Diallyl trisulfide inhibits 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung cancer via modulating gut microbiota and the PPARγ/NF-κB pathway†
Zhuo
Qu‡
a,
Jiahui
Tian‡
a,
Jiachen
Sun
c,
Ying
Shi
 a,
Jianqiang
Yu
a,
Jianqiang
Yu
 a,
Wannian
Zhang
ab and
Chunlin
Zhuang
a,
Wannian
Zhang
ab and
Chunlin
Zhuang
 *ab
*ab
aCollege of Pharmacy, Ningxia Medical University, 1160 Shengli Street, Yinchuan, Ningxia 750004, China. E-mail: zclnathan@163.com
bSchool of Pharmacy, Second Military Medical University, 325 Guohe Road, Shanghai 200433, China
cSchool of Biotechnology and Food Science, Tianjin University of Commerce, 409 Guangrong Road, Tianjin 300134, China
First published on 28th November 2023
Abstract
Smoking is the primary risk factor for developing lung cancer. Chemoprevention could be a promising strategy to reduce the incidence and mortality rates of lung cancer. Recently, we reported that A/J mice exposed to tobacco smoke carcinogens displayed the reshaping of gut microbiota. Additionally, garlic oil was found to effectively inhibit the carcinogenic effects of tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in lung tumorigenesis. Diallyl trisulfide (DATS), which is the predominant compound in garlic oil, exhibits various biological activities. To further explore the chemopreventive action and potential mechanism of DATS on lung tumorigenesis, we established a lung adenocarcinoma model in A/J mice stimulated by NNK. Subsequently, we employed multi-omics combined molecular biology technologies to clarify the mechanism. The results indicated that DATS significantly decreased the number of lung tumors in NNK induced A/J mice. Interestingly, we discovered that DATS could modulate gut microbiota, particularly increasing the abundance of F. rodentium, which has inhibitory effects on tumor growth. Mechanistically, DATS could activate the PPARγ pathway, leading to the negative regulation of the NF-κB signaling pathway and subsequent suppression of NF-κB-mediated inflammatory factors. Collectively, these findings provide support for DATS as a potential novel chemopreventive agent for tobacco carcinogen-induced lung cancer.
1. Introduction
Globally, lung cancer is the primary cause of cancer-related deaths.1 It is well-established that smoking is the most significant risk factor for developing lung cancer. In fact, smokers have a 6 to 10 times higher risk for developing lung cancer compared with non-smokers.2 Additionally, approximately 90% of lung cancer patients have a history of smoking.3 Therefore, smoking cessation is a crucial and effective strategy for reducing the incidence and mortality rates of lung cancer. However, due to the challenge of tobacco control, tobacco-related lung carcinogenesis remains widespread.4 Furthermore, the lack of reliable early detection methods and effective treatment contributes to the low 5-year survival rate (less than 20%) among lung cancer patients.5 As a result, the management of lung cancer has become a significant public health concern. It is noteworthy that Nature News report highlighted that almost 50% of cancers are preventable.6 Consequently, the development of chemopreventive agents against lung tumorigenesis is of utmost importance.The World Health Organization (WHO) recognizes that exposure to cigarette smoke is a significant environmental health issue that affects both active smokers and passive smokers, especially young children. Cigarette smoke contains more than 60 carcinogenic components,7 including 4-(methylnitrosamine)-1-(3-pyridine)-1-butanone (NNK), which is considered one of the strongest carcinogens and a ubiquitous environmental pollutant. NNK has the ability to trigger lung adenoma and adenocarcinoma in different species, particularly in A/J mice, which are highly susceptible to developing lung tumor.8 The mechanism of NNK stimulated lung carcinogenesis involves its metabolic conversion to 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in the presence of cytochrome P450 (CYP450) enzyme. NNAL is then activated by CYP450 to form DNA adducts. If these adducts are not repaired or improperly repaired, gene mutations may occur, resulting in the development of tobacco-related lung carcinogenesis.9 Similarly, NNK could activate nuclear factor kappa B (NF-κB) to promote cell proliferation, survival, and angiogenesis.8 Interestingly, our previous studies have shown that exposure of A/J mice to a mixture of NNK and Benzo[a]pyrene (BaP) altered the gut microbiota and led to lung cancer formation.10 Subsequently, Bai and colleagues have demonstrated that tobacco smoking promoted colorectal cancer via modulating gut microbiota.11 These findings highlight that the protumorigenic role of gut microbiota dysbiosis triggered by tobacco smoking in cancer development. In conclusion, the development of preventive pharmacological interventions aimed at reducing NNK-induced DNA damage, inflammation, and microbiota dysbiosis has become a crucial strategy for preventing lung cancer.
Natural products, particularly phytochemical rich foods, have shown enormous potential for cancer prevention.12 Long-term administration of phytochemical rich foods, which have relatively low toxicity, has been a well-established and effective approach in delaying the progression of carcinogenesis.13 Research conducted by Xing's group has demonstrated that dietary kava, a commonly used drink in the South Pacific islands, significantly inhibits tobacco-induced lung tumorigenesis.14 Further investigations by Xing and colleagues led to the identification of a highly bioactive compound in kava called dihydromethysticin (DHM), which can effectively block lung tumorigenesis induced by cigarette carcinogens in A/J mice.15 In addition, other dietary phytochemicals, such as haskap berry, Phyllanthus emblica L., Psoralea corylifolia L., 5-demethylnobiletin, and nobiletin, have also been reported to block tobacco carcinogen-induced lung cancer.13 However, many of these phytochemicals have failed phase III clinical trials for chemoprevention, primarily due to issues related to their poor bioavailability, reproducibility, or adverse effects.16 Therefore, there is a strong demand for exploring more novel and effective chemopreventive agents for tobacco-induced lung carcinogenesis.
Based on the aforementioned findings, our group recently conducted a screening of various phytochemical rich foods to investigate their potential chemopreventive effects. Among them, garlic oil emerged as a highly promising candidate that significantly reduced NNK-triggered DNA damage and effectively prevented lung cancer via modulating phase II detoxification enzymes.17 Numerous studies have consistently demonstrated the various benefits of garlic oil, such as its antioxidant, anti-inflammatory, antimicrobial, and detoxification properties.18 Phytochemical analysis has revealed the abundant organosulfur compounds presented in garlic oil, such as allicin, alliin, ajoene, diallyl sulfide (DAS), diallyl disulfide (DADS), and diallyl trisulfide (DATS).18 Notably, the content of DATS was found to be the highest, accounting for more than 40%.17 DATS, as the most abundant component in garlic oil, has been attributed to many biological effects. Studies have shown that DATS can protect against inflammation and oxidative stress induced by doxorubicin,19 alleviate cisplatin-induced oxidative injuries,20 trigger apoptosis in cancer cells,21 and even overcome drug resistance in breast cancer.22 Given the significant presence and potential beneficial effects of DATS in garlic oil, it is essential to investigate whether DATS also possesses preventive properties against lung tumorigenesis stimulated by tobacco carcinogens. Therefore, the primary purpose of our current study is to explore the chemopreventive effects of DATS on NNK induced lung tumorigenesis and elucidate the possible mechanisms by using a multi-omics approach.
2. Materials and methods
2.1. Chemicals and reagents
DATS and NNK (Fig. 1A) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). AIN-93G and AIN-93M diets were obtained from Beijing HuaFuKang Biotechnology Co., Ltd (Beijing, China). The enzyme linked immunosorbent assay (ELISA) kits for interleukin 1β (IL-1β), tumor necrosis factor α (TNFα), and interleukin 6 (IL-6) were purchased from MultiSciences (Lianke) Biotech Co., Ltd (Hangzhou, China). Antibodies used in this study included anti-NF-κB p65 (p65) antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, Proteintech, Catalogue numbers: 10745-1-AP), anti-peroxisome proliferator activated receptor γ (PPARγ, 1
1000, Proteintech, Catalogue numbers: 10745-1-AP), anti-peroxisome proliferator activated receptor γ (PPARγ, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000, Proteintech, Catalogue numbers: 16643-1-AP), anti-IκBα (1
5000, Proteintech, Catalogue numbers: 16643-1-AP), anti-IκBα (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, Affinity, Catalogue numbers: AF5002), anti-phospho-NF-κB p65 (Ser536) (p-p65) antibody (1
1000, Affinity, Catalogue numbers: AF5002), anti-phospho-NF-κB p65 (Ser536) (p-p65) antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, Affinity, Catalogue numbers: AF2006), anti-GAPDH (1
1000, Affinity, Catalogue numbers: AF2006), anti-GAPDH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000, Proteintech, Catalogue numbers: 10494-1-AP), and goat anti-rabbit IgG (1
5000, Proteintech, Catalogue numbers: 10494-1-AP), and goat anti-rabbit IgG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000, Proteintech, Catalogue numbers: SA00001-2). The primers were provided by Sangon Biotech Co., Ltd (Shanghai, China).
5000, Proteintech, Catalogue numbers: SA00001-2). The primers were provided by Sangon Biotech Co., Ltd (Shanghai, China).
2.2. Animals and treatments
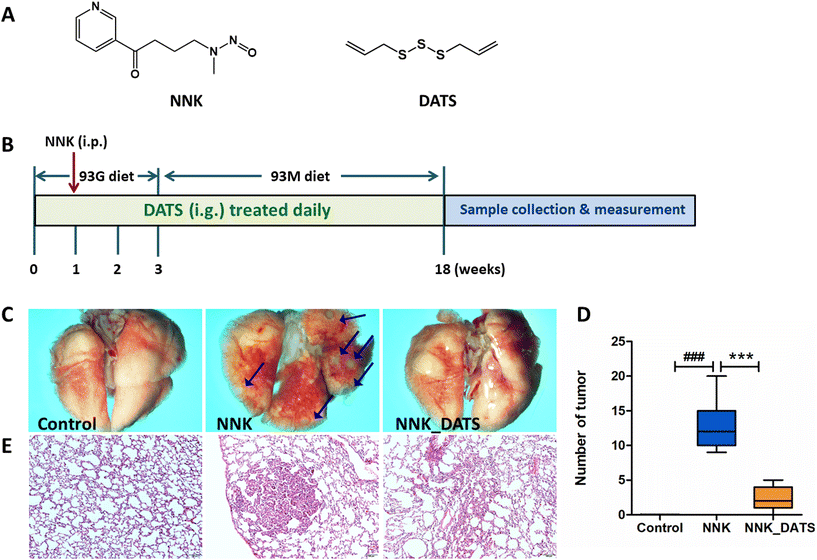
Female A/J mice (specific pathogen-free, SPF) aged 5–6 weeks were obtained from Jackson Laboratory. The mice were housed at the Laboratory Animal Center, Ningxia Medical University (Yinchuan, China). The mice were group-housed in ventilated cages under the condition of 23 ± 1 °C, 50% relative humidity, and a 12 h light/dark cycle, and free access to water. All animal experiments complied with National Guidelines for the Care and Use of Laboratory Animals (Laboratory Animal Guidelines for Ethical Review of Animal Welfare) (GB/T35892-2018, China) and approved by the Ningxia Medical University Animal Care and Use Committee (Certificate number no: 2022-N123).The procedure for the animal experiment is displayed in Fig. 1B. In brief, mice were initially fed a powdered diet called AIN-93G for the first three weeks. On the day 22, they were switched to another powdered diet called AIN-93M for the remainder of the treatment. After one week of acclimation, all mice were grouped (n = 12) and co-housed in cages of 3 mice per cage. The groups included Control group (mice received an oral administration of a solvent), NNK group (100 mg kg−1 NNK administrated intraperitoneally once on the day 8 of the experiment), and NNK_DATS group (mice were treated with NNK in the same way as the NNK group, but additionally received a daily dose of 23 mg kg−1 of DATS via intragastric gavage for the entire 18-week duration of the experiment). The dosage of DATS was established by our previous studies.17 Mice were sacrificed on the last day of the experiment, and lung tissues were obtained. The number of tumors on the surface of the lungs was counted. Images of the lungs were captured using an SMZ18 stereomicroscope (Nikon Microsystems, Japan). Some lung tissues were preserved in 4% paraformaldehyde. The remaining tissues were stored at −80 °C condition.
2.3. Hematoxylin–eosin (HE) staining
The lung tissues were fixed in 4% paraformaldehyde, and then embedded in paraffin. The paraffin blocks were cut into thin sections measuring 4 μm in thickness. These sections were then dewaxed and rehydrated. To visualize the tissue structures, the sections were stained with HE. The histological images were captured using an optical microscope (Olympus, Japan).2.4. Gut microbiota 16S rRNA sequencing
For gut microbiota 16S rRNA sequencing, two mice were taken from each cage and placed on an ultra-clean workbench to collect feces, respectively. A total of 6 stool samples from 6 mice were collected. The 16S rRNA sequencing was performed by Genesky Biotechnologies Inc. (Shanghai, China). The genomic DNA was extracted using the Toptaq DNA polymerase kit (Transgen, China), and its concentration was determined using ultraviolet spectroscopy. The V3–V4 hypervariable region of the 16S rRNA gene was amplified using the primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′) for each sample. The amplified DNA fragments were then sequenced using the Illumina MiSeq platform, generating 2 × 250 bp paired-end reads. The analysis of the sequencing data was based on the obtained reads and operational taxonomic units (OTUs) with a 97% similarity. The classification and annotation of the sequences were performed using Ribosomal Database Project (RDP). The numbers of sequences for each classification level were calculated and summarized to compare the abundance and diversity of gut microbiota between the two groups. Alpha diversities were calculated using mothur software. Principal component analysis (PCA) was applied for beta analysis. The bioinformatics analyses of the microbial data were performed by Genesky Biotechnologies, Inc.2.5. Metabolomic analysis
The metabolomic analysis was conducted by Genesky Biotechnologies Inc. (Shanghai, China). The experimental process involved metabolite extraction, quality control (QC) sample preparation, LC-MS analysis and data analysis. Briefly, freeze-dried feces were extracted to obtain samples for LC-MS analysis. The MS raw data files were then converted to the mzXML format using the MSConvert tool, which generated a data matrix that consisted of the retention time (RT), mass-to-charge ratio (m/z) values, and peak intensity to identify the metabolites. The first step in the identification process was to confirm the precise molecular weight of the metabolites with a molecular weight error less than 30 ppm. Following this, the fragment information obtained by the MS/MS model was further matched and annotated in various databases, including the Human Metabolome Database (HMDB) (https://www.hmdb.ca), Metlin (https://metlin.scripps.edu), massbank jp (https://www.massbank), Lipid Maps (https://www.lipidmaps.org), mzcloud (https://www.mzcloud.org), and self-built standard database, to obtain accurate information on the metabolites. Subsequently, multivariate statistical analysis, specifically partial least squares discriminant analysis (PLS-DA), was performed on the metabolites to reveal the differences between the different groups. Differentiated metabolites were identified based on specific criteria: those with an adjusted p-value less than 0.05, and a variable importance in projection (VIP) score greater than 1.0. These differentiated metabolites were then annotated by the KEGG pathway database. Enrichment analysis and cluster analysis were conducted using MetaboAnalyst 3.0. Genesky Biotechnologies Inc. conducted the bioinformatics analysis for the metabolomic data and provided these analyses as part of their services.2.6. Transcriptome sequencing of lung tissues
The transcriptome sequencing service was provided by Biotree Biotechnology Co., Ltd (Shanghai, China). Briefly, total RNAs were extracted from the lung tissues of mice using the Trizol reagent (Thermo Fisher, 15596018). The quality of RNA was assessed using a NanoDrop ND-1000 (NanoDrop, Wilmington, DE, USA). The integrity of RNA was evaluated using a Bioanalyzer 2100 (Agilent, CA, USA). The RNA samples with a concentration greater than 50 ng μL−1, an RNA Integrity Number (RIN) value greater than 7.0, and a total RNA mount exceeding 1 μg were considered suitable for downstream experiments. Dynabeads Oligo (dT) beads (cat.25-61005, Thermo Fisher, USA) were used for specific capture of mRNA containing PolyA by two rounds of purification. The captured mRNA was fragmented under high temperature using the NEBNextR Magnesium RNA Fragmentation Module kit (cat.E6150S, USA) at 94 °C for 5–7 min. cDNA was synthesized from the fragmented RNA by using reverse transcriptase (Invitrogen SuperScript™ II Reverse Transcriptase, cat.1896649, CA, USA). E. coli DNA polymerase I (NEB, cat.m0209, USA) and RNase H (NEB, cat.m0297, USA) were used for two-strand synthesis to convert these complex double strands of DNA and RNA into DNA double strands. At the same time, dUTP solution (Thermo Fisher, cat.R0133, CA, USA) was added into the two-stranded DNA, and the end of the double-stranded DNA was completed to the flat end. An A base is added to each end so that it can be connected to the terminal joint with a T base, and magnetic beads are used to screen and purify the fragment size. The two chains were digested by UDG enzyme (NEB, cat.m0280, MA, US), followed by pre-denaturation by PCR at 95 °C for 3 min, denaturation at 98 °C for a total of 8 cycles of 15 seconds each, annealing at 60 °C for 15 seconds, extension at 72 °C for 30 seconds, and finally extension at 72 °C for 5 minutes, to form a fragment size of 300 bp ± 50 bp library (chain specific library). Finally, an Illumina Novaseq™ 6000 was used for double-end sequencing in PE150 mode according to the standard procedure.After obtaining the sequencing data, the following analysis steps were performed. Low-quality reads (reads with Qphred ≤ 25 bases accounting for more than 60% of the entire reads) and reads with ambiguous nucleotides (n ≥ 5%) were removed to obtain clean reads. Differential expression analysis was conducted using DESeq2 and genes with a P < 0.05 and |log2-foldchange| ≥ 1.5 were considered differentially expressed. Functional enrichment analysis of Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways was performed using Fisher's exact test to identify enriched biological functions associated with the differentially expressed genes. The top 20 KEGG signaling pathways were selected for further investigation.
2.7. Real-Time quantitative PCR (RT-qPCR)
Total RNA of lung tissues was obtained using a buffer (AXY GEN, China). The cDNA was obtained using an All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (One-Step gDNA Removal) kit (TrsanBiotechgen, China). The primer pair sequences are as follows: IL-1β with forward TGAAGTTGACGGACCCCAAAAGATG and reverse GTTGATGTGCTGCTGCGAGA TTTG, TNFα with forward CAGGTTCTCTTCAAGGGACAAGGC and reverse TGACGGCAGAGAG GAGGTTGAC, IL-6 with forward AGACTTCCATCCAGTTGCCTTCTTG and reverse TCTGTTGGG AGTGGTATCCTCTGTG, p65 with forward AGACCCAGGAGTGTTCACAGACC and reverse GTCACCAGGCGAGTTATAGCTTCAG, GAPDH with forward GGTTGTCTCCTGCGACTTCA and reverse TGGTCCAGGGTTTCTTACTCC. RT-qPCR was performed by using the Tip Green qPCR SuperMix (TrsanBiotechgen, China). Relative expression levels of target genes were calculated using the 2−ΔΔCt method.2.8. Enzyme-linked immunosorbent assay (ELISA)
Lung tissues were homogenized and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g at 4 °C for 15 min. Protein concentration was detected by using the BCA kit (KeyGEN, China). The contents of IL-1β, IL-6 and TNFα were measured by ELISA kit according to the manufacturer's protocol.
000g at 4 °C for 15 min. Protein concentration was detected by using the BCA kit (KeyGEN, China). The contents of IL-1β, IL-6 and TNFα were measured by ELISA kit according to the manufacturer's protocol.
2.9. Western blotting
Lung tissues were homogenized according to the instructions of the whole cell lysis assay (KeyGEN, China). Then the supernatants were collected. The protein concentration was quantified using the BCA kit (KeyGEN, China). Proteins (50 μg) were separated on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels and electroblotted onto polyvinylidene fluoride (PVDF) membranes. 5% non-fat milk was used to block blots for 1 h, followed by primary antibody (PPARγ, p65, p-p65, and IκBα) incubation at 4 °C overnight. After washing with TBST, the blots were incubated with the secondary antibodies for 2 h. The intensity of the bands was quantified via ImageJ software.2.10. Immunofluorescence staining
The paraffin sections underwent deparaffinization and rehydration before antigen retrieval. The sections were then blocked at room temperature for 30 min with 3% BSA, followed by incubation with the primary antibody overnight at 4 °C and then the secondary antibody at room temperature for 50 min. The nuclei were stained with DAPI solution at room temperature for 10 min. Images were captured using a confocal microscope.2.11. Statistical analysis
All data are expressed as mean ± standard deviation (SD). One-way ANOVA with Tukey's test was applied to analyze the differences by Graphpad Prism 8 software (San Diego, USA). P values < 0.05 were considered statistically significant. Analysis of differential expression of metabolites was performed using P values and VIP values; P < 0.05 and VIP > 1 were selected as differentially altered metabolites.3. Results
3.1. DATS attenuates the progression of lung carcinogenesis in NNK induced A/J mice
To investigate the chemopreventive effect of DATS on NNK-induced lung carcinogenesis in A/J mice, the lungs were photographed to document their appearance and the number of lung tumors was counted. Additionally, HE staining was performed to observe the pathological changes in the lung tissue. As shown in Fig. 1C, the lung surface of mice in the Control group appeared smooth with no nodules or tumors observed. In contrast, the NNK group exhibited a significant number of tumors on the lung surface. However, after DATS intervention, the lung surface appeared improved with no obvious tumor present. The number of tumors in each group was counted and statistically analyzed. The results in Fig. 1D demonstrated that NNK significantly induced the occurrence of lung tumors in mice (P < 0.001), with an average number of 12.83 ± 3.54 tumors per mouse. However, DATS intervention was able to block the occurrence and development of lung tumors caused by NNK, dramatically reducing the number of tumors to approximately 2.42 ± 1.68 (P < 0.001). Moreover, representative images of HE staining from each group were examined (Fig. 1E). The lung tissue of the NNK group lost the normal alveolar architecture. As expected, DATS attenuated NNK-induced lung histopathological changes, resulting in a weakened degree of tissue differentiation and no significant loss of alveolar architecture. In summary, these findings suggest that DATS plays a chemoprotective role in NNK-induced lung carcinogenesis in A/J mice.3.2. DATS improves the alteration of gut microbiota composition in NNK induced A/J mice
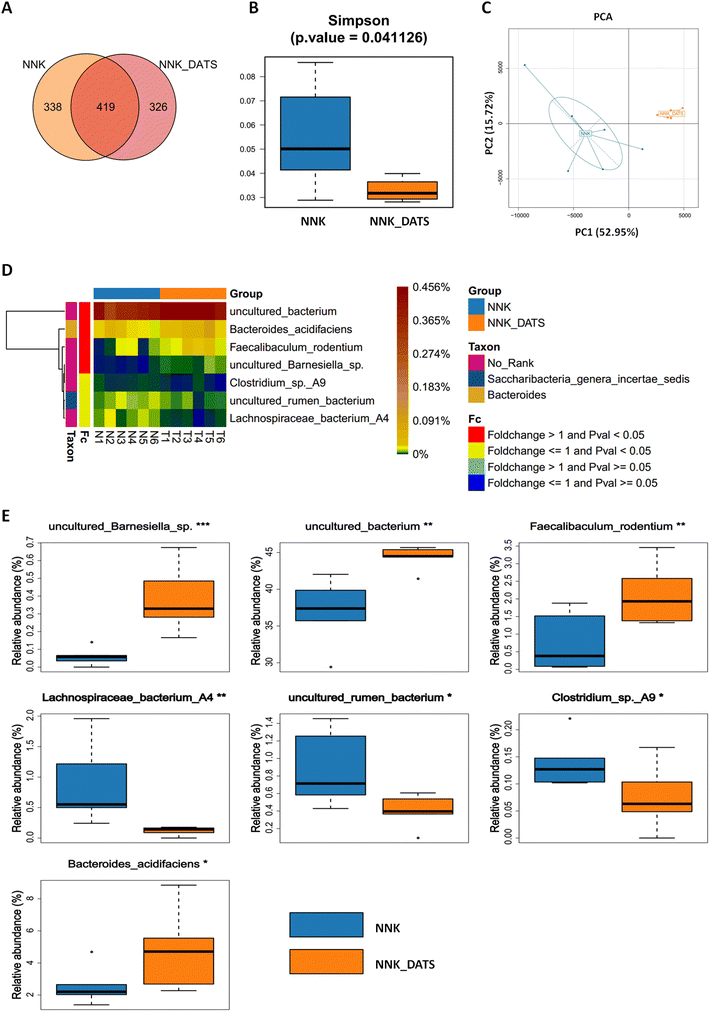
To investigate the impact of DATS on the gut microbiota of lung cancer mice exposed to NNK, we performed 16S rRNA sequencing on faecal samples. A total of 1083 OTUs were identified. Among these, 338 OTUs were unique to the NNK group, 326 were unique to the NNK-DATS group, and 463 OTUs were shared in both groups (Fig. 2A). Alpha diversity analysis revealed that mice treated with DATS had lower diversity compared to those exposed to NNK alone (Fig. 2B). Beta diversity analysis using PCA analysis exhibited remarkable differences in the clustering of gut microbiomes between NNK-exposed and DATS treated groups (Fig. 2C). Further analysis identified seven bacteria that were notably altered in the DATS treated mice as compared with NNK exposure (P < 0.05, Fig. 2D). The bacteria uncultured Barnesiella sp., uncultured Bacterium, Faecalibaculum rodentium (F. rodentium), and Bacteroides acidifaciens (B. acidifaciens) were enriched after DATS intervention, while Lachnospiraceae bacterium A4, uncultured Rumen bacterium, and Clostridium sp A9 were depleted (Fig. 2E). These findings suggest that DATS could reshape the gut microbiota composition in NNK-induced lung cancer mice.3.3. DATS modulates gut microbiota-related metabolites in faeces after NNK exposure
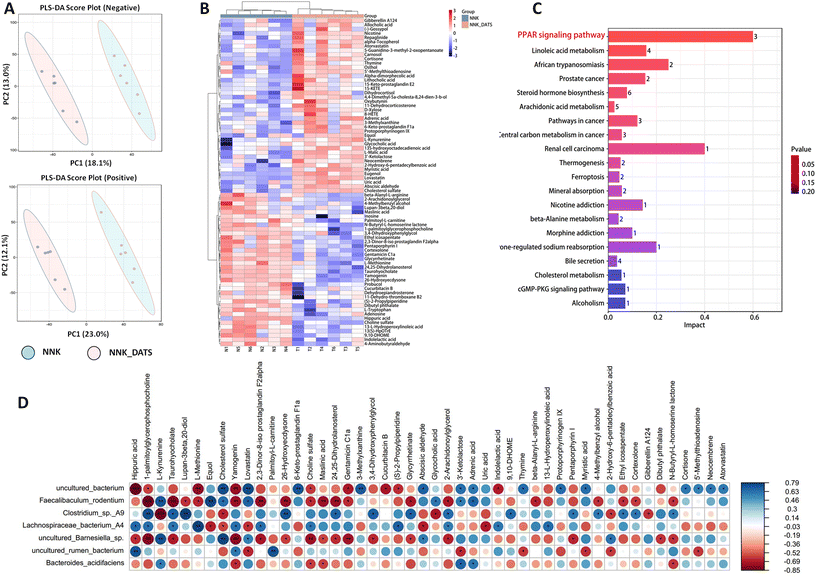
To investigate the impact of DATS on faecal metabolic changes in NNK-exposed mice, we conducted an untargeted metabolomics to determine the alterations in faecal metabolites. The PLS-DA scores clearly showed a distinct separation between DATS and NNK groups (Fig. 3A). Through data integration, a total of 78 metabolites (adjusted P < 0.05) were found to have significant alterations in the faeces of DATS-treated mice compared to NNK-exposed mice. To comprehensively analyze the levels of these differential metabolites, we applied a heat map to visualize their relative levels in each individual. In Fig. 3B, it displays the differential metabolites, representing up-regulated (red) or down-regulated (blue) intensities. Additionally, KEGG analysis was conducted to identify the pathways that were significantly enriched markedly in both groups. Twenty pathways were found to be mostly involved, including linoleic acid metabolism, arachidonic acid, cholesterol, and more. Among these pathways, the PPAR signaling pathway was identified as the most significant enriched pathway in DATS-treated mice compared to NNK-exposed mice (Fig. 3C). This suggests that the regulation of PPAR signaling might be one of the crucial pathways of DATS preventing NNK-stimulated lung cancer.To further determine potential relationships between stool metabolites and gut microbiota, we generated correlation matrices based on Spearman's coefficient. Our results revealed that uncultured Barnesiella sp, uncultured Bacterium, F. rodentium, and B. acidifaciens, which were enriched in DATS-treated mice, exhibited the strongest positive correlation with metabolites such as cholesterol sulfate, 2,3-dinor-8-iso prostaglandin F2α, cortexolone, and 13-L-hydroperoxylinoleic acid. Conversely, Lachnospiraceae bacterium A4, uncultured Rumen bacterium, and Clostridium sp A9 depleted in DATS treated mice showed a negative correlation with these metabolites (Fig. 3D). Of note, these metabolites are associated with important metabolic pathways identified through KEGG enrichment analysis, including linoleic acid metabolism, steroid hormone biosynthesis, and arachidonic acid metabolism. These correlations imply a strong connection between changes in intestinal structure, the related metabolites, and hosting metabolism. Consequently, the dysbiosis in the gut microbiome and alterations in metabolite profiles may synergistically contribute to the development of tobacco carcinogen-induced lung tumorigenesis.
3.4. Comprehensive transcriptome sequencing analysis reveals molecular insights into DATS-mediated chemoprevention of lung cancer in NNK-exposed mice
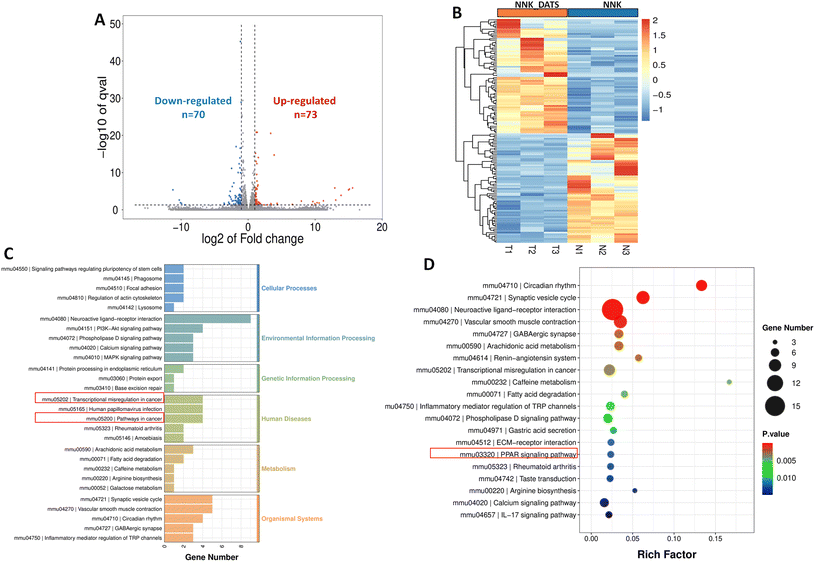
In order to gain a more comprehensive understanding of the molecular mechanisms underlying the chemopreventive effects of DATS against lung cancer, we conducted RNA-seq analysis on mouse lung tissue. The volcano diagram revealed that there were a total of 73 up-regulated genes and 70 down-regulated genes in the DATS-treated mice (Fig. 4A). The heat map visually showed the marked differences in gene expression between the NNK group and NNK-DATS group (Fig. 4B). To further analyze the functional significance of these differentially expressed genes, we performed KEGG analysis to identify enriched pathways. The KEGG analysis categorized pathways into six main groups, including cellular processes, environmental information processing, genetic information processing, human diseases, metabolism, and organismal systems. We observed that DATS had a significant impact on cancer-related pathways in the human disease section, particularly the pathway in cancer and pathway of transcriptional misregulation in cancer (Fig. 4C). Furthermore, we narrowed down the top 20 pathways influenced by DATS. Intriguingly, in line with the metabolomic findings, we found that DATS had a significant effect on the PPAR pathway (Fig. 4D). This suggests that the PPAR signaling pathway might play a crucial role in mediating the chemopreventive properties of DATS against lung tumourigenesis.3.5. DATS activates the PPARγ signaling pathway in NNK-exposed mice
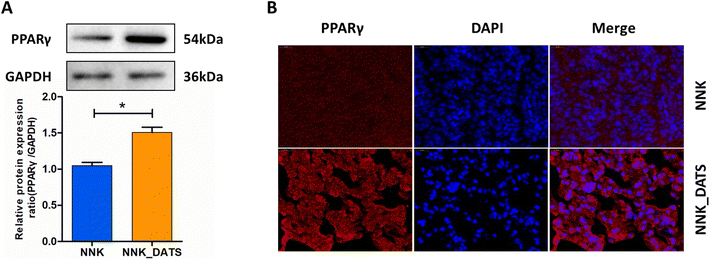
To confirm the activation of PPAR signaling in the prevention of lung tumorigenesis by DATS, we conducted additional experiments. Firstly, we assessed the expression of PPARγ protein using western blotting. The results in Fig. 5A revealed that the expression of PPARγ protein elevated remarkably in the DATS treated group compared to the NNK group. Furthermore, we examined the subcellular localization of the PPARγ protein in lung tissue using immunofluorescence staining. Consistent with the western blotting results, mice exposed to DATS exhibited increased PPARγ protein expression (red staining) and nuclear accumulation (colocalization with blue DAPI staining) in comparison to the NNK group (Fig. 5B). Overall, these experiments provide compelling evidence to support the activation of the PPARγ signaling by DATS contributing to the prevention of lung tumorigenesis induced by NNK exposure.3.6. DATS suppresses the NF-κB signaling pathway in NNK-exposed mice
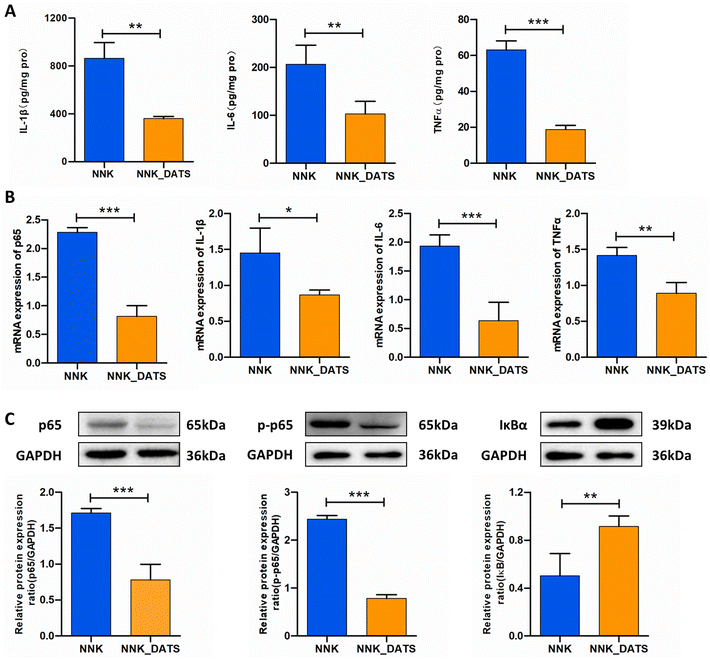
PPARγ has the ability to bind to NF-κB and inhibit its activation, leading to a reduction in the production of pro-inflammatory cytokines. In order to evaluate the anti-inflammatory activity of DATS, we performed ELISA and RT-qPCR assays to detect the protein and mRNA levels of pro-inflammatory cytokines, respectively. The ELISA results demonstrated that mice exposed to NNK exhibited increased protein expression of TNF-α (P < 0.05), IL-1β (P < 0.05), and IL-6 (P < 0.01). However, DATS treatment significantly attenuated the elevated protein levels induced by NNK (Fig. 6A). Additionally, the RT-qPCR results confirmed the decreased expressions of TNF-α, IL-1β, and IL-6 in DATS treated mice compared to NNK-exposed mice (Fig. 6B). Since these cytokines are closely associated with NF-κB activation, we further investigated proteins related to the NF-κB pathway. As revealed in Fig. 6C, NNK-treated mice showed significantly increased protein expression of NF-κB p65 and p-p65, indicating activation of the NF-κB pathway in the lungs. However, this effect was notably reduced in DATS-treated mice. Furthermore, DATS promoted the expression of IκBα. Collectively, these findings demonstrate that DATS suppresses inflammation and inhibits the activation of NF-κB induced by NNK, suggesting that the chemopreventive effects of DATS against NNK-induced lung tumorigenesis may be attributed to its inhibition of the NF-κB signaling pathway.4. Discussion
Lung cancer is one of the most prevalent cancer forms worldwide, with smoking being a major risk factor. Hence, the development of preventive strategies is crucial in combating this disease. Epidemiology analysis has demonstrated that daily diet adjustments can effectively prevent lung cancer.23 Our previous research focused on the potential chemopreventive effects of phytochemical rich foods against tobacco-induced lung cancer.13 Garlic (Allium sativum L.), a member of the genus Allium in the Liliaceae family, is native to Central Asia and now is cultivated worldwide.24 Numerous epidemiological studies have highlighted the various health benefits associated with garlic consumption, leading to its popularity as a food and health supplement. In 2013, clinical reports indicated that raw garlic intake could potentially prevent lung cancer induced by smoking or exposure to high temperature cooking fumes, indicating that garlic may serve as a chemopreventive agent against cancer.25 Garlic contains a rich array of chemical compounds, and garlic oil is considered to be the primary bioactive ingredients in garlic. This class of compounds exerts diverse biological activities, including anti-oxidant, anti-bacteria, anti-inflammatory, and detoxification properties.18 Our recent work has revealed the inhibitory effects of garlic oil, particularly its most abundant compound DATS, on lung tumorigenesis induced by the tobacco carcinogen NNK in A/J mice or MRC-5 cells.17 To further elucidate the chemopreventive action and underlying mechanisms of the DATS in lung tumorigenesis, we established an NNK-stimulated lung adenocarcinoma animal model in A/J mice.17 Subsequently, we employed multi-omics approach combined molecular biology to clarify the underlying mechanisms.In our study, we successfully established a lung adenocarcinoma model in A/J mice stimulated by NNK to investigate the chemopreventive effects of DATS against lung cancer. The A/J mouse strain is known to be highly susceptible to develop lung tumorigenesis.26 Exposure to cigarette smoke or its constituents, such as NNK, has been widely used to establish in vivo models for studying the chemoprevention of lung tumorigenesis. It has been shown that lung tumors can develop in A/J mice after exposure to NNK with an incidence of 100% and a high multiplicity.27 In our experiments, we observed the appearance of tumor loci on the lung surface of mice exposed to NNK, while no tumor loci were observed in the control group. As expected, treatment with DATS significantly reduced the number of lung tumors in NNK induced A/J mice compared to NNK treatment alone. Accordingly, DATS attenuated the histopathological changes stimulated by NNK in the lungs, showing a reduction in the degree of tissue differentiation and preservation of the alveolar architecture. Importantly, the dosage of DATS used in this study was determined based on our previous research.17 We previously demonstrated that A/J mice administrated with 50 mg kg−1 garlic oil exhibited a chemoprotective effect against NNK induced lung carcinogenesis. As DATS constitutes 46.35% ± 0.32% of garlic oil,17 we administrated DATS at a dose of 23 mg kg−1 in the current study. Collectively, our findings demonstrate that DATS exhibits a chemoprotective effect against NNK-induced lung carcinogenesis in A/J mice, highlighting its potential as a chemopreventive agent for lung cancer.
In order to further elucidate the potential mechanism of DATS in the chemoprevention of lung cancer induced by cigarette smoke carcinogens, we analyzed the composition of gut microbiota using 16S rRNA gene sequencing in the NNK and NNK-DATS groups. Increasing research has highlighted the pathogenic role of the gut microbiome in cancer progression.28 The gut microbiome influences cancer development by regulating metabolic pathways, suppressing immune cell function, and producing pro-inflammatory factors.29,30 For example, pattern recognition receptors (PRRs), such as toll-like receptors (TLRs) and nucleotide-bound oligomeric domain-like receptors (NLRs), activate the NF-κB signaling pathway, leading to the production of inflammatory cytokines. Short-chain fatty acids (SCFAs) derived from gut microbial metabolism can reduce inflammation by interacting with G protein-coupled receptors.31 Clinical trials have also shown that microbiome signatures can serve as diagnostic biomarkers of early disease detection.32 The lung microbiome, in particular, has shown promise as a diagnostic marker and a therapeutic target for lung cancer, especially in the early stages of cancers.33,34 Gut microbiome characteristics have also been explored as potential markers for predicting early lung cancer.35 In summary, disturbances in the gut microbiome can promote lung cancer progression through various mechanisms, making the gut microbiome a potential diagnostic marker and therapeutic target for lung cancer.36 Interestingly, we previously discovered that tobacco carcinogens, such as NNK and BaP, can disturb gut microbiota in lung cancer mice.10 Subsequent studies by Bai et al. further revealed that cigarette smoke exposure promoted colorectal cancer through modulation of the gut microbiota.11 These findings highlight the protumorigenic role of gut microbiota dysbiosis induced by cigarette smoke exposure. Consequently, targeting the microbiome could be an important strategy for the prevention or treatment of lung cancer. It is worth noting that dietary hydrogen sulfide (H2S) derived from plants has been shown to modulate the abundance and function of the microbiome.37 Garlic, a natural dietary source of H2S,38 has been investigated to regulate gut microbiome and ameliorate various diseases, including hypertension,39 atherosclerosis,40 and metabolic disorders.41 DATS, as one of the main active components of garlic, may, therefore, modulate the gut microbiota and play a crucial role in preventing lung cancer. To investigate this hypothesis, we performed 16S rRNA sequencing analysis to elucidate the composition of the gut microflora in mice treated with DATS and exposed to NNK. Interestingly, we found that DATS administration improved the alteration of gut microbiota composition induced by NNK exposure, leading to significant changes in the abundance of seven different bacteria. Among these bacteria, uncultured Barnesiella sp, uncultured Bacterium, F. rodentium, and B. acidifaciens were enriched, while Lachnospiraceae bacterium A4, uncultured Rumen bacterium, and Clostridium sp A9 were depleted with the DATS intervention group. Of particular interest was the enrichment of F. rodentium, which is known to be strongly under-represented during tumourigenesis. F. rodentium and its metabolic products have been shown to reduce tumor growth by producing SCFAs that regulate protein acetylation and tumor cell proliferation, inhibiting calcineurin and nuclear factor of activated T cells cytoplasmic 3 (NFATc3) activation.42 Similarly, Rescigno and co-workers identified the anti-tumourigenic effects of the human homologue of F. rodentium, named Holdemanella biformis (H. biformis), which was found to be reduced in the faeces of patients with large adenomas.42 Therefore, H. biformis may serve as a potential biomarker for early tumor detection. In our present study, we also observed an increased abundance of F. rodentium in the faeces of mice treated with DATS, indicating that DATS may exert its lung cancer preventive role through alteration in F. rodentium abundance. Follow-up experiments, such as antibiotic treatment and fecal microbiota transplantation (FMT), are needed to further validate the therapeutic effect of DATS on the gut microbiota.
Accumulating evidence indicates that cellular intrinsic metabolites play a crucial role in bacteria-induced cancer development.43 In this study, we conducted an untargeted metabolomics to determine alterations in faecal metabolites by DATS. We observed significant changes in the linoleic acid metabolism pathway, which is consistent with our previous findings that linoleic acid levels were higher in NNK plus BaP exposed A/J mice compared to control mice.10 Additionally, we found a correlation between stool metabolites and gut microbiome, revealing that certain bacteria, including uncultured Barnesiella sp, uncultured Bacterium, F. rodentium, and B. acidifaciens enriched in DATS treated mice were positively correlated with specific metabolites, including cholesterol sulfate, 2,3-dinor-8-iso prostaglandin F2α, cortexolone, and 13-L-hydroperoxylinoleic acid, while others were negatively correlated with these metabolites. These metabolites are implicated in metabolic pathways like steroid hormone biosynthesis, linoleic acid and arachidonic acid metabolism, indicating that gut microbial dysbiosis and altered metabolites may contribute to tobacco carcinogen-induced lung tumorigenesis.
Furthermore, our untargeted metabolome profiling results revealed that the PPAR signaling was significantly enriched in DATS-treated mice. This finding was confirmed by RNA-seq analysis, which also showed that the PPAR signaling pathway was enriched in DATS-treated mice compared to the NNK group. These findings indicate that DATS may suppress NNK induced lung tumourigenesis by modulating the PPAR pathway. It is worth noting that gut microbiota-derived metabolites, such as pathogen-associated molecular patterns (PAMPs) like SCFAs, bile acids (BAs), and lipopolysaccharides (LPS), play a vital role in crosstalk between microbes and hosts, impacting host metabolic health. These molecules can act on host receptors, including PPARγ, to regulate host signaling pathways and modulate host health and disease physiology.44 Therefore, the chemopreventive property of DATS on NNK-triggered lung cancer may be closely related to the PPAR signaling pathway.
To further verify the function of the PPAR pathway in the chemopreventive effect of DATS on lung cancer, we examined the PPARγ expression in lung tissues using western blotting and immunofluorescence. PPAR has three identified and cloned subtypes, including PPARα, PPARβ, and PPARγ.45 PPARγ is a transcription factor that regulates various cellular processes. It is known to negatively regulate inflammation and has been implicated in various diseases.46 Several studies have revealed that PPARγ plays a crucial role in lung cancer.47,48 PPARγ activation can inhibit proliferation through its differentiation-promoting effects.45 In our study, we discovered that DATS raised the expression of PPARγ protein remarkably, indicating that DATS may activate the PPARγ signaling pathway, induce lung cell differentiation, suppress proliferation, and prevent NNK-induced lung tumorigenesis. These findings are consistent with previous reports that demonstrate the activation of PPARγ by DATS and its role in suppressing lung inflammation.49
Because PPARγ is a negative regulator of inflammation, we further evaluated the anti-inflammatory property of DATS. It is well established that PPARγ interacts with and negatively regulates NF-κB, which is the basis of anti-inflammatory effect of PPARγ.46 By binding to NF-κB, PPARγ can inhibit its activation, leading to reduced production of pro-inflammatory cytokines.50 These cytokines are known to be involved in cancer development.
Moreover, activation of inflammatory signaling pathways, such as NF-κB, is involved in the development of cigarette carcinogen-stimulated lung cancer or lung injury.50,51 Therefore, we investigated the mRNA and protein levels of these pro-inflammatory factors and found that DATS significantly attenuated their expression induced by NNK. Additionally, DATS decreased protein expression of p65 and p-p65, while increasing the expression of IκBα protein. In other words, DATS was able to prevent the NNK-induced tumor inflammatory microenvironment, indicating that DATS may target carcinogen initiation. On the whole, these results indicate that the chemoprotective effect of DATS against NNK-triggered lung cancer is achieved, at least in part, by inhibiting the NF-κB signaling pathway and the inflammatory response.
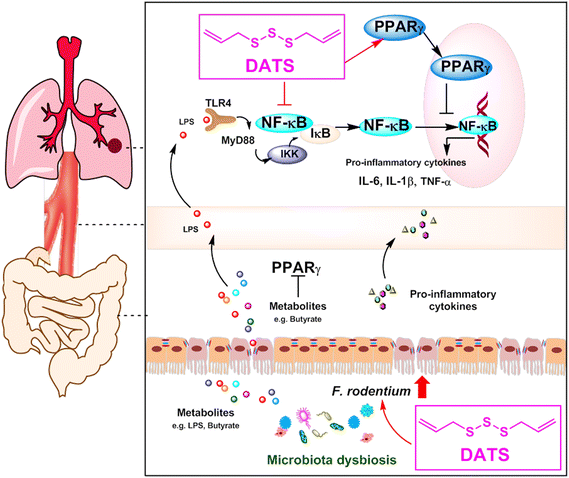
In conclusion, our study highlights the significant chemopreventive potential of DATS for lung cancer. We propose that DATS ameliorates NNK-induced lung tumorigenesis by modulating gut microbiota, specifically F. rodentium, which has an inhibitory effect on tumor growth. Mechanistically, DATS can regulate the PPARγ/NF-κB signaling pathway, leading to a reduction in the accumulation of inflammatory cytokines (Fig. 7). These findings indicate that DATS may be a promising candidate for the chemoprevention of tobacco carcinogen-induced lung cancer.
Author contributions
Zhuo Qu and Jiahui Tian: methodology, investigation, writing original draft. Jiachen Sun and Ying Shi: writing – review & editing. Jianqiang Yu and Wannian Zhang: supervision, project administration. Chunlin Zhuang: writing – review & editing, funding acquisition.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was funded by grants from the Natural Science Foundation of Ningxia Province (2023AAC05031), Key Research and Development Program of Ningxia (2021BEG03103, 2020BEG03011 and 2020BEB04020), Shanghai Shuguang Program (21SG38), and West Light Foundation of the Chinese Academy of Sciences (XAB2020YW15).References
- R. L. Siegel, K. D. Miller, N. S. Wagle and A. Jemal, Cancer statistics, 2023, CA Cancer J. Clin., 2023, 73, 17–48 CrossRef PubMed.
- T. S. Mok, Q. Zhou and Y.-L. Wu, Research and standard care: lung cancer in China, Am. Soc. Clin. Oncol. Educ. Book, 2012, 32, 432–436 CrossRef PubMed.
- P. Anand, A. B. Kunnumakara, C. Sundaram, K. B. Harikumar, S. T. Tharakan, O. S. Lai, B. Sung and B. B. Aggarwal, Cancer is a preventable disease that requires major lifestyle changes, Pharm. Res., 2008, 25, 2097–2116 CrossRef CAS PubMed.
- P. Nanavaty, M. S. Alvarez and W. M. Alberts, Lung cancer screening: advantages, controversies, and applications, Cancer Control, 2014, 21, 9–14 CrossRef PubMed.
- K. D. Miller, L. Nogueira, T. Devasia, A. B. Mariotto, K. R. Yabroff, A. Jemal, J. Kramer and R. L. Siegel, Cancer treatment and survivorship statistics, 2022, CA Cancer J. Clin., 2022, 72, 409–436 CrossRef PubMed.
- G. Guglielmi, Almost half of cancer deaths are preventable, Nature, 2022 DOI:10.1038/d41586-022-02355-x.
- S. S. Hecht, Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention, Lancet Oncol., 2002, 3, 461–469 CrossRef CAS PubMed.
- G.-Z. Ge, T.-R. Xu and C. Chen, Tobacco carcinogen NNK-induced lung cancer animal models and associated carcinogenic mechanisms, Acta Biochim. Biophys. Sin., 2015, 47, 477–487 CrossRef CAS PubMed.
- Y. Wang, S. Narayanapillai, Q. Hu, N. Fujioka and C. Xing, Contribution of tobacco use and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone to three methyl DNA adducts in urine, Chem. Res. Toxicol., 2018, 31, 836–838 Search PubMed.
- Z. Qu, L. Zhang, R. Hou, X. Ma, J. Yu, W. Zhang and C. Zhuang, Exposure to a mixture of cigarette smoke carcinogens disturbs gut microbiota and influences metabolic homeostasis in A/J mice, Chem.-Biol. Interact., 2021, 344, 109496 CrossRef CAS PubMed.
- X. Bai, H. Wei, W. Liu, O. O. Coker, H. Gou, C. Liu, L. Zhao, C. Li, Y. Zhou and G. Wang, Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites, Gut, 2022, 71, 2439–2450 CrossRef CAS PubMed.
- B. Xia, W. Yang, H. Liang, S. Liu, D. Wang and J. Huang, Cancer Prevention Effects of Foods, Food Groups, Nutrients, and Their Underlying Mechanisms, ACS Food Sci. Technol., 2022, 2, 437–454 CrossRef CAS.
- Y. Ding, R. Hou, J. Yu, C. Xing, C. Zhuang and Z. Qu, Dietary Phytochemicals as Potential Chemopreventive Agents against Tobacco-Induced Lung Carcinogenesis, Nutrients, 2023, 15, 491 CrossRef CAS PubMed.
- P. Leitzman, S. C. Narayanapillai, S. Balbo, B. Zhou, P. Upadhyaya, A. A. Shaik, M. G. O'Sullivan, S. S. Hecht, J. Lu and C. Xing, Kava blocks 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in association with reducing O6-methylguanine DNA adduct in A/J mice, Cancer Prev. Res., 2014, 7, 86–96 CrossRef CAS PubMed.
- S. C. Narayanapillai, S. H. Lin, P. Leitzman, P. Upadhyaya, C. J. Baglole and C. Xing, Dihydromethysticin (DHM) Blocks Tobacco Carcinogen 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-Induced O(6)-Methylguanine in a Manner Independent of the Aryl Hydrocarbon Receptor (AhR) Pathway in C57BL/6 Female Mice, Chem. Res. Toxicol., 2016, 29, 1828–1834 Search PubMed.
- E. Szabo, J. T. Mao, S. Lam, M. E. Reid and R. L. Keith, Chemoprevention of lung cancer: diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines, Chest, 2013, 143, e40S–e60S CrossRef CAS PubMed.
- L. Zhang, Z. Qu, A. Song, J. Yang, J. Yu, W. Zhang and C. Zhuang, Garlic oil blocks tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis by inducing phase II drug-metabolizing enzymes, Food Chem. Toxicol., 2021, 157, 112581 CrossRef CAS PubMed.
- D. De Greef, E. M. Barton, E. N. Sandberg, C. R. Croley, J. Pumarol, T. L. Wong, N. Das and A. Bishayee, Anticancer potential of garlic and its bioactive constituents: A systematic and comprehensive review, Semin. Cancer Biol., 2021, 73, 219–264 CrossRef CAS PubMed.
- W. S. Leung, W. W. Kuo, D. T. Ju, T. D. Wang, W. S. T. Chen, T. J. Ho, Y. M. Lin, B. Mahalakshmi, J. Y. Lin and C. Y. Huang, Protective effects of diallyl trisulfide (DATS) against doxorubicin-induced inflammation and oxidative stress in the brain of rats, Free Radicals Biol. Med., 2020, 160, 141–148 CrossRef CAS PubMed.
- X. Jiang, X. Zhu, N. Liu, H. Xu, Z. Zhao, S. Li, S. Li, J. Cai and J. Cao, Diallyl Trisulfide Inhibits Growth of NCI-H460 in Vitro and in Vivo, and Ameliorates Cisplatin-Induced Oxidative Injury in the Treatment of Lung Carcinoma in Xenograft Mice, Int. J. Biol. Sci., 2017, 13, 167–178 CrossRef CAS PubMed.
- R. Marni, D. B. Kundrapu, A. Chakraborti and R. Malla, Insight into drug sensitizing effect of diallyl disulfide and diallyl trisulfide from Allium sativum L. on paclitaxel-resistant triple-negative breast cancer cells, J. Ethnopharmacol., 2022, 296, 115452 CrossRef CAS PubMed.
- R. Malla, R. Marni, A. Chakraborty and M. A. Kamal, Diallyl disulfide and diallyl trisulfide in garlic as novel therapeutic agents to overcome drug resistance in breast cancer, J. Pharm. Anal., 2022, 12, 221–231 CrossRef PubMed.
- P. A. Williams, S. K. Zaidi and R. Sengupta, AACR cancer disparities progress report 2022, Cancer Epidemiol. Biomarkers Prev., 2022, 31, 1249–1250 CrossRef PubMed.
- Y. Zhang, X. Liu, J. Ruan, X. Zhuang, X. Zhang and Z. Li, Phytochemicals of garlic: Promising candidates for cancer therapy, Biomed. Pharmacother., 2020, 123, 109730 CrossRef.
- Z. Y. Jin, M. Wu, R. Q. Han, X. F. Zhang, X. S. Wang, A. M. Liu, J. Y. Zhou, Q. Y. Lu, Z. F. Zhang and J. K. Zhao, Raw garlic consumption as a protective factor for lung cancer, a population-based case-control study in a Chinese population, Cancer Prev. Res., 2013, 6, 711–718 CrossRef PubMed.
- S. S. Hecht, Carcinogenicity studies of inhaled cigarette smoke in laboratory animals: old and new, Carcinogenesis, 2005, 26, 1488–1492 CrossRef CAS.
- L. A. Peterson and S. S. Hecht, O 6-Methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in A/J mouse lung, Cancer Res., 1991, 51, 5557–5564 CAS.
- G. D. Sepich-Poore, L. Zitvogel, R. Straussman, J. Hasty, J. A. Wargo and R. Knight, The microbiome and human cancer, Science, 2021, 371, eabc4552 CrossRef CAS PubMed.
- C. Jin, G. K. Lagoudas, C. Zhao, S. Bullman, A. Bhutkar, B. Hu, S. Ameh, D. Sandel, X. S. Liang, S. Mazzilli, M. T. Whary, M. Meyerson, R. Germain, P. C. Blainey, J. G. Fox and T. Jacks, Commensal Microbiota Promote Lung Cancer Development via γδ T Cells, Cell, 2019, 176, 998–1013 CrossRef CAS PubMed.
- Y. Ge, X. Wang, Y. Guo, J. Yan, A. Abuduwaili, K. Aximujiang, J. Yan and M. Wu, Gut microbiota influence tumor development and Alter interactions with the human immune system, J. Exp. Clin. Cancer Res., 2021, 40, 1–9 CrossRef PubMed.
- Q. Mao, F. Jiang, R. Yin, J. Wang, W. Xia, G. Dong, W. Ma, Y. Yang, L. Xu and J. Hu, Interplay between the lung microbiome and lung cancer, Cancer Lett., 2018, 415, 40–48 CrossRef CAS PubMed.
- J. J. Tsay, B. G. Wu, I. Sulaiman, K. Gershner, R. Schluger, Y. Li, T. A. Yie, P. Meyn, E. Olsen, L. Perez, B. Franca, J. Carpenito, T. Iizumi, M. El-Ashmawy, M. Badri, J. T. Morton, N. Shen, L. He, G. Michaud, S. Rafeq, J. L. Bessich, R. L. Smith, H. Sauthoff, K. Felner, R. Pillai, A. M. Zavitsanou, S. B. Koralov, V. Mezzano, C. A. Loomis, A. L. Moreira, W. Moore, A. Tsirigos, A. Heguy, W. N. Rom, D. H. Sterman, H. I. Pass, J. C. Clemente, H. Li, R. Bonneau, K. K. Wong, T. Papagiannakopoulos and L. N. Segal, Lower Airway Dysbiosis Affects Lung Cancer Progression, Cancer Discovery, 2021, 11, 293–307 CrossRef CAS PubMed.
- E. A. Marshall, F. S. Filho, D. D. Sin, S. Lam, J. M. Leung and W. L. Lam, Distinct bronchial microbiome precedes clinical diagnosis of lung cancer, Mol. Cancer, 2022, 21, 68 CrossRef PubMed.
- M. Bou Zerdan, J. Kassab, P. Meouchy, E. Haroun, R. Nehme, M. Bou Zerdan, G. Fahed, M. Petrosino, D. Dutta and S. Graziano, The Lung Microbiota and Lung Cancer: A Growing Relationship, Cancers, 2022, 14, 4813 CrossRef CAS PubMed.
- Y. Zheng, Z. Fang, Y. Xue, J. Zhang, J. Zhu, R. Gao, S. Yao, Y. Ye, S. Wang and C. Lin, Specific gut microbiome signature predicts the early-stage lung cancer, Gut Microbes, 2020, 11, 1030–1042 CrossRef PubMed.
- M. R. Fernandes, P. Aggarwal, R. G. Costa, A. M. Cole and G. Trinchieri, Targeting the gut microbiota for cancer therapy, Nat. Rev. Cancer, 2022, 22, 703–722 CrossRef CAS PubMed.
- A. G. Buret, T. Allain, J. P. Motta and J. L. Wallace, Effects of Hydrogen Sulfide on the Microbiome: From Toxicity to Therapy, Antioxid. Redox Signaling, 2022, 36, 211–219 CrossRef CAS PubMed.
- G. A. Benavides, G. L. Squadrito, R. W. Mills, H. D. Patel, T. S. Isbell, R. P. Patel, V. M. Darley-Usmar, J. E. Doeller and D. W. Kraus, Hydrogen sulfide mediates the vasoactivity of garlic, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 17977–17982 CrossRef CAS PubMed.
- C. N. Hsu, C. Y. Hou, G. P. Chang-Chien, S. Lin and Y. L. Tain, Maternal garlic oil supplementation prevents high–fat diet–induced hypertension in adult rat offspring: Implications of H2S–generating pathway in the gut and kidneys, Mol. Nutr. Food Res., 2021, 65, 2001116 CrossRef CAS PubMed.
- S. Panyod, W. K. Wu, P. C. Chen, K. V. Chong, Y. T. Yang, H. L. Chuang, C. C. Chen, R. A. Chen, P. Y. Liu, C. H. Chung, H. S. Huang, A. Y. Lin, T. D. Shen, K. C. Yang, T. F. Huang, C. C. Hsu, C. T. Ho, H. L. Kao, A. N. Orekhov, M. S. Wu and L. Y. Sheen, Atherosclerosis amelioration by allicin in raw garlic through gut microbiota and trimethylamine-N-oxide modulation, npj Biofilms Microbiomes, 2022, 8, 4 CrossRef CAS PubMed.
- K. Chen, Y. Nakasone, S. Yi, H. R. Ibrahim, K. Sakao, M. A. Hossain and D. X. Hou, Natural Garlic Organosulfur Compounds Prevent Metabolic Disorder of Lipid and Glucose by Increasing Gut Commensal Bacteroides acidifaciens, J. Agric. Food Chem., 2022, 70, 5829–5837 CrossRef CAS PubMed.
- E. Zagato, C. Pozzi, A. Bertocchi, T. Schioppa, F. Saccheri, S. Guglietta, B. Fosso, L. Melocchi, G. Nizzoli, J. Troisi, M. Marzano, B. Oresta, I. Spadoni, K. Atarashi, S. Carloni, S. Arioli, G. Fornasa, F. Asnicar, N. Segata, S. Guglielmetti, K. Honda, G. Pesole, W. Vermi, G. Penna and M. Rescigno, Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth, Nat. Microbiol., 2020, 5, 511–524 CrossRef CAS PubMed.
- W. Y. Cheng, C.-Y. Wu and J. Yu, The role of gut microbiota in cancer treatment: friend or foe?, Gut, 2020, 69, 1867–1876 CrossRef CAS PubMed.
- W. M. de Vos, H. Tilg, M. Van Hul and P. D. Cani, Gut microbiome and health: mechanistic insights, Gut, 2022, 71, 1020–1032 CrossRef CAS PubMed.
- V. G. Keshamouni, R. C. Reddy, D. A. Arenberg, B. Joel, V. J. Thannickal, G. P. Kalemkerian and T. J. Standiford, Peroxisome proliferator-activated receptor-gamma activation inhibits tumor progression in non-small-cell lung cancer, Oncogene, 2004, 23, 100–108 CrossRef CAS PubMed.
- M. Y. Li, Z. H. Zhang, Z. Wang, H. X. Zuo, J. Y. Wang, Y. Xing, C. H. Jin, G. H. Xu, L. X. Piao, J. Ma and X. Jin, Convallatoxin protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-κB signaling through activation of PPARγ, Pharmacol. Res., 2019, 147, 104355 CrossRef CAS PubMed.
- J. Tian, L. Hu, X. Li, J. Geng, M. Dai and X. Bai, MicroRNA-130b promotes lung cancer progression via PPARgamma/VEGF-A/BCL-2-mediated suppression of apoptosis, J. Exp. Clin. Cancer Res., 2016, 35, 105 CrossRef PubMed.
- L. N. Ge, L. Yan, C. Li and K. Cheng, Bavachinin exhibits antitumor activity against non-small cell lung cancer by targeting PPARgamma, Mol. Med. Rep., 2019, 20, 2805–2811 CAS.
- M. K. Marimuthu, A. Moorthy and T. Ramasamy, Diallyl Disulfide Attenuates STAT3 and NF-kappaB Pathway Through PPAR-gamma Activation in Cerulein-Induced Acute Pancreatitis and Associated Lung Injury in Mice, Inflammation, 2022, 45, 45–58 CrossRef CAS PubMed.
- Q. Li, J. Sun, N. Mohammadtursun, J. Wu, J. Dong and L. Li, Curcumin inhibits cigarette smoke-induced inflammation via modulating the PPARgamma-NF-kappaB signaling pathway, Food Funct., 2019, 10, 7983–7994 RSC.
- Y. Lou, Z. Guo, Y. Zhu, M. Kong, R. Zhang, L. Lu, F. Wu, Z. Liu and J. Wu, Houttuynia cordata Thunb. and its bioactive compound 2-undecanone significantly suppress benzo(a)pyrene-induced lung tumorigenesis by activating the Nrf2-HO-1/NQO-1 signaling pathway, J. Exp. Clin. Cancer Res., 2019, 38, 242 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo03914e |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |