Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of biobased amines via Pd-catalysed telomerisation of the renewable β-myrcene in a water/ethanol multiphase system: catalyst recycling enabled by a self-separating product phase†
Anna
Kampwerth
 ,
Michael
Terhorst
,
Michael
Terhorst
 ,
Nils
Kampling
,
Dieter
Vogt
,
Nils
Kampling
,
Dieter
Vogt
 and
Thomas
Seidensticker
and
Thomas
Seidensticker
 *
*
TU Dortmund University, Department for Biochemical and Chemical Engineering, Laboratory of Industrial Chemistry, Emil-Figge-Straße 66, 44227 Dortmund, Germany
First published on 25th July 2023
Abstract
We developed an aqueous multiphase system for the synthesis of biobased alkyl amines via palladium-catalysed telomerisation based on the terpene β-myrcene. Ethanol was employed as a harmless co-solvent. With this “green” switchable multiphase solvent system, the recycling of the expensive homogeneous catalyst was successfully enabled by self-separating products at room temperature. The total turnover number (TON) was increased to almost 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 over 9 runs, with high selectivities towards the desired amine telomer products between 80 and 93% in each run. Furthermore, various amine nucleophiles were successfully used allowing to produce a wide variety of long-chain unsymmetrical alkyl amines with potential applications as, for instance, surfactant precursors or lubricants. In each case, the products self-separated after reaction from the aqueous multiphase system containing the homogeneous palladium catalyst allowing straightforward isolation by simple decantation. Finally, we proved the successful use of spirits (vodka) as solvents in this sustainable amine synthesis.
000 over 9 runs, with high selectivities towards the desired amine telomer products between 80 and 93% in each run. Furthermore, various amine nucleophiles were successfully used allowing to produce a wide variety of long-chain unsymmetrical alkyl amines with potential applications as, for instance, surfactant precursors or lubricants. In each case, the products self-separated after reaction from the aqueous multiphase system containing the homogeneous palladium catalyst allowing straightforward isolation by simple decantation. Finally, we proved the successful use of spirits (vodka) as solvents in this sustainable amine synthesis.
Introduction
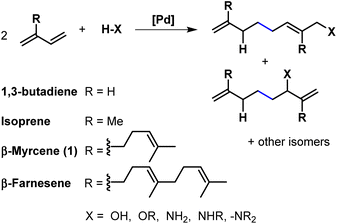
Telomerisation is a versatile catalytic tool in which 1,3-dienes and Brønsted-acidic nucleophiles can be freely selected as starting materials from its construction kit to generate a C–C as well as a C–X bond (Fig. 1). Common catalysts for this unique dimerisation, combined with functionalisation, are usually based on palladium. These are mostly used as homogeneous transition catalysts, although heterogenised ones are also known.1Telomerisation, characterised by its 100% atom economy, is already performed on an industrial scale with 1,3-butadiene as starting material. The process developed by Dow uses the telomerisation with methanol as a nucleophile towards the selective production of 1-octene.2 In addition, 1-octanol is obtained in the process of Kuraray using water as a nucleophile.3,4 Besides these industrial examples, the telomerisation is also part of current research, which is evident by the numbers of reviews and publications.1,5,6 Nevertheless, there are only a few examples where more complex 1,3-dienes than 1,3-butadiene or isoprene were used. Renewable raw materials, however, also offer this unique structure of 1,3-dienes, such as β-myrcene7–11 (1) and β-farnesene12 and thus are promising substrates in telomerisation. Especially 113 is an attractive substitute to fossil dienes because there are no ethical problems caused by competition with food, as it may be with, for example, plant oils. 1 is produced by pyrolysis of β-pinene,14 which is part of turpentine, a side product in paper manufacturing.15 Besides the discussed 1,3-dienes as starting materials, various nucleophiles can be used. As reflected by the two industrial processes, O-nucleophiles such as alcohols, water, and carboxylic acids, are most commonly used. N-Nucleophiles are also used in telomerisation, mainly in the form of amines, which commonly have a high reactivity compared to O-nucleophiles. In the telomerisation, two bonds (C–C and C–X) are formed simultaneously. Since the catalyst is also capable of forming only one of these bonds (C–C or C–X), two side reactions must be considered, the hydroamination (HA) and the dimerisation. Therefore, selective synthesis is particularly important and makes high demands on the active catalytic species. The most important factors determining chemo- and regioselectivity in telomerization are the ratio of metal to ligand, the ratio of 1,3-diene to nucleophile, and the reaction temperature.16,17 In addition, there is already an established reaction mechanism described by Jolly et al. for the telomerization of 1,3-butadiene with methanol, as well as various proposed modifications of this mechanism, also for application to different nucleophiles, like amines.16,18–21 In Fig. 2, the reaction network of the telomerisation of β-myrcene (1) with an amine (2) to the telomer products (3) is shown together with the two most prominent side reactions, the hydroamination (HA), and the dimerization to the corresponding side products (4 and 5).
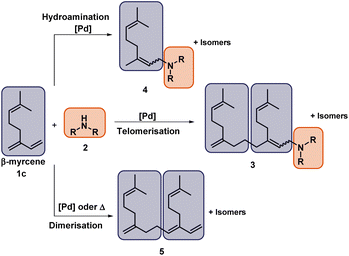 | ||
| Fig. 2 Reaction network of the telomerisation of β-myrcene (1) with an amine (2). R = H, alkyl, aryl. | ||
In addition to these difficult requirements on the reaction side, the recyclability of the expensive catalyst is also extremely important for designing a sustainable process. This ensures that the products are not contaminated with heavy metals, and a higher productivity can be achieved by reusing the catalyst. The importance of the telomerisation as a promising tool becomes further evident when considering that recycling concepts already exist, including telomerisation with renewable raw materials.
In 2010, Behr et al. used a thermomorphic multiphase system consisting of DMF/n-heptane in the first telomerisation of β-myrcene (1) with diethylamine (2a) to separate the catalyst from the products. However, recycling of the catalyst was not carried out.7 In 2018, Vorholt and coworkers performed the first telomerisation of β-farnesene with amines.12 A thermomorphic multiphase system, which formed a nearly pure product phase after cooling the reaction mixture to 0 °C, was achieved using only DMF as solvent. However, a ligand make-up was necessary. Recently, we successfully performed the catalyst recycling in telomerisation of 1 using the reactive ionic liquid (RIL) dimethylammonium-dimethyl carbamate (DimCarb),22 which acts as polar non-aqueous solvent and dimethylamine precursor.9 The catalyst phase was reused in 13 consecutive cycles, resulting in a total turn-over number (TTON) of about 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. However, this recycling concept is limited to the unique RIL and is not easily transferable to other carbamates, since the properties change greatly when the amine component is exchanged.
000. However, this recycling concept is limited to the unique RIL and is not easily transferable to other carbamates, since the properties change greatly when the amine component is exchanged.
Consequently, there is a need of an attractive recycling concept in the telomerisation of challenging substrates that allows the use of different amines to exploit the full potential of this powerful synthesis method. Furthermore, this recycling concept should also offer additional desirable features. The formation of a pure product phase eliminates the need for an extracting agent, which would require energy- and cost-intensive separation from the products in subsequent downstream processes. The polar catalyst phase can be easily separated from the products and directly reused without further steps. Also, a homogeneous system under reaction conditions omits mass transfer limitation that otherwise inhibit the reaction. Moreover, it should be possible to choose the amine concentration independently of the amount of solvent, which simplifies the control of selectivity towards telomer products. In addition, to be sustainable, only environmentally friendly, “green” solvents should be used, e.g., water. Aqueous biphasic systems are already used as a recycling strategy in the chemical industry, like in the Kuraray process. Some examples of water-based biphasic telomerisations that allow catalyst recycling exist in the literature, e.g., the telomerisation of butadiene with ammonia.23,24 However, unlike butadiene, 1 has very low solubility in water, leading to mass transfer limitations. A co-solvent that acts as a phase mediator is, therefore, essential. For a sustainable system, of course, the co-solvent must as well be environmentally friendly. Short-chain alcohols, in particular, have already provided good results for this purpose.10,25–28 Although both alcohol and water may act as nucleophiles in telomerisation, only amine telomers should form since amines are more nucleophilic. This has already been demonstrated in some publications.10,29,30
Thus, we present here the development of a sustainable catalyst recycling concept for the telomerisation of β-myrcene with various amines, using water as the “green” solvent and a short-chain alcohol as co-solvent to achieve the formation of a pure product phase and ideally a single-phase reaction mixture at the reaction temperature. This system exploits the full potential of the versatile catalytic tool: Telomerisation.
Results and discussion
The initial conditions used for the aqueous telomerisation of β-myrcene (1) were based on our publication with the RIL DimCarb.9 Because DimCarb was used as a source of dimethylamine in the previous work, the use of dimethylamine in this work would be advantageous for the sake of comparability. However, since dimethylamine is gaseous and sold in liquid form only as an aqueous or alcoholic solution, the independence of two parameters, the amount of amine and water, would not be given. Therefore, diethylamine (2a) as N-nucleophile was used. Since one amine is consumed per two dienes in telomerisation, the ratio was initially set to 0.7 equivalents of 2a to 1 to allow quantitative conversion of 1. As the catalyst system, a combination of the precursor Pd(acac)2 and the water-soluble ligand triphenylphosphine trisulfonate (TPPTS) was used. The Pd/P ratio is one of the key parameters in telomerisation. The more ligand is used, the lower the activity of the catalyst system, but the higher the long-term stability.17 The initially used Pd/P ratio in this work was set to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, which showed promising results in the previous work.9 In Fig. 3 the reaction system for the following screening experiments is shown.
4, which showed promising results in the previous work.9 In Fig. 3 the reaction system for the following screening experiments is shown.
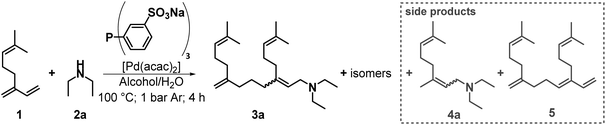 | ||
| Fig. 3 Telomerisation of β-myrcene (1) with diethylamine (2a) forming the telomer products (3a) and the possible side products of the hydroamination (4a) and the dimerization (5). | ||
As the miscibility of water and organic non-polar compounds such as 1 is poor, a phase mediator is needed. It has to provide sufficient phase transfer, while ensuring low catalyst leaching in the non-polar product phase. With reference to the CHEM21 selection guide,31 short-chain alcohols were used with water in a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mass ratio as the solvent in the telomerisation. For comparison, a reaction without co-solvent was also conducted (Fig. 4).
50 mass ratio as the solvent in the telomerisation. For comparison, a reaction without co-solvent was also conducted (Fig. 4).
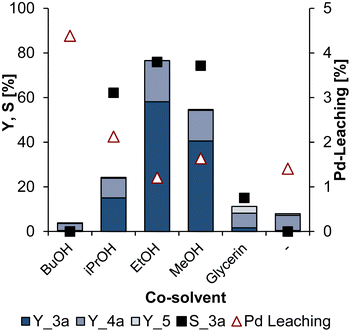
As expected, only the desired N-telomers 3a were formed and no reaction of the present O-nucleophiles alcohol and water was observed. All reaction mixtures were biphasic after the reaction, which is necessary for the desired recycling strategy. Without co-solvent, no telomers were formed. With n-butanol, even less 1c was converted than without any co-solvent, and the Pd leaching is also higher with over 4% of the initial amount of catalyst used. This could be related to the miscibility gap between water and n-butanol.32 Using isopropanol, ethanol as well as methanol and glycerin as phase mediator, 3a were formed. With glycerol as a co-solvent, only 2% of 3a was obtained, and no Pd-leaching could be determined because of the risk of nitroglycerin formation in the digestion procedure for ICP-OES. Highest yield of 58% and selectivity of 76% to 3a were achieved with ethanol. No increase in the selectivity or yield could be achieved when applying isopropanol or methanol. Instead, more catalyst leached into the non-polar product phase. Therefore, ethanol was chosen for further optimisation experiments.
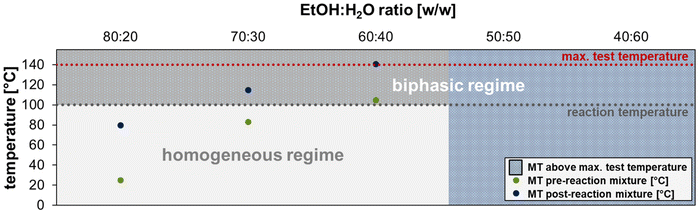
To gain deeper insights into the phase behavior, miscibility studies were carried out with different ethanol–water ratios. These experiments provide information about the mixing temperatures (MT) of the reaction mixtures and were carried out for the pre and post reaction mixtures, whereby complete conversion of 1 with 100% selectivity to the telomers 3a was assumed for the post-reaction mixtures. Ethanol–water ratios of over 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/w) lead to monophasic reaction mixtures at 20 °C and are therefore not considered. A biphasic system is necessary as we focus on the recyclability of the active catalyst species. The reaction temperature was set at 100 °C because selectivity decreases at higher temperatures due to increasing dimerisation (Fig. 5).9,33
10 (w/w) lead to monophasic reaction mixtures at 20 °C and are therefore not considered. A biphasic system is necessary as we focus on the recyclability of the active catalyst species. The reaction temperature was set at 100 °C because selectivity decreases at higher temperatures due to increasing dimerisation (Fig. 5).9,33
No MT could be determined applying the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (w/w) mixture of ethanol and water until 140 °C, as in the initial experiments. A decrease of the ethanol amount also didn't show any promising results. This suggests that we were always in the biphasic regime in the alcohol screening, which is why the reactions had low conversions. By increasing the ethanol amount up to a ratio of 80
50 (w/w) mixture of ethanol and water until 140 °C, as in the initial experiments. A decrease of the ethanol amount also didn't show any promising results. This suggests that we were always in the biphasic regime in the alcohol screening, which is why the reactions had low conversions. By increasing the ethanol amount up to a ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/w), the MT from both, the pre and post reaction mixture decreases. But only with the highest amount of ethanol both MTs were below the reaction temperature of 100 °C. One advantage of this ratio could be that the mass transfer limitations that occur in two-phase systems are avoided since the reaction system is always homogeneous under reaction conditions. However, with increasing amount of phase mediator the Pd-leaching could increase. For a ratio of 70
20 (w/w), the MT from both, the pre and post reaction mixture decreases. But only with the highest amount of ethanol both MTs were below the reaction temperature of 100 °C. One advantage of this ratio could be that the mass transfer limitations that occur in two-phase systems are avoided since the reaction system is always homogeneous under reaction conditions. However, with increasing amount of phase mediator the Pd-leaching could increase. For a ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 the reaction mixture will start monophasic and get biphasic with increasing conversion. Due to this phase behaviour, mass transfer limitations could be kept low as well as efficient phase separation after reaction could be achieved with low Pd leaching into the non-polar product phase. All ratios of 60
30 the reaction mixture will start monophasic and get biphasic with increasing conversion. Due to this phase behaviour, mass transfer limitations could be kept low as well as efficient phase separation after reaction could be achieved with low Pd leaching into the non-polar product phase. All ratios of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and below are biphasic throughout the reaction, with mixing temperatures for the pre-reaction mixture just above 100 °C with the ratio of 60
40 and below are biphasic throughout the reaction, with mixing temperatures for the pre-reaction mixture just above 100 °C with the ratio of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.
40.
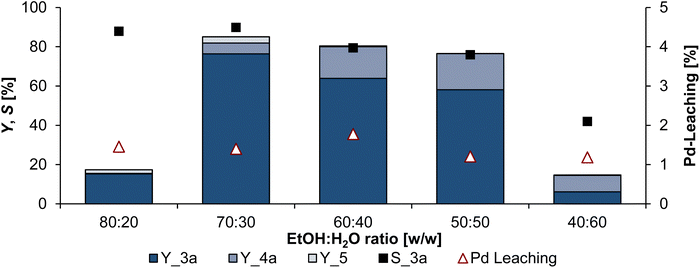
However, a particular phase behaviour does not necessarily mean that this is optimal for the reaction. Thus, all ratios were tested in further experiments (Fig. 6), to see the influence on (catalytic) activity, as well as the Pd-leaching. It was expected that conversion and Pd-leaching increases with increasing ethanol content.
Contrary to our expectations, leaching does not increase with increasing mass fraction of phase mediator but remains constant between 1.2–1.8% of the initial catalyst amount over all ethanol–water ratios investigated. Therefore, the ratio can be adjusted to obtain the highest possible yield. Since the ratios of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 to 40
40 to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 are always biphasic, which we know thanks to the miscibility studies, phase transfer problems should occur here. The hydroamination requires only one molecule of β-myrcene (1) and one amine molecule, whereas the telomerisation requires two molecules of 1 to coordinate to the catalyst. Since the catalyst resides in the polar aqueous phase and 1 is non-polar, the probability of two molecules of 1 coordinating at the catalyst decreases as the amount of co-solvent acting as a phase mediator is reduced. Consequently, more hydroamination products (4a) were formed in these constantly biphasic reaction mixtures, and the yield of telomers 3a increases with an increasing amount of ethanol. The highest selectivity and conversion were obtained with an ethanol–water ratio of 70
60 are always biphasic, which we know thanks to the miscibility studies, phase transfer problems should occur here. The hydroamination requires only one molecule of β-myrcene (1) and one amine molecule, whereas the telomerisation requires two molecules of 1 to coordinate to the catalyst. Since the catalyst resides in the polar aqueous phase and 1 is non-polar, the probability of two molecules of 1 coordinating at the catalyst decreases as the amount of co-solvent acting as a phase mediator is reduced. Consequently, more hydroamination products (4a) were formed in these constantly biphasic reaction mixtures, and the yield of telomers 3a increases with an increasing amount of ethanol. The highest selectivity and conversion were obtained with an ethanol–water ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, which indicates that a thermomorphic behaviour is advantageous for telomerisation. Here, the reaction is homogeneous at the beginning and enters the biphasic regime with increasing conversion to 3a. This system provides excellent selectivity towards 3a of 90% and a yield of 76.5%. Since with an ethanol–water ratio of 80
30, which indicates that a thermomorphic behaviour is advantageous for telomerisation. Here, the reaction is homogeneous at the beginning and enters the biphasic regime with increasing conversion to 3a. This system provides excellent selectivity towards 3a of 90% and a yield of 76.5%. Since with an ethanol–water ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 the reaction mixture is homogeneous throughout the reaction, it is reasonable to assume that this ratio allows excellent yield and selectivity to telomers 3a. However, only a conversion of 1 of less than 20% is achieved here. After the reaction, a yellowish residue was found at the bottom of the reactor, which suggests that the solubility of the catalyst in this reaction mixture is insufficient.
20 the reaction mixture is homogeneous throughout the reaction, it is reasonable to assume that this ratio allows excellent yield and selectivity to telomers 3a. However, only a conversion of 1 of less than 20% is achieved here. After the reaction, a yellowish residue was found at the bottom of the reactor, which suggests that the solubility of the catalyst in this reaction mixture is insufficient.
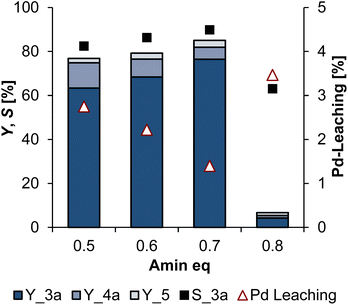
With the selected ethanol–water ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, the amine equivalent was investigated in the next step (Fig. 7). Since 2a also has a phase-mediating effect, a variation might have an influence on the phase behaviour and thus the reaction performance. In addition, more amine could favour the undesired hydroamination.
30, the amine equivalent was investigated in the next step (Fig. 7). Since 2a also has a phase-mediating effect, a variation might have an influence on the phase behaviour and thus the reaction performance. In addition, more amine could favour the undesired hydroamination.
With the selected ethanol–water ratio, the amine equivalent of 0.7 is optimal. With less 2a, the selectivity and conversion decrease. Furthermore, the formation of 4a increases. This indicates that the reduction in amine equivalents leads to a biphasic reaction mixture, leading to mass transfer limitations. A higher amine equivalent is also not beneficial and leads to more Pd-leaching.
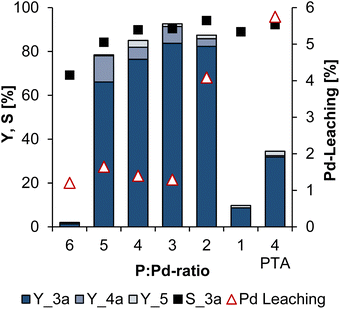
In theory, the selectivity towards 3a should increase with less ligand, because two molecules of 1 need to coordinate at the Pd-catalyst to undergo the telomerisation. Therefore, the more ligand is present in the solution, the more the substrate 1 has to compete for the vacant coordination sites on the catalyst. Thus, the ratio of phosphor to palladium (P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd ratio) is a key parameter for shifting the chemoselectivity to the telomerisation and was next screened (Fig. 8).
Pd ratio) is a key parameter for shifting the chemoselectivity to the telomerisation and was next screened (Fig. 8).
As expected, the selectivity towards the telomers 3a and the conversion increases with decreasing amount of ligand, except for the lowest ratio of one, in which conversion of 1 is only 10%. The highest selectivity of 94% was achieved with a P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd ratio of 2. However, this amount of TPPTS is apparently insufficient to immobilise and stabilise the catalyst in the aqueous polar phase after the reaction, as palladium particles were present in the reaction mixture, which was confirmed by ICP-OES analysis. The most promising results were obtained with a P
Pd ratio of 2. However, this amount of TPPTS is apparently insufficient to immobilise and stabilise the catalyst in the aqueous polar phase after the reaction, as palladium particles were present in the reaction mixture, which was confirmed by ICP-OES analysis. The most promising results were obtained with a P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd ratio of 3. This ratio achieved 84% yield of 3a, corresponding to a selectivity to 3a of 90%, a palladium leaching of about 1%, and a productivity (moles of substrate converted to the desired product per mole of Pd) of around 2.500. In addition to the already well known TPPTS ligand, the water-soluble ligand 1,3,5-triaza-7-phosphaadamantane (PTA) was also tested in this multiphase system. PTA has already been used with 1,3-butadiene in water, and both telomeres and oligomers were obtained.34 In the reaction system developed here, 32% telomeres 3a were formed with a selectivity of 92% when PTA was used as a ligand. Thus, compared to the reaction with TPPTS, the reaction with PTA as ligand is slower and the Pd-leaching is higher at about 6%.
Pd ratio of 3. This ratio achieved 84% yield of 3a, corresponding to a selectivity to 3a of 90%, a palladium leaching of about 1%, and a productivity (moles of substrate converted to the desired product per mole of Pd) of around 2.500. In addition to the already well known TPPTS ligand, the water-soluble ligand 1,3,5-triaza-7-phosphaadamantane (PTA) was also tested in this multiphase system. PTA has already been used with 1,3-butadiene in water, and both telomeres and oligomers were obtained.34 In the reaction system developed here, 32% telomeres 3a were formed with a selectivity of 92% when PTA was used as a ligand. Thus, compared to the reaction with TPPTS, the reaction with PTA as ligand is slower and the Pd-leaching is higher at about 6%.
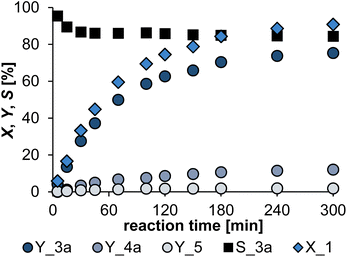
To confirm the stability of this system, a scale-up from a 25 mL to a 300 mL reactor was successfully conducted resulting in a slightly lower selectivity of 85%. A reaction profile of the reaction was recorded (Fig. 9).
At a conversion of less than 20% the selectivity to 3a is close to 90%. Already at a conversion of 40%, the selectivity drops to 86%, and at the end of the reaction (X = 91%) the selectivity is 84.5%. This shows that the selectivity decreases with increasing conversion, which is due to the decreasing concentration of 1a in the reaction mixture. From a process engineering point of view, a low conversion with high selectivity should be selected, since the unreacted starting material can then be recirculated by purification. Nevertheless, as expected, the products self-separated after reaction from the aqueous multiphase system allowing straightforward isolation of the bio-based amine. Subsequent vacuum distillation of the product phase allowed isolation of the desired telomer 3a (ca. 70% isolated yield, 83 mmol, 28.7 g).
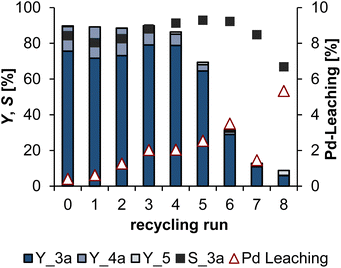
After proving that the system is stable for a scale up, it must obviously be verified that the recycling of the catalyst is possible with this solvent system. Not only to simplify the separation in the reactor after the reaction, but also to allow observation of the phase behavior during the reaction, a homemade 40 mL glass reactor was used for the subsequent recycling experiments, in which the reproducibility was checked beforehand. Detailed information on the exact setup is presented in the ESI.† With a P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd ratio of 3, which obtained excellent results in the batch experiments, the recycling of the catalyst was only stable over three runs with steadily decreasing conversion of 1 after the initial run (see in ESI, Fig. S5†). Already after the first recycling run, black residues appeared at the reactor edge, which were precipitated palladium particles. To stabilise the catalyst and achieve better retention in the polar phase, the P
Pd ratio of 3, which obtained excellent results in the batch experiments, the recycling of the catalyst was only stable over three runs with steadily decreasing conversion of 1 after the initial run (see in ESI, Fig. S5†). Already after the first recycling run, black residues appeared at the reactor edge, which were precipitated palladium particles. To stabilise the catalyst and achieve better retention in the polar phase, the P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd ratio was next changed to 5 (Fig. 10).
Pd ratio was next changed to 5 (Fig. 10).
With the changed P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd ratio of 5, the recycling of the catalyst was able over 4 stable runs with yields of the telomers 3a between 73 and 79%. The yield of 3a as well as the conversion of 1 is about 10% higher compared to the batch experiments in Fig. 8. However, the selectivity to 3a is comparable. This indicates that the reaction proceeds faster in the glass reactor than in the smaller 25 mL steel reactors. From the fifth recycling run onwards, the yield drops to 65%, until only 6% 3a is obtained in run 8. However, the selectivities are high, as usual, with the exception of run 8. The catalyst leaching increases already in the second recycling run to more than 1% of the palladium mass used at the beginning. By run 6, this then increases further to 3.5%. After the 8th run, the total loss of palladium via recycling amounts to just under 20% of the initial mass. Reasons for the increase of palladium leaching could be, for example, the contamination with oxygen. This would led to oxidation of the ligand, resulting in the leaching of uncoordinated palladium. However, the catalyst phase was checked by 31P-NMR after completion of the recycling, and only a small amount of oxidised phosphorus was detected. After run 4, the formation of a black deposit on the reactor glass was observed in this recycling experiment, as in the first recycling experiment. Using ICP-OES, this could be identified as precipitated palladium particles. Thus, the loss of activity is not exclusively due to the leaching of palladium, but also to the formation of non-active palladium particles which are not included in the leaching results. Over the entire recycling experiment, a TTON of 11
Pd ratio of 5, the recycling of the catalyst was able over 4 stable runs with yields of the telomers 3a between 73 and 79%. The yield of 3a as well as the conversion of 1 is about 10% higher compared to the batch experiments in Fig. 8. However, the selectivity to 3a is comparable. This indicates that the reaction proceeds faster in the glass reactor than in the smaller 25 mL steel reactors. From the fifth recycling run onwards, the yield drops to 65%, until only 6% 3a is obtained in run 8. However, the selectivities are high, as usual, with the exception of run 8. The catalyst leaching increases already in the second recycling run to more than 1% of the palladium mass used at the beginning. By run 6, this then increases further to 3.5%. After the 8th run, the total loss of palladium via recycling amounts to just under 20% of the initial mass. Reasons for the increase of palladium leaching could be, for example, the contamination with oxygen. This would led to oxidation of the ligand, resulting in the leaching of uncoordinated palladium. However, the catalyst phase was checked by 31P-NMR after completion of the recycling, and only a small amount of oxidised phosphorus was detected. After run 4, the formation of a black deposit on the reactor glass was observed in this recycling experiment, as in the first recycling experiment. Using ICP-OES, this could be identified as precipitated palladium particles. Thus, the loss of activity is not exclusively due to the leaching of palladium, but also to the formation of non-active palladium particles which are not included in the leaching results. Over the entire recycling experiment, a TTON of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 618 is achieved. The same catalyst system of Pd(acac)2 and TPPTS had also achieved a TTON of nearly 12
618 is achieved. The same catalyst system of Pd(acac)2 and TPPTS had also achieved a TTON of nearly 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in the DimCarb system published by our group in 2020.9 Thus, both solvent systems have almost identical productivity. However, the selectivity to the telomers in the water/ethanol system with over 80% is significantly better than in the DimCarb system with around 70%. In addition, the water/ethanol system offers the possibility to vary the amine used easily, which we investigated next.
000 in the DimCarb system published by our group in 2020.9 Thus, both solvent systems have almost identical productivity. However, the selectivity to the telomers in the water/ethanol system with over 80% is significantly better than in the DimCarb system with around 70%. In addition, the water/ethanol system offers the possibility to vary the amine used easily, which we investigated next.
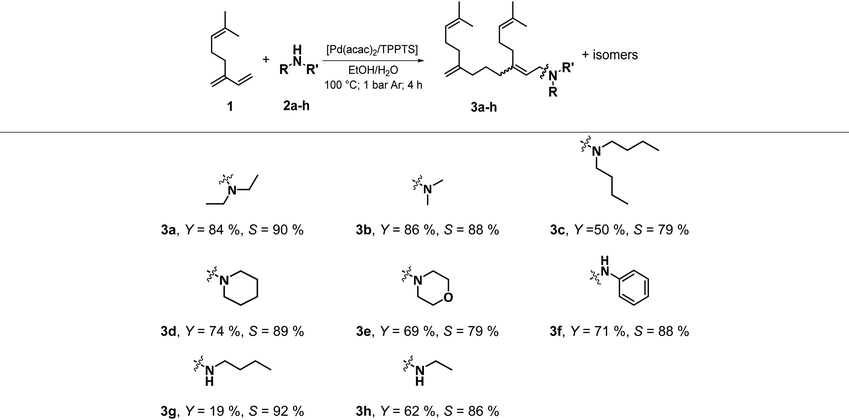
With the optimised reaction conditions, different classes of amines (2) were employed in the telomerisation to prove the general applicability of the multiphase system. Different alkylamines, cyclic and heterocyclic amines, as well as ammonia, primary and secondary amines were used (Table 1).
In addition to the diethylamine (2a) investigated at the beginning, with which a yield of 3a of 84% was achieved, the telomerisation of 1 was also successfully carried out with other dialkyl amines in the aqueous ethanol system. With dimethylamine (2b), a yield of telomer 3b of 86% and with di-n-butylamine (2c) of 50% to 3c was reached. However, no telomerisation was observed with the branched di-iso-propylamine. Telomers were also obtained with the heterocyclic amines piperidine (2d) and morpholine 2e, with yields of 74% 3d and 69% 3e, respectively. Using the primary amine aniline (2f), the secondary amine 3f was also synthesised with excellent yield of 71%. With the two linear primary amines, ethylamine (2g) and butylamine (2h), the corresponding telomeres 3g and 3h were obtained with 19% and 62% yield. In addition, telomerisation with the monopeptide sarcosine, para-aminobenzoic acid and with ammonia was also investigated, but no conversion to the telomer products were observed. In all reactions where telomerisation occurred, palladium leaching was between 2 and 4% of the initial amount of catalyst used. The only exceptions were the reaction with aniline with a palladium leaching of 64% and butylamine with a leaching of 12%. For all amines used, the product phase contained between 5 and 11% of the ethanol used. Only with aniline as nucleophile the amount was 77%, which is the reason for the high palladium leaching.
Not only the amine used can be exchanged in the telomerization, but also the 1,3-diene. Instead of β-myrcene, β-farnesene was also used in the telomerization to further prove the general applicability of our multiphase system. Under the same reaction conditions (see Table 1) and with the diethylamine (2a) as nucleophile, a yield of the β-farnesene telomers of 65% was achieved with a selectivity of 88%.
The overall aim of this work was to develop a sustainable reaction concept for the telomerisation of 1 with different amines allowing for easy product separation and potential catalyst recycling. This was to be realised using a green solvent system. The ethanol–water solvent used here is probably one of the “greenest” systems imaginable, as it is practically drinkable. To further illustrate this, we performed the reaction in commercially available Rasputin brand vodka with 70 vol% (Fig. 11), which is equivalent to about 61 w% (see in ESI, Fig. S6†).
In vodka, the same selectivity towards the telomers 3a of 88% was achieved as in the 70 w% ethanol–water solvent system, mixed in the laboratory from ultrapure water and 99.8% ethanol absolute. Only the conversion with 85% is slightly lower than in the 70 w% mixture with 95%, which is probably due to the reduced amount of co-solvent, acting as a phase mediator. In comparison to the reaction with 60 w% ethanol–water solvent system, the conversion is with 83% in a similar range. However, the selectivity is significantly lower, since a P/Pd ratio of 4 was used. This shows that even in conventional drinkable alcohol, such as vodka, telomerisation can be carried out in excellent yields.
Conclusion
In this work, an aqueous multiphase system was successfully developed for the palladium-catalysed amino-telomerisation of the renewable β-myrcene (1). With this water/ethanol solvent system, the recycling of the expensive homogeneous catalyst was enabled by the self-separating products at room temperature (Fig. 11). Detailed phase studies also showed that the best results were obtained with a phase behavior in which the reaction mixture is initially monophasic at reaction conditions and then becomes biphasic35 with increasing conversion. With this “green” switchable multiphase solvent system, catalyst recycling was achieved, and the total turnover number was increased to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 618 over 9 runs, with selectivity towards the telomer products 3a between 80 and 93% in each run. Furthermore, various other amines could be used in the reaction to produce a wide variety of long-chain unsymmetrical secondary and tertiary amines 3 based on the renewable raw material β-myrcene. For each product, easy separation from the homogeneous palladium catalyst was achieved by simple decantation of the pure product phase. To demonstrate the robustness and greeness of our approach, commercial spirit (vodka) was successfully used as solvent.
618 over 9 runs, with selectivity towards the telomer products 3a between 80 and 93% in each run. Furthermore, various other amines could be used in the reaction to produce a wide variety of long-chain unsymmetrical secondary and tertiary amines 3 based on the renewable raw material β-myrcene. For each product, easy separation from the homogeneous palladium catalyst was achieved by simple decantation of the pure product phase. To demonstrate the robustness and greeness of our approach, commercial spirit (vodka) was successfully used as solvent.
Experimental section
Preparation of chemicals
All substrates used, as well as the catalyst, ligand, and solvents, are commercially available and were purchased from ABCR, Acros Organics, TCI, Merck, VWR, OXEA or Umicore. Each week, β-myrcene was purified over an alumina column and stored under argon. To perform all reactions under strictly inert conditions, the substrates (diethylamine, β-myrcene) and solvents (ethanol, water) were each transferred to Schlenk's vessel. Afterwards, oxygen was removed from the substrates using the freeze-pump-thaw method. The vessel containing the substrate was sealed and connected to a Schlenk line via a hose. The valve of the Schlenk's vessel was closed, and the substrate was frozen with liquid nitrogen. A vacuum was then applied to the vessel for approx. 15 min. Especially for diethylamine, care must be taken to ensure that the substrate is completely frozen before opening the valve to vacuum; otherwise, the substrate would be removed. The valve of the Schlenk's vessel was then closed again, removed from the liquid nitrogen, and placed in a warm water bath to remelt the substrate. The formation of gas bubbles was observed. After the substrate was completely melted again, it was frozen again with liquid nitrogen and finally vacuumed again. This procedure was performed four times for each substrate and solvent.Screening experiments in 25 mL stainless steel autoclaves
The screening experiments were performed in custom made 25 mL stainless steel autoclaves (cf. ESI†). Under argon atmosphere, the palladium precursor and the ligand were first introduced into the reactors. The autoclaves were sealed and inerted by applying vacuum and purging with argon four times. Ethanol, water, amine, and β-myrcene were then added sequentially to the reactor in argon counter-flow. The reaction mixture was heated to 100 °C and stirred at 500 rpm for 4 h. After the reaction time, the autoclaves were cooled, depressurised, and emptied. The samples were prepared for further investigations via GC-FID.Recycling experiments in 40 mL glass autoclave
Under argon atmosphere, the catalyst and ligand were first added to an inert Schlenk vessel. Ethanol and water were then added in argon counter-flow. The solution was homogenised in an ultrasonic bath. The glass autoclave (cf. ESI†) was inerted by applying vacuum and then purged with argon. In argon counter-flow, the catalyst phase was introduced into the reactor. Diethylamine and β-myrcene were then added in argon counter-flow. The reactor was heated to 100 °C with an oil bath and stirred at 500 rpm for 4 h. After the reaction, the reactor was cooled with an ice bath. In argon counter-flow, the upper phase (product phase) was removed with a cannula. β-Myrcene, diethylamine, and a small amount of ethanol were added again, and the reactor was again stirred at 100 °C with 500 rpm for 4 h. The product phase weighed, and analysed via GC-FID.Conflicts of interest
■■■■Acknowledgements
Gefördert durch die Deutsche Forschungsgemeinschaft (DFG)–TRR 63 “Integrierte chemische Prosesse in flüssigen Mehrphasensystemen” (Teilprojekt A11) – 56091768 – funded by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)–TRR 63 “Integrated Chemical Processes in Liquid Multiphase Systems” (subproject A11) – 56091768. The authors are very thankful to the German Federal Ministry of Food and Agriculture (Bundesministerium für Ernährung und Landwirtschaft) represented by the FNR (Fachagentur Nachwachsende Rohstoffe) for financial support of the junior research group “Renewlysis” (Project No. 2219NR355). Support through the research network SusChemSys 2.0 is gratefully acknowledged. We thankfully acknowledge Umicore AG & Co. KG for donation of the palladium precursors and OQ Chemicals (formerly OXEA GmbH) for the donation of Na-TPPTS solution.References
- T. A. Faßbach, A. J. Vorholt and W. Leitner, The Telomerization of 1,3-Dienes - A Reaction Grows Up, ChemCatChem, 2019, 11, 1153–1166 CrossRef.
- R. C. Bohley, G. B. Jacobsen, H. L. Pelt, B. J. Schaart, M. Schenk and D. A. G. van Oeffelen, Process for producing 1-octene, WO1992010450A1, 1992.
- N. Yoshimura and M. Tamura, Process for producing normal-octanol, US4417079A, 1983.
- T. Maeda, Y. Tokitoh and N. Yoshimura, Phosphonium salts and processes for production of and uses for the same, EP0296550A2, 1988.
- A. Behr, M. Becker, T. Beckmann, L. Johnen, J. Leschinski and S. Reyer, Telomerization: advances and applications of a versatile reaction, Angew. Chem., Int. Ed., 2009, 48, 3598–3614 CrossRef CAS PubMed.
- S. Bouquillon, J. Muzart, C. Pinel and F. Rataboul, Palladium-catalyzed telomerization of butadiene with polyols: from mono to polysaccharides, in Carbohydrates in Sustainable Development II - A mine for functional molecules and materials, ed. A. P. Rauter, P. Vogel and Y. Queneau, Springer, Berlin, 2010, vol. 295 Search PubMed.
- A. Behr, L. Johnen and A. J. Vorholt, Telomerization of myrcene and catalyst separation by thermomorphic solvent systems, ChemCatChem, 2010, 2, 1271–1277 CrossRef CAS.
- J. M. Lopes, Z. Petrovski, R. Bogel-Łukasik and E. Bogel-Łukasik, Heterogeneous palladium-catalyzed telomerization of myrcene with glycerol derivatives in supercritical carbon dioxide: a facile route to new building blocks, Green Chem., 2011, 13, 2013–2016 RSC.
- M. Terhorst, A. Kampwerth, A. Marschand, D. Vogt, A. Vorholt and T. Seidensticker, Facile Catalyst Recycling by Thermomorphic Behaviour Avoiding Organic Solvents – A Reactive Ionic Liquid in the Homogeneously Pd-Catalysed Telomerisation of the Renewable β-Myrcene, Catal. Sci. Technol., 2020 Search PubMed.
- T. A. Faßbach, F. O. Sommer, A. Behr, S. Romanski, D. Leinweber and A. J. Vorholt, Non-ionic surfactants from renewables-amphiphilic ligands in biphasic reactions, Catal. Sci. Technol., 2017, 7, 1650–1653 RSC.
- D. Vogelsang, M. Dittmar, T. Seidensticker and A. J. Vorholt, Palladium-catalysed carboxytelomerisation of β-myrcene to highly branched C 21 -esters, Catal. Sci. Technol., 2018, 8, 4332–4337 RSC.
- D. Vogelsang, T. A. Faßbach, P. P. Kossmann and A. J. Vorholt, Terpene-Derived Highly Branched C30-Amines via Palladium-Catalysed Telomerisation of \beta-Farnesene, Adv. Synth. Catal., 2018, 360, 1984–1991 CrossRef CAS.
- A. Behr and L. Johnen, Myrcene as a natural base chemical in sustainable chemistry: A critical review, ChemSusChem, 2009, 2, 1072–1095 CrossRef CAS PubMed.
- L. A. Goldblatt and T. R. Savich, Process for producing myrcene from beta-pinene, US2507546A, 1948.
- J. C. Roberts, The Chemistry of Paper, Royal Society of Chemistry, Cambridge, 1996 Search PubMed.
- F. Vollmüller, J. Krause, S. Klein, W. Mägerlein and M. Beller, Control of Chemo- and Regioselectivity in the Palladium-Catalyzed Telomerization of Butadiene with Methanol – Catalysis and Mechanism, Chem. Ber., 2000, 2000, 1825–1832 Search PubMed.
- N. Yoshimura, Typical Reactions. Hydrodimerization, in Aqueous–Phase Organometallic Catalysis, ed. B. Cornils and W. A. Herrmann, Wiley-VCH, Weinheim, 2004 Search PubMed.
- S. M. Maddock and M. G. Finn, Palladium-catalyzed head-to-head telomerization of isoprene with amines, Organometallics, 2000, 19, 2684–2689 CrossRef CAS.
- P. W. Jolly, η3-Allylpalladium-Verbindungen, Angew. Chem., Int. Ed. Engl., 1985, 97, 279–291 CrossRef CAS.
- P. W. Jolly, R. Mynott, B. Raspel and K. P. Schick, Intermediates in the palladium-catalyzed reactions of 1,3-dienes. The reaction of (.eta.1,.eta.3-octadiendiyl)palladium complexes with acidic substrates, Organometallics, 1986, 5, 473–481 CrossRef CAS.
- R. Benn, P. W. Jolly, R. Mynott, B. Raspel, G. Schenker, K. P. Schick and G. Schroth, Intermediates in the palladium-catalyzed reactions of 1,3-dienes. 2. Preparation and structure of (.eta.1,.eta.3-octadienediyl)palladium complexes, Organometallics, 1985, 4, 1945–1953 CrossRef CAS.
- W. Schroth, H.-D.-D. Schädler and J. Andersch, Struktur und Aggregation von Dimethylammonium–dimethylcarbamat (Dimcarb) und analogen Dialkylammonium–dialkylcarbamaten, Z. Chem., 1989, 29, 129–135 CrossRef CAS.
- T. Prinz and B. Driessen-Hölscher, Biphasic Catalyzed Telomerization of Butadiene and Ammonia: Kinetics and New Ligands for Regioselective Reactions, Chem. – Eur. J., 1999, 5, 2069–2076 CrossRef CAS.
- T. Prinz, W. Keim and B. Driessen-Hölscher, Two-Phase Catalysis: A Strategy for Avoiding Consecutive Reactions as Exemplified in the Telomerization of Butadiene and Ammonia, Angew. Chem., Int. Ed. Engl., 1996, 35, 1708–1710 CrossRef CAS.
- N. Herrmann, J. Bianga, T. Gaide, M. Drewing, D. Vogt and T. Seidensticker, Aqueous biphasic hydroformylation of methyl oleate: a green solvent-only strategy for homogeneous catalyst recycling, Green Chem., 2019, 21, 6738–6745 RSC.
- T. Rösler, T. A. Faßbach, M. Schrimpf, A. J. Vorholt and W. Leitner, Toward Water-Based Recycling Techniques: Methodologies for Homogeneous Catalyst Recycling in Liquid/Liquid Multiphase Media and Their Implementation in Continuous Processes, Ind. Eng. Chem. Res., 2019, 58, 2421–2436 CrossRef.
- P. Purwanto and H. Delmas, Gas-liquid-liquid reaction engineering: hydroformylation of 1-octene using a water soluble rhodium complex catalyst, Catal. Today, 1995, 24, 135–140 CrossRef CAS.
- J. Mesnager, C. Quettier, A. Lambin, F. Rataboul and C. Pinel, Telomerization of butadiene with starch under mild conditions, ChemSusChem, 2009, 2, 1125–1129 CrossRef CAS PubMed.
- A. Grotevendt, M. Bartolome, D. J. Nielsen, A. Spannenberg, R. Jackstell, K. J. Cavell, L. A. Oro and M. Beller, Efficient catalysts for telomerization of butadiene with amines, Tetrahedron Lett., 2007, 48, 9203–9207 CrossRef CAS.
- T. A. Faßbach, S. Püschel, A. Behr, S. Romanski, D. Leinweber and A. J. Vorholt, Towards a process for the telomerization of butadiene with N-methylglucamine, Chem. Eng. Sci., 2018, 181, 122–131 CrossRef.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, CHEM21 selection guide of classical- and less classical-solvents, Green Chem., 2016, 18, 288–296 RSC.
- R. Stephenson and J. Stuart, Mutual binary solubilities: water-alcohols and water-esters, J. Chem. Eng. Data, 1986, 31, 56–70 CrossRef CAS.
- A. Behr and A. J. Vorholt, Homogeneous Catalysis with Renewables, Springer International Publishing, Cham, 2017 Search PubMed.
- J. Čermák, M. Kvíčalová and V. Blechta, Nickel(0) and Palladium(0) Complexes with 1,3,5-Triaza-7-phosphaadamantane. Catalysis of Buta-1,3-diene Oligomerization or Telomerization in an AqueousBiphasic System, Collect. Czech. Chem. Commun., 1997, 62, 355–363 CrossRef.
- J. Bianga, K. U. Künnemann, T. Gaide, A. J. Vorholt, T. Seidensticker, J. M. Dreimann and D. Vogt, Thermomorphic Multiphase Systems: Switchable Solvent Mixtures for the Recovery of Homogeneous Catalysts in Batch and Flow Processes, Chem. – Eur. J., 2019, 25, 11586–11608 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc00453h |
| This journal is © The Royal Society of Chemistry 2023 |