajcsy
eg,
Michael A.
Pope
 ah and
Mustafa
Yavuz
*a
ah and
Mustafa
Yavuz
*a
aWaterloo Institute for Nanotechnology (WIN), University of Waterloo, 200 University Ave. West, Waterloo, ON N2L 3G1, Canada. E-mail: bbastuga@uwaterloo.ca; myavuz@uwaterloo.ca
bDepartment of Physics and Astronomy, University of Waterloo, 200 University Ave. West, Waterloo, ON N2L 3G1, Canada
cDepartment of Physics and Energy Engineering, Amirkabir University of Technology (Tehran Polytechnic), P.O. Box 15875-4413, Tehran, Iran
dDepartment of Nanotechnology Engineering, University of Waterloo, 200 University Ave. West, Waterloo, ON N2L 3G1, Canada
eDepartment of Electrical and Computer Engineering, University of Waterloo, 200 University Ave. West, Waterloo, ON N2L 3G1, Canada
fDepartment of Systems Design Engineering, University of Waterloo, 200 University Ave. West, Waterloo, ON N2L 3G1, Canada
gInstitute for Quantum Computing, University of Waterloo, 200 University Ave. West, Waterloo, ON N2L 3G1, Canada
hDepartment of Chemical Engineering, University of Waterloo, 200 University Ave. West, Waterloo, ON N2L 3G1, Canada
First published on 26th January 2023
Abstract
Engineering defects in titanium dioxide (TiO2) is becoming increasingly important to enhance its photocatalytic performance by increasing active sites and lowering its band gap from the UV region to the visible light region. Herein, we demonstrate a simple method to create stable surface defects by exposing TiO2 dispersions to femtosecond laser irradiation. When using an 800 nm wavelength source, little change to the TiO2 dispersion is observed. However, including the second harmonic of this source (400 nm wavelength) creates stable, dark colored, defective TiO2, also known as black TiO2. This occurs without the formation of a rutile phase, previously thought to be necessary for black TiO2 formation. We demonstrate that the surface oxygen vacancy concentration can be tuned to a maximum of 8% with irradiation time. Beyond this, defect annihilation is observed. Unlike irradiation using longer pulse width lasers (e.g. nanosecond and picosecond lasers), which are known to create more bulk defects, modification using femtosecond pulse widths only modifies a thin shell at the surface, leaving the bulk of the particles unaffected. The most defective TiO2 exhibits enhanced photocatalytic performance under UV light owing to the formation of defects which act as trapping states for photogenerated electron/hole pairs.
Introduction
TiO2 is one of the most widely studied materials for various applications since Honda and Fujishima discovered its effective photocatalytic properties.1 In addition, it is an attractive material because of its low cost, chemical and thermal stabilities, low toxicity, natural abundance, and availability.2 Consequently, TiO2 is one of the most prominent materials for photoelectrochemical water splitting,3,4 dye-sensitized solar cells,5,6 photoelectrochemical sensors,7–12 photocatalysis in atmospheric chemistry,13 photocatalytic degradation of organic pollutants,14–16 and other photocatalytic applications.17,18 However, challenges with TiO2 include its wide band gap energy (3.0–3.2 eV), which restricts light absorption to the high-energy UV region19 and the typically fast recombination rate of photogenerated electron–hole pairs that reduces device quantum efficiency.20,21 To overcome these drawbacks, TiO2 has been modified using different strategies, such as metal doping22–24 and non-metal doping.25–28 Despite enhancing the visible light activity of TiO2, doping enhances electron–hole pair recombination.29,30 Moreover, it has been reported that doping of TiO2 also causes secondary phase formation and a decrease in crystallinity, affecting the lifetime of electron–hole pairs.31In 2011, Chen et al.32 produced black-colored TiO2 by generating disorder in the surface layers through a hydrogenation process. Since then, the so-called black TiO2 has become attractive for various applications such as photocatalytic dye degradation,33 photoelectrochemical applications,34 photocatalytic water splitting,35 the development of dye-sensitized solar cells,36 and Li-ion batteries.37 The black or other coloration of TiO2 results from at least one type of defect in the structure, such as the presence of Ti3+ species, oxygen vacancies, Ti–H bonds, surface hydroxyl groups (Ti-OH groups), and structural disorder at the surface such as the formation of Ti3+ and Ti2O3, at the outermost layer.38 The formation of these defects enhances solar absorption along with the electrical, optical, and photocatalytic properties. Enhanced quantum efficiency of black TiO2 thought to be caused by the reduced recombination rate of photogenerated charge carriers has also been reported.39
Black TiO2 has been synthesized by various techniques,38 such as hydrogenation,32,40 plasma treatment,41 chemical reduction,42 and electrochemical reduction.43 However, these techniques require either high-temperature, high-pressure reactions, or even toxic chemicals.44 A green and environmentally friendly synthesis technique is needed for black TiO2 production. Recently, pulsed laser irradiation techniques have been used as a method of nanomaterials synthesis to obtain metals, metal oxides, core–shell structures of metal oxides, and doped oxides.45 In particular, femtosecond laser pulses have been effectively used to either generate new nanostructures,46,47 or modify the physical and chemical properties of existing nanoparticles.48 For example, the pulse laser ablation of a Ti target in liquids has been used to produce TiO2 with various properties and defect densities.49,50 By using TiO2 nanoparticles, laser irradiation in liquids was used to produce disorder on the surface of TiO2. Russo et al.51 studied the effects of femtosecond laser (800 nm, 35 fs, 1 kHz) irradiation of P25 powders in water. They reported a color change, but they observed that with an increase in the irradiation time, the blue color decreased. In addition to this, they reported a phase transformation from anatase to rutile and then back to anatase with an increase in the laser irradiation time. Chen et al.52 prepared black TiO2 nanospheres by irradiating third harmonic pulses of a nanosecond Nd:YAG laser (355 nm, 8 ns, 10 Hz), which exhibited good photocatalytic performance under visible light. Zuniga-Ibarra et al.53 used second harmonic pulses of a nanosecond Nd:YAG laser (532 nm, 10 ns, 10 Hz) to synthesize black TiO2 nanoparticles using pulsed laser irradiation in liquid dispersions. They reported an enhancement in visible light absorption by decreasing the band gap energy from 2.98 eV to 1.84 eV. Lau et al.54 explained how surface and bulk defects were generated by laser irradiation by comparing the ns-355 nm laser and the ps-532 nm laser. They obtained more than 2-fold enhancement in the photoelectrochemical activity of ps-532 nm laser irradiated TiO2 nanoparticles, even without observing any color change. They explained the color difference between these two samples with the location of defects. Black samples were obtained by irradiating with ns-355 nm and generated mainly bulk defects, while white samples were produced by processing with ps-532 nm laser which created more surface defects. All these studies52–54 state that the color change is linked to rutile phase formation, which is observed with high pulse width lasers (nanosecond lasers) because of high thermal loads causing bulk defects.
In this work, we study the use of femtosecond laser pulsed irradiation to obtain defective TiO2 using both the fundamental (800 nm) and the second harmonic (400 nm) pulses from a Ti:sapphire laser with a 1 kHz repetition rate and a pulse duration of 35 fs. For the first time, we observe a color change without rutile phase formation suggesting a novel defect formation mechanism related to the wavelength used and the femtosecond pulse duration. We systematically study the structure and optical properties of the TiO2 produced and determine that, through this approach, defects are localized to the surface while the bulk of the material remains unchanged. The degradation of the methylene blue dye is used to demonstrate the improved photocatalytic performance of the modified TiO2.
Experimental
Synthesis of blue TiO2 by laser irradiation
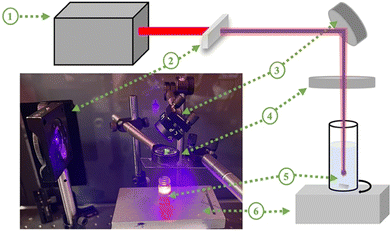
20 mg of pure TiO2 (Sigma Aldrich, anatase <25 nm) were added to 1 mL of ultra-pure de-ionized (DI) water and ultrasonicated for 30 minutes. Then, it was irradiated with a combination of fundamental (800 nm) and second harmonic generated pulses (λ = 400 nm) of a Ti:sapphire pulsed laser with a pulse duration of 35 fs and a repetition rate of 1 kHz. The spectra of the laser before and after barium borate (BBO) non-linear crystal are shown in Fig. S1 (ESI†). The irradiation was performed by constantly mixing the solution with a magnetic stirrer to obtain a homogeneous and uniform solution. A focus lens with a 50 mm focal length was used to focus the beam at a depth of 2 mm inside the solution. The solution was irradiated with average powers of 1 W and 0.8 W at an 800 nm wavelength and 0.2 W at a 400 nm wavelength. The laser fluences of 800 and 400 nm wavelength outputs were estimated as 4074 J cm−2 and 1018 J cm−2, respectively. The total laser irradiation time is n minutes. The laser setup is shown in Fig. 1.After laser irradiation, the samples were left under vacuum to dry at ambient temperature. The prepared samples are indicated as TiO2-n, where n is the laser irradiation duration in minutes, n = 15, 30, 60, 90, and 120.
Characterization
Powder X-ray diffraction (XRD) was carried out using a Bruker D8-Focus with Cu-Kα radiation at a wavelength of 1.5406 Å, an acceleration voltage of 40 kV, and an emission current of 40 mA.Ultraviolet/visible (UV-Vis) diffuse reflectance spectroscopy was carried out using a Shimadzu UV-2501PC spectrometer. The absorbance was calculated from the diffuse reflectance values using eqn (1).
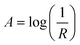 | (1) |
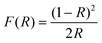 | (2) |
The chemical states of the samples and the valence band (VB) were identified by X-ray photoelectron spectroscopy (XPS) using a Thermo ESCALAB 25 using an Al-Kα X-ray source. XPS analyses were performed using OriginPro 2021b and Shirley XPS baseline, and Gaussian fitting was performed. The oxygen vacancy content can be calculated from XPS analyses using eqn (3).
 | (3) |
Photocatalytic reaction
The photocatalytic activity of laser irradiated samples was measured by methylene blue (MB) photodegradation. For a typical reaction, 5 mg of photocatalytic powder was added to 15 mL of a 5 mg L−1 concentration of MB solution. Before starting the reaction, the solution was stirred using a magnetic stirrer in the dark for 1 h to establish the equilibrium absorption of MB. Then, the solution was exposed to UV light (Analytik Jena) with a 6 W power with a center wavelength of 365 nm. The pH of suspensions was stable at 7 ± 0.1, the temperature was maintained at 25 ± 1 °C and they were stirred at 400 rpm. Samples with a volume of 2 mL were collected at designated time intervals and centrifuged at 3000 rpm for 20 minutes to remove the dispersed TiO2 particles. The concentration of MB was obtained by recording the absorbance spectra of the solution in the range of 200–800 nm using a UV-Vis spectrometer with a quartz cuvette. The change in the absorption of MB solution at a 664 nm wavelength during the photodegradation process was monitored. The Lambert–Beer law was used to correlate the absorption to the MB concentration using eqn (4).| A = εcl | (4) |
Results and discussion
Morphological and structural properties of TiO2 nanoparticles
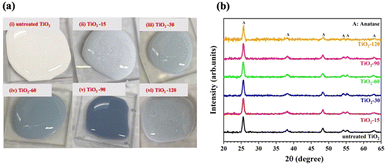
In previous studies,52–54 it has been suggested that a color change cannot be observed with irradiation of lasers with shorter than nanosecond pulse widths (<ns) because the color change is associated with rutile phase formation. In this study, the stable color change was not observed when pulses of pure 800 nm wavelength radiation were used similar to the study of Russo et al.51 They reported that the increase in the ablation time resulted in a less blue colour intensity, but they did not mention the stability of the blue TiO2 samples. In this study, when the 800 nm wavelength output was used, samples were observed to turn blue, but returned to a white on the time scale of minutes. On the other hand, after generating the second harmonic (400 nm) using a BBO crystal and combining it with the fundamental (800 nm) light, a significant and stable color change was observed without any rutile phase formation, as shown in Fig. 2b. The effect of the laser wavelength on the defect formation mechanism has not been fully explained in the literature yet. We did not observe any stable color and structural change with laser irradiation at an 800 nm wavelength. However, when we created the second harmonic, the combination of 800 nm and 400 nm wavelengths resulted in the defect formation. We also attempted to filter out the 800 nm wavelength and ablate TiO2 solution with only a 400 nm wavelength to understand the effect of each wavelength well. However, this reduced the intensity of the 400 nm light significantly. This yielded no observable change either due to the low power or due to the absence of the 800 nm wavelength.As shown in Fig. 2a, the color of the TiO2 dispersions changed from white to blue-gray upon laser irradiation for a 15 minute duration. Further increasing the laser irradiation time darkened the coloration, reaching a maximum darkness after 90 minutes. However, extending the irradiation time further, to 120 minutes, resulted in a lighter colored dispersion. The color of the samples suggests that they can absorb visible light partially or totally.
The crystalline structure of samples before and after laser irradiation was studied by XRD analysis. Laser irradiation did not affect the phases present in the structure, as shown in Fig. 2b. The XRD patterns of all samples are composed of diffraction peaks at 25.2, 36.9, 37.7, 38.5, 47.9, 53.8, 54.9, 61.9, and 62.5 degrees, corresponding to the (101), (103), (004), (112), (200), (105), (211), (213) and (204) lattice planes of the anatase phase of TiO2 (JCPDS Card No. 21-1272).
This result is new for femtosecond irradiated TiO2 as previously only nanosecond irradiation was able to induce a color change.45,54 In the previous ns irradiation works, the observed color change has always been associated with rutile phase formation. The authors argued that this phase transformation occurs via recrystallization after isochoric melting occurs. On the other hand, lower energy but focused femtosecond laser pulses can cause ablative surface ionization.45 Light–matter interactions with femtosecond lasers are usually athermal and thus melting and the associated recrystallization process cannot occur. Only defect formation occurs, and these defects are localized at the surface due to surface ionization. Here, we have shown that not only the pulse width but also the wavelength plays a role in the color change mechanism induced by femtosecond laser irradiation. One possible explanation for this could be the introduction of photoinduced defects through the introduction of electron/hole pairs generated by the single photon absorption of 400 nm radiation as follows:
| TiO2 + hν → eCB− + hVB+ | (5) |
| eCB− + Ti4+ + Ti3+ | (6) |
| hVB+ + O2− → O− | (7) |
The morphological analyses of nanoparticles before and after laser irradiation were performed by scanning electron microscopy (SEM), as shown in Fig. 3. As seen from the SEM images, there was no significant morphological change after laser irradiation. It was also seen that the femtosecond laser irradiation does not impact the particle size. There are some agglomerates and nanoparticles in the structure before and after laser irradiation. This behavior does not match with nanosecond laser irradiated TiO2 as well. Chen et al.52 reported that as the laser irradiation time increased, the particle size decreased. Similarly, Zuñiga-Ibarra et al.53 showed a morphological change with laser irradiation, and they demonstrated an increased number of agglomerates and small nanoparticles with an increase in the laser irradiation time. They explained this behavior by applying enough energy to melt TiO2 and the nucleation and growth of modified TiO2 nanoparticles, which is also explained by rutile phase formation. In this study, no morphological and crystallinity changes were observed, and this can be concluded that femtosecond laser might only form defects on the surface of nanoparticles without melting TiO2, leading to the nucleation and growth of modified nanoparticles.
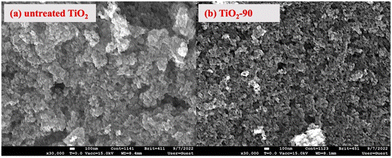 | ||
| Fig. 3 SEM micrographs of TiO2 before and after laser irradiation: (a) untreated TiO2 and (b) TiO2-90. | ||
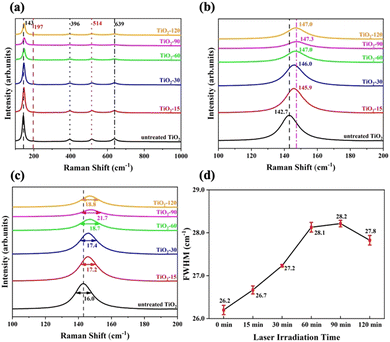
Raman spectroscopy was performed to determine the crystallinity and molecular interactions of untreated and laser irradiated samples. The spectra shown in Fig. 4a show peaks at the major Raman bands at around 143 cm−1, 197 cm−1, 396 cm−1, 514 cm−1, and 639 cm−1 which correspond to the six Raman active modes of anatase, which are Eg, Eg, B1g, A1g/B1g, and Eg, respectively.57–59 The strongest peak at around 143 cm−1 in the neat sample is caused by symmetric vibrations of oxygen atoms in the O–Ti–O bond. The position of this peak shifts positively, increasing to 147.3 cm−1 for TiO2-90 and then decreasing with further treatment time to 120 minutes, as shown in Fig. 4b. The intensity of the peak decreased and broadened. This suggests that the O–Ti–O bond amount decreased in the structure by increasing the laser irradiation time up to 90 minutes. After 90 minutes, the peak intensity increased, the peak narrowed, and a negative shift was observed from TiO2-90. The full-width-at-half-maximum (FWHM) values were calculated for each sample by the Gaussian fitting curve, as shown in Fig. 4c.
Raman spectroscopy is an effective tool for determining the surface oxygen vacancies on the structure.60 It was reported that the broadening of A1g/B1g mode by a FWHM value of 0.25 cm−1 corresponds to the increment of 1% oxygen vacancy in the lattice structure.61 A1g/B1g peaks were Gaussian fitted, and the FWHM values were calculated for untreated and laser irradiated samples, as shown in Fig. 4d. The data indicate that TiO2-90 has the highest amount of oxygen vacancies, which is 8% more than untreated TiO2. The oxygen vacancy concentration of TiO2-15 was estimated to be 2% more than untreated TiO2, the oxygen vacancy of TiO2-30 was 4% more than untreated TiO2, TiO2-60 had 7.6% more oxygen vacancy than untreated TiO2, and the oxygen vacancy of TiO2-120 was 6.4% more than untreated TiO2. This showed that increasing the laser irradiation time after 90 minutes led to a decrease in the oxygen vacancy content. By femtosecond laser irradiation, oxygen atoms were removed from the TiO2 surface because of localized heating. With an increase in the laser irradiation time from 90 to 120 minutes, further oxygen removal could not occur, and oxygen atoms were bonded with Ti to form Ti4+–O, resulting in a decrease in the oxygen vacancy concentration.
Optical properties of TiO2 nanoparticles
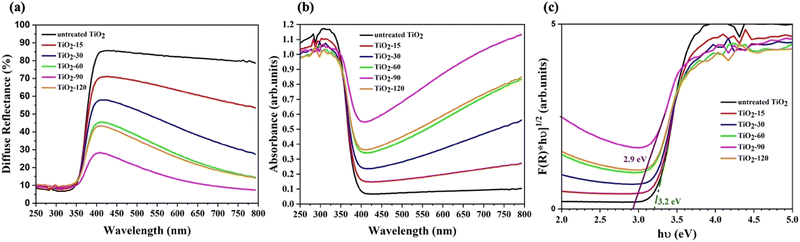
The UV-Vis diffuse reflectance spectra of the various samples are shown in Fig. 5a while Fig. 5b shows absorbance spectra. After laser irradiation, all laser irradiated TiO2 samples exhibit absorption throughout the visible light region when compared with untreated TiO2. Furthermore, the absorption of visible light above the 400 nm wavelength increased as the laser irradiation time increased up to 90 minutes.The band gap energies of untreated and laser irradiated TiO2 nanomaterials are evaluated by the Kubelka–Munk function method using eqn (2). The determined band gap energy values are shown in Fig. 5c. The band gap energy of TiO2 shifts from 3.2 eV to 2.9 eV by laser irradiation for 90 minutes. The oxygen vacancies and other defects might have created additional energy levels between the valence band (VB) and the conduction band (CB) of TiO2. TiO2-90 has 8% more oxygen vacancies than untreated TiO2, and the band gap energy of TiO2-90 decreased from 3.2 eV to 2.9 eV. This supports the previous studies that reported oxygen vacancies to cause enhanced visible light absorption below the direct band gap.29,52
The lowest band gap energy obtained from laser irradiated samples is 2.9 eV. After increasing the laser irradiation time from 90 to 120 minutes, the band gap energy increased from 2.9 eV to 3 eV. This also matches the oxygen vacancy concentration obtained from the FWHM values of A1g/B1g mode, as shown in Fig. 4d. The oxygen vacancy of TiO2-90 is 0.4% more than that of TiO2-120.
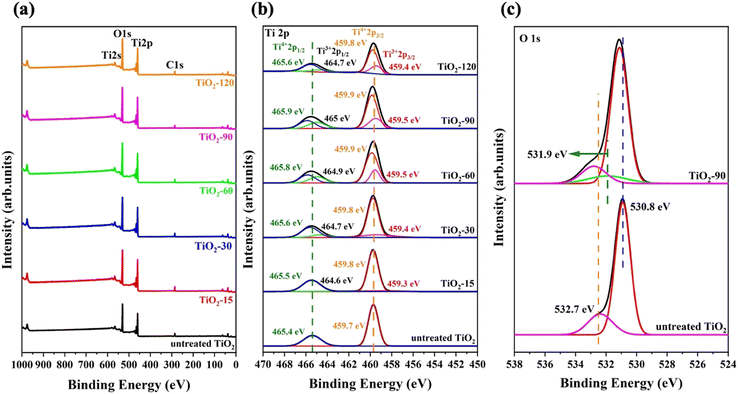
Chemical state analysis of TiO2 nanoparticles
XPS analysis was carried out to determine the change of surface chemical constituents caused by laser irradiation. The surface of the samples is clean from impurities except for a trace amount of C. Ti, O, and C elements were found from the survey scan (Fig. 6a). The Ti 2p XPS spectra of untreated TiO2, TiO2-15, TiO2-30, TiO2-60, TiO2-90 and TiO2-120 are shown in Fig. 6b. The energy and area of each peak for every sample are given in Table 1. The peaks at 459.7 eV and 465.4 eV are Ti4+ 2p3/2 and Ti4+ 2p1/2 XPS peaks, respectively. For laser irradiated samples, two new peaks appeared between 459.3 and 459.5 eV and 464.7 and 465 eV, depending on the laser irradiation time, and they are assigned to Ti3+ 2p3/2 and Ti3+ 2p1/2, respectively. The binding energy of Ti4+ 2p1/2 shifted to 0.5 eV for TiO2-90 compared to that of untreated TiO2, which is the maximum shift for laser irradiated samples. Similarly, the positive shifts of Ti4+ 2p3/2, Ti3+ 2p3/2, and Ti3+ 2p1/2 were observed, which are probably because of the formation of oxygen vacancies which causes the bond length of Ti–O to shorten, resulting in a positive shift in the XPS peaks.61| Sample | Ti4+ 2p1/2 | Ti3+ 2p1/2 | Ti4+ 2p3/2 | Ti3+ 2p3/2 | ||||
|---|---|---|---|---|---|---|---|---|
| Peak energy (eV) | Peak area | Peak energy (eV) | Peak area | Peak energy (eV) | Peak area | Peak energy (eV) | Peak area | |
| Untreated TiO2 | 465.4 | 21433.74 | — | — | 459.7 | 49213.07 | — | — |
| TiO2-15 | 465.5 | 14563.02 | 464.6 | 1473.82 | 459.8 | 31530.21 | 459.3 | 2511.86 |
| TiO2-30 | 465.6 | 12297.05 | 464.7 | 3244.98 | 459.8 | 30809.21 | 459.4 | 5635.04 |
| TiO2-60 | 465.8 | 10653.7 | 464.9 | 8783.12 | 459.9 | 33499.92 | 459.5 | 10379.01 |
| TiO2-90 | 465.9 | 11736.76 | 465 | 9820.84 | 459.9 | 35794.13 | 459.5 | 12735.9 |
| TiO2-120 | 465.6 | 19588.54 | 464.7 | 6004.09 | 459.8 | 42541.09 | 459.4 | 16197.21 |
Eqn (3) was used to calculate the oxygen vacancy content for all laser irradiated samples and the values are provided in Table 2. The oxygen vacancy contents calculated from XPS analysis are consistent with the Raman results.
| Samples | VO |
|---|---|
| Untreated TiO2 | — |
| TiO2-15 | 1.99% |
| TiO2-30 | 4.27% |
| TiO2-60 | 7.57% |
| TiO2-90 | 8.05% |
| TiO2-120 | 6.58% |
Fig. 6c shows the XPS O 1s peaks of untreated TiO2 and TiO2-90. For untreated TiO2, two peaks appear, and the one at 530.8 eV is assigned to the lattice oxygen of TiO2 because of the Ti4+–O bond, while the peak at 532.7 eV is attributed to adsorbed oxygen. For TiO2-90, these peaks show positive peaks by 0.2 eV and 0.1 eV to 531 eV and 532.8 eV, respectively. In addition, a new peak also appears at 531.9 eV and corresponds to hydroxyl oxygen (Ti–OH). The O 1s XPS spectra of TiO2-15, TiO2-30, TiO2-60, and TiO2-120 are given in Fig. S2 in the ESI.†
The elemental composition of the bulk structure of untreated and laser modified TiO2 was determined by EDS analysis. Ti, O, and C elements were found in the samples. The atomic percentages of each element for all samples are given in Table 3. The O/Ti ratio of all samples is close to 2 with no systematic variations. This suggests that significant oxygen vacancies are not introduced into the bulk of the material since it is known that the EDS analysis gives information about the bulk of the sample of the large interaction volume of high energy electrons which produce the X-rays generated. Since the XPS and Raman spectra are surface sensitive and EDS is not, the laser treated samples have a defective shell with an unmodified core, and core–shell defective TiO2 has been produced.
| Sample | Ti (at%) | O (at%) | C (at%) | O/Ti ratio |
|---|---|---|---|---|
| Untreated TiO2 | 32.15 | 62.71 | 5.14 | 1.95 |
| TiO2-15 | 32.35 | 62.55 | 5.1 | 1.93 |
| TiO2-30 | 32.11 | 62.86 | 5.03 | 1.96 |
| TiO2-60 | 31.91 | 62.97 | 5.12 | 1.97 |
| TiO2-90 | 32.31 | 63.11 | 4.58 | 1.95 |
| TiO2-120 | 32.05 | 62.87 | 5.08 | 1.96 |
The high-resolution VB XPS spectra of untreated TiO2 and TiO2-90 are given in Fig. 7a. The VB maximum values of untreated TiO2 and TiO2-90 are 3.49 eV and 3.74 eV, which shows a 0.4 eV shift of the maximum value above by the laser irradiation of TiO2. The VB XPS spectra of TiO2-15, TiO2-30, TiO2-60, and TiO2-120 are given in Fig. S3 in the ESI.†
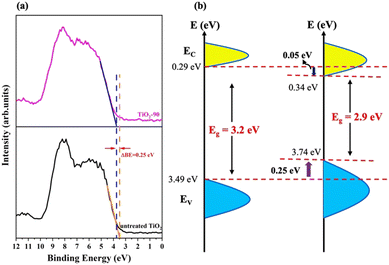 | ||
| Fig. 7 (a) High-resolution valence band XPS spectra and (b) schematic illustration of the electronic density of states of untreated TiO2 and TiO2-90. | ||
The band gap energies obtained from the Kubelka–Munk function from the UV-Vis diffuse reflectance measurement (Fig. 5c) suggest a downward shift of the CB minimum of 0.025 eV. With this information, a schematic illustration of the band structure of untreated TiO2 before and after femtosecond laser modification is shown in Fig. 7b. The formation of Ti3+ species, oxygen vacancies, Ti–OH bonds, and surface disorder caused by femtosecond laser irradiation are probably for band tail states around the VB maximum and the CB minimum.
Photocatalytic performance of TiO2 nanoparticles
Many researchers have identified that surface and bulk defects, i.e., Ti3+ and oxygen vacancies, can improve the photocatalytic performance of TiO2 by narrowing the band gap and providing more catalytic adsorption sites. However, surface defects are more efficient at trapping and separating photogenerated electron–hole pairs, whereas bulk defects can act as recombination centers for such photogenerated charge carriers.62–64 The Raman and XPS spectra show the oxygen vacancies and Ti3+ species formation on the surface, suggesting that laser modified TiO2 can potentially exhibit better photocatalytic performance. To test this, the breakdown of the MB dye was compared with untreated TiO2 and TiO2-90 as the photocatalytic materials. TiO2-90 was chosen to compare with untreated TiO2 since it has the most oxygen vacancies according to the Raman and XPS results. The untreated TiO2 and TiO2-90 were mixed with MB, and the degradation of MB was measured under ultraviolet light by recording the C/C0 ratio, as shown in Fig. 8. A decrease in the MB concentration was observed with the increasing ultraviolet light exposure time. It was also found that when no light was used, the decrease in the concentration was insignificant, indicating that only the photocatalytic process was responsible for the decrease. The degradation rates of MB were 80% for TiO2-90 and 50% for untreated TiO2 after exposure to ultraviolet light for 3 hours.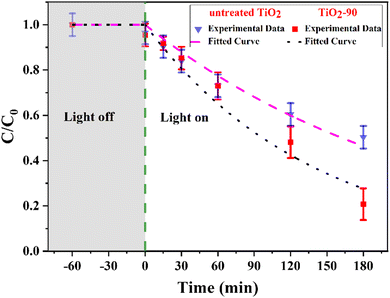 | ||
| Fig. 8 Photocatalytic degradation of methylene blue under UV light irradiation of untreated TiO2 and TiO2-90. | ||
During the femtosecond laser irradiation process, oxygen vacancies were formed on the surface. To maintain the charge neutrality, some of the Ti4+ were transformed to Ti3+. Since the reaction occurred in an aqueous environment, some Ti3+ species tended to bond with H2O and resulted in the formation of surface functional groups such as Ti–OH as obtained from the XPS analysis. Oxygen vacancies, Ti3+, Ti–OH, and general surface disorder might act as trapping centers by locally shifting energy levels below the CB and above the VB, and they can reduce the recombination rate of photogenerated electron–hole pairs.65 It was stated that oxygen vacancies acted as electron donors and contributed to the increased donor density.40 This can improve the charge transport and shift the CB and the VB, as shown in this study. These shifts can make electron/hole separation easier, resulting in better photocatalytic activity.66
Conclusions
In this study, the combination of fundamental and second harmonic radiations from a femtosecond laser was used to produce core–shell, defective TiO2. Unlike the literature, a new defect formation mechanism has been introduced relating the defect generation to the wavelength of the laser. The influence of laser irradiation on the oxygen vacancy concentration and photocatalytic performance was revealed. The femtosecond laser surface irradiation led to the formation of Ti3+, oxygen vacancies, and Ti–OH on the TiO2 surface without deforming the bulk of the sample. According to the Raman and XPS spectra analyses, a maximum of 8% oxygen vacancies were produced on the surface by femtosecond laser irradiation, which narrowed the band gap energy of TiO2 from 3.2 eV to 2.9 eV by shifting the VB maximum and CB minimum values. The EDS analysis showed that the bulk of the sample is stoichiometric TiO2, which suggests that by femtosecond laser irradiation, TiO2 nanoparticles with an undefective core with a defective shell could be formed. The femtosecond laser modified defective core–shell structured TiO2 exhibited better photocatalytic performance under ultraviolet light exposure owing to the formation of oxygen vacancies, Ti3+ and surface disorders, which might act as trapping centers for photogenerated charge carriers and can result in a reduction in the recombination rate. To further explain the defect formation mechanism, different wavelengths with different pulse width lasers should be used to demonstrate the formation of surface defects and bulk defects in TiO2 and other metal oxides. Their effect on photocatalytic performance under various light sources should be studied. In the literature, it is known that surface defects positively affect the electronic properties of TiO2, which suggests that the surface defective TiO2 can be a promising material for the functionalization of different sensing applications such as biosensing, gas sensing, and electrochemical sensing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the CMC Microsystems, Canadian Mathematics of Information Technology and Complex Systems Agency (MITACS) [Grant No: IT17161 and 2000-2024], the NSERC Alliance [Grant No: ALLRP 554872-20], and the Discovery [Grant No: GPIN-2020-06053] research programs. The University of Waterloo's QNFCF (Quantum-Nano Fabrication and Characterization Facility) facility was used for this work.Notes and references
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS PubMed.
- K. Nakata and A. Fujishima, J. Photochem. Photobiol., C, 2012, 13, 169 CrossRef CAS.
- G. Wang, H. Wang, Y. Ling, Y. Tang, X. Yang, R. C. Fitzmorris, C. Wang, J. Z. Zhang and Y. Li, Nano Lett., 2011, 11, 3026 CrossRef CAS PubMed.
- Z. Yu, H. Liu, M. Zhu, Y. Li and W. Li, Small, 2021, 17, 1903378 CrossRef CAS PubMed.
- Z. Wang, H. Kawauchi, T. Kashima and H. Arakawa, Coord. Chem. Rev., 2004, 248, 1381 CrossRef CAS.
- M. S. Ahmad, A. K. Pandey and N. A. Rahim, Renewable Sustainable Energy Rev., 2017, 77, 89 CrossRef.
- S. Zhang, S. Zhang, B. Peng, H. Wang, H. Yu, H. Wang and F. Peng, Electrochem. Commun., 2014, 40, 24 CrossRef CAS.
- R. Wang, M. Zu, S. Yang, S. Zhang, W. Zhou and Z. Mai, Sens. Actuators, B, 2018, 270, 270 CrossRef CAS.
- M. Zu, M. Zheng, S. Zhang, C. Xing, M. Zhou, H. Liu, X. Zhou and S. Zhang, Sens. Actuators, B, 2020, 321, 128504 CrossRef CAS.
- X. Wang, S. Zhang, H. Wang, H. Yu, H. Wang, S. Zhang and F. Peng, RSC Adv., 2015, 5, 76315 RSC.
- Y. Wang, M. Zu, X. Zhou, H. Lin, F. Peng and S. Zhang, Chem. Eng. J., 2020, 381, 122605 CrossRef CAS.
- B. Bastug Azer, A. Gulsaran, J. R. Pennings, R. Saritas, S. Kocer, J. L. Bennett, Y. Devdas Abhang, M. A. Pope, E. Abdel-Rahman and M. Yavuz, J. Electroanal. Chem., 2022, 918, 116466 CrossRef CAS.
- H. Chen, C. E. Nanayakkara and V. H. Grassian, Chem. Rev., 2012, 112, 5919 CrossRef CAS PubMed.
- D. Chen, Y. Cheng, N. Zhou, P. Chen, Y. Wang, K. Li, S. Huo, P. Cheng, P. Peng, R. Zhang, L. Wang, H. Liu, Y. Liu and R. Ruan, J. Cleaner Prod., 2020, 268, 121725 CrossRef CAS.
- H. Dong, G. Zeng, L. Tang, C. Fan, C. Zhang, X. He and Y. He, Water Res., 2015, 79, 128 CrossRef CAS PubMed.
- P. Pascariu, C. Cojocaru, M. Homocianu, P. Samoila, A. Dascalu and M. Suchea, Ceram. Int., 2022, 48, 4953 CrossRef CAS.
- K. Nakata and A. Fujishima, J. Photochem. Photobiol., C, 2012, 13, 169 CrossRef CAS.
- Q. Guo, C. Zhou, Z. Ma and X. Yang, Adv. Mater., 2019, 31, 1901997 CrossRef CAS PubMed.
- S. G. Ullattil, S. B. Narendranath, S. C. Pillai and P. Periyat, Chem. Eng. J., 2018, 343, 708 CrossRef CAS.
- V. Binas, D. Venieri, D. Kotzias and G. Kiriakidis, J. Materiomics, 2017, 3, 3 CrossRef.
- W. Fang, M. Xing and J. Zhang, J. Photochem. Photobiol., C, 2017, 32, 21 CrossRef CAS.
- M. Zhang, S. Yuan, Z. Wang, Y. Zhao and L. Shi, Appl. Catal., B, 2013, 134, 185 CrossRef.
- B. Gao, T. Wang, X. Fan, H. Gong, H. Guo, W. Xia, Y. Feng, X. Huang and J. He, Inorg. Chem. Front., 2017, 4, 898 RSC.
- M. Pelaez, N. T. Nolan, S. C. Pillai, M. K. Seery, P. Falaras, A. G. Kontos, P. S. M. Dunlop, J. W. J. Hamilton, J. A. Byrne, K. O’Shea, M. H. Entezari and D. D. Dionysiou, Appl. Catal., B, 2012, 125, 331 CrossRef CAS.
- Y. Chen, A. Li, Q. Li, X. Hou, L. N. Wang and Z. H. Huang, J. Mater. Sci. Technol., 2018, 34, 955 CrossRef CAS.
- P. S. Basavarajappa, S. B. Patil, N. Ganganagappa, K. R. Reddy, A. V. Raghu and C. V. Reddy, Int. J. Hydrogen Energy, 2020, 45, 7764 CrossRef CAS.
- W. Yuan, L. Cheng, Y. An, S. Lv, H. Wu, X. Fan, Y. Zhang, X. Guo and J. Tang, Adv. Sci., 2018, 5, 1700870 CrossRef PubMed.
- S. B. Patil, P. S. Basavarajappa, N. Ganganagappa, M. S. Jyothi, A. V. Raghu and K. R. Reddy, Int. J. Hydrogen Energy, 2019, 44, 13022 CrossRef CAS.
- F. Zuo, L. Wang, T. Wu, Z. Zhang, D. Borchardt and P. Feng, J. Am. Chem. Soc., 2010, 132, 11856 CrossRef CAS PubMed.
- H. U. Lee, S. C. Lee, S. H. Choi, B. Son, S. J. Lee, H. J. Kim and J. Lee, Appl. Catal., B, 2013, 129, 106 CrossRef CAS.
- S. G. Ullattil and P. Periyat, J. Mater. Chem. A, 2016, 4, 5854 RSC.
- X. Chen, L. Liu, P. Y. Yu and S. S. Mao, Science, 2011, 331, 746 CrossRef CAS PubMed.
- T. Leshuk, R. Parviz, P. Everett, H. Krishnakumar, R. A. Varin and F. Gu, ACS Appl. Mater. Interfaces, 2013, 5, 1892 CrossRef CAS PubMed.
- H. Cui, W. Zhao, C. Yang, H. Yin, T. Lin, Y. Shan, Y. Xie, H. Gu and F. Huang, J. Mater. Chem. A, 2014, 2, 8612 RSC.
- B. Wang, S. Shen and S. S. Mao, J. Materiomics, 2017, 3, 96 CrossRef.
- S. G. Ullattil, A. V. Thelappurath, S. N. Tadka, J. Kavil, B. K. Vijayan and P. Periyat, Sol. Energy, 2017, 155, 490 CrossRef CAS.
- Y. Yan, B. Hao, D. Wang, G. Chen, E. Markweg, A. Albrecht and P. Schaaf, J. Mater. Chem. A, 2013, 1, 14507 RSC.
- S. G. Ullattil, S. B. Narendranath, S. C. Pillai and P. Periyat, Chem. Eng. J., 2018, 343, 708 CrossRef CAS.
- T. S. Rajaraman, S. P. Parikh and V. G. Gandhi, Chem. Eng. J., 2020, 389, 123918 CrossRef CAS.
- A. Naldoni, M. Allieta, S. Santangelo, M. Marelli, F. Fabbri, S. Cappelli, C. L. Bianchi, R. Psaro and V. Dal Santo, J. Am. Chem. Soc., 2012, 134, 7600 CrossRef CAS PubMed.
- Z. Wang, C. Yang, T. Lin, H. Yin, P. Chen, D. Wan, F. Xu, F. Huang, J. Lin, X. Xie and M. Jiang, Adv. Funct. Mater., 2013, 23, 5444 CrossRef CAS.
- Q. Kang, J. Cao, Y. Zhang, L. Liu, H. Xu and J. Ye, J. Mater. Chem. A, 2013, 1, 5766 RSC.
- H. Zhou and Y. Zhang, J. Phys. Chem. C, 2014, 118, 5626 CrossRef CAS.
- X. Chen, L. Liu and F. Huang, Chem. Soc. Rev., 2015, 44, 1861 RSC.
- V. Amendola, D. Amans, Y. Ishikawa, N. Koshizaki, S. Scirè, G. Compagnini, S. Reichenberger and S. Barcikowski, Chem. – Eur. J., 2020, 26, 9206 CrossRef CAS PubMed.
- K. Ibrahim, I. Novodchuk, K. Mistry, M. Singh, C. Ling, J. Sanderson, M. Bajcsy, M. Yavuz and K. P. Musselman, Small, 2019, 15, 1904415 CrossRef CAS PubMed.
- Y. Taguchi, H. Endo, T. Kodama, Y. Achiba, H. Shiromaru, T. Wakabayashi, B. Wales and J. H. Sanderson, Carbon, 2017, 115, 169 CrossRef CAS.
- I. Novodchuk, M. Kayaharman, K. Ibrahim, S. Al-Tuairqi, M. Irannejad, E. Abdel-Rahman, J. Sanderson, M. Bajcsy and M. Yavuz, Carbon, 2020, 158, 624 CrossRef CAS.
- A. Singh, J. Vihinen, E. Frankberg, L. Hyvärinen, M. Honkanen and E. Levänen, Nanoscale Res. Lett., 2016, 11, 1 CrossRef CAS PubMed.
- A. S. Nikolov, P. A. Atanasov, D. R. Milev, T. R. Stoyanchov, A. D. Deleva and Z. Y. Peshev, Appl. Surf. Sci., 2009, 255, 5351 CrossRef CAS.
- P. Russo, R. Liang, R. X. He and Y. N. Zhou, Nanoscale, 2017, 9, 6167 RSC.
- X. Chen, D. Zhao, K. Liu, C. Wang, L. Liu, B. Li, Z. Zhang and D. Shen, ACS Appl. Mater. Interfaces, 2015, 7, 16070 CrossRef CAS PubMed.
- V. A. Zuñiga-Ibarra, S. Shaji, B. Krishnan, J. Johny, S. Sharma Kanakkillam, D. A. Avellaneda, J. A. A. Martinez, T. K. D. Roy and N. A. Ramos-Delgado, Appl. Surf. Sci., 2019, 483, 156 CrossRef.
- M. Lau, S. Reichenberger, I. Haxhiaj, S. Barcikowski, A. M. Müller and A. C. S. Appl, Energy Mater., 2018, 1, 5366 CAS.
- J. Yan, G. Wu, N. Guan, L. Li, Z. Li and X. Cao, Phys. Chem. Chem. Phys., 2013, 15, 10978 RSC.
- A. K. Kunti, K. C. Sekhar, M. Pereira, M. J. M. Gomes and S. K. Sharma, AIP Adv., 2017, 7, 015021 CrossRef.
- H. Berger, H. Tang and F. Lévy, J. Cryst. Growth, 1993, 130, 108 CrossRef CAS.
- T. Sekiya, S. Ohta, S. Kamei, M. Hanakawa and S. Kurita, J. Phys. Chem. Solids, 2001, 62, 717 CrossRef CAS.
- H. C. Choi, Y. M. Jung and S. B. Kim, Vib. Spectrosc., 2005, 37, 33 CrossRef CAS.
- Q. Liu, F. Wang, H. Lin, Y. Xie, N. Tong, J. Lin, X. Zhang, Z. Zhang and X. Wang, Catal. Sci. Technol., 2018, 8, 4399 RSC.
- X. Bi, G. Du, A. Kalam, D. Sun, Y. Yu, Q. Su, B. Xu and A. G. Al-Sehemi, Chem. Eng. Sci., 2021, 234, 116440 CrossRef CAS.
- L. Hou, Z. Guan, T. Liu, C. He, Q. Li and J. Yang, Int. J. Hydrogen Energy, 2019, 44, 8109 CrossRef CAS.
- X. Xing, M. Zhang, L. Hou, L. Xiao, Q. Li and J. Yang, Int. J. Hydrogen Energy, 2017, 42, 28434 CrossRef CAS.
- M. Kong, Y. Li, X. Chen, T. Tian, P. Fang, F. Zheng and X. Zhao, J. Am. Chem. Soc., 2011, 133, 16414 CrossRef CAS PubMed.
- Y. Zou, K. Yang, Q. Chen, H. Wang and X. Meng, RSC Adv., 2018, 8, 36819 RSC.
- H. Tan, Z. Zhao, M. Niu, C. Mao, D. Cao, D. Cheng, P. Feng and Z. Sun, Nanoscale, 2014, 6, 10216 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The spectrum of the BBO crystal before and after the application of femtosecond laser. The O1s XPS spectra of untreated TiO2, TiO2-15, TiO2-30, TiO2-60, TiO2-90 and TiO2-120 samples. The VB XPS spectra of untreated TiO2, TiO2-15, TiO2-30, TiO2-60, TiO2-90 and TiO2-120 samples. See DOI: https://doi.org/10.1039/d3ma00019b |
| This journal is © The Royal Society of Chemistry 2023 |