Open Access Article
Open Access ArticleHigh-yield synthesis of HMF from glucose and fructose by selective catalysis with water-tolerant rare earth metal triflates assisted by choline chloride†
Fabrizio
Olivito
 *,
Vincenzo
Algieri
*,
Vincenzo
Algieri
 ,
Matteo Antonio
Tallarida
,
Matteo Antonio
Tallarida
 ,
Antonio
Jiritano
,
Paola
Costanzo
,
Antonio
Jiritano
,
Paola
Costanzo
 ,
Loredana
Maiuolo
,
Loredana
Maiuolo
 and
Antonio De
Nino
and
Antonio De
Nino
 *
*
Department of Chemistry and Chemical Technologies, University of Calabria, Via P. Bucci, Cubo 12C, Rende, CS, Italy. E-mail: denino@unical.it; fabrizio.olivito@unical.it
First published on 26th January 2023
Abstract
The conversion of naturally occurring organic substances into value-added platform chemicals by simple, green, and efficient procedures represents one of the most accessible and sought-after routes towards sustainable chemistry. In the present work, we report the remarkable catalytic activity of rare-earth metal triflates in conjunction with choline chloride, a natural, low-cost, and available organic compound to selectively convert glucose and fructose into hydroxymethylfurfural (HMF). The hypothesized mechanism is based on the initial glycosylation of glucose assisted by scandium(III) triflate and choline chloride to produce a glycoside, which can evolve through an intramolecular rearrangement and subsequent dehydration to produce the final product HMF. A comparison with other types of catalysts is carried out with particular focus on the side reactions. The apparatus consists of a closed biphasic system and the excellent capacity of methyl propyl ketone (MPK) to extract HMF in only one cycle is proved. The process was conducted at 150 °C using 1.5 molar equivalents of choline chloride in which glucose was converted into HMF after three hours using the catalyst in 8% molar quantity, while fructose was converted in one hour employing the catalyst in 4% molar quantity. The best performance was obtained by employing scandium(III) triflate as a catalyst with an yield of 94% and 99% of HMF from glucose or fructose, respectively. We assumed a first-order reaction model for both glucose and fructose conversion into HMF. The R-squared values are greater than 0.9, demonstrating that our kinetic model fitted well with the experimental results. In addition, activation energies are 16.9 kJ mol−1 for glucose and 9.31 kJ mol−1 for fructose due to the longer reaction path of glucose. The catalytic system can be recycled up to five times with a HMF yield of over 80% for glucose and over 90% for fructose, maintaining the same selectivity.
1. Introduction
The growing limitations of fossil resources have turned the spotlight onto innovative and sustainable technologies.1,2 Renewable sources have gained enormous attention over the past years and this is largely due to the constant demand related to the expansion of the world population.3–5 Biomass-derived substances represent the main source to draw renewable raw materials to be converted into useful products with a similar value to those obtained from fossil feedstocks.6–8 Carbohydrate research grew fast in this direction and it is now a consolidated starting point to develop alternative chemistry processes.9–11 Glucose is not only abundant in nature and food-related products, but it also represents important energy storage for different living organisms.12,13 It can be recovered from natural sources or can be produced from naturally occurring polymers, for instance, and non-food waste, like cellulose.14–16 Fructose is less abundant than glucose but is also conspicuously present in nature as a renewable feedstock.17–19 Furan biomass-derived molecules represent important targets for carbohydrate research.20 Over the years, an extraordinary number of breakthroughs have been achieved regarding synthetic strategies and industrial applications, which have allowed these products to gain a precious market value.21–23 Given the increasing use and multiple connections in various sectors, such as biofuels, textile industry, medical, pharmaceutical, and construction, these compounds have acquired the name of platform molecules.24–26 HMF is one of the most investigated molecules belonging to this category with a broad network of interactions in different sectors.27,28 Furthermore, as a platform molecule, HMF represents a versatile intermediate to obtain various high-value derivatives, for example, deriving from its oxidation. In fact, 2,5-diformylfuran and 2,5-furandicarboxylic acid are promising intermediates for pharmaceuticals and very useful polymer-building blocks for the synthesis of functional polymers.29–31HMF is a well-known product of sugar dehydration, studied and researched for decades.32,33 Catalysis has played a central role in this process from the past years to the present day.34,35Conventionally, when the starting material is glucose, a Lewis acid is usually employed for its isomerization to fructose and then a Brønsted acid is necessary to push the dehydration towards the product.36,37 Other reports have also hypothesized the direct dehydration of glucose to HMF by Brønsted acid without passing through fructose.38,39 The interest in the selective production of HMF is always growing and this increases the publication of scientific papers.40,41 A valid experimental procedure developed in this field consists of a biphasic system, in which the extraction solvent plays a central role together with other factors such as the catalyst loading, temperature, and reaction time.42,43 Hydroxymethyl furfural is known to be unstable under aqueous conditions in the presence of Brønsted acids, because it can undergo rehydration to levulinic acid and formic acid or produce humin by auto-condensation.44,45 Metal halides usually employed as Lewis acids easily decompose in the presence of water or under harsh reaction conditions producing inorganic acids, such as hydrochloric acid, that can decompose HMF.46,47 Choline chloride-based DES together with metal halide have been proven to be efficient methods to produce HMF from carbohydrates, but the main drawbacks are the need for large amounts of solvents to extract the product from the ionic phase and the toxicity of Lewis acid metals, for example, the use of chromium.48,49 Rare earth metal halides and triflate have proved to be excellent Lewis acids as the catalytic system for HMF synthesis from glucose and fructose but the use of harmful solvents like N,N′-dimethylacetamide (DMA), and DMSO was necessary to reach efficient yields.50,51 In continuity with respect to the well-documented catalytic activity and water-tolerability of triflate lanthanides,52–54 in this work, we proved the stability and the exceptional catalytic activity of rare-earth metal triflates to produce HMF through isomerization-dehydration of carbohydrates, with the participation of choline chloride, an abundant, cheap and biodegradable substrate that allows Fisher-type glycosylation on fructose before the dehydration steps. The used reaction apparatus consists of a biphasic system in which methyl propyl ketone (MPK) was proved to be an excellent extraction solvent for the recovery of HMF with respect to conventional ones. A comparison employing the relative metal halides was carried out and the yields and reaction by-products are discussed. The methodology furnished optimum selectivity and yields. Screening of different rare-earth metal cations was carried out, with the analysis of the used molar ratio, catalyst loading, temperature, and reaction time. The catalytic mechanism has been hypothesized and discussed. The mechanism is explained through the high affinity of scandium for oxygen, the tendency of glucose to form the glucoside with choline, conditions suitable for dehydration, and numerous NMR and kinetic tests. HMF was extracted after only one reaction cycle while the recyclability of the catalytic system was proved to furnish excellent yields up to five times.
2. Experimental
2.1 Materials and methods
All chemicals and solvents were purchased from common commercial sources (see ESI†). Solvents were distilled before use. The analytical standard of HMF was purchased in analytical grade from Sigma Aldrich. All reactions were monitored by GC/MS analysis (Shimadzu, Kyoto, Japan). The GC-MS Shimadzu workstation was constituted by a GC 2010 (equipped with a 30 m-QUADREX 007-5MS capillary column, operating in the “split” mode, 1 mL min−1 flow of He as carrier gas) and a 2010 quadrupole mass-detector. 1H and 13C NMR spectra were recorded on Brüker spectrometers (Bruker Instrument, Inc., Zurich, Switzerland) at 300 MHz and 126 MHz, respectively, in CDCl3 or D2O. Chemical shifts were reported in δ units (ppm) with tetramethylsilane (TMS) as a reference.Samples were analysed using HPLC Agilent series 1100 equipped with an isocratic pump (Agilent technologies 1200 series) and UV-vis detector for HMF analysis and a refractive index detector for the detection of unreacted glucose and fructose. The reversed phase analytical column was a C18 Jupiter, with dimensions of 300 A, 250 × 4.60 mm, and 10 microns for particle size (Phenomenex, USA). The wavelength of the reference was 360 nm with a peak width of 100, while the wavelength for HMF analysis was set to 385 nm with a peak width of 8 mm. The chosen mobile phase was methanol (50%) and acetonitrile (50%), in the isocratic elution mode at a flow rate of 1 mL min−1. The sample injection volume was 20 μL. The column was washed with methanol after 5 injections for 30 minutes. The quantitative analysis of HMF was performed by HPLC with a UV-vis detector, using the external analytical standard method. The unknown sample was diluted in water, filtered, and injected after removing the reaction solvent. The unreacted quantities of glucose and fructose were analysed by HPLC, dissolving the solid at the bottom of the biphasic system in water, using HPLC with a refractive index detector and a solution of 5 mM H2SO4 as the mobile phase. Each test was done in triplicate and the values were expressed as mean values. Qualitative analysis of the products and the relative by-products in the solvent phase was evaluated by GC-MS, using a gradient temperature from 70 °C to 250 °C with an increase of 16 °C min−1.
Glucose conversion or fructose conversion (x), HMF yield (y), and HMF selectivity (s) were calculated based on the following eqn (1)–(3), in accordance with the previously reported studies:55
 | (1) |
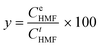 | (2) |
 | (3) |
For the recycling test, the catalytic system consisting of choline chloride/rare earth metal triflate was used without any pre-treatment for the next cycle, after removing the extraction solvent.
2.2 Preparation of rare earth metal triflates
Cerium(III) triflate, lanthanum(III) triflate, and ytterbium(III) triflate were prepared freshly in accordance with a procedure reported in the literature.56 Cerium chloride heptahydrate, lanthanum chloride heptahydrate, and ytterbium chloride hexahydrate, were dissolved in distilled water and trifluoromethanesulfonic acid (3 molar equivalents) was added drop-wise at room temperature. The reaction was allowed to stir at room temperature for two hours and after that, the solvent was removed under a vacuum. The solid was washed several times with diethyl ether and dried in an oven at 80 °C for one day and after that, the product was kept in a chemical drier for 15 days. Traces of water remained in the FT-IR spectrum in accordance with the literature.51 The FT-IR spectra of the freshly synthesized cerium(III) triflate, lanthanum(III) triflate, and ytterbium(III) triflate were acquired using the Shimadzu IRAffinity-1S spectrometer (Shimadzu Corporation) in the spectral region of 375 and 4000 cm−1 with a resolution of 1 cm−1, setting 50 scans for a single analysis and using the KBr pellet technique. The KBr pellets were obtained by mixing the sample with KBr powder (ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) and pressing with a hydraulic press, at the pressure of 10 tons for 5 minutes. The resulting pellets were placed in the appropriate compartment of the instrument and exposed to the FT-IR light beam for analysis.
100) and pressing with a hydraulic press, at the pressure of 10 tons for 5 minutes. The resulting pellets were placed in the appropriate compartment of the instrument and exposed to the FT-IR light beam for analysis.
2.3 Reaction procedure
Glucose (40 mg) was mixed with choline chloride (1.5 molar equivalents) and the relative metal triflate (8% molar quantity with respect to glucose) in a closed Pyrex vial. Methyl propyl ketone (4 ml) was added, the vial was closed with a cap and the biphasic system was allowed to stir for three hours by using a magnetic bar at 150 °C. The solution was decanted and filtered through a sintered glass filter and the solvent was removed under a vacuum. The product in the form of a yellowish oil was analysed by GC-MS and HPLC to detect any trace of by-products and isolated with a yield of 94%. The unreacted solid was dissolved in water and analysed by HPLC to detect the trace of unreacted glucose. The purity of the product was also confirmed by 1H NMR spectroscopy in accordance with the spectra previously reported.57Fructose (40 mg) was mixed with choline chloride (1.5 molar equivalents) and the relative metal triflate (4% molar quantity with respect to fructose) in a vial. Methyl propyl ketone (4 ml) was added, the vial was closed with a cap and the biphasic system was allowed to stir for one hour by using a magnetic bar at 150 °C. The solution was decanted and filtered through a sintered glass filter and the solvent was removed under a vacuum. The product in the form of yellowish oil was analysed by GC-MS and HPLC to detect any trace of by-products and isolated with a yield of 99%. The unreacted solid was dissolved in water and analysed by HPLC to detect the traces of unreacted fructose. The purity of the product was also confirmed by 1H NMR spectroscopy in accordance with the spectra previously reported.48
3. Results and discussion
We focused our attention on the development of a synthetic strategy to produce HMF in high yields and selectivity, starting from fully renewable sources. Choline chloride is biocompatible and cheap molecule that was proved recently to possess a stabilizing effect for HMF production.58 In particular, there are different reports on the physicochemical investigation of choline chloride-based DES with glucose and fructose or other sugars.59 One of the most important limitations of the use of DES as a solvent, involves the difficulty to extract HMF quantitatively, due to the strong affinity of this molecule for these hydrogen-bond-rich phases.60 K. De Oliveira Vigier et al., recently focused attention on the role of choline chloride not only as a stabilizing agent but mostly as a reactive participant in the conversion of xylose into furfural.61 With these important considerations in mind, we started the optimization of the process.3.1 Optimization of the catalytic system
Initially, starting from glucose and fructose, we used an equimolar quantity of choline chloride in which these sugars were completely solubilized at 150 °C after some time. To evaluate any conversion, we used a closed vial, MIBK (methyl isobutyl ketone) as a conventional extraction solvent, to create a biphasic system,62 as illustrated in Fig. 1. The latter is only a representative scheme, in which the real used volumes are not shown.After three hours, no trace of HMF was detected by GC-MS in the solvent phase. HMF is commonly produced by the dehydration reaction of sugars and for this reason, with the aim of creating a stable catalytic system, we used rare earth metal triflates because the water-tolerability of these species has been known for a long time.63,64 We started with the optimization of the reaction conditions using glucose, choline chloride, and Er(OTf)3 with MIBK as the extraction solvent. The results are reported in the following Table 1.
Using one equivalent of choline chloride, the glucose conversion into HMF increases with the increase of the percentage of the catalyst (entries 1–4). The percentage of the product is the same using 8% of the catalyst instead of 10% (entries 4 and 5). When half of the choline chloride equivalents are used (entry 6), the glucose conversion is lower. This result may demonstrate that choline chloride plays an essential role in the catalysis of this reaction and that it is not merely a reaction medium. In any case, the optimal equivalents of choline chloride are 1.5 because when 2 equivalents were used, the extraction process becomes worse, probably because the product has a stronger affinity with choline chloride with respect to the solvent (entries 7 and 8). Starting from fructose, the optimized conditions consist of 1.5 equivalent of choline chloride, 4% molar of the catalyst, and the quantitative yield is obtained after one hour. Both for glucose and fructose, we did not detect any by-products in the reaction phase by HPLC analysis.
At this point, a screening of different metal triflates starting from glucose and choline chloride was carried out and the resulting HMF yield is reported in Table 2.
The HMF yields increase passing from lanthanum to ytterbium and scandium (entries 1–6), demonstrating that Lewis acid catalysis is fundamental in this process and is linked to electrons and the ionic radius of the element.65
The same evaluation of the different metal triflates was conducted starting from fructose and the yield of HMF is reported in Table 3.
The same trend of the metals is obtained from the reaction starting from fructose, with a yield increasing from lanthanum to scandium (entries 1–6). As can be seen, the yield in HMF is higher with respect to all the metals tested under the same reaction conditions on glucose.
In this reaction process, given the production of water molecules, the Lewis acidity of rare earth metals is also influenced by hydrolysis constants and high order of exchange rate constants for the substitution of inner-sphere water ligands (WERC), as reported in many studies, which are in agreement with the observed trend. In accordance with the literature,66 the parameters are shown in Table 4.
| Entry | Cations | pKh | WERC |
|---|---|---|---|
| 1 | La3+ | 7.6–8.5 | 106–108 |
| 2 | Ce3+ | 8.3 | 2.7 × 108 |
| 3 | Ho3+ | 8 | 6.1 × 107 |
| 4 | Er3+ | 7.9 | 1.4 × 108 |
| 5 | Yb3+ | 7.7 | 8 × 107 |
| 6 | Sc3+ | 4.3 | 4.8 × 107 |
Metal cations with pKh values from 4.3 to 10.08 and WERC higher than 3.2 × 106 M−1s−1 show excellent performances in the reactions in which they act as Lewis acids. When the values are below this limit, metals are easily hydrolysable, while beyond this limit they are too stable to catalyse a reaction.67,68
From entries 1–6, it is evident that all the rare earth metals used are in this range of values and can be considered optimal Lewis acids.
At this point, considering the very good results obtained from both glucose and fructose, we decided to employ scandium(III) triflate as a catalyst for HMF production.
It is noteworthy that, in the presence of triflates as a catalyst, formic acid as a by-product was not detected. All of this is evident by the GC-MS chromatograms of crude that have showed only formation of HMF (see ESI†), also by using the operative conditions reported in literature to perform the quantitative detection of formic acid.69,70
3.2 The effect of the solvent
Starting from the promising results achieved with MIBK, we tested different solvents on the model reaction starting from glucose, choline chloride, and scandium(III) triflate. The results are presented in Fig. 2.When polar aprotic solvents like acetone and acetonitrile were used, the yield of HMF was 41% and 30%, respectively. Probably the greater polarity of the solvent prevents the product formation because the reagents are solubilized and their contact was restricted. This deduction was confirmed when ethanol and water were used as polar protic solvents. The yields of 10% and 8%, respectively, are due to the complete dissolution and solvation of the reagents without the formation of a biphasic system in the vial.
When methyl propyl ketone (MPK) was used, the reaction yield increased to 94% (Table 5, entry 1) with respect to 84% (Table 2, entry 6) deriving from the use of conventional methyl isobutyl ketone (MIBK). This effect is justifiable because MPK is slightly more polar than MIBK, but has no solvation effect compared to the reactants. In addition, it has a lower boiling point, which allows easier removal from the product once formed. The reaction was also conducted in MPK for fructose, observing the same quantitative yield of 99%. Table 5 summarizes the obtained results for glucose and fructose as the starting materials.
| Entry | Reagent | Conversion (%) | HMF yield (%) |
|---|---|---|---|
| 1 | Glucose | 95 | 94 |
| 2 | Fructose | 100 | 99 |
To demonstrate the ability of MPK to extract the product, we calculated the partition coefficients of HMF (PHMF) in MPK/water and MIBK/water systems. Therefore, 5 mL of MPK or MIBK, and 5 mL of water containing 100 mM HMF, were mixed together and heated to the desired temperature. The partition coefficient (PHMF) was calculated by dividing the concentration of HMF (CHMF) in the extraction phase by CHMF in the aqueous phase. The concentration was analysed by HPLC coupled with a UV detector. In Table 6 we reported the obtained data from 25 °C to 60 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Vorganic/Vaq and 0.1 mM HMF)
1 Vorganic/Vaq and 0.1 mM HMF)
| Entry | T (°C) | P HMF (MPK) | P HMF (MIBK) |
|---|---|---|---|
| 1 | 25 | 1.55 | 1.34 |
| 2 | 40 | 1.54 | 1.33 |
| 3 | 60 | 1.54 | 1.33 |
As reported in Table 6, the partition coefficient of HMF in MPK/water is higher than that of MIBK/water at all the tested temperatures (entries 1–3). This is another confirmation of the potential that this new solvent has if compared to the conventional ones, such as MIBK.71
3.3 Effect of reaction temperature
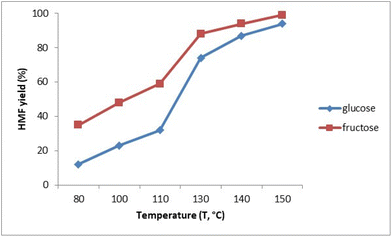
We evaluated the effect of temperature on the conversion of glucose and fructose into HMF, starting from the optimized reaction conditions. The values are reported in Fig. 3.The production of HMF in this catalytic process is strongly dependent on the temperature. For glucose, the yield is about 12% at 80 °C until the maximum yield of 94% is reached 150 °C. From 150 °C onwards, the graph reached a plateau. It is remarkable how the product remains stable in the extraction solvent under the optimized reaction conditions even at high temperatures. The same trend was proved to start from fructose with reaction yields of HMF higher at all the tested reaction times. The yield is about 35% at 80 °C until a maximum yield of 99% at 150 °C and the value remained constant at higher temperatures. Both for glucose and fructose, a significant increase in the HMF yield between 110 °C and 130 °C was evident. Excellent selectivity was confirmed by no detection of by-products through the analysis of the reaction phase by HPLC for both carbohydrates.
3.4 Effect of reaction time
The dependence of the HMF yield on the reaction times is another critical parameter; the plots relative to the reaction starting from glucose (A) and fructose (B) at three different temperatures are shown in Fig. 4.We began the investigation of the reaction kinetics relative to HMF production from glucose (Fig. 4A). The slope of the graph is higher at 130 °C and 150 °C respect to 110 °C, this is proof that temperature is crucial in this process. From 120 to 180 minutes, the process shows the best yield of 94% at 150 °C. Over the time of 180 minutes, the graph reached a plateau at all the temperatures used.
For the reaction conducted starting from fructose, the kinetics are remarkably different (Fig. 4B). The slope of the graph is similar at 130 °C and 150 °C and just a little lower at 110 °C. The reaction starting from fructose is already efficient in terms of yield. After only five minutes, the yield of HMF produced was 40% at 150 °C, the optimized yield of 99% was reached after 60 minutes at the same temperature. An identical yield was obtained after 90 minutes, demonstrating the stability of the product in the reaction medium. The difference in the reaction kinetics starting from glucose and fructose will be discussed in sections 3.6 and 3.7.
3.5 Product analysis using metal halides
At this point, we carried out an investigation about the use of the metal halides instead of triflates on the optimized reaction starting from glucose. The percentage of the products is reported in Fig. 5. | ||
| Fig. 5 Glucose conversion using metal halides. Reaction conditions: glucose (40 mg, 0.22 mmol), choline chloride (46 mg, 0.33 mmol), 8% of the catalyst, 4 ml of MPK, after 3 hours at 150 °C. | ||
From the reported results, it is evident that the production of chloromethyl furfural (CMF) is the main compound in all obtained mixtures. This molecule is another important platform molecule with high potential.72 The production of CMF can be rationalized with the fact that such catalysts are unstable under the conditions used in this study. The production of water together with high temperature and pressure promotes the decomposition of the metal halides.73 This decomposition is due to the hydrolysis of the species with the consequent production of hydrochloric acid. The presence of an inorganic acid together with the availability of chloride ions coming from both the acid and the choline chloride converts the produced HMF into the respective halogenated molecule CMF (see ESI†). Another indicator of this result is the presence of levulinic acid in the mixture. This acid is a common degradation product of HMF, mostly in the presence of water and a Brønsted acid like hydrochloric acid.74
3.6 Hypothesis of the catalytic mechanism
After we have analysed and discussed the critical parameters of the process, we supposed the mechanism underlying this new synthetic method for the production of HMF starting from glucose or fructose in the presence of Sc(III) triflate/choline chloride as a catalytic system, as depicted in Fig. 6.Due to the substantial difference between the kinetics of the reaction starting from glucose and the same reaction starting from fructose, we supposed an initial isomerization of glucose to fructose in the first step, which justifies the longer reaction times.75
Furthermore, considering the known affinity of lanthanides and rare earth elements, in general, to coordinate with oxygen atoms,76 we supposed the initial chelation of scandium(III) triflate with hydroxyls on C1 and C2 of glucose. Then, choline chloride can react with glucose anomeric hydroxyl by Fisher-type glycosidation to produce glycoside 1, which can evolve to fructofuranosyl oxonium ion 2 through an intramolecular rearrangement.77 In the subsequent dehydration step, scandium(III) triflate is the fundamental key to promoting double water elimination and producing the final product HMF 3.
To support this hypothesis, we recorded 13C NMR spectra on the glucose/Sc(OTf)3/choline chloride mixture in which we did not observe any C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the open-chain form of glucose.78 Instead, in this mixture, we noted a slight shift of signal of the glucose anomeric carbon with respect to the classical value of only glucose, which may confirm the formation of glucoside 1 (see ESI for spectra†).
O group of the open-chain form of glucose.78 Instead, in this mixture, we noted a slight shift of signal of the glucose anomeric carbon with respect to the classical value of only glucose, which may confirm the formation of glucoside 1 (see ESI for spectra†).
3.7 Reaction kinetic models of HMF synthesis from glucose and fructose
There are few studies on the production of HMF from glucose, fructose, or other carbohydrates in a biphasic system.79,80 In other procedures starting from cellulose, the production of HMF passing from glucose is described through consecutive first-order reaction models and the rate constant for glucose isomerization to fructose is commonly neglected.81,82 We used a biphasic system and no trace of fructose was detected during the reaction investigation. In addition, we did not detect other common by-products such as levulinic acid or formic acid.83 For these reasons, we assumed that the production of HMF from glucose and fructose follows a first-order model with the relative rate constants k1 and k2, reported as follows:The experiments were conducted at three different reaction temperatures of 110, 130, and 150 °C for the conversion of both glucose and fructose. Due to the different reaction kinetics, we decided to use a different sampling interval, from 0 to 60 minutes for fructose and from 0 to 200 minutes for glucose. We reported the derived reaction rate equations for glucose conversion into HMF:
 | (4) |
 | (5) |
Due to our assumption, the equations are similar for fructose conversion:
 | (6) |
 | (7) |
The activation energy was calculated using the Arrhenius equation:
 | (8) |
In Fig. 7, we reported the Arrhenius plots for glucose and fructose conversion into HMF.
 | ||
| Fig. 7 Arrhenius plot for glucose conversion into HMF (blue) and fructose conversion into HMF (red). | ||
The R-squared values for both glucose and fructose conversions are greater than 0.9, which demonstrates that our assumed model fits well with the experimental results. The value is just a little lower for glucose, despite the assumption of neglecting the isomerization step. The values of the activation energies are reported in Table 7.
| Temperature (K) | 383.15 | 403.15 | 423.15 |
| Rate constant (min −1 ) | |||
| K 1 | 3.6 × 10−3 | 4.7 × 10−3 | 5.4 × 10−3 |
| K 2 | 1.4 × 10−2 | 1.5 × 10−2 | 1.7 × 10−2 |
| Activation energy (kJ mol −1 ) | |||
| E a1 | 16.9 | ||
| E a2 | 9.31 | ||
The value of the activation energy of 16.9 kJ mol−1 is higher for glucose conversion with respect to fructose conversion (9.31 kJ mol−1). These findings are in accordance with our hypothesized mechanism in which glucose passes for fructose through isomerization and then dehydrates, while for fructose, the reaction path is the direct dehydration to HMF. These values are of the same order but lower than the ones reported in similar studies.75,84–87
3.8 Recycling test
To demonstrate the further use of the method, we evaluated the recyclability of the catalytic system for up to five cycles.After the fresh reaction, a polar phase containing the catalytic system (scandium(III) triflate/choline chloride) was added with sugar (glucose or fructose) and MPK, and the process was repeated four times. The results are shown in Fig. 8.
The reaction yield for glucose is 90% after three cycles and 82% after five cycles, while for fructose, the yield is 95% after three cycles and 90% after five cycles. The different behaviour of the catalytic system for glucose may be due to a greater probability of deactivation due to prolonged reaction temperature and pressure. Anyway, the results are satisfactory with respect to the literature values reported in the following paragraph.
3.9 A comparison with literature data
In Table 8, we show a comparison with significant works from the past years on the catalytic conversion of glucose or fructose into HMF.| Entry | Reagent | Catalyst | Time (min) | T (°C) | HMF yield (%) | Ref. |
|---|---|---|---|---|---|---|
| a Reactions in which biphasic systems are employed. | ||||||
| 1 | Glucose | NbO/NbP | 120 | 152 | 26.5 | 88 |
| 2 | Glucose | HNbWO6 | 180–1440 | 120–140 | 36–44 | 89 |
| 3 | Fructose | WO3/SnO2 | 120 | 120 | 93 | 90 |
| 4 | Fructose | PWAl-200 | 240 | 170 | 61.7 | 91 |
| 5 | Glucose | DES/CrCl3 | 40 | 70 | 80 | 92 |
| 6 | Fructose | HCl/KBr | 1 | (MW) 160 | 91 |
93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| 7 | Glucose | AlCl3/HCl | 20 | 175 | 54.5 |
94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| 8 | Glucose | Phosphated titania | 60 | 150 | 66 | 95 |
| 9 | Fructose | [C4SO3Hmim] [HSO4] | 180 | 120 | 94.6 |
96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| 10 | Fructose | Sulfur-doped peanut shell | 120 | 130 | 94.6 | 97 |
| 11 | Glucose | Sc(OTf)3 | 180 | 150 | 94 | This study |
| 12 | Fructose | Sc(OTf)3 | 60 | 150 | 99 | This study |
The HMF yield from glucose is lower for all the reported studies using different solid acid catalysts (entries 1 and 2), metal halides (entries 5 and 7), and a microreactor with phosphated titania catalyst (entry 8) with respect to the present work (entries 11 and 12). The same result was obtained considering the study on fructose conversion, using solid acid catalysts (entries 3 and 4), water-soluble inorganic acid (entry 6), acid-functionalized ionic liquid (entry 9), and sulphur-doped peanut shell catalysts in ionic liquid (entry 10) with respect to the results of the present study (entries 11 and 12).
4. Conclusions
In this work, we presented an innovative strategy to produce HMF with excellent yields and selectivity starting from glucose or fructose. The methodology employs a reusable catalytic system consisting of rare earth metal(III) trifluoromethanesulfonates (i.e. scandium(III) triflate) and choline chloride. We tested different solvents and methyl propyl ketone furnished the best performance. This solvent has several advantages, including a low boiling point, which allows easy removal from the product. In addition, it is stable under the temperature and pressure used in this study and can be recycled after evaporation. During the discussion, we analysed all the critical parameters related to this synthetic procedure, including the choice and catalyst loading, reaction time, and temperature. Due to the difference in the reaction kinetics for glucose and fructose conversion, we hypothesized a catalytic mechanism. The role of the rare-earth metal Lewis acid is crucial, both for glucose isomerization into fructose and also for fructose dehydration. We also hypothesized the important role of choline chloride in glucose glycosylation. The best results in HMF yield were obtained using scandium(III) triflate. In this work, we have provided a method for obtaining HMF from glucose and fructose in short reaction times and in very high yields. Rare earth metal triflates can be purchased or produced from their relative metal halides, while choline chloride is an abundant and cheap reagent. The high selectivity of the developed methodology avoids further purification practices, since after the reaction, it is sufficient to remove only the extraction solvent to obtain pure HMF that can be used in subsequent applications. Finally, this methodology can also be applied to biomass-derived sugars, demonstrating the high eco-compatibility of the process.Author contributions
Conceptualization, A. D. N., F. O.; methodology, F. O., V. A., A. J. and M. A. T.; validation, F. O., and P. C.; formal analysis, F. O., V. A. and P. C.; investigation, F. O., V. A., A. J. and M. A. T.; resources, A. D. N., L. M., and P. C.; data curation, F. O., A. J., L. M.; writing—original draft preparation, A. D. N., F. O., and L. M.; writing—review and editing, A. D. N., F. O. and L. M.; supervision, A. D. N., and L. M.; project administration, A. D. N., and L. M.; funding acquisition, A. D. N., L. M., and P. C. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the University of Calabria and Calabria Region (PAC CALABRIA 2014–2020-Asse Prioritario 12, Azione B 10.5.12 CUP: H28D19000040006) for financial support. Moreover, we thank the Italian Ministry of University and Research (MUR) for a doctoral grant.References
- P. Marion, B. Bernela, A. Piccirilli, B. Estrine, N. Patouillard, J. Guilbot and F. Jérôme, Green Chem., 2017, 19, 4973 RSC.
- V. G. Zuin, I. Eilks, M. Elschami and K. Kümmerer, Green Chem., 2021, 23, 1594 RSC.
- J. Kühlborn, J. Groß and T. Opatz, Nat. Prod. Rep., 2020, 37, 380 RSC.
- C. Claver, M. B. Yeamin, M. Reguero and A. M. Masdeu-Bultó, Green Chem., 2020, 22, 7665 RSC.
- S. E. Motika and P. J. Hergenrother, Nat. Prod. Rep., 2020, 37, 1395 RSC.
- M. Hiloidhari, D. C. Baruah, M. Kumari, S. Kumari and I. S. Thakur, Cleaner Prod. Lett., 2019, 220, 931 CrossRef.
- F. Shen, X. Xiong, J. Fu, J. Yang, M. Qiu, X. Qi and D. C. W. Tsang, Renewable Sustainable Energy Rev., 2020, 130, 109944 CrossRef CAS.
- K. Alper, K. Tekin, S. Karagöz and A. J. Ragauskas, Sustainable Energy Fuels, 2020, 4, 4390 RSC.
- P. R. Jensen and S. Meier, Chem. Commun., 2020, 56, 6245 RSC.
- A. Kumar and R. Srivastava, Sustainable Energy Fuels, 2019, 3, 2475 RSC.
- J. Remón, F. Santomauro, C. J. Chuck, A. S. Matharu and J. H. Clark, Green Chem., 2018, 20, 4507 RSC.
- A. Cazor, C. Deborde, A. Moing, D. Rolin and H. This, J. Agric. Food Chem., 2006, 54, 4681 CrossRef CAS PubMed.
- T. Bauchop and S. R. Elsden, J. Gen. Microbiol., 1960, 23, 457 CAS.
- A. Nayak, I. N. Pulidindi and C. S. Rao, Renewable Energy, 2020, 159, 215 CrossRef CAS.
- I. Romeo, F. Olivito, A. Tursi, V. Algieri, A. Beneduci, G. Chidichimo, L. Maiuolo, E. Sicilia and A. De Nino, RSC Adv., 2020, 10, 34738 RSC.
- J. Wang, J. Xi and Y. Wang, Green Chem., 2015, 17, 737 RSC.
- S. R. Taylor, S. Ramsamooj and R. J. Liang, et al. , Nature, 2021, 597, 263 CrossRef CAS PubMed.
- A. Kriebs, Nat. Rev. Endocrinol., 2020, 16, 402 CrossRef PubMed.
- S. R. Taylor, S. Ramsamooj and R. J. Liang, et al. , Nature, 2021, 597, 263 CrossRef CAS PubMed.
- Z. Zhang and G. W. Huber, Chem. Soc. Rev., 2018, 47, 1351 RSC.
- R. Gogar, S. Viamajala, P. A. Relue and S. Varanasi, ACS Sustainable Chem. Eng., 2021, 9, 3428 CrossRef CAS.
- B. Saha and M. M. Abu-Omar, ChemSusChem, 2015, 8, 1133 CrossRef CAS PubMed.
- H. Chang, M. S. Kim, G. W. Huber and J. A. Dumesic, Green Chem., 2021, 23, 9479 RSC.
- L. Maiuolo, F. Olivito, V. Algieri, P. Costanzo, A. Jiritano, M. A. Tallarida, A. Tursi, C. Sposato, A. Feo and A. De Nino, Polymers, 2021, 13, 2802 CrossRef CAS PubMed.
- R. S. Assary, T. Kim, J. J. Low, J. Greeley and L. A. Curtiss, Phys. Chem. Chem. Phys., 2012, 14, 16603 RSC.
- M. Mascal, ACS Sustainable Chem. Eng., 2019, 7, 5588 CrossRef CAS.
- A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754 RSC.
- M. G. Davidson, S. Elgie, S. Parsons and T. J. Young, Green Chem., 2021, 23, 3154 RSC.
- P. Kisszekelyi, R. Hardian, H. Vovusha, B. Chen, X. Zeng, U. Schwingenschlçgl, J. Kupai and G. Szekely, ChemSusChem, 2020, 13, 3127 CrossRef CAS PubMed.
- G. Totaro, L. Sisti, P. Marchese, M. Colonna, A. Romano, C. Gioia, M. Vannini and A. Celli, ChemSusChem, 2022, 15, e202200501 CrossRef CAS PubMed.
- A. Al Ghatta and J. P. Hallett, Green Chem., 2022, 24, 3309 RSC.
- D. Mastrocola and M. Munari, J. Agric. Food Chem., 2000, 48, 3555 CrossRef CAS PubMed.
- J. Y. G. Chan and Y. Zhang, ChemSusChem, 2009, 2, 731 CrossRef CAS PubMed.
- C. Carlini, M. Giuttari, A. M. Raspolli Galletti, G. Sbrana, T. Armaroli and G. Busca, Appl Catal A Gen, 1999, 183, 295 CrossRef CAS.
- C. Lin, C. Chai, Y. Li, J. Chen, Y. Lu, H. Wu, L. Zhao, F. Cao, K. Chen, P. Wei and P. Ouyang, Green Chem., 2021, 23, 2058 RSC.
- B. Saha and M. M. Abu-Omar, Green Chem., 2014, 16, 24 RSC.
- H. Li, Z. Xia, P. Yan and Z. C. Zhang, Catal. Today, 2022, 402, 10 CrossRef CAS.
- W. Guo, T. Kortenbach, W. Qi, E. Hensen, H. J. Heeres and J. Yue, Appl. Catal., 2022, 301, 120800 CrossRef CAS.
- T. Istasse and A. Richel, RSC Adv., 2020, 10, 23720 RSC.
- G. Parameswaram and S. Roy, RSC Adv., 2018, 8, 28461 RSC.
- J. Slak, B. Pomeroy, A. Kostyniuk, M. Grilc and B. Likozara, Chem. Eng. J., 2022, 429, 132325 CrossRef CAS.
- Q. Hou, W. Li, M. Zhen, L. Liu, Y. Chen, Q. Yang, F. Huang, S. Zhang and M. Ju, RSC Adv., 2017, 7, 47288 RSC.
- B. Saha and M. M. Abu-Omar, Green Chem., 2014, 16, 24 RSC.
- B. Girisuta, L. P. B. M. Janssena and H. J. Heeres, Green Chem., 2006, 8, 701 RSC.
- R. Weingarten, W. C. Conner Jr. and G. W. Huber, Energy Environ. Sci., 2012, 5, 7559 RSC.
- M. Hartman, O. Trnka and O. Šolcová, Ind. Eng. Chem. Res., 2005, 44, 6591 CrossRef CAS.
- C. W. Koch, A. Broido and B. B. Cunningham, J. Am. Chem. Soc., 1952, 74, 2349 CrossRef CAS.
- X. Guo, H. Zhu, Y. Si, X. Lyu, Y. Cheng, L. Zheng, L. Wang and X. Li, Ind. Eng. Chem. Res., 2022, 61, 7216–7224 CrossRef CAS.
- C. Rosenfeld, J. Konnerth, W. Sailer-Kronlachner, P. Solt, T. Rosenau and H. W. G. van Herwijnen, ChemSusChem, 2020, 13, 3544 CrossRef PubMed.
- F. Wang, A.-W. Shi, X.-X. Qin, C.-L. Liu and W.-S. Dong, Carbohydr. Res., 2011, 346, 982–985 CrossRef CAS PubMed.
- K. Beckerle and J. Okuda, J. Mol. Catal. A: Chem., 2012, 356, 158–164 CrossRef CAS.
- O. Bortolini, A. De Nino, A. Garofalo, L. Maiuolo, A. Procopio and B. Russo, Appl. Catal., A, 2010, 372, 124 CrossRef CAS.
- A. Procopio, R. Dalpozzo, A. De Nino, M. Nardi, B. Russo and A. Tagarelli, Synthesis, 2006, 2, 332 CrossRef.
- S. Kobayashi, S. Nagayama and T. Busujima, J. Am. Chem. Soc., 1998, 120, 8287 CrossRef CAS.
- P. Zhao, Y. Zhang, Y. Wang, H. Cui, F. Song, X. Suna and L. Zhanga, Green Chem., 2018, 20, 155 Search PubMed.
- P. H. Smith, Z. E. Reyes, C.-W. Lee and K. N. Raymond, Inorg. Chem., 1988, 27, 4154 CrossRef CAS.
- A. Dibenedetto, M. Aresta, L. di Bitonto and C. Pastore, ChemSusChem, 2016, 9, 118 CrossRef CAS.
- A. R. Mankar, A. Pandey, A. Modak and K. K. Pant, Renewable Energy, 2021, 177, 643 CrossRef.
- R. Häkkinen and A. Abbott, Green Chem., 2019, 21, 4673 RSC.
- P. Makoś, E. Słupek and J. Gębicki, J. Mol. Liq., 2020, 308, 113101 CrossRef.
- S. Jiang, C. Verrier, M. Ahmar, J. Lai, C. Ma, E. Muller, Y. Queneau, M. Pera-Titus, F. Jérôme and K. De Oliveira Vigier, Green Chem., 2018, 20, 5104 RSC.
- A. Pande, P. Niphadkar, K. Pandare and V. Bokade, Energy Fuels, 2018, 32, 3783 CrossRef CAS.
- S. Meier, Catal. Sci. Technol., 2020, 10, 1724 RSC.
- S. Kobayashi and I. Hachiya, J. Org. Chem., 1994, 59, 3590 CrossRef CAS.
- T. Kishida, T. Yamauchi, Y. Kubota and Y. Sugi, Green Chem., 2004, 6, 57 RSC.
- S. Kobayashi, S. Nagayama and T. Busujima, J. Am. Chem. Soc., 1998, 120, 8287 CrossRef CAS.
- C. F. Baes Jr. and R. Mesmer, The Hydrolysis of Cations, Wiley, New York, 1976 Search PubMed.
- K. B. Yatsimirksii and V. P. Vasil'ev, Instability Constants of Complex Compounds, Pergamon, New York, 1960 Search PubMed.
- M.-A. del Barrio, J. Hu, P. Zhou and N. Cauchon, J. Pharm. Biomed. Anal., 2006, 41, 738 CrossRef CAS PubMed.
- M. Stupak, V. Kocourek, I. Kolouchova and J. Hajslova, Food Control, 2017, 80, 307 CrossRef CAS.
- E. Weingart, L. Teevs, R. Krieg and U. Prϋße, Energy Technol., 2018, 6, 432 CrossRef CAS.
- M. Mascal, ACS Sustainable Chem. Eng., 2019, 7, 5588 CrossRef CAS.
- M. Hartman, O. Trnka and O. Šolcová, Ind. Eng. Chem. Res., 2005, 44, 6591 CrossRef CAS.
- R. Weingarten, J. Cho, R. Xing, W. C. Conner Jr. and G. W. Huber, ChemSusChem, 2012, 5, 1280 CrossRef CAS PubMed.
- I. Delidovich and R. Palkovits, ChemSusChem, 2016, 9, 547 CrossRef CAS PubMed.
- T. Moeller, D. F. Martin, L. C. Thompson, R. Ferrús, G. R. Feistel and W. J. Randall, Chem. Rev., 1965, 65, 1 CrossRef CAS.
- S. Jiang, C. Verrier, M. Ahmar, J. Lai, C. Ma, E. Muller, Y. Queneau, M. Pera-Titus, F. Jérôme and K. De Oliveira Vigier, Green Chem., 2018, 20, 5104 RSC.
- X. Zhang, P. Murria, Y. Jiang, W. Xiao, H. I. Kenttämaa, M. M. Abu-Omarb and N. S. Mosier, Green Chem., 2016, 18, 5219 RSC.
- L. Atanda, M. Konarova, Q. Ma, S. Mukundan, A. Shrotri and J. Beltramini, Catal. Sci. Technol., 2016, 6, 6257–6266 RSC.
- W. Guo, Z. Zhang, J. Hacking, H. J. Heeres and J. Yue, Chem. Eng. J., 2021, 409, 128182 CrossRef CAS.
- N. S. Mosier, A. Sarikaya, C. M. Ladisch and M. R. Ladisch, Biotechnol. Prog., 2001, 17, 474–480 CrossRef CAS PubMed.
- L. Vanoye, M. Fanselow, J. D. Holbrey, M. P. Atkins and K. R. Seddon, Green Chem., 2009, 11, 390–396 RSC.
- H. S. Kim, S.-K. Kim and G.-T. Jeong, J. Ind. Eng. Chem., 2018, 63, 48–56 CrossRef CAS.
- N. A. S. Ramli and N. A. S. Amin, Chem. Eng. J., 2018, 335, 221–230 CrossRef CAS.
- K. Kumar, S. Pathak and S. Upadhyayula, J. Cleaner Prod., 2020, 256, 120292 CrossRef CAS.
- K.-L. Chang, Q. T. Huynh, C.-T. Zhong, W.-R. Chen, H.-Y. Wang, P. Phitsuwan, Y.-C. Lin and G. C. C. Yang, Environ. Technol., 2022, 28, 102844 CAS.
- J. Wang, H. Cui, J. Wang, Z. Li, M. Wang and W. Yi, Chem. Eng. J., 2021, 415, 128922 CrossRef CAS.
- M. N. Catrinck, E. S. Ribeiro, R. S. Monteiro, R. M. Ribas, M. H. P. Barbosa and R. F. Teófilo, Fuel, 2017, 210, 67 CrossRef CAS.
- A. Takagaki, Catalysts, 2019, 9, 818 CrossRef CAS.
- G. Raveendra, M. Surendarb and P. S. Sai Prasad, New J. Chem., 2017, 41, 8520 RSC.
- X. Wang, T. Lv, M. Wu, J. Sui, Q. Liu, H. Liu, J. Huang and L. Jia, Appl. Catal., A, 2019, 574, 87 CrossRef CAS.
- Q. Li, Q. Li, Q. Li, K. Sun, Y. Shao, S. Zhang, Z. Yan, L. Zhang, Q. Liu, Y. Wang and X. Hu, Ind. Eng. Chem. Res., 2020, 59, 17554 CrossRef CAS.
- P. Wrigstedt, J. Keskiväli and T. Repo, RSC Adv., 2016, 6, 18973 RSC.
- C. Wang, L. Zhang, T. Zhou, J. Chen and F. Xu, Sci. Rep., 2017, 7, 40908 CrossRef PubMed.
- W. Guo, T. Kortenbach, W. Qi, E. Hensen, H. Jan Heeres and J. Yue, Appl. Catal., B, 2022, 301, 120800 CrossRef CAS.
- F. Tao, H. Song and L. Chou, RSC Adv., 2011, 1, 672 RSC.
- K.-L. Chang, S. C. Muega, B. Ivan, G. Ofrasio, W.-H. Chen, E. G. Barte, R. R. M. Abarca, M. Daniel and G. de Luna, Chemosphere, 2022, 291, 132829 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc04046h |
| This journal is © The Royal Society of Chemistry 2023 |