Defluorophosphorylation of fluoroalkyl peroxides for the synthesis of highly substituted furans†
Abstract
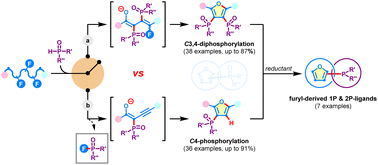
Transformation of multifunctional materials with control over site-selectivity and chemical diversity remains challenging. Herein, we present a metal-free, one-pot strategy for the defluorophosphorylation of polyfluoroalkyl peroxides that enables expedient construction of structurally diverse phosphoryl-containing heterocyclic libraries. By judicious choice of reaction conditions, C3,4-diphosphoryl furans and C4-monophosphoryl furans can be easily accessed. In addition, synthetic derivatization of the obtained organophosphorus heteroarenes to value-added monodentate and bidentate phosphines has been demonstrated. Mechanistic studies revealed that regioselective defluorophosphorylation allows divergent product formation in two reaction modes.


 Please wait while we load your content...
Please wait while we load your content...