Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A non-covalent supramolecular dual-network polyelectrolyte evaporator based on direct-ink-writing for stable solar thermal evaporation†
Sihan
Tang
a,
Xinyue
Lu
a,
Peng
Geng
a,
Daobing
Chen
a,
Yunsong
Shi
c,
Jin
Su
a,
Yan
Zhou
*b,
Bin
Su
 *a and
Shifeng
Wen
*a and
Shifeng
Wen
 *a
*a
aState Key Laboratory of Materials Processing and Die & Mold Technology, School of Materials Science and Engineering, Huazhong University of Science and Technology, Wuhan 430074, China. E-mail: roya_wen@hust.edu.cn
bDepartment of Construction Management, School of Civil & Hydraulic Engineering, Huazhong University of Science and Technology, Wuhan 430074, China
cDepartment of Orthopaedics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 430022 Wuhan, China
First published on 25th November 2022
Abstract
Polymers possessing highly adjustable physicochemical properties have been accepted as ideal flexible materials for solar thermal evaporation, which is considered as an eco-friendly and progressive strategy for water reclamation. Conventional dual-network polymers based on the construction of covalent and non-covalent interactions usually involve toxic reactive monomers, initiators and organic solvents in 3D printing. The integration of additive manufacturing into the construction of supramolecular non-covalent dual-networks to achieve structural and performance optimisation remains a noteworthy part. Here we developed a 3D-printed anti-swelling polyelectrolyte evaporator consisting of k-carrageenan (CG), poly(diallyldimethylammoniumchloride) (PDADMAC), and carbon nanotubes (CNTs). CG-PDADMAC-CNT (CGP-CNT) was constructed by non-covalent supramolecular interactions (hydrogen bonds and electrostatic complexation). The exhibited “sol–gel” transition based on thermo reversible hydrogen bonds and helical structure reconfiguration facilitated three-dimensional shaping, while strong electrostatic complexation stabilized the evaporator structure. As a proof-of-concept, the 3D-printed evaporator was intrinsically antibacterial and exhibited remarkable swelling resistance (water bath at 100 °C for 14 days, pH = 13) to a harsh water environment and stable solar thermal evaporation (2.3 kg m−2 h) under 1 sun irradiation, which was one of the prominent values among the evaporators prepared by direct-ink-writing (DIW).
1. Introduction
Effective strategies have been proposed to exploit eco-friendly water reclamation technology. Solar-thermal energy is recognized as a sustainable and clean energy, and increasingly novel photothermal materials have been developed for interfacial solar thermal evaporation.1–4 High hydrophilicity and robust structure of evaporators are generally pivotal for continuous water permeation and evaporation.5–9 The structure10,11 and surface properties12,13 of materials are important for functional applications. Recent research studies have proved that various functional materials and intelligent design structures are instrumental in solar thermal evaporation,14–16 and three-dimensional structures based on 3D-printing are gradually being developed for more effective photothermal conversion.17–22 Zheng et al.23 designed a new 3D hydrogel evaporator with hierarchical microstructures, which showed effective thermal insulation to water, while the vertical radial structure ensured water transportation. Liu et al.24 reported a 3D-printed integrated evaporator with a vertical array structure and lattice structures, which were functional for heat collection and water transfer, respectively. Cheng et al.25 prepared a hierarchical micro-nano-porous hydrogel evaporator by 3D layer-by-layer printing, which showed stable photothermal evaporation performance. DIW as one of the simplistic additive manufacturing technologies is suitable for building macroscopic multiscale structures, which has become a promising trend for preparing three-dimensional evaporators recently.DIW has the advantages of low cost, simple process and wide material applicability, and printing inks generally are non-Newtonian fluids with yield stress, which can form rapidly through extrusion due to the rheological behaviour of shear thinning.26,27 Conventional dual-network polymers based on DIW were constructed by the synergism of covalent and non-covalent supramolecular interactions, indicating that the precursor inks generally require the addition of reactive monomers, initiators, toxic solvents, etc., for further crosslinking. The integration of 3D printing technology into multiple non-covalently interacting polymers for versatile, structured assemblies is the current focus for developing novel three-dimensional evaporators.
Despite that many polymers based on covalent structural support with intriguing architectures and specific properties have been reported,27–29 few studies have been reached when it comes to DIW applicable materials with non-covalent supramolecular dual-network design (including host–guest recognition, π–π stacking, hydrogen bonds, hydrophobicity or electrostatic complex) for solar thermal evaporation. Polyelectrolytes with abundant hydrophilic groups (hydroxyl, carboxyl and amino groups, etc.) and charge properties are ideal evaporating substrates for building non-covalent supramolecular dual-networks.30–32 For instance, hydroxyl groups can form a large number of heat-regulatable dynamic hydrogen bonds to meet the fast phase transformation of DIW, while insoluble and refractory electrostatic complexation triggered by oppositely charged groups guaranteed the structural integrity.33–36 In this dual-network, weaker non-covalent supramolecular interactions were used as “sacrificial bonds”, which consumed a lot of energy while breaking, and stronger bonds protected the integrity of the structure after first network breaking.28,37–42 Polyelectrolytes composed of multiple non-covalent bonds not only match DIW processes but also can expand the range of 3D printable composites.
Here we reported a strategy to construct a non-covalent supramolecular dual-network polyelectrolyte evaporator for stable solar thermal evaporation. CG is a bio-based thermosensitive hydrogel which was chosen as the DIW substrate due to its rapid “sol–gel” phase transition, and this thermally reversible behaviour is owing to the rich hydrogen bonds and helical structure reconfiguration.43,44 CNT as a rigid filler with a wide range of light absorption effectively improved solar thermal conversion efficiency,45 and also stabilized hierarchical and porous structures.23 Further, electrostatic complexation serving as the second crosslinking supramolecular network assured evaporator structural integrity. As a result, reasonable structural design and functional coordination endowed CGP-CNT with remarkable properties such as intrinsic antibacterial activity, thermo and alkali resistance, and exceptional solar thermal evaporation performance among the evaporators prepared by DIW (1 sun irradiation, 2.3 kg m−2 h).
2. Results and discussion
As shown in Fig. 1, a homogeneous CGP-CNT ink was consecutively extruded by a laboratory DIW printer with a thermostatic sleeve (60 °C), and swift set on the glass substrate attributed to the low-thermal self-gelling property (Fig. S1, ESI†). The interpenetrating condensation of hydrogen bonds and helical reconfiguration lead to the interlayer coalescence without involving volatilization of toxic solvents. Ink concentration and printing process were obtained by further experimental optimization (Fig. S2 and Table S1, ESI†). The formed 3D hydrogel was freeze-dried to remove water, and then soaked in PDADMAC aqueous solution (10 wt%) for further electrostatic complexation. Sulfonic acid groups (–SO3−) in CG complexed with positive charges in PDADMAC to form a second cross-linking network. It is worth noting that the swelling and complexation should reach a relative kinetic equilibrium in this process, complexation of opposite charges stabilized the polymer microstructure,46,47 while dilute PADMAC solution may lead to excessive swelling and insufficient complexation of CG-CNT (Fig. S3, ESI†).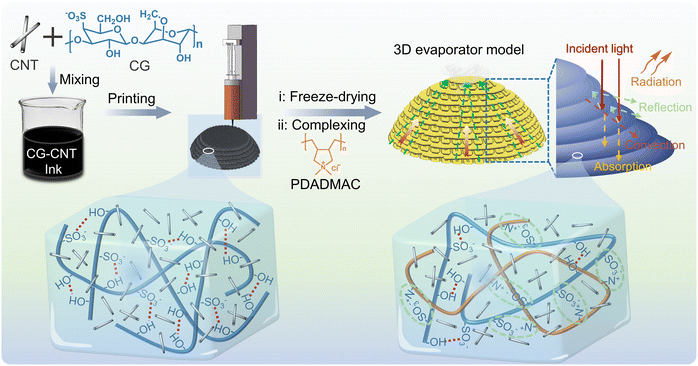 | ||
| Fig. 1 The preparation of the CGP-CNT evaporator by DIW printing and schematic diagram of the CGP-CNT non-covalent supramolecular dual-network. | ||
Multiple hydrogen bonds endowed CG with thermally reversible behaviour,48 CG-CNT ink interpenetrated and condensed layer by layer with the assistance of rich hydrogen bonds and helical reconfiguration regulated by thermo difference, and reversible conversion of “sol–gel” also increased composite printability (Movie S1, ESI†). After freeze-drying, CG-CNT complexed with PDADMAC for further cross-linking, and the printed wiring trace was also retained (Fig. S4, ESI†). Firm electrostatic interaction served as a protect key for the structure of the CGP-CNT evaporator, while hydrogen bonds acted as a sacrifice for energy dissipation when it comes to harsh challenges. Relevant literature studies have reported that the three-dimensional stacking construction and the existence of CNT can effectively increase the scattering and absorption of light.49 The hierarchical structure left on the CGP-CNT surface by DIW was conducive to light absorption.
Continuous ink extrusion was ensured by a thermostatic control device, while gel transition should be fast enough to prevent the ink from depositing on the substrate. Herein, the CG-CNT ink was evaluated by rheological experiments. As shown in Fig. 2a, CG-CNT ink was a gel at low temperature, where storage modulus (G′) was higher than loss modulus (G′′). Then the gel network was gradually destroyed with the increasing temperature, and both G′ and G′′ decreased. When it reached the critical value of about 51 °C, a sudden change occurred (G′ < G′′), while the ink was in a fluid state, which may be conducive to extrusion and shaping. This “sol–gel” transition was a typical rheological behaviour of physical cross-linking networks.50 The continuous DIW forming process was based on the shear thinning behaviour of CGP-CNT in Fig. 2b. Fast sol–gel transition and narrow gel temperature range allow CG-CNT solutions to be used directly as an ideal three-dimensional forming ink for DIW.
The optical photograph in Fig. 3a showed that the freeze-dried CGP-CNT evaporator can readily stand on the surface of a flexible plant without external disturbance. CNTs were added as a light-absorbing composite and exhibited an excellent photothermal conversion performance. The surface SEM morphology in Fig. 3b showed fine dispersion of CNTs, which were tightly embedded in the CGP matrix, and it was illustrated that CNTs could serve as rigid fillers, which help to stabilize pores during the freeze drying process.51 As shown in Fig. 3c, the multichannel structure indicated by arrows in CGP-CNT cross-sectional morphology ensured efficient water transmission. Micro-Computed Tomography (Micro-CT) images in Fig. 3d demonstrated the distribution of pores in the CGP-CNT evaporator, and the presence of a polymer network with a porous structure facilitated the continuous water transfer for evaporation.
Fig. 3e shows that CG revealed a characteristic FT-IR peak at 1065 cm−1 (blue line), which was assigned to –SO3− groups. When compared to CG-CNT, the 1012 cm−1 peak can be clearly observed (orange line), which was owing to the quaternary ammonium groups in PDADMAC. This result indicated the complexation of CG and PDADMAC. Tensile strength curves illustrated that the presence of complexation and nanocomposites effectively enhanced the mechanical strength of CGP-CNT (16.6 MPa), when compared to CG-CNT (7.1 MPa) and CGP (9.5 MPa) (Fig. S5, ESI†). Moreover, elemental analysis in Fig. 3f revealed the content changes of nitrogen (N) and sulphur (S) after complexation. Mole ratio (N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 1.45) in CGP-CNT increased almost tenfold compared to CG-CNT (0.146), further corroborating the complexation of CG and PDADMAC, and mass content of PDADNMAC in CGP-CNT was 26.3 wt%. EDS analysis showed that both nitrogen and sulphur elements were homogeneously distributed in the CGP-CNT evaporator, respectively, which illustrated the uniformity of complexation. The dynamic contact angle pictures in Fig. 3g showed fast water absorption of the CGP-CNT evaporator, which benefited water transfer for solar thermal evaporation. CGP-CNT was stably formed by two supramolecular dual-networks consisting of hydrogen bonds and electrostatic complexation. The rich polyhydroxy and –SO3− groups in CG interactive cross-linked to form a hydrogen bonding network, and –SO3− groups complexed with positive charges in PDADMAC to form a second interpenetrating network.
S = 1.45) in CGP-CNT increased almost tenfold compared to CG-CNT (0.146), further corroborating the complexation of CG and PDADMAC, and mass content of PDADNMAC in CGP-CNT was 26.3 wt%. EDS analysis showed that both nitrogen and sulphur elements were homogeneously distributed in the CGP-CNT evaporator, respectively, which illustrated the uniformity of complexation. The dynamic contact angle pictures in Fig. 3g showed fast water absorption of the CGP-CNT evaporator, which benefited water transfer for solar thermal evaporation. CGP-CNT was stably formed by two supramolecular dual-networks consisting of hydrogen bonds and electrostatic complexation. The rich polyhydroxy and –SO3− groups in CG interactive cross-linked to form a hydrogen bonding network, and –SO3− groups complexed with positive charges in PDADMAC to form a second interpenetrating network.
As a proof-of-concept, the utility of CGP-CNT was evaluated by solar thermal tests (Fig. S6, ESI†). As shown in Fig. 4a, high light absorptivity (98%) of wet CGP-CNT was attributed to the fine dispersion of CNTs compared to wet CGP (45%) and multiple reflections of light on the inner graded pores.52,53 Under 1 sun irradiation, the surface temperature of CGP-CNT (wet) gradually increased to ca. 53 °C (Fig. 4b), which was higher than that of CGP (ca. 43 °C) and bulk water (ca. 38 °C). Infrared images (Fig. S7, ESI†) also confirmed the high-efficiency solar-thermal conversion capability of CGP-CNT. The mass of water decreased linearly with irradiation time, and the evaporation rate of CGP-CNT (2.3 kg m−2 h) in Fig. 4c was higher than that of CGP (1.1 kg m−2 h) and bulk water (0.8 kg m−2 h) under 1 sun irradiation. The hydrophilic groups in CGP-CNT formed hydrogen bonds with water molecules while breaking the hydrogen bonds between free water, thus reducing the energy for evaporation5,6,54,55 (Fig. S8, ESI†).
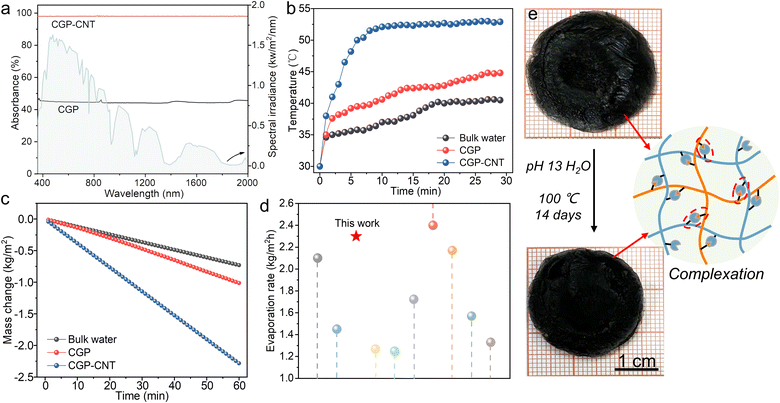 | ||
| Fig. 4 (a) UV-vis-NIR absorption spectra of wet CGP and CGP-CNT. (b) Surface temperature and (c) evaporation rate of bulk water, CGP and CGP-CNT under 1 kW m−2 irradiation. (d) Comparison of the evaporation rate of CGP-CNT with reported 3D photothermal materials by DIW (Table S2, ESI†). (e) Photographs of CGP-CNT after swelling in 100 °C water (pH 13) for 14 days. | ||
The equivalent evaporation enthalpies of water, CGP and CGP-CNT were determined by dark evaporation experiments, which were 2.34, 1.86 and 1.5 kJ g−1, respectively (Fig. S9 and S10, ESI†). Further data on the enthalpy of evaporation of the samples were characterised using a differential scanning calorimeter (DSC), which showed that water evaporation enthalpy of CGP-CNT (1.1 kJ g−1) reduced by 54% compared to that of bulk water (2.4 kJ g−1) (Fig. S11, ESI†). The energy conversion efficiency was calculated by the following equation:
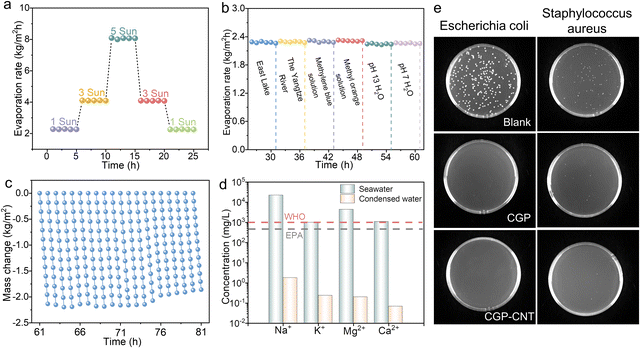
The effect of evaporator height and CNT content on solar evaporation performance has also been explored (Fig. S12 and S13, ESI†). The charge properties of polyelectrolytes and existence of abundant hydrophilic functional groups were instrumental in absorbing water, while layered pores facilitated water transport. The evaporation rate of CGP-CNT was also one of the prominent values among the evaporators prepared by DIW (Fig. 4d and Table S2, ESI†). The CGP-CNT evaporator possessed extraordinarily heat and swelling resistance (Fig. S14, ESI†), and it remained relatively intact after continuous operation in an alkaline water bath (pH 13) at 100 °C for 14 days (the diameter of the sample only reduced 0.5 cm), which was attributed to the stable electrostatic complexation between strong ions (Fig. 4e). This outstanding feature of resistance to harsh water environments is beyond the reach of many hydrogen bonded solar-thermal materials. FTIR confirmed the structural stability of CGP-CNT after swelling in 100 °C water (Fig. S15, ESI†).
Notably, compared to the performance in other reports,19,56,57 CGP-CNT exhibited excellent solar thermal evaporation stability in continuous operation (81 h) under different irradiation and water samples. Firstly, as shown in Fig. 5a, the CGP-CNT evaporator was continuously cycled under 1, 3, and 5 sun irradiation for 25 h, and then exposed to the East Lake, Yangtze River, methylene blue, methyl orange, pH 13 and neutral samples for further evaporation tests under one sun irradiation (36 h), successively (Fig. 5b). Optical pictures and UV absorption curves of different water samples after evaporation illustrated the effective purification (Fig. S16, ESI†). As a proof of concept, solar thermal desalination experiment of the CGP-CNT evaporator was operated with real seawater collected from the Bohai Sea (China) for 20 h cycle testing (Fig. 5c). With the increase of test time, salt particles began to crystallize on the surface at the tenth seawater cycle and were obviously enriched at the top of the evaporator by the thirteenth cycle (Fig. S17, ESI†), which led to a rapid decrease in the solar-thermal evaporation rate, consistent with that reported in the literature.51,58Fig. 5d shows that all four primary ions (i.e. Na+, Mg2+, K+ and Ca2+ in condensate water) were 3–4 orders of magnitude lower than pristine seawater and significantly lower than the drinking water standard defined by the World Health Organization (WHO, i.e. 1000 mg L−1) or the United States Environmental Protection Agency (EPA, i.e. 500 mg L−1). In this work, the desalination stability of the evaporator in seawater was added as an explored system in cyclic experiments. In the swelling experiment, CGP and CGP-CNT both reached swelling equilibrium (∼10 wt%) in real seawater for 11 h, respectively. The mass loss degree of CGP was 10.5 wt%, which was 2.3-fold that of CGP-CNT (4.5 wt%); although rich salt ions in seawater shielded the polyelectrolyte complexation, mass loss of CGP-CNT after swelling was still within an acceptable limit due to the addition of CNT and hydrogen bonds (Fig. S18 and S19, ESI†). What's more, the CGP-CNT evaporator possessed the intrinsic antibacterial property (Fig. 5e), and guaranteed the purification of different water samples. Compared with the blank group, CGP and CGP-CNT have obvious antibacterial activity on Escherichia coli and Staphylococcus aureus, which was attributed to quaternary ammonium ions.59
3. Conclusions
CGP-CNT possessed intrinsic antibacterial, remarkable anti-swelling, and stable solar thermal evaporation performance, and the cost-effective and simple operation based on 3D printing can be applied in additive manufacturing of various functional composites. Layer-by-layer printing processes endowed CGP-CNT with a hierarchical pore structure, while CNTs facilitated solar light absorption and energy conversion. Notably, CGP-CNT has prominent solar thermal evaporation performance under different sun irradiation and water samples for 81 h consecutively (e.g., 2.3 kg m−2 h evaporation rate under one sun irradiation) and excellent stability in a harsh water environment; for instance, it can anti-swell cycle for 14 days in aqueous solution (pH = 13, 100 °C). Overall, assembling non-covalent supramolecular dual-networks to meet the 3D printing process and stable solar thermal evaporation, which can be applied to various polyelectrolytes and nano-composites, expands the scope of functional materials accessible by DIW.4. Experimental
Materials
Poly(diallyldimethylammoniumchloride) (Mw = 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–500
000–500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, 20 wt% aqueous solution) was purchased from Sigma-Aldrich Industrial Co. Ltd (China). k-Carrageenan, methyl orange and methylene blue were purchased from Aladdin Industrial Co. Ltd (China). CNTs were purchased from Shenzhen nanoport Co. Ltd (China). Sodium hydroxide (NaOH) was purchased from Sinopharm Chemical Reagent Co. Ltd (China).
000 g mol−1, 20 wt% aqueous solution) was purchased from Sigma-Aldrich Industrial Co. Ltd (China). k-Carrageenan, methyl orange and methylene blue were purchased from Aladdin Industrial Co. Ltd (China). CNTs were purchased from Shenzhen nanoport Co. Ltd (China). Sodium hydroxide (NaOH) was purchased from Sinopharm Chemical Reagent Co. Ltd (China).
Preparation of the CGP-CNT evaporator
Printing ink: 2.04 g of CG and 0.306 g CNT were dissolved in 100 mL H2O, then stirred in a 60 °C water bath (600 rpm) and sonicated for 1 h to form a homogeneous, black CG-CNT ink.The procedure of DIW:CG-CNT ink was heated to a flowable state in a 60 °C water bath, then transferred to a 5 ml syringe and fixed in the printer (Shangpu Boyuan Biotechnology Co. Ltd China) with a thermal sleeve (60 °C) to ensure continuous extrusion. After extrusion, CGP-CNT ink was solidified layer by layer on a clean glass substrate (room temperature). The computer controlled the printing path, including printing speed, extrusion speed, line distance, layer distance, etc.
CGP-CNT evaporator: The printed CG-CNT hydrogel was freeze-dried for 12 h to remove most water, and then swollen in 10 wt% PDADMAC solution (3 h, appropriate ultrasound could be used to accelerate complexation) for enough complexation (Table S3, ESI†). The cross-linked CGP-CNT was washed 3 times with deionized water to remove excess PDADMAC and salts. The method can apply to different composites and polyelectrolyte complexation systems (Fig. S20, ESI†).
Solar thermal evaporation experiments
A solar light simulator (CEL-S500L) was used to conduct the solar thermal steaming measurement at ca. 35 ± 1 °C room temperature and 35 ± 1% relative humidity. The evaporator was placed on the surface of PS foam covered with hydrophilic cotton, which effectively prevented the heat from transferring to water, and hydrophilic cotton also ensured the transport of water. Seawater for solar thermal tests was collected from the Bohai Sea (China). Surface temperature of samples was monitored using an infrared camera. An electronic balance (JA2003, Soptop) was used for recording water mass changes. The evaporator sample was a three-dimensional cone-shaped porous aerogel, approximately 3 cm in diameter and 1 cm in height, respectively. The evaporation rate R (kg m−2 h) was calculated by the following equation:| R = m/(s·t) |
| S = π·r·1 |
The swelling degree (SD) was calculated using the following equation:
| SD = 100%·(m1 − m0)/m0 |
Characterization
The morphologies of CGP-CNT were observed by electron microscopy (FESEM, Hitachi, SU-8010). Fourier transform infrared spectroscopy (FT-IR) was performed using a BRUKER Vertex 80 FT-IR. The dynamic water contact angle was measured using a contact angle meter (DATAPPHYSICS, OCA20). Element analyses (C, N, S and H) were performed using an element analyzer (PERKIN ELMER, Optima 5300-DV). The absorption spectrum, pore distribution and mechanical property for CGP-CNT were measured using a UV-Vis-NIR spectrophotometer (PerkinElmer, Lambda 750S), Micro-CT (GE, Vtomex) and electronic universal testing machine (Sansi, SHT4206), respectively. The rheological properties were analyzed using a rheometer (TA, DHR-2). Ion contents were measured using an inductively coupled plasma emission spectrometer (ICP-OES, PerkinElmer 8300). pH values were measured using a digital pH meter (SARTORIUS, PB-10).Author contributions
Shifeng Wen, Bin Su and Yan Zhou designed the experimental project. Sihan Tang performed the experiments and analysed the data. Sihan Tang wrote the paper. All the authors discussed and interpreted the results.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (Grant No. U1808216, 52105296, 52105339). S. H., S. F., and B. S. supervised the project. S. H. is responsible for experiments and grateful to J. S. for providing the DIW printer. All authors wrote the work.Notes and references
- L. Wu, Z. Dong, Z. Cai, T. Ganapathy, N. X. Fang, C. Li, C. Yu, Y. Zhang and Y. Song, Nat. Commun., 2020, 11, 521 CrossRef CAS
.
- Y. Shi, O. Ilic, H. A. Atwater and J. R. Greer, Nat. Commun., 2021, 12, 2797 CrossRef CAS
.
- J. Li, X. Wang, Z. Lin, N. Xu, X. Li, J. Liang, W. Zhao, R. Lin, B. Zhu, G. Liu, L. Zhou, S. Zhu and J. Zhu, Joule, 2020, 4, 928–937 CrossRef CAS
.
- Y. Guo, X. Zhao, F. Zhao, Z. Jiao, X. Zhou and G. Yu, Energy Environ. Sci., 2020, 13, 2087–2095 RSC
.
- F. Zhao, X. Zhou, Y. Shi, X. Qian, M. Alexander, X. Zhao, S. Mendez, R. Yang, L. Qu and G. Yu, Nat. Nanotechnol., 2018, 13, 489–495 CrossRef CAS
.
- F. Zhao, Y. Guo, X. Zhou, W. Shi and G. Yu, Nat. Rev. Mater., 2020, 5, 388–401 CrossRef
.
- Y. Xia, Q. Hou, H. Jubaer, Y. Li, Y. Kang, S. Yuan, H. Liu, M. W. Woo, L. Zhang, L. Gao, H. Wang and X. Zhang, Energy Environ. Sci., 2019, 12, 1840–1847 RSC
.
- F. Zhao, X. Zhou, Y. Guo, B. Rosenberger and G. Yu, Sci. Adv., 2019, 5, eaaw5484 CrossRef PubMed
.
- Y. Shi, R. Li, Y. Jin, S. Zhuo, L. Shi, J. Chang, S. Hong, K.-C. Ng and P. Wang, Joule, 2018, 2, 1171–1186 CrossRef CAS
.
- X. Zhang, Q. Wang, R. Zou, B. Song, C. Yan, Y. Shi and B. Su, Engineering, 2022, 15, 196–205 CrossRef
.
- Z. Ma, Q. Wang, Z. Wu, D. Chen, C. Yan, Y. Shi, M. D. Dickey and B. Su, Adv. Mater., 2022, 34, e2203814 CrossRef
.
- Z. Wu, H. Sun, Z. Xu, H. Chi, X. Li, S. Wang, T. Zhang and Y. Zhao, ACS Appl. Nano Mater., 2021, 4, 8979–8989 CrossRef CAS
.
- H. Chi, Z. Xu, Z. Wei, T. Zhang, H. Wang, T. Lin and Y. Zhao, ACS Appl. Mater. Interfaces, 2021, 13, 29150–29157 CrossRef CAS PubMed
.
- P. Wu, X. Wu, H. Xu and G. Owens, EcoMat, 2021, 3, e12140 CAS
.
- Y. Wang, X. Wu, P. Wu, J. Zhao, X. Yang, G. Owens and H. Xu, Sci. Bull., 2021, 66, 2479–2488 CrossRef CAS
.
- Y. Wang, X. Wu, P. Wu, H. Yu, J. Zhao, X. Yang, Q. Li, Z. Zhang, D. Zhang, G. Owens and H. Xu, J. Mater.
Chem. A, 2022, 10, 14470–14478 RSC
.
- Y. Wang, X. Wu, T. Gao, Y. Lu, X. Yang, G. Y. Chen, G. Owens and H. Xu, Nano Energy, 2021, 79, 105477 CrossRef CAS
.
- Z. Wang, W. Tu, Y. Zhao, H. Wang, H. Huang, Y. Liu, M. Shao, B. Yao and Z. Kang, J. Mater. Chem. A, 2020, 8, 14566–14573 RSC
.
- S. Zhou, S. Huang, Y. Ming, Y. Long, H. Liang, S. Ruan, Y.-J. Zeng and H. Cui, J. Mater. Chem. A, 2021, 9, 9909–9917 RSC
.
- M. Zou, Y. Zhang, Z. Cai, C. Li, Z. Sun, C. Yu, Z. Dong, L. Wu and Y. Song, Adv. Mater., 2021, 33, e2102443 CrossRef
.
- T. Gao, X. Wu, Y. Wang, G. Owens and H. Xu, Sol. RRL, 2021, 5, 2100053 CrossRef CAS
.
- Y. Lu, D. Fan, Z. Shen, H. Zhang, H. Xu and X. Yang, Nano Energy, 2022, 95, 107016 CrossRef CAS
.
- X. Liu, F. Chen, Y. Li, H. Jiang, D. D. Mishra, F. Yu, Z. Chen, C. Hu, Y. Chen, L. Qu and W. Zheng, Adv. Mater., 2022, e2203137 CrossRef
.
- Y. Yang, W. Fan, S. Yuan, J. Tian, G. Chao and T. Liu, J. Mater. Chem. A, 2021, 9, 23968–23976 RSC
.
- J. Yuan, X. Lei, C. Yi, H. Jiang, F. Liu and G. J. Cheng, Chem. Eng. J., 2022, 430, 132765 CrossRef CAS
.
- X. Li, H. Wang, D. Li, S. Long, G. Zhang and Z. Wu, ACS Appl. Mater. Interfaces, 2018, 10, 31198–31207 CrossRef CAS PubMed
.
- F. Zhu, L. Cheng, J. Yin, Z. L. Wu, J. Qian, J. Fu and Q. Zheng, ACS Appl. Mater. Interfaces, 2016, 8, 31304–31310 CrossRef CAS
.
- H. Li, C. Tan and L. Li, Mater. Des., 2018, 159, 20–38 CrossRef CAS
.
- H. Yang, C. Li, M. Yang, Y. Pan, Q. Yin, J. Tang, H. J. Qi and Z. Suo, Adv. Funct. Mater., 2019, 1901721 CrossRef
.
- Z. Chen, D. Zhao, B. Liu, G. Nian, X. Li, J. Yin, S. Qu and W. Yang, Adv. Funct. Mater., 2019, 29, 1900971 CrossRef
.
- D. Chimene, R. Kaunas and A. K. Gaharwar, Adv. Mater., 2020, 32, e1902026 CrossRef
.
- Y. Zhou, M. Layani, S. Wang, P. Hu, Y. Ke, S. Magdassi and Y. Long, Adv. Funct. Mater., 2018, 28, 1705365 CrossRef
.
- F. Luo, T. L. Sun, T. Nakajima, T. Kurokawa, Y. Zhao, K. Sato, A. B. Ihsan, X. Li, H. Guo and J. P. Gong, Adv. Mater., 2015, 27, 2722–2727 CrossRef CAS PubMed
.
- P. Schaaf and J. B. Schlenoff, Adv. Mater., 2015, 27, 2420–2432 CrossRef CAS
.
- H. C. Yu, S. Y. Zheng, L. Fang, Z. Ying, M. Du, J. Wang, K. F. Ren, Z. L. Wu and Q. Zheng, Adv. Mater., 2020, 32, e2005171 CrossRef PubMed
.
- M. J. Yin, Q. Zhao, J. Wu, K. Seefeldt and J. Yuan, ACS Nano, 2018, 12, 12551–12557 CrossRef CAS
.
- Z. Bao, C. Xian, Q. Yuan, G. Liu and J. Wu, Adv. Healthcare Mater., 2019, 8, e1900670 CrossRef PubMed
.
- M. A. Darabi, A. Khosrozadeh, R. Mbeleck, Y. Liu, Q. Chang, J. Jiang, J. Cai, Q. Wang, G. Luo and M. Xing, Adv. Mater., 2017, 29, 1700533 CrossRef PubMed
.
- F. Gao, Z. Xu, Q. Liang, B. Liu, H. Li, Y. Wu, Y. Zhang, Z. Lin, M. Wu, C. Ruan and W. Liu, Adv. Funct. Mater., 2018, 28, 1706644 CrossRef
.
- D. L. Taylor and M. In Het Panhuis, Adv. Mater., 2016, 28, 9060–9093 CrossRef CAS
.
- S. Van Belleghem, L. Torres, Jr., M. Santoro, B. Mahadik, A. Wolfand, P. Kofinas and J. P. Fisher, Adv. Funct. Mater., 2020, 30, 1907145 CrossRef CAS PubMed
.
- Y. Wang, Q. Chang, R. Zhan, K. Xu, Y. Wang, X. Zhang, B. Li, G. Luo, M. Xing and W. Zhong, J. Mater. Chem. A, 2019, 7, 24814–24829 RSC
.
- S. Liu and L. Li, ACS Appl. Mater. Interfaces, 2017, 9, 26429–26437 CrossRef CAS
.
- D. J. Adams, J. Am. Chem. Soc., 2022, 144, 11047–11053 CrossRef CAS
.
- Y. Ni, X. Zhou, J. Gong, L. Xue and Q. Zhao, Adv. Funct. Mater., 2021, 31, 2103818 CrossRef CAS
.
- C. F. J. Faul and M. Antonietti, Adv. Mater., 2003, 15, 673–683 CrossRef CAS
.
- L. Li, A. M. Rumyantsev, S. Srivastava, S. Meng, J. J. de Pablo and M. V. Tirrell, Macromolecules, 2020, 54, 105–114 CrossRef
.
- L. Li, B. Yan, J. Yang, L. Chen and H. Zeng, Adv. Mater., 2015, 27, 1294–1299 CrossRef CAS
.
- Y. Qiao, S. Xu, T. Zhu, N. Tang, X. Bai and C. Zheng, Polymer, 2020, 186, 121994 CrossRef CAS
.
- S. Hietala, P. Mononen, S. Strandman, P. Järvi, M. Torkkeli, K. Jankova, S. Hvilsted and H. Tenhu, Polymer, 2007, 48, 4087–4096 CrossRef CAS
.
- F. Guo, Y. Jiang, Z. Xu, Y. Xiao, B. Fang, Y. Liu, W. Gao, P. Zhao, H. Wang and C. Gao, Nat. Commun., 2018, 9, 881 CrossRef PubMed
.
- Y. Li, X. Cui, M. Zhao, Y. Xu, L. Chen, Z. Cao, S. Yang and Y. Wang, J. Mater. Chem. A, 2019, 7, 704–710 RSC
.
- D.-D. Qin, Y.-J. Zhu, F.-F. Chen, R.-L. Yang and Z.-C. Xiong, Carbon, 2019, 150, 233–243 CrossRef CAS
.
- N. Liu, L. Hao, B. Zhang, R. Niu, J. Gong and T. Tang, Energy Environ. Mater., 2021, 5, 1–10 Search PubMed
.
- H. Bai, N. Liu, L. Hao, P. He, C. Ma, R. Niu, J. Gong and T. Tang, Energy Environ. Mater., 2021, 0, 1–10 CAS
.
- Z. Wang, H. Liu, F. Chen and Q. Zhang, J. Mater. Chem. A, 2020, 8, 19387–19395 RSC
.
- X. Ming, A. Guo, Q. Zhang, Z. Guo, F. Yu, B. Hou, Y. Wang, K. P. Homewood and X. Wang, Carbon, 2020, 167, 285–295 CrossRef CAS
.
- N. Cao, S. Lu, R. Yao, C. Liu, Q. Xiong, W. Qin and X. Wu, Chem. Eng. J., 2020, 397, 125522 CrossRef CAS
.
- A. Jain, L. S. Duvvuri, S. Farah, N. Beyth, A. J. Domb and W. Khan, Adv. Healthcare Mater., 2014, 3, 1969–1985 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00927g |
| This journal is © The Royal Society of Chemistry 2023 |