Open Access Article
Open Access ArticlePhotochemical aerobic upcycling of polystyrene plastics to commodity chemicals using anthraquinone as the photocatalyst†
Nikolaos F.
Nikitas
,
Elpida
Skolia
,
Petros L.
Gkizis
,
Ierasia
Triandafillidi
and
Christoforos G.
Kokotos
 *
*
Laboratory of Organic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis 15771, Athens, Greece. E-mail: ckokotos@chem.uoa.gr
First published on 26th May 2023
Abstract
Since the United Nations set goals dealing with climate change, the chemical industry has focused on recycling the already-used polymers, targeting the reinsertion of plastic waste to market via new products through reforming. Upcycling of polystyrene plastic waste is becoming one of the hottest fields of research in plastic upconversion. Herein, we introduce a novel, green, organocatalytic and photochemical aerobic upcycling process of polystyrene to benzoic acid, utilizing anthraquinone as the photocatalyst, LED 390 nm as the irradiation source and air as the sole oxidant. The developed protocol was applied successfully to the upcycling of daily-life used polystyrene products, leading to yields varying from 25% to 58%. Moreover, the obtained upcycled product from the polystyrene materials was employed to the successful synthesis of bioactive molecules, such as acetylsalicylic acid.
Introduction
During the last century, the quality of every-day life of humanity has improved exponentially and chemistry has played a vital role in this upgrading process via its key developments in the fields of pharmaceuticals, agrochemicals, plastics, every-day commodities, etc. A pivotal innovation in this process was the introduction of polymers, and plastics in particular, in basic every-day activities. Nowadays, the global economy is based on polymers, since they present numerous uses, such as in health-care products, packaging, electronics, transportation, constructions, etc. Plastics, due to their low cost, light weight, diverse properties and easy manufacturing procedures, have the lion's share in polymer's global market and constitute most frequently the material of choice for the production of daily-life products.1 Unfortunately, the exponential burst in massive production of plastics, along with their usual single-use, short-time of use after purchase (for example, food packaging) and slow decomposition,2 have contributed significantly to the planet's pollution and climate change.3–5 Since the 1950s, when the annual production of plastics was 1.5 million metric tonnes, a sharp and constant increase in the production of plastics has been observed and in 2021, the production of plastics was 380 million tonnes,6 while being projected to rise to 900 million tonnes by 2050.6 This sharp increase in production has constituted the vast accumulation of plastic waste as a matter of great concern, which has already created environmental implications, economic problems and waste management crises. In order to address these issues, the recycling of plastic waste [close-loop, mechanical, chemical and energy recovery],7 became the key weapon in scientist's armor to tackle these problems.7–9 Unfortunately, only 16% of polymer waste is recycled, while around 40% of it is ending up in waste landfills,8 producing in 2015, 4.9 billion tonnes of plastic waste,6 which are projected to rise to 12 billion tonnes of plastic waste by 2030,6 creating a myriad of problems in the environment and the planet.10–12 There are different ways to perform the recycling of plastics.13–17 However, it has not provided the desired results, since in most cases, recycling of plastic waste leads to downgraded materials, which inevitably end up as plastic waste as well in waste landfills, not providing a solution to the problem. In an effort to overcome this issue, the substitution of plastic commodities by paper or other-type products was envisaged. However, again, the final recycled product is a downgraded material, while in some cases, the plastic substitute does not exhibit the same properties as the original plastics. In another attempt to solve these problems, the last few years, the idea of plastic upcycling, the recycling of plastics to high-added value chemicals, has become very popular,6,18–20 ensuing that the basic principles of circular economy are followed.19Among different plastics, polystyrene (PS) is one of the most frequently-used polymers in every-day life products and along with polyethylene (PE) and polypropylene (PP) are accounting for more than 50% (around 60%) of the world plastic production.11 Polystyrene polymers are widely used in cutlery, food containers and drinking cups, having a short life cycle and their demand increases significantly year by year. Thus, there is an elevated need for more efficient and sustainable recycling methods, while humanity urgently requires to focus on routes of re-entering these types of waste, back to the chemical industry as sources of raw materials. In order to face these challenges, the chemical community has created opportunities for polystyrene recycling, via pyrolysis, which constitutes the most frequent approach used to date.21–24 In these processes, polystyrene can be transformed to arene derivatives via catalysed processes. Along these lines, in 2021, Wang, Yan and coworkers reported a catalytic protocol for the reductive upcycling of aromatic polymers, such polystyrene (PS), polyethylene terephthalate (PET) and polycarbonate (PC) over a Ru/Nb2O5 catalyst (Scheme 1A).24 The process was effective for the formation of reduced aromatic species, which were obtained as a mixture, with indane being the major product. Despite the efficiency of the method, the need of high temperature (200 °C) and the high hydrogen pressure is a concern for the adaptability of the process in the chemical industry. Alternatively, microwave chemistry was employed to promote the recycling of polystyrene and other plastic materials,25 while in an alternative approach for upcycling of polystyrenes, Leibfarth and coworkers reported the modification of plastics via a C–H fluoroalkylation process.26 Photochemistry, the use of light to promote organic transformations, is not a new concept, since Ciamician proved that sunlight can promote organic reactions.27 Since then, a century passed, in order the field to receive massive attention and a literature explosion on photochemical-promoted reactions occurred.28,29 In particular, the area of photochemistry dealing with radical species to perform Hydrogen Atom Transfer (HAT) processes, creates the opportunity to cleave strong C–H bonds.30 The use of light in polystyrene upcycling has been known since 1984, when Mita, Horie and coworkers reported the use of a benzophenone-catalysed process using a high-pressure mercury lamp at 60 °C, studying mainly the mechanism of action of the process regarding benzophenone and reporting that products of lower molecular weight were obtained.31,32 In 2021, two different research groups, on their way to study the Fe-catalysed photochemical aerobic oxidation of alkyl aromatics, they reported that the catalytic system FeCl3-TBACl (tetrabutyl ammonium chloride)33 or FeCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 can promote the degradation of polystyrene into benzoic acid. In both cases, an oxygen atmosphere was necessary to promote the photochemical degradation, while an irradiation reaction time of 3–5 days was necessary. Benzoic acid is an important commodity chemical.35 In 2021, the global market of benzoic acid surpassed 1 billion USD, while there is a projection that will reach 2 billion USD by 2028. It is a valuable feedstock that can be used as a food additive in almost all kind of food products.35 Benzoic acid is the starting raw material for the synthesis of various bioactive compounds, since the benzene ring is abundant in nature. In 2022, Oh and Stache built on earlier contributions and utilised FeCl3 as the photocatalyst for the upcycling of polystyrenes (Scheme 1B).36 This elegant contribution ensured the formation of chlorine radicals, which are known to be capable of performing HAT of strong C–H bonds (ca. 103 kcal mol−1),30 and via this indirect HAT process, the authors were able to upcycle polystyrene into benzoic acid.36 The process enabled the use of 10 wt% FeCl3 as the photocatalyst under white LED irradiation for 20 h, using an oxygen atmosphere. Xiao and coworkers proposed the use of p-toluenesulfonic acid as the photochemical promoter to perform the oxidative upcycling of polystyrene, under 405 nm light irradiation and an oxygen atmosphere (Scheme 1C).37 The authors supported that a [PS⋯H+] adduct is the species that gets excited and further decomposes to furnish the final arene derivatives. Similar yields (36–51%) of benzoic acid were observed, when every-day polystyrene plastics were employed, while a large-scale synthesis (18.7 g) was demonstrated.37 Also in 2022, Das and coworkers reported another photocatalytic system that employs an indirect-HAT protocol to afford the products of the aerobic upcycling of PS (Scheme 1D).38 The authors proposed the use of N-bromosuccinimide (NBS) (15 mol%), along with sodium triflinate (CF3SO2Na) (50 mol%) (Scheme 1D). The catalytic system, upon irradiation at 390 nm, can form various radicals, which are able to perform a HAT with PS, upon an oxygen atmosphere. The need though for the presence of both radical precursors is crucial for the fate of the reaction. A recent example by Reisner and coworkers introduced the use of fluorenone (20 mol%) as the photocatalyst, in order to achieve the upcycling of polystyrene to benzoic acid (Scheme 1E).39 The protocol employs an oxygen atmosphere as the source of the oxidant and one equivalent of sulfuric acid is essential as the acidic additive and irradiation at blue LEDs region (450 nm), leading to the oxidation of polystyrene in good yields.
34 can promote the degradation of polystyrene into benzoic acid. In both cases, an oxygen atmosphere was necessary to promote the photochemical degradation, while an irradiation reaction time of 3–5 days was necessary. Benzoic acid is an important commodity chemical.35 In 2021, the global market of benzoic acid surpassed 1 billion USD, while there is a projection that will reach 2 billion USD by 2028. It is a valuable feedstock that can be used as a food additive in almost all kind of food products.35 Benzoic acid is the starting raw material for the synthesis of various bioactive compounds, since the benzene ring is abundant in nature. In 2022, Oh and Stache built on earlier contributions and utilised FeCl3 as the photocatalyst for the upcycling of polystyrenes (Scheme 1B).36 This elegant contribution ensured the formation of chlorine radicals, which are known to be capable of performing HAT of strong C–H bonds (ca. 103 kcal mol−1),30 and via this indirect HAT process, the authors were able to upcycle polystyrene into benzoic acid.36 The process enabled the use of 10 wt% FeCl3 as the photocatalyst under white LED irradiation for 20 h, using an oxygen atmosphere. Xiao and coworkers proposed the use of p-toluenesulfonic acid as the photochemical promoter to perform the oxidative upcycling of polystyrene, under 405 nm light irradiation and an oxygen atmosphere (Scheme 1C).37 The authors supported that a [PS⋯H+] adduct is the species that gets excited and further decomposes to furnish the final arene derivatives. Similar yields (36–51%) of benzoic acid were observed, when every-day polystyrene plastics were employed, while a large-scale synthesis (18.7 g) was demonstrated.37 Also in 2022, Das and coworkers reported another photocatalytic system that employs an indirect-HAT protocol to afford the products of the aerobic upcycling of PS (Scheme 1D).38 The authors proposed the use of N-bromosuccinimide (NBS) (15 mol%), along with sodium triflinate (CF3SO2Na) (50 mol%) (Scheme 1D). The catalytic system, upon irradiation at 390 nm, can form various radicals, which are able to perform a HAT with PS, upon an oxygen atmosphere. The need though for the presence of both radical precursors is crucial for the fate of the reaction. A recent example by Reisner and coworkers introduced the use of fluorenone (20 mol%) as the photocatalyst, in order to achieve the upcycling of polystyrene to benzoic acid (Scheme 1E).39 The protocol employs an oxygen atmosphere as the source of the oxidant and one equivalent of sulfuric acid is essential as the acidic additive and irradiation at blue LEDs region (450 nm), leading to the oxidation of polystyrene in good yields.
Our group has a long experience in photochemical processes and during the last years, we have demonstrated the power of small organic molecules as promoters of photochemical processes.40 Following our interest in merging photochemistry with aerobic processes,40c,f we questioned whether a commercially available small organic molecule can be employed as a potential promoter for the photochemical aerobic upcycling of polystyrenes (Scheme 1F). Herein, we present a mild protocol that is free of metal additives, strong acidic additives or toxic intermediates such as bromine, for the photochemical aerobic upconversion of polystyrene to benzoic acid in good yield. Utilizing as low as 5 mol% of anthraquinone as the catalyst, LED 390 nm as the irradiation source and air as the oxidant, a variety of every-day plastic products were converted into benzoic acid. Furthermore, we studied the potential of post-upcycling modification of benzoic acid to every-day commodities, like salicylic acid and acetylsalicylic acid.
Results and discussion
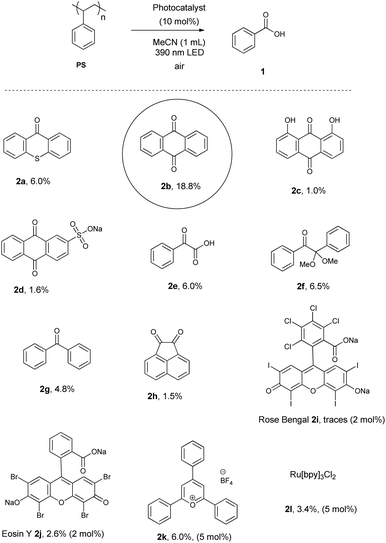
We initiated our studies using a commercially available polystyrene as the model substrate and screened a variety of photocatalysts–photoinitiators in a search for the ideal molecule that can promote the photochemical aerobic cleavage of the benzylic moieties of the polystyrene chain (Scheme 2). The efficiency of the protocol was followed by monitoring the formation of the benzoic acid, even though it is not the sole oxidation product but it is stable, not volatile and it can be easily extracted from the crude reaction mixture. We employed acetonitrile as the solvent and air as the oxidant, using a LED 390 nm irradiation source. At the beginning, we employed aromatic ketones that are known to perform HAT reactions, like thioxanthone (2a)41 or benzophenone (2g), which was presented in literature to work (under mercury lamp irradiation though)31 or acenaphthoquinone (2h), but in all cases, the yield of benzoic acid was 1.9–6.0% (Scheme 2). Aryl ketones that perform as photoinitiators, such as phenylglyoxylic acid (2e)42 or 2,2-dimethoxy-2-phenylacetophenone (2f)43 led to a slight increase in yield (Scheme 2). Organic dyes that are known as singlet oxygen generators or can perform HAT processes were also tested,44 however similar low yields were obtained (Scheme 2, 2i or 2j). The family of anthraquinones was tested as well, and anthraquinone (2b) proved to be the optimum photocatalyst, leading to 18.8% yield of isolated benzoic acid (1) (Scheme 2). Other derivatives of anthraquinone did not perform as well as 2b. Other typical photocatalysts, like 2k and 2l were tested for comparison purposes. In all cases, the desired benzoic acid was isolated after acid–base wash and extractions, without the need for further purification.After identifying the optimum photocatalyst, we studied the importance of the irradiation source (Table 1). First, household lamps were used, but proved ineffective (Table 1, entry 1). Similarly, no other LED irradiation led to similar high yield of benzoic acid as the 390 nm (Table 1, entry 3 vs. entries 2 and 4–7). Following the discovery of the optimum irradiation source, we probed the optimum solvent for the photochemical aerobic upcycling of polystyrene (Table 2). We observed that when we doubled the amount of the solvent, the yield of benzoic acid increased (Table 2, entry 2 vs. entry 1). Among the tested solvents, acetonitrile (Table 2, entry 2) and benzene (Table 2, entry 7) led to the best results. We decided to use acetonitrile instead of benzene in our studies, since acetonitrile is a less toxic solvent compared to benzene. The optimum reaction conditions were found after a series of experiments and employ 5 mol% of 2b, LED 390 nm irradiation for 48 h under open air, leading to 28.2% yield of benzoic acid (1) (Table 2, entry 10). Since the addition of CH2Cl2 as the co-solvent facilitates the solubility of the aggregates that are formed during the addition of extra amount of PS, a similar yield was obtained (Table 2, entry 11), which proved to be the best conditions for real-life polystyrene plastics (the use of MeCN alone proved sluggish).
| Entry | Solvent | Yieldb (%) |
|---|---|---|
| a In an open Schlenk flask, PS (100 mg, 0.96 mmol), 2b (20 mg, 0.096 mmol) and solvent (2 mL) were added and irradiated under 390 nm LED for 20 h. b Yield of isolated benzoic acid. c Reaction performed with 1 mL of MeCN. d The reaction was performed with 2b (10 mg, 0.048 mmol, 5 mol%) for 48 h. | ||
| 1c | MeCN | 18.8 |
| 2 | MeCN | 21.3 |
| 3 | CH2Cl2 | 6.0 |
| 4 | CHCl3 | Traces |
| 5 | AcOEt | Traces |
| 6 | Acetone | 4.4 |
| 7 | Benzene | 21.3 |
| 8 | DMF | n.d. |
| 9 | DMSO | n.d. |
| 10 | MeCN | 28.2 |
| 11 |
MeCN/CH
2
Cl
2
(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
28.1 |
After finding the optimum reaction conditions for the photochemical aerobic upcycling of polystyrenes, we decided to probe its substrate scope. We initially tested the use of other commercially available resins that are based on polystyrene (Scheme 3). Aminomethylpolystyrene resin, as well as the commonly employed Wang resin, were used successfully, leading to a similar yield of benzoic acid as our model substrate. However, the use of commercially available chlorotrityl chloride resin did not perform equally well and led to a diminished yield of 13.1%.
 | ||
| Scheme 3 Photochemical aerobic upcycling of commercially available resins, used in organic synthesis and are based on polystyrene. | ||
Since the use of commercially available resins based on polystyrene was amenable in our photochemical aerobic upcycling process, we then decided to employ every-day products that are polystyrene-based (Scheme 4). Interestingly, the commercially available plastic commodities proved to work better than our model polymer substrate and could be perfectly incorporated to our protocol, furnishing the desired oxidation product, benzoic acid, after an aerobic cleavage promoted by light (Scheme 4). The yields refer to isolated benzoic acid after the reaction took place, using acid–base wash and extractions, without taking into consideration the formation of other volatile oxidation products. The extraction of benzoic acid was performed with CH2Cl2 as the solvent, while ethyl acetate was also tested as the extraction solvent, but the final yield was low (3% yield). Polystyrene-based plastic knife worked well, leading to 31.5% yield of benzoic acid, while the corresponding plastic spoon led to an excellent 51.4% yield. In the market, a variety of different polystyrene-based plastic cups exist. We started by employing a small transparent plastic cup and a high yield of 50.6% was obtained. Similarly, using a big transparent plastic cup led to a further increase in the yield and benzoic acid was isolated in 58.8%. However, the use of colored plastic cups (yellow or red) led to similar or higher yields than our model polymer substrate (38.8% and 26.8%, respectively), but lower than the transparent plastic cups. The various colors of the commercial plastic materials were not present in the final isolated benzoic acid, probably due to their existence in low amount, but its presence or their potential photodecomposition under the irradiation could account for the lower yields that were obtained. The use of polystyrene-based plastic coffee cup led to 29.6% yield, while the use of a microwavable food container afforded benzoic acid in 31.1% yield. Two different kinds of polystyrene foams that are employed in packaging industries led to 43.5% and 49.5% yields, respectively. The use of a polystyrene CD case led to a decreased yield of 24.9%. Finally, the use of polystyrene beads or commercially available polystyrene polymer (MW 10.000) led to 41.8% and 49.2%, yield respectively. Benzoic acid is not the sole product of oxidation and in some cases, there are also lower molecular weight oxidized polystyrene products.
In order to probe the industrial application of the process, a large-scale experiment was performed (Scheme 5). We utilized a 2 g scale and the reaction time had to be prolonged to 96 h, along with the use of 2 × 35 W LED 390 nm irradiation source. A similar yield was obtained as in the smaller scale for benzoic acid. Interestingly, herein, we were also able to monitor the reaction by 1H-NMR and probe the presence of formic acid (3) and benzaldehyde (4). In the former case, a significant amount of 3 was observed, in line with literature observations.37 Furthermore, from the initial organic layer, after quenching, we were able to isolate via column chromatography a small amount of acetophenone (5).
In an effort to further expand the applicability of this protocol, we envisaged the application of the delivered upcycled product into pharmaceuticals, like salicylic acid 6 and acetylsalicylic acid 7 (Scheme 6). Salicylic acid 6 constitutes a plant hormone46 and it has a long story as a bioactive compound, which dates back in ancient Greece.47 Even though it demonstrates a potent activity against cardiovascular diseases, its modern application is not recommended due to side-effects47 although, it is employed against acne.48 The side effects of 6 were surpassed by a simple acetylation, forming the corresponding acetylsalicylic acid, a compound which later became extremely popular under the name aspirin. Since then, it is being used to treat, apart from cardiovascular issues, pain, fever, inflammation and others and constitutes one of the most consumable drugs around the globe as its consumption rises to 40 hundred tons per year.49 Using a modified literature procedure for the C–H hydroxylation of benzoic acids,50 we were able to convert benzoic acid to salicylic acid in a single step and in 60% yield (Scheme 6). Then, a simple acetylation with acetic anhydride led to 7 (Scheme 6).
We then turned our attention in elucidating the reaction mechanism of the photochemical aerobic upcycling of polystyrene plastics. As in earlier studies,36–38 when the reaction was performed either under argon, or under dark or without catalyst, the reaction did not proceed. Furthermore, the radical nature of the process was probed with the presence of BHT or TEMPO. Anthraquinone (2b) is a well-known photocatalyst, used in literature for the production of peroxide, a process known as the anthraquinone process.51 We performed a control experiment, where polystyrene was treated with hydrogen peroxide, but no photodegradation occurred, stating that hydrogen peroxide, generated in the process, is not responsible for the upcycling process. Also, anthraquinone is known to participate in HAT processes,30 while it can also generate singlet oxygen.40f Fluorescent quenching studies were then performed (Fig. 1). After irradiation of anthraquinone (2b) (1 mM in MeCN) at 358 nm, its fluorescence was measured at 404 nm. Increasing the added amount of polystyrene, a decrease in the fluorescence was observed. The corresponding Stern–Volmer plot is also presented in Fig. 1. Thus, there is a clear interaction between excited anthraquinone and polystyrene, which indicates that the direct HAT from the excited catalyst to the polystyrene is occurring. The low slope in the Stern–Volmer plot can be explained by the small quantum yield of the reaction (Φ = 0.08).45 This is in agreement with the mechanisms that are proposed in the literature.37,39
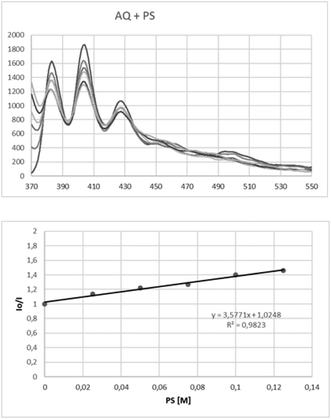 | ||
| Fig. 1 Fluorescence quenching studies of anthraquinone (2b) by polystyrene and the corresponding Stern–Volmer plot. | ||
In order to understand better the mechanism of the reaction, studies of the oxidation of the model substrate 1,3-diphenylbutane (8) were performed (Scheme 7). The model substrate behaves as a styrene dimer. In literature, it is supported through DFT calculations that the most substituted benzylic position of model compound 8 is more reactive in HAT processes.37 Apart from the formation of benzoic acid (1) and acetophenone (5), in our case though, the formation of oxidation product 9 was also detected (Scheme 7A). This product is not reported in any previous literature examples. It is formed, probably through a direct HAT from the excited form of anthraquinone (AQ), followed by oxygen incorporation and oxidation.45 The amount of this oxidation product is lower than that of benzoic acid (1) or acetophenone (5), probably due to further oxidation processes that can take place and transform 9 to 1 and 5.45 Furthermore, Direct Infusion High Resolution Mass Spectrometry (DI-HRMS) studies52 were performed with model substrate 8 to probe the intermediates generated in this protocol (Scheme 7B).45 Initially, we monitored the photochemical aerobic upcycling reaction using DI-HRMS. Compound 9, as well as intermediates that bear the hydroxy (10) or peroxy (11) moiety were detected (Scheme 7B).45 In addition, peaks that could be attributed to acetophenone (5), benzaldehyde (4), benzoic acid (1), cumene, ethyl benzene, as well as hydroxylated cymene were observed. These compounds verify that oxygen can be inserted in multiple positions and β-scission and oxidation is occurring. Furthermore, we studied by DI-HRMS, the photochemical aerobic upcycling process in the presence of TEMPO.45 We observed peaks that could be attributed to hydroxy and peroxy radicals (trapped with TEMPO), as well as peaks that correspond to alkyl radicals (trapped with TEMPO).45
In addition, when the reaction was performed in the presence of singlet oxygen quenchers, such as sodium azide or DABCO, the reaction was completely inhibited, stating that singlet oxygen is involved, which is in accordance with literature.37 Bringing all these data together, a plausible reaction mechanism is proposed in Scheme 8. The various radical intermediates are known in literature and supported by EPR trapping experiments and DFT calculation.37 After irradiation, triplet state anthraquinone I is capable of performing a hydrogen atom abstraction, forming anthraquinone ketyl radical II and alkyl benzyl radical III (Scheme 8A). II can perform another HAT via reaction with O2 that leads to anthraquinone regeneration and H2O2 formation or perform additional HAT events with either PS or IV38,39 or IX.39III reacts with ground state oxygen, forming peroxy radical intermediate IV. Upon further irradiation, the unstable intermediate decomposes to intermediate V and then through a β-scission into two polymeric chains VI and VII. This is in accordance with the literature.36–38 Polymer chain VI reacts with a second excited molecule of anthraquinone, entering in a new cycle of oxidation, leading finally to benzoic acid (1). Intermediate VII upon oxygen incorporation and rearrangement furnishes benzaldehyde (4), which can be further oxidized to benzoic acid (1). Since our control experiments showed that singlet oxygen could also play a role in the process, a second pathway is also proposed (Scheme 8B). The triplet state anthraquinone is capable of generating singlet oxygen,40f leading to the mechanism proposed in Scheme 8B. Singlet oxygen is capable of performing a direct insertion at the activated benzylic position or react similarly via a HAT to the benzylic C–H bond of PS,37 leading to IX. The decomposition of these high energy peroxy intermediates can lead to reactive oxygen species (ROS), including V, hydroxyl radical and superoxide radical anion, which are highly oxidative and potent hydrogen atom abstractors. Then, a similar β-scission can occur leading to VI and VII, following then the same pathway as in Scheme 8A.
Conclusions
In conclusion, a green, mild, metal-free photochemical aerobic upcycling process of polystyrene was developed using anthraquinone (2b) (5 mol%) as the photocatalyst and LED 390 nm as the irradiation source. In contrast to recent developed methodologies, this protocol does not require the use of an oxygen atmosphere and the process is occurring under air. There is no need for acidic additives, just the addition of the catalyst (lower catalyst loading than in literature) under 390 nm irradiation. An easy-to-work procedure leads to the isolation of benzoic acid from the polystyrene degradation process in good yields, after simple base-acid wash and extractions. The protocol can be performed in a large-scale, while the use of every-day polystyrene-based commodities led to higher yields than the model substrate, which could be up to 58%. Moreover, to the upcycling of polystyrene to benzoic acid, which is a key food additive, the process was applied in the simple and straightforward synthesis of two pharmaceuticals, salicylic acid and acetylsalicylic acid. We believe this process can have a huge impact in recycling processes and upconversion of plastic waste, which could help in the fight of mankind against pollution and climate change.Author contributions
C.G.K. conceived and directed the project. N.F.N., E.S., P.L.G. and I.T. conducted the experiments and analysed experimental data. N.F.N., E.S., P.L.G. and I.T. performed mechanistic studies and P.L.G. and I.T. performed DI-HRMS studies. C.G.K. wrote the first draft of the manuscript and E.S. and C. G. K. made the final editing of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the Hellenic Foundation for Research and Innovation (HFRI) for financial support through a grant, which is financed by 1st Call for H.F.R.I. Research Projects to Support Faculty Members & Researchers and the procurement of high-cost research equipment grant (grant number 655).References
- A. Tennakoon, X. Wun, A. L. Paterson, S. Patnaik, Y. Pei, A. M. LaPointe, S. C. Ammal, R. A. Hackler, A. Heyden, I. I. Slowing, G. W. Coates, M. Delferro, B. Peters, W. Huang, A. D. Sadow and F. A. Perras, Nat. Catal., 2020, 3, 893–901 Search PubMed.
- A. Chamas, H. Moon, J. Zheng, Y. Qiu, T. Tabassum, J. H. Jang, M. Abu-Omar, S. L. Scott and S. Suh, ACS Sustainable Chem. Eng., 2020, 8, 3494–3511 CrossRef CAS.
- N. Simon, K. Raubernheimer, N. Urho, S. Unger, D. Azoulay, T. Farrelly, J. Sousa, H. Van Asselt, G. Carlini, C. Sekomo, M. L. Schulte, P.-O. Busch, N. Wienrich and L. Weiand, Science, 2021, 373, 43–47 CrossRef CAS PubMed.
- M. MacLeod, H. P. H. Arp, M. B. Tekman and A. Jahnke, Science, 2021, 373, 61–65 CrossRef CAS PubMed.
- D. K. A. Barnes, F. Galgani and R. C. Thompson, Philos. Trans. R. Soc., B, 2009, 364, 1985–1998 Search PubMed.
- Editorial, Making plastics sustainable isn't the whole solution, Nature, 2021, 590, 363–364 CrossRef PubMed.
- A. Rahimi and J. M. Garcia, Nat. Rev. Chem., 2017, 1, 0046 Search PubMed.
- Z. O. G. Schyns and M. P. Shaver, Macromol. Rapid Commun., 2021, 42, 2000415 CrossRef CAS PubMed.
- B. Singh and N. Sharma, Polym. Degrad. Stab., 2008, 93, 561–584 CrossRef CAS.
- J. R. Jambeck, R. Geyer, C. Wilcox, T. R. Siegler, M. Perryman, A. Andrady, R. Narayan and K. L. Law, Science, 2015, 347, 768–771 CrossRef CAS PubMed.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, e1700782 CrossRef PubMed.
- J. M. Garcia and M. L. Robertson, Science, 2017, 358, 870–872 CrossRef CAS PubMed.
- A. Pifer and A. Sen, Angew. Chem., Int. Ed., 1998, 37, 3306–3308 CrossRef CAS PubMed.
- S. M. Al-Sale, P. Lettieri and J. Baeyens, Waste Manage., 2009, 29, 2625–2643 CrossRef PubMed.
- I. A. Ignatyev, W. Thielemans and B. Van der Beke, ChemSusChem, 2014, 7, 1578–1593 CrossRef PubMed.
- I. Vollmer, M. J. F. Jenks, M. C. P. Roelands, R. J. White, T. van Harmelen, P. de Wild, G. P. van der Laan, F. Meirer, J. T. F. Keurntjes and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2020, 59, 15402–15423 CrossRef CAS PubMed.
- S. C. Kosloski-Oh, Z. A. Wood, Y. Manjarrez, J. P. de los Rios and M. E. Fieser, Mater. Horiz., 2021, 8, 1084–1129 RSC.
- Editorial, Plastic upcycling, Nat. Catal., 2019, 2, 945–946 Search PubMed.
- L. S. T. J. Korley, T. H. Epps III, B. A. Helms and A. J. Ryan, Science, 2021, 373, 66–69 CrossRef CAS PubMed.
- C. Jehanno, J. W. Alty, M. Roosen, S. De Meester, A. P. Dove, E. Y.-X. Chen, F. A. Leibfarth and H. Sardon, Nature, 2022, 603, 803–814 CrossRef CAS PubMed.
- Z. Zhang, T. Hirose, S. Nishio, Y. Morioka, N. Azuma, A. Ueno, H. Ohkita and M. Okada, Ind. Eng. Chem. Res., 1995, 34, 4514–4519 CrossRef CAS.
- D. S. Achilias, I. Kanellopoulou, P. Megalokonomos, E. Antonakou and A. A. Lappas, Macromol. Mater. Eng., 2007, 292, 923–934 CrossRef CAS.
- M. Marczewski, E. Kaminska, H. Marczewska, M. Godek, G. Rokicki and J. Sokolowski, Appl. Catal., B, 2013, 129, 236–246 Search PubMed.
- Y. Jing, Y. Wang, S. Furukawa, J. Xia, C. Sun, M. J. Hulsey, H. Wang, Y. Guo, X. Liu and N. Yan, Angew. Chem., Int. Ed., 2021, 60, 5527–5535 CrossRef CAS PubMed.
- X. Jie, W. Li, D. Slocombe, Y. Gao, I. Banerjee, S. Gonzalez-Cortez, B. Yao, H. AlMegren, S. Alsihri, J. Dilworth, J. Thomas, T. Xiao and P. Edwards, Nat. Catal., 2020, 3, 902–912 Search PubMed.
- S. E. Lewis, B. E. Wilhelmy Jr. and F. A. Leibfarth, Chem. Sci., 2019, 10, 6270–6277 RSC.
- G. Ciamician, Science, 1912, 36, 385–394 Search PubMed.
- For selected reviews, see: (a) K. L. Skubi, T. R. Blum and T. P. Yoon, Chem. Rev., 2016, 116, 10035–10074 CrossRef CAS PubMed; (b) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS PubMed; (c) D. Cambié, C. Bottecchia, N. J. W. Straathof, V. Hessel and T. Noël, Chem. Rev., 2016, 116, 10276–10341 CrossRef PubMed; (d) J. Twilton, C. Le, P. Zhang, M. H. Shaw, R. W. Evans and D. W. C. MacMillan, Nat. Rev. Chem., 2017, 1, 0052 CrossRef CAS.
- For selected papers, see: (a) D. A. Nicewicz and D. W. C. MacMillan, Science, 2008, 322, 77–80 CrossRef CAS PubMed; (b) T. P. Yoon, M. A. Ischay and J. Du, Nature, 2010, 2, 527–532 CAS; (c) R. Brimioulle and T. Bach, Science, 2013, 342, 840–843 CrossRef CAS PubMed; (d) Y. Y. Loh, K. Nagao, A. J. Hoover, D. Hesk, N. R. Rivera, S. L. Coletti, I. W. Davies and D. W. C. MacMillan, Science, 2017, 358, 1182–1187 CrossRef CAS PubMed; (e) A. Fawcett, J. Pradeilles, Y. Wang, T. Mutsuga, E. L. Myers and V. K. Aggarwal, Science, 2017, 357, 283–286 CrossRef CAS PubMed; (f) T. Patra, S. Mukherjee, J. Ma, F. Strieth-Kalthoff and F. Glorius, Angew. Chem., Int. Ed., 2019, 58, 10514–10520 CrossRef CAS PubMed; (g) A. Ruffoni, F. Julia, T. D. Svejstrup, A. J. McMillan, J. J. Douglas and D. Leonori, Nat. Chem., 2019, 11, 426–433 Search PubMed; (h) Visible Light Photocatalysis in Organic Chemistry, ed. C. R. J. Stephenson, T. P. Yoon and D. W. C. MacMillan, Wiley-VCH, Weinheim, 2018 Search PubMed; (i) J. Schwarz and B. König, Green Chem., 2018, 20, 323–361 RSC; (j) M. Silvi and P. Melchiorre, Nature, 2018, 554, 41–49 CrossRef CAS PubMed.
- L. Capaldo, D. Ravelli and M. Fagnoni, Chem. Rev., 2022, 122, 1875–1924 CrossRef CAS PubMed.
- I. Mita, T. Takagi, K. Horie and Y. Shindo, Macromolecules, 1984, 17, 2256–2260 CrossRef CAS.
- For a recent highlight on polystyrene photochemical upcycling, see: E. Skolia, O. G. Mountanea and C. G. Kokotos, Trends Chem., 2023, 5, 116–120 CrossRef CAS.
- G. Zhang, Z. Zhang and R. Zeng, Chin. J. Chem., 2021, 39, 3225–3220 CrossRef CAS.
- M. Wang, J. Wen, Y. Huang and P. Hu, ChemSusChem, 2021, 14, 5049–5056 CrossRef CAS PubMed.
- M. Zeece, Introduction to the Chemistry of Food, Academic Press, San Diego, 2020 Search PubMed.
- S. Oh and E. E. Stache, J. Am. Chem. Soc., 2022, 144, 5745–5749 CrossRef CAS PubMed.
- Z. Huang, M. Shanmugam, Z. Liu, A. Brookfield, E. L. Bennett, R. Guan, D. E. Vega Herrera, J. A. Lopez-Sanchez, A. G. Slater, E. J. L. McInnes, X. Qi and J. Xiao, J. Am. Chem. Soc., 2022, 144, 6532–6542 CrossRef CAS PubMed.
- Y. Qin, T. Zhang, H. Y. V. Ching, G. S. Raman and S. Das, Chem, 2022, 8, 2472–2484 CAS.
- T. Li, A. Vijeta, C. Casadevall, A. S. Gentleman, T. Euser and E. Reisner, ACS Catal., 2022, 12, 8155–8163 CrossRef CAS PubMed.
- (a) N. Kaplaneris, A. Bisticha, G. N. Papadopoulos, D. Limnios and C. G. Kokotos, Green Chem., 2017, 19, 4451–4456 RSC; (b) E. Voutyritsa and C. G. Kokotos, Angew. Chem., Int. Ed., 2020, 59, 1735–1741 CrossRef CAS PubMed; (c) N. F. Nikitas, D. I. Tzaras, I. Triandafillidi and C. G. Kokotos, Green Chem., 2020, 22, 471–477 RSC; (d) N. Spiliopoulou and C. G. Kokotos, Green Chem., 2021, 23, 546–551 RSC; (e) C. S. Batsika, C. Mantzourani, D. Gkikas, M. G. Kokotou, O. G. Mountanea, C. G. Kokotos, P. K. Politis and G. Kokotos, J. Med. Chem., 2021, 64, 5654–5666 Search PubMed; (f) E. Skolia, P. L. Gkizis, N. F. Nikitas and C. G. Kokotos, Green Chem., 2022, 24, 4108–3118 RSC.
- N. F. Nikitas, P. L. Gkizis and C. G. Kokotos, Org. Biomol. Chem., 2021, 19, 5237–5253 RSC.
- N. F. Nikitas and C. G. Kokotos, C-C and C-Heteroatom bonds photocatalyzed and photoinitiated by carbonyls in Photochemistry, ed. S. Crespi and S. Protti, Royal Society of Chemistry, Cambridge, 2022, vol. 49, ch. 10, pp. 270–291 Search PubMed.
- N. Spiliopoulou, P. L. Gkizis, I. Triandafillidi, N. F. Nikitas, C. S. Batsika, A. Bisticha and C. G. Kokotos, Chem. – Eur. J., 2022, 28, e202200023 CrossRef CAS PubMed.
- A. Bosveli, T. Montagnon, D. Kalaitzakis and G. Vassilikogiannakis, Org. Biomol. Chem., 2021, 19, 3303–3317 RSC.
- For full reaction conditions, optimization, data characterization and mechanistic studies, see ESI.†.
- Salicylic Acid: A Plant Hormone, ed. S. Hayat and A. Ahmad, Springer, Dordrecht, 2007 Search PubMed.
- D. B. Jack, Lancet, 1997, 350, 437–439 CrossRef CAS PubMed.
- T. Arif, Clin., Cosmet. Invest. Dermatol., 2015, 8, 455–461 CrossRef CAS PubMed.
- T. D. Warner and J. A. Mitchell, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 13371–13373 CrossRef CAS PubMed.
- F. Luo, S. He, Q. Gou, J. Chen and M. Zhang, Tetrahedron Lett., 2021, 84, 153434 CrossRef CAS.
- H.-G. Korth and P. Mulder, J. Org. Chem., 2020, 85, 2560–2574 CrossRef CAS PubMed.
- For selected studies using DI-HRMS to study reaction mechanisms, see: (a) E. Voutyritsa, A. Theodorou, M. G. Kokotou and C. G. Kokotos, Green Chem., 2017, 19, 1291–1298 Search PubMed; (b) I. Triandafillidi, M. G. Kokotou and C. G. Kokotos, Org. Lett., 2018, 20, 36–39 CrossRef CAS PubMed; (c) I. Triandafillidi, M. G. Kokotou, D. Lotter, C. Sparr and C. G. Kokotos, Chem. Sci., 2021, 12, 10191–10196 RSC; (d) C. S. Batsika, C. Koutsilieris, G. S. Koutoulogenis, M. G. Kokotou, C. G. Kokotos and G. Kokotos, Green Chem., 2022, 24, 6224–6231 RSC; (e) O. G. Mountanea, C. Mantzourani, M. G. Kokotou, C. G. Kokotos and G. Kokotos, Eur. J. Org. Chem., 2023, e202300046 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental data, 1H and 13C NMR data, UV-Vis, fluorescence, mechanistic and DI-HRMS studies. See DOI: https://doi.org/10.1039/d3gc00986f |
| This journal is © The Royal Society of Chemistry 2023 |