Efficient synthesis of 5-methyl-2-furancarboxylic acid via selective hydrogenolysis of bio-renewable 5-hydroxymethyl-2-furancarboxylic acid on Pd/C catalysts at ambient temperature†
Abstract
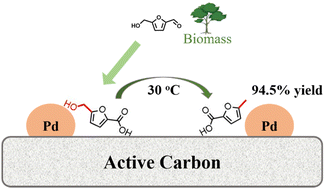
5-Methyl-2-furancarboxylic acid (MFA) is an important substituted furoic acid with versatile applications. However, its synthesis processes are not green and efficient. Here, we report a novel route to the sustainable synthesis of MFA from bio-renewable 5-hydroxymethyl-2-furancarboxylic acid (HMFA) by the direct cleavage of the C–OH bond in HMFA at ambient temperature. Active carbon (C)-supported Pd catalysts exhibited high efficiency and stability in HMFA hydrogenolysis to MFA, providing a high yield of 94.5% at 30 °C and 3.0 MPa H2 in tetrahydrofuran. Such a high efficiency of Pd/C was found to be related to the strong adsorption of HMFA on the C support surfaces, most likely via their π–π interactions. This work provides an efficient strategy for the selective cleavage of the α-C–OH bonds in the furan ring under mild conditions and the sustainable production of MFA and its derivatives from biomass resources.


 Please wait while we load your content...
Please wait while we load your content...