 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recycling process development with integrated life cycle assessment – a case study on oxygen transport membrane material†
Melanie
Johanning‡
 a,
Marc
Widenmeyer
a,
Marc
Widenmeyer
 *a,
Giamper
Escobar Cano
*a,
Giamper
Escobar Cano
 b,
Vanessa
Zeller
b,
Vanessa
Zeller
 c,
Sebastian
Klemenz
c,
Sebastian
Klemenz
 d,
Guoxing
Chen
d,
Armin
Feldhoff
d,
Guoxing
Chen
d,
Armin
Feldhoff
 b and
Anke
Weidenkaff
ad
b and
Anke
Weidenkaff
ad
aTechnical University of Darmstadt, Research Division of Materials & Resources, 64287 Darmstadt, Germany. E-mail: marc.widenmeyer@mr.tu-darmstadt.de
bLeibniz University Hannover, Institute of Physical Chemistry and Electrochemistry, 30167 Hannover, Germany
cTechnical University of Darmstadt, Research Division of Material Flow Management and Resource Economy, 64287 Darmstadt, Germany
dFraunhofer Research Institution for Material Recycling and Resource Strategies IWKS, 63755 Alzenau, Germany
First published on 23rd May 2023
Abstract
The transformation towards a circular economy based on sustainable technologies requires future-oriented materials development, which considers materials recycling with a minimum environmental impact (EI). This demands a holistic approach towards materials design, including a combined assessment of functional and environmental performance. Scientific methods for environmental assessment, e.g., life cycle assessment (LCA), are well established but rarely integrated into the chemical process development at early stages. Consequently, sustainability claims often lack scientific verification. Here, we test the approach of integrating a screening LCA into the development of a chemical (recycling) process. As a relevant use case, we selected the recently developed oxygen transport membrane (OTM) material (La0.9Ca0.1)2Ni0.75Cu0.25O4±δ (LCNC). An initial LCA identified the consumption of primary metal nitrates as a major contributor to the EI of the primary synthesis. To address this issue, a Pechini-based chemical recycling process for LCNC was developed, which involves microwave-heated dissolution and subsequent re-gelation. Experimental results demonstrate the synthesis of recycled LCNC powder with primary-like properties, similar reaction behaviour, and >96% yield. Based on the LCA results, the EI of recycling is reduced by up to 76% compared to the primary synthesis in 12 of 14 impact categories. Measures for the simultaneous improvement of the process functionality and environmental performance were identified. The approach of integrating LCA in chemical process development is discussed critically based on the given use case. The results strongly encourage the integration of LCA as a standard method into the future development of sustainable chemical processes.
1. Introduction
The world's current linear, fossil–fuel-based value chain and increasing resource consumption has severe impacts on ecosystems, human health implications, and resource scarcity.1–10 Overcoming these challenges requires a holistic system change, including a circular economy based on “renewable” energies and complete recycling of materials using sustainable technologies.11–15 To establish these technologies, researchers must develop sustainable materials and (chemical) processing methods.2,12,16–18 In materials design, integrating sustainability criteria into the performance evaluation is thus imperative,16,19–21 but currently only seldomly implemented. To be future-oriented, sustainability can no longer be an add-on to conventional performance evaluation. The awareness of these requirements among researchers is increasing and concepts are evolving.22–24 Consequently, many novel processes and materials are claimed as more sustainable, but, as selected examples demonstrate, often without scientific verification.25–34 For instance, wet chemistry processes are considered advantageous compared to solid state processes due to the lower processing temperature in a recent study.35 Data ensued proof for this sustainability statement is lacking, e.g., the additional presence of process chemicals and emissions is briefly mentioned but not further evaluated.35 Without a quantitative assessment, an effective, transparent process selection and improvement for enhanced material sustainability remain impossible. Even worse, isolated improvement of one aspect can lead to burden shifting.36,37To guide the simultaneous improvement of technical and environmental performance along with other sustainability aspects during materials development, sustainability criteria must be integrated taking a holistic approach.19,24,38,39 Holistic material design criteria were proposed by Klemenz et al. for the example of catalysts for water electrolysis.19 Their approach unites criteria of sustainability (e.g., ecology, criticality), performance (e.g., efficiency, durability), and economics (e.g., investment costs) in the material design process. Evaluation of such criteria during material development requires standardised scientific methods, which are still under development.19,39–42 Often based on semi-quantitative and qualitative data for the foreground system, such as the method proposed by Patel et al.,42 holistic approaches do not allow an in-depth analysis of the chemical process design. For such purposes, life cycle assessment (LCA) is already established as a valuable scientific method to evaluate environmental impacts (EIs).43 Based on the standards ISO 14040![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 and ISO 14044,45 LCA provides a quantitative, science-based, and multi-dimensional assessment with a life cycle perspective.36 LCA was applied successfully to chemical processes46–49 and materials for new technologies.50–55 Adaptations of chemical processes are especially effective in early-stage research when the methods are still flexible compared to established procedures.56 Researchers thus demand the early integration of LCA in chemical process design.40,56,57
44 and ISO 14044,45 LCA provides a quantitative, science-based, and multi-dimensional assessment with a life cycle perspective.36 LCA was applied successfully to chemical processes46–49 and materials for new technologies.50–55 Adaptations of chemical processes are especially effective in early-stage research when the methods are still flexible compared to established procedures.56 Researchers thus demand the early integration of LCA in chemical process design.40,56,57
In order to determine EIs of emerging technologies or processes under development, prospective LCA (p-LCA) should be performed.58 p-LCAs are future-oriented LCA studies that focus either on upscaling methods for foreground processes or scenario analyses for background processes (e.g., modelling of the future electricity mix). In all cases, it is necessary to perform an LCA first at an early developmental stage (e.g., at the concept, laboratory, or pilot stage). The review by Thonemann et al. includes 44 prospective LCA case studies covering the application fields of nanomaterials (11 studies), chemical production (11 studies), energy (7 studies), wastewater treatment (6 studies), biofuel, biomass, and food production (11 studies) as well as mobility (2 studies).58 Cossutta et al.59 united all three components, i.e., laboratory scale, upscaling, and energy scenarios, in their comparative LCA of graphene production. Other LCA studies have examined emerging technologies at a laboratory scale (without performing an upscaling) and developed guidelines for screening LCAs.60,61 Thus, early-stage LCAs have been applied to diverse sectors and sector-specific guidance has been developed.
In current practice, the environmental assessment of chemical processes is usually conducted in separate LCA studies, which can already provide valuable insights.40,49,56 Unfortunately, LCA is only applied to a minor fraction of chemical processes, presumably due to insufficient awareness and resources. As a result, there is a time delay between the process development and the EI assessment, which leads to a need for more scientific information during process improvement. The separation of LCA can cause an information asymmetry. Data availability and process understanding are significant challenges for early-stage LCA studies in chemistry.62 For instance, many studies lack sufficient consideration of the process chemicals and emissions. In the study of Agarski et al.,47 it remains unclear why NOx process emissions are not considered for all the product systems with the presence of nitrate ions. In another study, Lee and Hong50 did not include upstream activities and process emissions from the decomposition of organic process chemicals in their cradle-to-gate study. The conduction of a screening LCA during the process development is expected to guide research towards the simultaneous improvement of the environmental and functional performance.40,56
The following studies showed how the integration of LCA allows to include the profound process understanding of the developing researcher, helps to collect primary data efficiently, and avoids unsupported claims of environmental benefits. Samori et al.63 performed a simplified and preliminary LCA for applying switchable hydrophilicity solvents for a developed recycling process of multilayer plastic packaging. For silicon and silver recovery from solar cells, Deng et al.64 recently demonstrated the lower EI of a developed processing route compared to conventional recycling processes reported in literature. These studies indicate the feasibility and benefits of integrating LCA in early-stage process development. In the field of process development for chemical recycling of functional ceramic oxides, the integration of such an early-stage LCA is yet to be demonstrated. Consequently, this work is aimed to test the applicability of a combined experimental process development and early-stage LCA study in the above-mentioned research field.
An interesting use case for such a combined material design process is oxygen transport membranes (OTMs). OTMs are a versatile tool for more sustainable chemical and energy conversion processes.65 This includes the production of hydrogen and oxygen, carbon capture and utilization, and cathode materials for solid oxide fuel cells.65–67 In membrane reactors, OTMs can control the oxygen partial pressure of a chemical reaction.68 In this context, OTMs enhance the process efficiency of the emerging technology plasma-assisted CO2 splitting and conversion.69,70 For maximum economic and ecological benefit, high material quality and minimum EI are required for the membrane material.65 Recycling end-of-life membranes could prevent the membranes from becoming waste and reduce the EI of the material synthesis.3 End-of-life membranes would be an easily recoverable, high-quality waste with well-defined composition and valuable components.71,72 After the chemical failure of membranes (e.g., formation of carbonates or binary phases),73–76 closed-loop chemical recycling appears favourable to fulfil the high quality and security standards.77–79
A promising Ruddlesden–Popper (RP) phase membrane material (La0.9Ca0.1)2Ni0.75Cu0.25O4±δ (LCNC) was recently developed by Chen et al.80 LCNC showed a sufficient oxygen permeation flux of 0.63 mL min−1 cm−2 at 900 °C for a 0.65 mm thick membrane under both, air/helium and air/CO2 gradient.80 Its chemical stability towards CO2 and CO has been demonstrated.80 LCNC is synthesised by a Pechini-based sol–gel process from primary metal nitrates with ethylenediaminetetraacetic acid (EDTA) and citric acid (CA) as chelation agents. This enables the formation of the complex metal oxide. Exploring recycling as an alternative route for the synthesis of LCNC can contribute to establishing a circular use of OTMs and is hence hypothesised to reduce their EI.
To the best of the authors’ knowledge, no process for chemical recycling of complex metal oxides in a closed-loop has been reported. Previous research on the recycling of metal-containing compounds mainly focused on the recovery of selected metal ions81–87 for open-loop recycling by hydrometallurgy,82,84 pyrometallurgy, mechanical, and physical recycling.81,83 However, practical challenges nowadays arise due to the complex and undefined composition of scrap and the high effort for purification.81,83,85,88 The awareness for a more holistic consideration of the design of recycling processes is rising.89 Elemental separation steps can become unnecessary if materials are designed for closed-loop circularity with processes for waste recovery and recycling techniques in place.71 The first processes for chemical recycling of metal-containing functional materials have been published25,77,78,90–93 with a sole focus on the scientific evaluation of the process functionality. These processes involve reactive transformations or the production of primary-like precursors by the dissolution of the waste material. A sol–gel-based recycling method was developed by Dixini et al.25 for recycling Zn–Mn–O2 battery cathodes. After extraction of metal ions by leaching, the gelation process was started by adding citric acid and controlling pH. A mixture of MnO2, Mn3O4, and ZnMn2O4 was synthesised as pseudocapacitor material.25 A comparable strategy of leaching and regelation was applied to battery anode material in a recent study.94 Leaching temperatures of 850 °C, 1100 °C, and 1200 °C were used. Energy-efficient dissolution by microwave-heated autoclaves has been demonstrated for other applications.95–100 Combining these strategies shows high potential for developing a chemical recycling method for LCNC.
In this work, we conducted an early-stage screening LCA while developing a chemical recycling process for the synthesis of LCNC. The results highly suggest the integration of LCA in the early-stage development of sustainable chemical processes as a future standard.
2. Results and discussion
The methodological approach of integrating LCA into the chemical process development is shown schematically in Fig. 1. As a reference baseline, the main contributors to the EI of the primary synthesis of LCNC (product system (1)) were identified by an attributional cradle-to-gate LCA at the laboratory scale (see section 2.1). The EI of the LCNC synthesis was assessed based on the International Reference Life Cycle Data System (ILCD) 2011 impact model.101 The LCA model and data collection are detailed in section 5 (Materials and methods) and the ESI.† The relevant information with respect to the used life cycle inventory models and data can be found in Tables S5–S11 (ESI)† and the surrounding explanatory text.The experimental steps and contributions to the life cycle inventory of the primary synthesis based on Chen et al.80 are shown in Fig. 2(a). Following the identified main contributors to the EI, chemical recycling is a promising approach to produce high quality LCNC membrane material with improved environmental performance. A Pechini-based chemical recycling process was developed (see Fig. 2(b)). It involves microwave-heated dissolution in an aqueous solution of CA and nitric acid, subsequent re-gelation, and further primary-like processing (for further experimental details, see section 5 and ESI†).
The results of experimental characterisation verify the functional performance of the chemical recycling process (see section 2.2). A comparative LCA evaluated the environmental performance of the developed chemical recycling process (product system (2)) compared to the reference primary synthesis (1) (see section 2.3). Combining LCA and experimental results, the performance of the recycling process is discussed in section 3.1. Based on the given use case results, the approach of integrating LCA into the chemical process development is evaluated critically in section 3.2.
2.1. Reference LCA of primary LCNC synthesis
The relative contributions to the cradle-to-gate EI of the primary synthesis (1) of LCNC powder at the laboratory-scale are shown in Fig. 3. The chosen impact categories for environmental assessment focus on the effects of process emissions and resource consumption, including upstream activities (for model description see section 5.3 and ESI†). In the following, the name of the product, e.g., “EDTA”, is used as a short expression for the EI associated with the production and provision of the named product. The main upstream contributors are clearly identified as consumed electricity and metal nitrates as reactants for the primary synthesis. The process emissions, (i.e., synthesis of LCNC) cause a significant share of the overall EI (up to 79%) in the foreground system. Upstream activities such as deionised (DI) water, ammonia, and nitric acid production are negligible, while CA and EDTA have considerable contributions of up to 8%. | ||
| Fig. 3 Reference LCA results for the primary synthesis of (La0.9Ca0.1)2Ni0.75Cu0.25O4±δ (LCNC) visualised as respective relative impact contributions to each individual impact category from the main contribution categories (e.g., electricity). For absolute values, see Table 2 in section 2.3 (H: hydrogen, eq.: equivalent, CTUe: comparative toxic unit for ecosystems, P: phosphorus, CTUh: comparative toxic unit for human, HH: human health, U235: uranium 235, N: nitrogen, Sb: antimony, CFC-11: trichlorofluoromethane, PM: particulate matter, NMVOC: non-methane volatile organic compound). | ||
Electricity consumption in the foreground system is the dominant contribution (>50%) in 10 of 14 impact categories. This high contribution is expectable due to the limited efficiency of the synthesis at the laboratory scale. When the processes are designed for scalability, the energy consumption would be expected to decrease by orders of magnitude in large-scale production. The modelling of efficient up-scaling or future energy scenarios was out of the scope of the current study.
The production and provision of metal nitrates significantly contribute to all impact categories and would scale directly proportional with the amount of linearly produced primary LCNC. The utilised background processes are less specific for the conducted synthesis than the foreground data but represent the average industrial production more accurately. The production of lanthanum oxide causes the main upstream contribution by metal nitrates (between 68% and 100%).
The consumption of the process chemicals CA, EDTA, and ammonia plays a crucial role despite their comparably low contribution from upstream activities. First, their contribution scales linearly with the system size. Second, their impact is not limited to upstream contributions but also entails the produced process emissions. The amount and type of process chemicals determine the process emissions, directly contributing to the EI (see combined thermal analysis in section 2.2.4 and ESI† for details). Due to the up-to-date linear scaling of emissions with the produced amount of LCNC, they are essential to consider. While often neglected in literature,47,50 the results clearly show that considering total process emissions is vital for building a representative LCA model.
At the industrial scale, the contribution from energy consumption can be reduced by upscaling through size scaling, technological and industrial learning, and circularity. In contrast, the high contribution of metal nitrate reactants would increase with the system size in the potential production of primary LCNC on an industrial scale. Chemical recycling of LCNC in a closed-loop hence appears promising to address this contribution and produce LCNC with a reduced EI.
2.2. Functional performance of the Pechini-based recycling process
A specifically developed Pechini-based chemical recycling process (see section 5) was used to produce recycled (i.e., secondary) LCNC powder from primary LCNC powder by dissolution and regelation. Various characterisation techniques were applied to evaluate the functional performance of the recycling process compared to the primary synthesis. The phase composition, sample morphology, elemental composition, reaction behaviour, and oxygen permeability were assessed by powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDXS), combined thermal analysis methods, and oxygen permeation measurements, respectively. A direct comparison of representative primary and recycled LCNC from the same batch is presented in the following. | ||
Fig. 5 SEM images of primary (a and b) and recycled (c and d) LCNC powder in two different magnifications of 2500× and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×. 000×. | ||
Instead, the similarity of optical appearance and grain size does not allow the distinction of the two powder types based on their morphology. The similarity further indicates the two sample types’ comparable reaction behaviour and sintering properties.
| Element | Theoretical | Primary | Deviation to theoretical | Recycling | Deviation to primary |
|---|---|---|---|---|---|
| at% metals | at% metals | % | at% metals | % | |
| La | 60.0 | 60.4 ± 0.3 | +1% | 60.6 ± 0.2 | <1% |
| Ca | 6.7 | 6.3 ± 0.1 | −6% | 6.3 ± 0.1 | <1% |
| Ni | 25.0 | 24.6 ± 0.3 | −2% | 24.4 ± 0.2 | −1% |
| Cu | 8.3 | 8.7 ± 0.1 | +4% | 8.8 ± 0.2 | +1% |
The composition of recycled powder and its primary reference match within the errors of measurement (given by the standard deviations). Hence, the developed recycling process retains the elemental composition and purity.
Recovery rates of >96% were achieved for the Pechini-based LCNC recycling. The recycling yield was even higher than in the primary synthesis (∼94%). A potential reason is the minor variation of the metal ion content of the hygroscopic metal nitrates. During chemical recycling (dissolution and re-gelation), all metal ions are expected to be conserved. The processing method hence does not cause any inherent loss of metal ions. Consequently, the processual losses are most likely caused by residuals on the equipment.
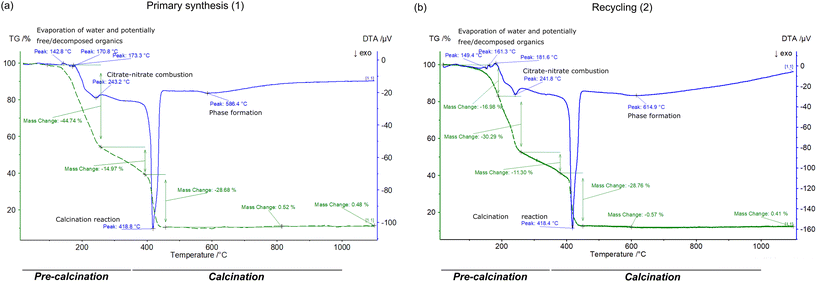 | ||
| Fig. 6 Results of TG-DTA of (a) primary and (b) recycled precursor gels measured from room temperature to 1100 °C. The temperature ranges of the pre-calcination and calcination process are indicated. | ||
Process emissions are released in a significant amount, as shown by the weight change of around −90 wt% during the thermal analysis. Thus, considering these gaseous emissions is crucial for a representative LCA. The emissions scale linearly with the batch size, and their amount differs for the primary and recycled gels. Due to the necessary addition of nitric acid for dissolution (see Fig. S3 in ESI†), recycled gels contain around 40% more nitrate ions than primary gels. Additional emissions from the higher nitrate content could be a drawback of the recycling process due to burden shifting. To identify the main emission species and build a quantitative emission model, combined thermal measurements were conducted. The MS and FTIR results (see Fig. S10–S13†) of this combined measurement match well with the corresponding TG-DTA (see Fig. S8 and S9†) and separately measured TG-DTA data shown in Fig. 6.
The emission peaks with the highest intensity are detected at the stages of organic–nitrate combustion (∼240 °C) and calcination (∼420 °C), which are also the stages with the highest weight changes. The main peaks can be assigned to NO, NO2, N2O, NH3, CO2, CO, and H2O emissions. Signals of other organic fragments (e.g., C2NH3, HNCO, CH4) were close to the detection limits. On the contrary, atmospheric oxygen shows a negative peak and is hence consumed during the calcination reaction in addition to the oxygen present in the precursor gel (e.g., from nitrates). The organic matrix of the gel reacts with oxygen to form the gaseous emissions. LCNC forms by the reaction of metal ions and oxygen. To enable the modelling of the process emissions for LCA, quantitative estimates of the emission shares were based on literature (see Table S9 in ESI†).
Open porosity was identified as the origin of high leakage and weak mechanical stability in both the membranes (from primary and recycled powder). The general requirement of >95% rel. Archimedes density has been reported for sufficiently gas-tight OTMs.111 The geometrical and Archimedes density of the recyclate-based membrane are 63% and 97%, respectively, with respect to the theoretical value of 6.82 g cm−3.80 Comparable relative densities of 69% and 90%, respectively, were obtained for a membrane from primary LCNC. The high discrepancies between the geometrical and Archimedes density indicate open porosity. SEM imaging further confirmed this (see Fig. S15 and S16 in ESI†). The open porosity and consequently high leakage for the recyclate-based membrane with 97% relative Archimedes density shows that the geometrical density should be incorporated as an additional membrane quality indicator. Suitable sintering conditions must be found to produce high-quality OTMs from LCNC (see ESI†). Nevertheless, the results overall indicate a similar quality for primary and recycled powders; thus, indicating that recycling does not compromise LCNC's intrinsic properties.
2.3. Comparative LCA of Pechini-based LCNC recycling
A comparative LCA was conducted to evaluate the potential of the chemical recycling process to reduce the EI from the production of LCNC-based OTM material. Applying the same modelling approach as the one chosen for the reference LCA enables a direct comparison of EIs of the absolute values for impact indicators listed in Table 2. A relative comparison of the EIs of the primary synthesis (1) and recycling (2) of LCNC powder at the laboratory-scale is shown in Fig. 7. The EI of recycling (2) is reduced by up to 76% compared to the primary synthesis (1) in 12 of 14 assessed impact categories.101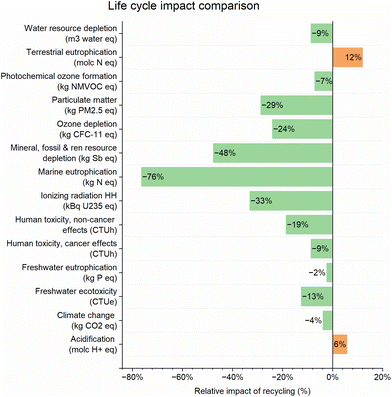 | ||
| Fig. 7 Relative comparison of life cycle impacts for the recycling of LCNC (2) to the value for the primary synthesis (1) per impact category. No normalisation or weighting was applied. | ||
| Impact category | Unit | Primary (1) | Recycling (2) |
|---|---|---|---|
| Acidification | molc H+ eq. | 1.1 × 10−2 | 1.2 × 10−2 |
| Climate change | kg CO2 eq. | 1.3 | 1.2 |
| Freshwater ecotoxicity | CTUe | 37.2 | 33.5 |
| Freshwater eutrophication | kg P eq. | 1.4 × 10−3 | 1.4 × 10−3 |
| Human toxicity, cancer effects | CTUh | 1.3 × 10−7 | 1.2 × 10−7 |
| Human toxicity, non-cancer effects | CTUh | 5.0 × 10−7 | 4.1 × 10−7 |
| Ionizing radiation human health (HH) | kBq U235 eq. | 2.6 × 10−1 | 1.7 × 10−1 |
| Marine eutrophication | kg N eq. | 5.5 × 10−3 | 1.3 × 10−3 |
| Mineral, fossil & renewable resource depletion | kg Sb eq. | 3.5 × 10−5 | 1.8 × 10−5 |
| Ozone depletion | kg CFC-11 eq. | 3.6 × 10−8 | 2.8 × 10−8 |
| Particulate matter | kg PM2.5 eq. | 2.9 × 10−4 | 2.1 × 10−4 |
| Photochemical ozone formation | kg NMVOC eq. | 2.4 × 10−3 | 2.2 × 10−3 |
| Terrestrial eutrophication | molc N eq. | 3.3 × 10−2 | 3.7 × 10−2 |
| Water resource depletion | m3 water eq. | 7.0 × 10−1 | 6.4 × 10−1 |
The most significant reductions are calculated in the categories “marine eutrophication”, “mineral, fossil, and renewable resource depletion”, and “ionizing radiation human health”. A higher EI for recycling (2) was calculated in the categories “terrestrial eutrophication” and “acidification”. Note that emissions of La ions to water are currently not implemented in the utilised ILCD 2011 impact model in Open LCA. Lanthanum emissions are thus only listed in the LCI (see Tables S10 and S11 in ESI†) but not considered for the EI.
The relative shares for the different contributions to the EI of the recycling process are shown in Fig. 8. As expected, the contributions of metal nitrates disappear in the recycling process and a negligible upstream contribution of nitric acid appears in addition. Electricity consumption remains the main upstream contributor to the recycling of LCNC. The additional step of microwave-heated dissolution increases the overall electricity consumption during recycling by only 4%. The relative contributions of CA and EDTA increase slightly despite a similar consumption due to the elimination of metal nitrates in recycling. In contrast, the process emissions (i.e., synthesis of LCNC) cause a significant share of the overall EI (up to 85%), which is higher than in the primary synthesis.
 | ||
| Fig. 8 Overview of the respective relative impact contributions to each individual impact category from the main contribution categories (e.g., electricity) for Pechini-based recycling of LCNC. | ||
In conclusion, the differences between the two systems can be mainly ascribed to using metal nitrates for the primary synthesis and higher process emissions during recycling. These higher emissions are caused by the ∼40% higher nitrate content in the recycled samples. To prevent underestimating the influence of the higher nitrate content, worst-case assumptions were used to build the emission model (see section 5 and ESI†). The increased emissions are weighted out in 12 of 14 impact categories by avoiding the utilisation of primary metal nitrates.
3. Discussion of methods
3.1. Pechini-based recycling process
Combining experimental and LCA results, the developed Pechini-based recycling process is a promising method to synthesise recycled LCNC powder with primary-like properties and reduced EI. The equivalence of oxygen permeability is indicated but needs further verification. The high yield and retained chemical composition after recycling highlight the advantages of such a solution-based one-pot approach for closed-loop chemical recycling. In large-scale production, recovery rates even closer to 100% are expected. Following the LCA results, the highest benefits are anticipated by reducing the consumption of electricity and process chemicals. Quantitative estimations of the electricity consumption after upscaling could estimate the actual relevance of electricity more accurately.59,112 A significant contribution can still be expected after upscaling. It can be argued that future energy mix changes towards more “renewable” electricity would reduce the EI. A scenario analysis could provide further insights. However, “renewable” energies are generally not free of EI113 and energy consumption always remain a cost factor. Consequently, in this early stage, possible measures should be taken to enhance the energy efficiency of both processes. A high potential to reduce the calcination temperature and time is indicated by the phase formation at 600 °C as determined by TG-DTA. The partial sintering of LCNC powder observed in SEM supports this statement. Adjusting the calcination parameters could significantly enhance the energy efficiency of the reaction and simultaneously improve the powder properties. The energy efficiency could be increased by extending the utilisation of microwave radiation to other processing steps.114–117The process emissions as the second hot spot can be addressed based on the chemical knowledge of the process. The synthesis with a reduced amount of CA and EDTA or utilisation of alternative chelation agents from renewable sources (e.g., waste apple pomace) or different chelation agents with fewer carbon and nitrogen atoms per molecule could help to reduce emissions. In addition, the consumption of solvents (e.g., DI water, nitric acid) should be reduced by re-usage and recycling methods as far as possible. For instance, consuming less water for dissolution would also reduce the required amount of nitric acid and ammonia solution and, ultimately, process emissions. These measures need to be explored experimentally by targeted parameter studies. For instance, the dissolution in alternative acids was not feasible for recycling in preliminary studies. Possibilities to reduce hazardous emissions by alternative temperature programs could also be assessed experimentally. The substitution of La in the primary LCNC synthesis could improve the environmental footprint of the materials synthesis but is limited by the material performance and stability.80
In application, the similarity of constituents, processing, and reaction behaviour is assumed to allow the mixing of primary and recycled precursors or compositional adjustments. In the current study, the primary powder was taken as the input material for recycling. This needs to be extended to chemically degraded material and utilised membranes in future studies to develop the recycling process further and ensure its applicability for closed-loop membrane recycling. Chemical degradation due to phase segregation and ageing during the membrane utilisation is expected to retain the metal ion composition since they are not volatile. An introduction of a significant amount of persistent impurity atoms is not anticipated. Therefore, the dissolution of chemically degraded membrane material is expected to produce a very similar processable precursor.
3.2. Approach of integrating LCA into chemical process development
Based on the presented use case of chemical recycling of the OTM material LCNC, a discussion of the advantages and drawbacks of the combined LCA approach is presented in the following. The combined approach makes the results of experimental characterisation and LCA merge synergistically. As discussed in the following, some limitations of separate LCA studies can be overcome by this approach, while other limitations remain. A few identified “success factors” for the smooth integration of LCA into chemical process development are presented within this section as well.Conventional chemical process development focuses on the process functionality and lacks a quantitative environmental assessment. Separate LCA studies provide this quantitative environmental perspective but are usually conducted independently from process development and with time delay. Hence, the process understanding can be insufficient but would be needed (e.g.: for the modelling of process emissions). For the presented use case, the experimental characterisation results demonstrate the successful recycling of LCNC and deepen the chemical understanding. Complementary, the LCA results approve a reduced EI from recycling compared to the primary synthesis, point out hot spots, and foster a more comprehensive system perspective. In this manner, the interrelations of the chemical reaction, process chemicals, emissions, and EIs can be explored. The LCA guides the researcher towards the next steps of process improvement. This can result in a direct feedback loop, as indicated in Fig. 1. Experimental results and chemical process understanding can verify the feasibility of potential measures to reduce the EI. For example, the experimental characterisation confirms the potential to reduce the electricity consumption suggested by the LCA by adjusting the calcination parameters. The feasibility of other measures (e.g., utilisation of microwave heating, reducing the solvent consumption), might only be recognised with sophisticated chemical knowledge.
A significant share of material research is designated to developing more sustainable technologies. However, actual improvements can only be verified with a quantitative assessment method and subsequent feasibility studies using the knowledge on the chemical process and EI. LCA is a well-established assessment method, but a conventional LCA lacks an absolute measure of the environmental performance, e.g., with regard to planetary boundaries,118 as well as economic and social factors of sustainability. The chosen methodology always involves a trade-off between accuracy and feasibility, thus, limiting the informative value of the LCA results. For instance, the given results do not indicate if any environmental benefit is created from the production of LCNC as OTM material. Integration of LCA is by no means an exhaustive measure for a holistic material design and equally integrable methods must be further developed. Compared to proposed holistic assessment methods,41,42 LCA is a more quantitative approach that can provide an in-depth understanding of the environmental performance.40,56 Prospective LCA (p-LCA) studies have begun manifesting in chemical research, but p-LCA is not yet a standard method applied during chemical process development. Consequently, a high potential for efficient reduction of EIs in the early stages remains unexploited. Early-stage LCA studies conducted by external parties or collaborators are difficult to be performed regularly. Reasons are many processes, insufficient available information, and deficient process understanding. If performed, separate LCA studies are associated with time delay, additional effort, and can be accompanied by an information asymmetry. With the integration of LCA in the chemical process development, the collection of primary data and building of the LCA model can be performed in parallel with the experiments. The evolving knowledge, e.g., about process emissions, is used directly for a sustainable process development and subsequent industrial scale up. Once developed, the LCA model could be integrated into the assessment of the overall technology, used for monitoring and predicting improvements, or transferred to other systems.
The changing requirements for material performance demand a systematic consideration of environmental performance in the material design.16,19–21,24 Despite the accessibility of primary data in laboratory experiments, building accurate LCA models and applying advanced LCA methodology can cause very high efforts. It is thus crucial to make consistent modelling choices that match the defined LCA goal and scope at such an early stage. For instance, this study chose a standard LCA methodology and neglected equipment but required an individual model for the process emissions. Furthermore, extensive sensitivity and uncertainty analysis were out-of-scope. Harmonised guidelines for the integration of LCA into chemistry and materials science should be developed to facilitate this process.24,40,56
Based on the presented use case, we conclude that integration of LCA in the chemical process development can be a fast track for the development of more sustainable and functional (circular) processing methods and materials in the field of chemical recycling of functional ceramic oxides.
4. Conclusions
A Pechini-based recycling process was developed to synthesise recycled LCNC with >96% yield and primary-like properties as confirmed by PXRD, SEM, EDXS, combined thermal analysis, and oxygen permeation measurements. The concept of dissolution and re-processing could be applied to other functional ceramic oxides synthesised by gelation-based processes. Such a recycling route holds high potential for establishing the required materials recycling for green technology applications. In such cases, infrastructure for material recovery is more straightforward to establish than for end-consumer deposits. Chemical recycling offers the benefit of high similarity to primary material and less dependence on the waste material's varying quality and chemical composition compared to mechanical recycling.The direct integration of LCA into the process development enables a consistent comparison of the primary synthesis and recycling methods in terms of their EIs. Compared to the primary synthesis, the developed recycling process produces LCNC powder with reduced EI by up to 76% in 12 of 14 impact categories. Processual adjustments to improve environmental performance can be most easily implemented in the early research stage. Integration of LCA thus saves resources by a direct collection of primary data, avoids time delays for environmental improvement, and provides profound processual knowledge to prevent information gaps. For instance, the results highlight the importance of direct process emissions, which are often neglected in separate LCA studies.
In the context of sustainability and circular economy, the combined approach of simultaneous development of more sustainable synthesis alternatives, material characterisation, and environmental impact evaluation has been proven valuable. Therefore, we recommend that LCA is integrated into chemical process development as an inherent part to effectively guide research towards more sustainable materials.
5. Materials and methods
5.1. Pechini-based synthesis and recycling of LCNC powder
A previously reported Pechini-based synthesis process using CA and EDTA as complexing agents80 was the basis for the developed chemical recycling process. It was used to synthesise LCNC powder from primary resources as reference material and input for recycling. The batch size was 10 g of total metal ions to produce primary powder for recycling and 5 g for LCA data collection.After gelation (pH = 9, 110 °C, 90–120 min) and pre-calcination (120 °C, 10 h and 350 °C, 5 h), an additional ball milling step (Fritsch Pulverisette 7) of the precursor (300 rpm for 10 min) was conducted. This produced a homogenous precursor powder with increased surface area. Black LCNC powder was obtained as the product after calcination at 1000 °C for 10 h (see ESI† for further experimental details).
A closed-loop chemical recycling process was developed, which is based on the primary synthesis process. To obtain a functional precursor for further Pechini-based processing, 6.01 g primary LCNC powder (5 g total metal ions) was dissolved in an aqueous solution of 200 mL DI water, CA, and 13 mL nitric acid using a microwave autoclave (MILESTONE SynthWAVE MA167). The molar ratio of CA to total metal ions M was identical to the primary synthesis (CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M = 2
M = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The dissolution agents were chosen due to their similarity with the constituents of the primary precursor. Highly acidic pH values <0.3 are required to obtain a homogeneous solution (see Fig. S3 and S4 in ESI†). The dissolution was conducted at 110 °C for 15 min after 5 min heating time.
1). The dissolution agents were chosen due to their similarity with the constituents of the primary precursor. Highly acidic pH values <0.3 are required to obtain a homogeneous solution (see Fig. S3 and S4 in ESI†). The dissolution was conducted at 110 °C for 15 min after 5 min heating time.
The precursor solution was concentrated under magnetic stirring in an oil bath at 110 °C for around 120 min. The gelation process was started, similar to the primary synthesis, with the addition of EDTA solution (pH = 9, EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M = 1.5
M = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). All further processing steps were conducted identically to the synthesis of primary LCNC.
1). All further processing steps were conducted identically to the synthesis of primary LCNC.
5.2. Materials characterisation
To evaluate the functionality of the Pechini-based recycling process, primary and recycled LCNC powders were compared. The phase composition, morphology, elemental composition, reaction progression, and oxygen permeability were assessed using various characterisation techniques. The recycled (i.e., secondary) powder was directly compared to the respective primary batch from which it was produced.Powder X-ray diffraction (PXRD) was conducted in transmission mode using a STOE STADI MP with Mo-Kα1 radiation (λ = 0.709317(4) Å). The phase composition and crystal structure of the products were assessed by Rietveld refinements using FullProf.2k119 and pseudo-Voigt functions to describe the profile of the diffraction peaks.
The sample morphology was observed by scanning electron microscopy (SEM) (PHILIPS XL30) at magnifications of 2500× and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×. The elemental composition and potential element loss after recycling were probed by energy-dispersive X-ray spectroscopy (EDXS) using PHILIPS XL30 equipped with an EDAX CDU Leap detector and Genesis Spectrum Software.120 The average composition of three different spots was calculated for each sample.
000×. The elemental composition and potential element loss after recycling were probed by energy-dispersive X-ray spectroscopy (EDXS) using PHILIPS XL30 equipped with an EDAX CDU Leap detector and Genesis Spectrum Software.120 The average composition of three different spots was calculated for each sample.
Thermal analysis of the reaction was conducted by thermogravimetric differential thermal analysis (TG-DTA) in purified air (Netzsch STA 409). The primary and recycled precursor gels were measured from room temperature to 1100 °C or 600 °C, respectively, with a heating and cooling rate of 3 K min−1. The formation of LCNC was confirmed by subsequent PXRD.
To build the emission model for LCA, combined TG-DTA (Netzsch STA 449C Jupiter), mass spectrometry (MS) (Netzsch QMS 403C Aeolos), and Fourier-transformed infrared spectroscopy (FTIR) (Bruker Optics Tensor 27) measurements were carried out from room temperature to 800 °C with a heating and cooling rate of 3 K min−1.
The material quality of recyclate-based LCNC membranes was assessed by comparing the oxygen permeability of a sintered membrane to the previously reported value for primary LCNC membranes. To enable comparison, the recyclate-based membrane was produced with a sintering procedure inspired by Chen et al. and measured using identical conditions under an air/CO2 gradient at 900 °C.80 The membrane leakage was corrected as reported previously.121 To complement the picture of membrane quality, primary and recyclate-based membranes were analysed by SEM (see ESI†). The density of the as-sintered discs was measured geometrically and by Archimedes method after vacuuming the samples.
5.3. Life cycle assessment (LCA) methodology
The goal of the integrated LCA study was identifying hot spot contributions to the EI of the primary synthesis of LCNC and enable an early-stage, quantitative comparison with the environmental performance of the developed recycling processes. Furthermore, the study aimed at identifying levers for reducing the EI of both processes, primary synthesis, and recycling. The described use case tested the direct integration of LCA into the early-stage chemical process development.Two product systems of LCNC membrane material, produced by the Pechini-based primary synthesis (1) and the developed chemical recycling process (2), were compared. The final function for both product systems is an OTM with an oxygen permeation flux of 0.63 mL min−1 cm−2 at 900 °C for 24 h for a 0.65 mm thick membrane under an air/CO2 gradient in a measurement set-up.80 Since the scope of the environmental impact analysis is cradle-to-gate and the membrane production not considered, the declared unit within this study is the production of 1 g LCNC powder. The results of the functional characterisation in Chapter 2.2 indicate the equivalence of performance for the primary and recycled membrane material.
An attributional cradle-to-gate assessment was conducted by taking the cut-off approach. The whole supply chain for reactants, process chemicals, and energy is included. This means that all upstream emissions and resource uses, e.g., from energy requirements to generate reactants such as metal nitrates, are included in the assessment of the primary synthesis. In the case of recycling, secondary raw material is considered “burden-free”, but all upstream contributions to process chemicals and energy are included.
The EI was assessed at the midpoint level based on the ILCD 2011 impact model101 focusing on EI from process emissions and resource consumption. The study aimed for a first assessment (screening LCA) of the EI to provide an early-stage indication if further development of the recycling process is promising. Therefore, uncertainty and sensitivity analysis were out-of-scope.
The LCA model framework and data structure are shown in Fig. 9. The similarity of the two chemical processes facilitates choosing a comparable LCA methodology with similar system boundaries. Due to the expected similarity and limited knowledge about further life cycle stages, only the synthesis process and upstream activities were considered. Due to the mono-functionality of both processes, no allocation method was needed. Assuming functional equivalence of primary (1) and recycled (2) LCNC (based on the presented results), all flows were scaled to a reference flow of 1 g LCNC powder produced in a 5 g metal ion batch. The conducted LCA study focused on chemical process design for material synthesis on the laboratory-scale. This included reactants, process chemicals, electricity consumption, and process emissions. Infrastructure, consumables waste, and equipment were neglected, which limits the representativeness for production on an industrial scale.
 | ||
| Fig. 9 LCA model of LCNC primary synthesis and recycling using foreground and background data. The modelling approach and data structure for the considered flows in the foreground and background system can be seen in Tables S5–S8 (ESI).† Manufacturing, use, and end-of-life stage were out of scope. Process emissions were calculated by a specific emission model (see Fig. S21 and Table S9, and surrounding explanatory text in ESI†). | ||
The life cycle inventory (LCI) was obtained from three sets of primary data for each synthesis process and available or modified background processes from the ecoinvent 3.8 database122 (see Tables S7 and S8 in ESI†). For the similar steps of pre-calcination and calcination, collected data points were averaged for all data sets of both systems to avoid distortion.
The following further assumptions were made in order to construct the LCA model. Average market processes for Germany or Europe were selected as supplier when available for the highest representativeness. The production of ammonia solution, nickel nitrate, copper nitrate, and lanthanum nitrate were modelled based on existing processes with similar production pathways (see ESI†).
A specific emission model was developed based on combined thermal analysis and literature (see Fig. S19 and Table S9 in ESI†). It was assumed that all organic emissions are emitted as gases to air and all metal ion losses are emitted to water. A nominal composition was considered for all chemicals. The theoretical reaction equation for a complete combustion served as the basis to estimate the quantity of emissions. Relevant emission species were identified by combined TG-MS-FTIR analysis and added into the equation in variable amounts. The atomic and mass balance must be fulfilled to solve the model equation. Unrestricted exchange of oxygen was allowed. The amount of starting materials was known from the life cycle inventory. The quantitative shares for each emission species were based on available literature, assuming sufficient transferability (see Table S9†). Direct evaporation was assumed for water and ammonia. Carbon and remaining nitrogen were assumed to react with oxygen to form COx, NOx, and N2O. The emission model consistently treats both product systems. A worst case of a reaction without the formation of N2 was assumed to prevent an underestimation of the impact from recycling due to higher nitrate content.
Modelling of the two systems and impact assessment was conducted using openLCA.123 The results are only valid for the chosen LCA model, batch size, and equipment. For further details on the LCA methodology and experiments, readers may kindly refer to the ESI.†
Author contributions
M. J. – Investigation, original draft preparation and editing, visualization; M. W. – Conceptualization, methodology, validation, Rietveld refinements, draft review and editing; V. Z. – Methodology, validation, draft review and editing; G. E. – Investigation, editing; S. K. – Draft review and editing; G. C. – Conceptualization; A. F. – Funding acquisition, resources, editing; A. W. – Funding acquisition, resources, conceptualization; All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the German Federal Ministry of Education and Research within the project NexPlas—project number 03SF0618B and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—project number 435833397. We acknowledge the assistance of Kerstin Lakus-Wollny in the conduction of SEM and EDXS. We also thank Claudia Fasel for the conduction of combined thermal analysis measurements. Open access funding enabled and organized by Projekt DEAL.References
- W. B. Meyer, Human impact on the earth, Cambridge University Press, Cambridge, 1996 Search PubMed.
- OECD, Global Material Resources Outlook to 2060: Economic Drivers and Environmental Consequences, OECD Publishing, Paris, 2019 Search PubMed.
- S. Kaza, L. Yao, P. Bhada-Tata and F. van Woerden, What a waste 2.0. A global snapshot of solid waste management to 2050, International Bank for Reconstruction and Development/The World Bank, Washington DC, 2018 Search PubMed.
- J. Rockström, W. Steffen, K. Noone, Å. Persson, F. S. Chapin III, E. Lambin, T. M. Lenton, M. Scheffer, C. Folke, H. J. Schellnhuber, B. Nykvist, C. A. de Wit, T. Hughes, S. van der Leeuw, H. Rodhe, S. Sörlin, P. K. Snyder, R. Costanza, U. Svedin, M. Falkenmark, L. Karlberg, R. W. Corell, V. J. Fabry, J. Hansen, B. Walker, D. Liverman, K. Richardson, P. Crutzen and J. Foley, Ecol. Soc., 2009, 14, 32 CrossRef.
- T. M. Lenton, Nat. Clim. Change, 2011, 1, 201–209 CrossRef.
- E. S. Poloczanska, C. J. Brown, W. J. Sydeman, W. Kiessling, D. S. Schoeman, P. J. Moore, K. Brander, J. F. Bruno, L. B. Buckley, M. T. Burrows, C. M. Duarte, B. S. Halpern, J. Holding, C. V. Kappel, M. I. O'Connor, J. M. Pandolfi, C. Parmesan, F. Schwing, S. A. Thompson and A. J. Richardson, Nat. Clim. Change, 2013, 3, 919–925 CrossRef.
- Eric Post, Ecology of Climate Change The Importance of Biotic Interactions, Princeton University Press, Princeton, 2013 Search PubMed.
- A. J. McMichael, R. E. Woodruff and S. Hales, Lancet, 2006, 367, 859–869 CrossRef PubMed.
- S. Kabisch, A. K. Kunath, P. Schweizer-Ries and A. Steinführer, Vulnerability, risks, and complexity: impacts of global change on human habitats, Hogrefe Publishing, Massachusetts, 2012 Search PubMed.
- M. Hofmann and H. J. Schellnhuber, Energy Environ. Sci., 2010, 3, 1883–1896 RSC.
- European Commission, A new circular economy action plan, available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1583933814386&uri=COM:2020:98:FIN, (accessed 31 January 2022).
- ELLEN MACARTHUR FOUNDATION, Completing the Picture – How the circular economy tackles climate change, available at: https://ellenmacarthurfoundation.org/completing-the-picture, (accessed 8 April 2022).
- ELLEN MACARTHUR FOUNDATION, Universal Circular Economy Policy Goals, available at: https://ellenmacarthurfoundation.org/universal-policy-goals/overview, (accessed 8 April 2022).
- J. Korhonen, A. Honkasalo and J. Seppälä, Ecol. Econ., 2018, 143, 37–46 CrossRef.
- W. R. Stahel, Nature, 2016, 531, 435–438 CrossRef CAS PubMed.
- D. S. Ginley and D. Cahen, Fundamentals of materials for energy and environmental sustainability, Cambridge University Press, Cambridge, 2011 Search PubMed.
- Pathways towards a German Circular Economy. Lessons from European Strategies (Preliminary Study), ed. T. Weber and M. Stuchtey, acatech – National Academy of Science and Engineering, Munich, 2019 Search PubMed.
- M. A. Omer and T. Noguchi, Sustainable Cities Soc., 2020, 52, 101869 CrossRef.
- S. Klemenz, A. Stegmüller, S. Yoon, C. Felser, H. Tüysüz and A. Weidenkaff, Angew. Chem., Int. Ed., 2021, 60, 20094–20100 CrossRef CAS PubMed.
- United Nations Environmental Programme, Green and Sustainable Chemistry: Framework Manual, 2020, available at: https://www.unep.org/explore-topics/chemicals-waste/what-we-do/policy-and-governance/green-and-sustainable-chemistry, (accessed 29 January 2022).
- A. Halpaap and J. Dittkrist, Curr. Opin. Green Sustainable Chem., 2018, 9, 25–29 CrossRef.
- C. Blum, D. Bunke, M. Hungsberg, E. Roelofs, A. Joas, R. Joas, M. Blepp and H.-C. Stolzenberg, Sustainable Chem. Pharm., 2017, 5, 94–104 CrossRef CAS.
- E. Bontempi, G. P. Sorrentino, A. Zanoletti, I. Alessandri, L. E. Depero and A. Caneschi, Molecules, 2021, 26, 1407 CrossRef CAS PubMed.
- DIN Deutsches Institut für Normung e. V., DKE Deutsche Kommission Elektrotechnik, Elektronik und Informationstechnik and VDI Verein Deutscher Ingenieure, Deutsche Normungsroadmap Circular Economy, Berlin/Offenbach/Düsseldorf, 2023.
- P. V. M. Dixini, B. B. Carvalho, G. R. Gonçalves, V. C. B. Pegoretti and M. B. J. G. Freitas, Ionics, 2019, 25, 4381–4392 CrossRef CAS.
- G. Zhang, Z. Du, Y. He, H. Wang, W. Xie and T. Zhang, Sustainability, 2019, 11, 2363–2374 CrossRef CAS.
- P. Gupta and A. Mahajan, Environ. Chem. Lett., 2019, 17, 879–895 CrossRef CAS.
- S. B. Peh, Y. Wang and D. Zhao, ACS Sustainable Chem. Eng., 2019, 7, 3647–3670 CrossRef CAS.
- W. S. Putro, A. Ikeda, S. Shigeyasu, S. Hamura, S. Matsumoto, V. Y. Lee, J.-C. Choi and N. Fukaya, ChemSusChem, 2021, 14, 842–846 CrossRef CAS PubMed.
- R. M. Rodrigues, D. A. Thadathil, K. Ponmudi, A. George and A. Varghese, ChemistrySelect, 2022, 7, e202200081 CrossRef CAS.
- R. S. Varma, ACS Sustainable Chem. Eng., 2016, 4, 5866–5878 CrossRef CAS PubMed.
- H. Zhai, C. Bian, Y. Yu, L. Zhu, L. Guo, X. Wang, Q. Yu, J. Zhu and X. Cao, Crystals, 2019, 9, 338 CrossRef CAS.
- N. Baccile, F. Babonneau, B. Thomas and T. Coradin, J. Mater. Chem., 2009, 19, 8537 RSC.
- D. Tan, P. Chen, G. Wang, G. Chen, T. Pietsch, E. Brunner, T. Doert and M. Ruck, RSC Adv., 2020, 10, 22250–22256 Search PubMed.
- E. Carlos, R. Martins, E. Fortunato and R. Branquinho, Chem. Eur., 2020, 26, 9099–9125 CrossRef CAS PubMed.
- Life Cycle Assessment. Theory and Practice, ed. M. Z. Hauschild, R. K. Rosenbaum and O. S. Irving, Springer International Publishing, Cham, 2018 Search PubMed.
- T. Uekert, J. S. DesVeaux, A. Singh, S. R. Nicholson, P. Lamers, T. Ghosh, J. E. McGeehan, A. C. Carpenter and G. T. Beckham, Green Chem., 2022, 24, 6531–6543 RSC.
- Z. Wang and S. Hellweg, ACS Sustainable Chem. Eng., 2021, 9, 6939–6951 CrossRef CAS.
- M. A. Gonzalez, S. Takkellapati, K. Tadele, T. Li and R. S. Varma, ACS Sustainable Chem. Eng., 2019, 7, 6744–6757 CrossRef CAS PubMed.
- J. Kleinekorte, L. Fleitmann, M. Bachmann, A. Kätelhön, A. Barbosa-Póvoa, N. von der Assen and A. Bardow, Annu. Rev. Chem. Biomol. Eng., 2020, 11, 203–233 CrossRef PubMed.
- X. Jia, Z. Li, F. Wang and Y. Qian, Clean Technol. Environ. Policy, 2016, 18, 1295–1306 CrossRef CAS.
- A. D. Patel, K. Meesters, H. den Uil, E. de Jong, K. Blok and M. K. Patel, Energy Environ. Sci., 2012, 5, 8430 RSC.
- S. Maranghi and C. Brondi, Life Cycle Assessment in the Chemical Product Chain, Springer International Publishing, Cham, 2020 Search PubMed.
- DIN Deutsches Institut für Normung e. V., Umweltmanagement – Ökobilanz – Grundsätze und Rahmenbedingungen (ISO 14040
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2006 + Amd 1
2006 + Amd 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2020). Deutsche Fassung EN ISO 14040
2020). Deutsche Fassung EN ISO 14040![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2006 + A1:2020.
2006 + A1:2020. - DIN Deutsches Institut für Normung e. V., Umweltmanagement – Ökobilanz – Anforderungen und Anleitungen (ISO 14044
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2006 + Amd 1
2006 + Amd 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2017 + Amd 2
2017 + Amd 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2020). Deutsche Fassung EN ISO 14044
2020). Deutsche Fassung EN ISO 14044![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2006 + A1:2018 + A2:2020.
2006 + A1:2018 + A2:2020. - X. Domènech, J. A. Ayllón, J. Peral and J. Rieradevall, Environ. Sci. Technol., 2002, 36, 5517–5520 CrossRef PubMed.
- B. Agarski, V. Nikolić, Ž. Kamberović, Z. Anđić, B. Kosec and I. Budak, J. Cleaner Prod., 2017, 162, 7–15 CrossRef CAS.
- O. G. Griffiths, J. P. O'Byrne, L. Torrente-Murciano, M. D. Jones, D. Mattia and M. C. McManus, J. Cleaner Prod., 2013, 42, 180–189 Search PubMed.
- A. A. Burgess and D. J. Brennan, Chem. Eng. Sci., 2001, 56, 2589–2604 CrossRef CAS.
- S.-S. Lee and T.-W. Hong, Materials, 2014, 7, 6677–6685 CrossRef PubMed.
- E. Sánchez-Cruces, E. Barrera-Calva, K. Lavanderos and F. González, Energy Procedia, 2014, 57, 2812–2818 CrossRef.
- A. Schreiber, J. Marx and P. Zapp, J. Membr. Sci., 2013, 440, 122–133 CrossRef CAS.
- S. Troy, A. Schreiber, T. Reppert, H.-G. Gehrke, M. Finsterbusch, S. Uhlenbruck and P. Stenzel, Appl. Energy, 2016, 169, 757–767 CrossRef.
- X. Tian, S. D. Stranks and F. You, Nat. Sustainability, 2021, 4, 821–829 CrossRef.
- R. Meys, F. Frick, S. Westhues, A. Sternberg, J. Klankermayer and A. Bardow, Resour., Conserv. Recycl., 2020, 162, 105010 CrossRef.
- D. Kralisch, D. Ott and D. Gericke, Green Chem., 2015, 17, 123–145 RSC.
- S. Weyand, C. Wittich and L. Schebek, Energies, 2019, 12, 4228 CrossRef CAS.
- N. Thonemann, A. Schulte and D. Maga, Sustainability, 2020, 12, 1192 CrossRef.
- M. Cossutta, J. McKechnie and S. J. Pickering, Green Chem., 2017, 19, 5874–5884 RSC.
- E. Gasafi and M. Weil, J. Cleaner Prod., 2011, 19, 1590–1600 CrossRef CAS.
- A. C. Hetherington, A. L. Borrion, O. G. Griffiths and M. C. McManus, Int. J. Life Cycle Assess., 2014, 19, 130–143 CrossRef.
- S. Righi, A. Dal Pozzo, A. Tugnoli, A. Raggi, B. Salieri and R. Hischier, in Life Cycle Assessment in the Chemical Product Chain, ed. S. Maranghi and C. Brondi, Springer International Publishing, Cham, 1st edn, 2020, pp. 3–32 Search PubMed.
- C. Samorì, D. Cespi, P. Blair, P. Galletti, D. Malferrari, F. Passarini, I. Vassura and E. Tagliavini, Green Chem., 2017, 19, 1714–1720 RSC.
- R. Deng, P. R. Dias, M. M. Lunardi and J. Ji, Green Chem., 2021, 23, 10157–10167 RSC.
- G. Chen, A. Feldhoff, A. Weidenkaff, C. Li, S. Liu, X. Zhu, J. Sunarso, K. Huang, X.-Y. Wu, A. F. Ghoniem, W. Yang, J. Xue, H. Wang, Z. Shao, J. H. Duffy, K. S. Brinkman, X. Tan, Y. Zhang, H. Jiang, R. Costa, K. A. Friedrich and R. Kriegel, Adv. Funct. Mater., 2022, 32, 2105702 CrossRef CAS.
- A. Jun, J. Kim, J. Shin and G. Kim, ChemElectroChem, 2016, 3, 511–530 CrossRef CAS.
- J. Sunarso, S. Baumann, J. M. Serra, W. A. Meulenberg, S. Liu, Y. S. Lin and J. C. Diniz da Costa, J. Membr. Sci., 2008, 320, 13–41 CrossRef CAS.
- A. Arratibel Plazaola, A. Cruellas Labella, Y. Liu, N. Badiola Porras, D. Pacheco Tanaka, M. Sint Annaland and F. Gallucci, Processes, 2019, 7, 128 CrossRef.
- G. Chen, F. Buck, I. Kistner, M. Widenmeyer, T. Schiestel, A. Schulz, M. Walker and A. Weidenkaff, Chem. Eng. J., 2020, 392, 123699 CrossRef CAS.
- G. J. van Rooij, H. N. Akse, W. A. Bongers and M. C. M. van de Sanden, Plasma Phys. Controlled Fusion, 2018, 60, 14019 CrossRef.
- J. Heelan, E. Gratz, Z. Zheng, Q. Wang, M. Chen, D. Apelian and Y. Wang, JOM, 2016, 68, 2632–2638 CrossRef CAS.
- G. A. Blengini, C. El Latunussa, U. Eynard, C. Torres de Matos, D. M. A. G. Wittmer, K. Georgitzikis, C. C. Pavel, S. Carrara, L. Mancini, M. Unguru, D. Blagoeva, F. Mathieux and D. Pennington, Study on the EU's list of critical raw materials (2020). Final report, Publications Office of the European Union, Luxembourg, 2020 Search PubMed.
- C. Zhang, J. Sunarso and S. Liu, Chem. Soc. Rev., 2017, 46, 2941–3005 RSC.
- B. Rutkowski, R. Kriegel and J. Malzbender, J. Membr. Sci., 2014, 462, 69–74 CrossRef CAS.
- K. Eichhorn Colombo, V. V. Kharton, A. P. Viskup, A. V. Kovalevsky, A. L. Shaula and O. Bolland, J. Solid State Electrochem., 2011, 15, 329–347 CrossRef CAS.
- A. A. Yaremchenko, V. V. Kharton, M. V. Patrakeev and J. R. Frade, J. Mater. Chem., 2003, 13, 1136–1144 RSC.
- P. Chhillar, B. P. Dhamaniya, V. Dutta and S. K. Pathak, ACS Omega, 2019, 4, 11880–11887 CrossRef CAS PubMed.
- J. M. Kadro, N. Pellet, F. Giordano, A. Ulianov, O. Müntener, J. Maier, M. Grätzel and A. Hagfeldt, Energy Environ. Sci., 2016, 9, 3172–3179 RSC.
- Circular Business Models: Overcoming Barriers, Unleashing Potential, ed. Circular Economy Initiative Deutschland, acatech/SYSTEMIQ, Munich/London, 2020 Search PubMed.
- G. Chen, M. Widenmeyer, B. Tang, L. Kaeswurm, L. Wang, A. Feldhoff and A. Weidenkaff, Front. Chem. Sci. Eng., 2020, 14, 405–414 CrossRef CAS.
- A. Tuncuk, V. Stazi, A. Akcil, E. Y. Yazici and H. Deveci, Miner. Eng., 2012, 25, 28–37 CrossRef CAS.
- M. K. Jha, J. Lee, M. Kim, J. Jeong, B.-S. Kim and V. Kumar, Hydrometallurgy, 2013, 133, 23–32 CrossRef CAS.
- K. Binnemans, P. T. Jones, B. Blanpain, T. van Gerven, Y. Yang, A. Walton and M. Buchert, J. Cleaner Prod., 2013, 51, 1–22 CrossRef CAS.
- S. Syed, Waste Manage., 2016, 50, 234–256 CrossRef CAS PubMed.
- W. Zhang, J. Yang, X. Wu, Y. Hu, W. Yu, J. Wang, J. Dong, M. Li, S. Liang, J. Hu and R. V. Kumar, Renewable Sustainable Energy Rev., 2016, 61, 108–122 CrossRef CAS.
- S. M. Abdelbasir, S. S. M. Hassan, A. H. Kamel and R. S. El-Nasr, Environ. Sci. Pollut. Res., 2018, 25, 16533–16547 CrossRef PubMed.
- Z. Wu, W. Yuan, J. Li, X. Wang, L. Liu and J. Wang, Front. Environ. Sci. Eng., 2017, 11, 8 CrossRef.
- L. Cassayre, B. Guzhov, M. Zielinski and B. Biscans, Renewable Sustainable Energy Rev., 2022, 170, 112983 CrossRef CAS.
- Y. Li, W. Lv, H. Huang, W. Yan, X. Li, P. Ning, H. Cao and Z. Sun, Green Chem., 2021, 23, 6139–6171 RSC.
- A. Binek, M. L. Petrus, N. Huber, H. Bristow, Y. Hu, T. Bein and P. Docampo, ACS Appl. Mater. Interfaces, 2016, 8, 12881–12886 CrossRef CAS PubMed.
- B. Chen, C. Fei, S. Chen, H. Gu, X. Xiao and J. Huang, Nat. Commun., 2021, 12, 5859 CrossRef CAS PubMed.
- P.-Y. Chen, J. Qi, M. T. Klug, X. Dang, P. T. Hammond and A. M. Belcher, Energy Environ. Sci., 2014, 7, 3659–3665 RSC.
- J. Yu, X. Wang, M. Zhou and Q. Wang, Energy Environ. Sci., 2019, 12, 2672–2677 RSC.
- A. A. L. Marins, L. M. Boasquevisque, E. J. B. Muri and M. B. Freitas, Mater. Chem. Phys., 2022, 280, 125821 CrossRef CAS.
- A. de La Hoz, A. Díaz-Ortiz and A. Moreno, Chem. Soc. Rev., 2005, 34, 164–178 RSC.
- D. Florian and G. Knapp, Anal. Chem., 2001, 73, 1515–1520 CrossRef CAS PubMed.
- M. Zischka, P. Kettisch and A. Schalk, Fresenius’ J. Anal. Chem., 1998, 361, 90–95 CrossRef CAS.
- K. J. Lamble and S. J. Hill, Analyst, 1998, 123, 103–133 RSC.
- S. Zhu, H. Zhang and R. Bai, Mater. Lett., 2007, 61, 16–18 CrossRef CAS.
- E. Tatár, I. Varga and G. Záray, Microchem. Acta, 1993, 111, 45–54 CrossRef.
- European Commission, Joint Research Centre, Institute for Environment and Sustainability, Characterisation factors of the ILCD Recommended Life Cycle Impact Assessment methods. Database and ESI, Publications Office of the European Union, Ispra, 2012.†.
- R. T. Downs and M. Hall-Wallace, Am. Mineral., 2003, 88, 247–250 CrossRef CAS.
- S. Gražulis, A. Daškevič, A. Merkys, D. Chateigner, L. Lutterotti, M. Quirós, N. R. Serebryanaya, P. Moeck, R. T. Downs and A. Le Bail, Nucleic Acids Res., 2012, 40, D420–D427 CrossRef PubMed.
- S. Gražulis, D. Chateigner, R. T. Downs, A. F. T. Yokochi, M. Quirós, L. Lutterotti, E. Manakova, J. Butkus, P. Moeck and A. Le Bail, J. Appl. Crystallogr., 2009, 42, 726–729 CrossRef PubMed.
- S. Gražulis, A. Merkys, A. Vaitkus and M. Okulič-Kazarinas, J. Appl. Crystallogr., 2015, 48, 85–91 CrossRef PubMed.
- A. Merkys, A. Vaitkus, J. Butkus, M. Okulič-Kazarinas, V. Kairys and S. Gražulis, J. Appl. Crystallogr., 2016, 49, 292–301 CrossRef CAS PubMed.
- M. Quirós, S. Gražulis, S. Girdzijauskaitė, A. Merkys and A. Vaitkus, J. Cheminf., 2018, 10, 23 Search PubMed.
- A. Vaitkus, A. Merkys and S. Gražulis, J. Appl. Crystallogr., 2021, 54, 661–672 CrossRef CAS PubMed.
- K. Efimov, M. Arnold, J. Martynczuk and A. Feldhoff, J. Am. Ceram. Soc., 2009, 92, 876–880 CrossRef CAS.
- G. Chen, Z. Zhao, M. Widenmeyer, R. Yan, L. Wang, A. Feldhoff and A. Weidenkaff, Membranes, 2020, 10, 183 CrossRef CAS PubMed.
- M. Reichmann, P.-M. Geffroy, J. Fouletier, N. Richet and T. Chartier, J. Power Sources, 2014, 261, 175–183 CrossRef CAS.
- N. Tsoy, V. Prado, A. Wypkema, J. Quist and M. Mourad, J. Cleaner Prod., 2019, 221, 365–376 CrossRef.
- F. Asdrubali, G. Baldinelli, F. D'Alessandro and F. Scrucca, Renewable Sustainable Energy Rev., 2015, 42, 1113–1122 CrossRef.
- J. Prado-Gonjal, Á. Arévalo-López and E. Morán, Mater. Res. Bull., 2011, 46, 222–230 CrossRef CAS.
- J. Valdés, D. Reséndiz, Á. Cuán, R. Nava, B. Aguilar, C. M. Cortés-Romero and O. Navarro, Materials, 2021, 14, 3876 CrossRef PubMed.
- K. Polychronopoulou, A. F. Zedan, M. S. Katsiotis, M. A. Baker, A. A. AlKhoori, S. Y. AlQaradawi, S. J. Hinder and S. AlHassan, J. Mol. Catal. A: Chem., 2017, 428, 41–55 CrossRef CAS.
- Y. Zhai, C. Ye, J. Xiao and L. Dai, J. Power Sources, 2006, 163, 316–322 CrossRef CAS.
- I. M. Algunaibet, C. Pozo, Á. Galán-Martín, M. A. J. Huijbregts, N. Mac Dowell and G. Guillén-Gosálbez, Energy Environ. Sci., 2019, 12, 1890–1900 RSC.
- J. Rodriguez-Carvajal, FullProf. 2k (v. 7.00), ILL, Grenoble, 2019 Search PubMed.
- EDAX INC., Genesis Spectrum (v. 5.21), Mahwah, New Jersey, 2007 Search PubMed.
- H. Luo, B. Tian, Y. Wei, H. Wang, H. Jiang and J. Caro, AIChE J., 2010, 56, 604–610 CAS.
- G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz and B. Weidema, The ecoinvent database version 3 (part I): overview and methodology, Int. J. Life Cycle Assess., 2016, 21, 1218–1230 CrossRef , available at: https://link.springer.com/10.1007/s11367-016-1087-8, (accessed 28 January 2022).
- GreenDelta GmbH, openLCA (v 1.10.3), Berlin, 2020 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc00391d |
| ‡ Present address: Ecole polytechnique fédérale de Lausanne EPFL, Laboratory for Molecular Engineering of Optoelectronic Nanomaterials LIMNO, 1015 Lausanne, Switzerland. |
| § Note that a small amount of Si impurities might be present in both samples but was not further investigated. It might have originated from the sand used for cleaning the ball milling equipment. |
| This journal is © The Royal Society of Chemistry 2023 |



