Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The generally recognized as safe (GRAS) microalgae Haematococcus pluvialis (wet) as a multifunctional additive for coloring and improving the organoleptic and functional properties of foods†
Aly
Castillo
 *abc,
Tiane C.
Finimundy
*abc,
Tiane C.
Finimundy
 de,
Sandrina A.
Heleno
de,
Sandrina A.
Heleno
 de,
Paula
Rodrigues
de,
Filipa A.
Fernandes
de,
Simón
Pereira
f,
Marta
Lores
de,
Paula
Rodrigues
de,
Filipa A.
Fernandes
de,
Simón
Pereira
f,
Marta
Lores
 b,
Lillian
Barros
b,
Lillian
Barros
 de and
Carmen
Garcia-Jares
ab
de and
Carmen
Garcia-Jares
ab
aCRETUS, Department of Analytical Chemistry, Nutrition and Food Science, Universidade de Santiago de Compostela, E-15782, Santiago de Compostela, Spain. E-mail: alyjesus.castillo.zamora@usc.es
bLIDSA, Department of Analytical Chemistry, Nutrition and Food Science, Universidade de Santiago de Compostela, E-15782, Santiago de Compostela, Spain
ci-Grape Laboratory, Edificio Emprendia, Avda Mestre Mateo s/n, 15702, Santiago de Compostela, Spain
dCentro de Investigação de Montanha (CIMO), Instituto Politécnico de Bragança, Campus de Santa Apolónia, Bragança, 5300-253, Portugal
eLaboratório Associado para a Sustentabilidade e Tecnologia em Regiões de Montanha (SusTEC), Instituto Politécnico de Bragança, Campus de Santa Apolónia, 5300-253 Bragança, Portugal
fInstitute of Aquaculture and Department of Microbiology and Parasitology, Universidade de Santiago de Compostela, Campus Vida, E-15782, Santiago de Compostela, Spain
First published on 22nd May 2023
Abstract
This work proposes the application of astaxanthin-rich H. pluvialis wet paste (HPW) as a partial substitute for wheat flour in the preparation of filloas, a dish that combines the basic ingredients of industrial bakery. The nutritional and color profile of HPW-enriched samples was evaluated by comparative analysis with a mixture of synthetic food dyes. The highest content of carotenoids (798 ± 12 μg g−1) and fatty acids (76 ± 2 mg g−1) was obtained for a filloa fortified with H. pluvialis in contrast to a non-significant dye response. Subsequently, the color stability of the fortified filloa was evaluated over time (3, 6 and 9 days), as well as its physicochemical properties and microbiological profile. As a result, HPW provided filloas with a longer shelf life, brightness (*L), and texture, in comparison with a mixture of synthetic dyes. In addition, an inhibitory effect of HPW towards mesophilic aerobic microorganisms in the food was obtained.
Introduction
Aesthetics as an enhancer of the final product in the food industry has a positioning equal to or greater than the nutritional value provided.1 Consumer choice has been proven to be highly dependent on the appearance, appearance-induced expectations, and visual quality of the food.2 These demands have focused efforts on whole foods that incorporate organoleptic and functional properties, generally requiring a wide number of additives and specific functional ingredients. As an alternative to the high costs of improving individual food properties, multifunctional additives are clearly more attractive as they improve more than one aspect of the final product. Among the most common family of additives, the use of synthetic additives prevails over natural additives, due to their lower production costs, giving them an 18% superiority in the global food market in the last 5 years.3 In contrast, their excessive use has been framed in a controversial environment due to the potential impact on the consumer, being associated with reactions such as asthma, allergies, and tumor development.4Within synthetic additives, food colors stand out for their high consumption, with a five-fold increase in intake over the last 60 years.5 Paradoxically, they generally do not provide any nutritional value, coupled with the growing concern about their adverse health effects.6 The main market for synthetic colors is focused on sectors such as confectionery, where children, the main consumers, are particularly affected by the intake of these substances. Artificial colors such as azorubine (E-122), tartrazine (E-102), erioglaucine (E-133), sunset (E-110), quinoline (E-104), allura (E129), and ponceau 4R (E-124) have been linked to possible effects on activity and attention in infants.7 Because of this, there is a trend in the industry to remove synthetic food colors and replace them with natural products.
Recent research on functional food ingredients has shown interest in developing foods containing algae or algal ingredients.8 Within this large group of living organisms, one of the most promising sources of bioactive ingredients and compounds for novel food products, which can be used to improve the nutritional value of foods due to their balanced chemical composition, are microalgae.9 Different approaches have increased the bioactive content of diverse food matrices, incorporating freeze-dried microalgae such as Arthrospira platensis, Chlorella vulgaris, and Spirulina sp. at concentrations of 1 to 10% gmicroalga gfood−1, obtaining high consumer acceptance and even a predilection for foods enriched with these microorganisms.10–12 Likewise, microalgae have several attractive characteristics for large-scale sustainable production, such as high biomass yields per unit area and the ability to be cultivated on a non-arable land using non-potable water or even salt water.13 In turn, because not all microalgae are suitable for consumption, approval as a Generally Recognized as Safe (GRAS) organism is necessary. Limited microalgae have the FDA-recognized GRAS status, mainly because this category requires costly and time-consuming safety testing, which has limited the number of algal species with the GRAS status.14
A microorganism characterized by its capacity to accumulate bioactive compounds, where carotenoids and fatty acids stand out, is the microalga Haematococcus pluvialis.15 This alga belongs to the GRAS category, being an indirect but essential coloring agent in providing the characteristic reddish hue to salmon. This coloring is due to its ability to store up to 6% dry weight (DW) of astaxanthin, and other carotenoids such as β-carotene and lutein.16 This makes the microalga not only a powerful coloring agent, but also a source of antioxidants with potential action in the prevention of various health disorders and metabolic diseases.17 In turn, H. pluvialis is cultivated to produce omega-3 and omega-6 fatty acids, as it can store up to 32% DW of these essential fatty acids.18 In addition to their beneficial effect in combating cardiovascular diseases, these fatty acids are linked to astaxanthin through esterification processes, giving it greater stability against degradation,19 maintaining its bioactive capacity and bioavailability, as well as prolonging its coloring capacity.
There is limited information on the use of microalgae as an additive in the confectionery industry, to enrich its organoleptic and functional properties. Only one study has used H. pluvialis as a partial substitute for whole grains to reduce the glycemic response in cookies, showing a healthy alternative with bioactive potential.20 However, the costly lyophilization process required to obtain flour from H. pluvialis and the subsequent dry milling, which favors the degradation of carotenoid compounds, make its use as a colorant on a commercial scale complex.21 Therefore, a direct method that takes advantage of the bioactive content of wet microalgae pastes by avoiding pre-treatment of the biomass would be an attractive approach.
In this way, this work shows a functional alternative for the enrichment of the organoleptic and bioactive properties of filloas (Galician pancakes), a typical dish from the northwest of the Iberian Peninsula, which combines the essential ingredients of the confectionery industry. The microalga in its wet state is proposed as a natural coloring agent in contrast to commercial synthetic colorants. Likewise, its functionality as a potential moderator of the physicochemical and microbiological degradation of foodstuffs is determined through a stability study.
Materials and methods
Haematococcus pluvialis wet paste biomass
The astaxanthin-rich Haematococcus pluvialis wet paste (above 50 mgastaxanthin gDW−1) was obtained through a two-step industrial process optimized for maximum astaxanthin concentration following the best set of parameters delimited by Pereira and Otero 2020.22 In summary, in a first stage, the vegetative cell growth of H. pluvialis is promoted until a defined density is reached (target value: 7.0 × 106 cells per mL). Then, the microalga is exposed to inductive conditions of irradiation and nitrate starvation, which induced the accumulation of astaxanthin. The wet biomass is centrifuged to remove free water until a paste containing about 25% solids is obtained. The paste was then stored at a temperature of −20 °C.Filloa preparation
The preparation of filloas was outlined by the American Association for Cereal Chemistry (AACC) 10-80.01 Baking Flour Quality in a Pancake-Making Method, with slight modifications applied to preserve the original organoleptic characteristics of filloas.23 The basic recipe requires flour (136 g), two eggs, milk (340 mL), salt (0.20 g) and sugar (10 g). The addition percentages of the microalgae paste were determined by a previous analysis (results not shown) of 50% inhibition of oxidative haemolysis (OxHLIA). Thus, a concentration that guarantees 50% inhibition of OxHLIA analysis was added as a lower limit; 7% (7 gmicroalgaeDW 100 gflour−1) (HPW7) as well as an inhibition higher than 95% using 13% (13 gmicroalgaeDW 100 gflour−1) (HPW13) have been considered. These concentrations provide a total free astaxanthin concentration in a 20 g portion below the intake suggested by the European Food Safety Authority of 8 mg day−1.24 For comparative analysis of the pigmentation obtained by the addition of HPW relative to a synthetic dye (ESI Table S1†), the volumes of the commercial colorants E122 (2.6 mL) and E102 (3.9 mL) were determined by colorimetric analysis to an equivalent hue between the colorant and HPW7 of ±4 (ESI Fig. S1†).For the preparation of the control filloas, eggs and milk were mixed with an electric mixer for 2 minutes. Then, salt, sugar, and flour were added and mixed for 3 minutes. HPW supplemented filloas were prepared by replacing the wheat flour by the corresponding dry weight of the algae paste (7 and 13%). The optimized volume of E122 red and E102 yellow was added for the comparative study with the addition of a commercial food dye. Since the H. pluvialis cells need to be broken to release their content, both the control and the supplemented mixtures were homogenized in an Ultra Turrax® T25 rotor-stator system at 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 minutes in an ice bath. The filloas were cooked on a 12 cm circular hotplate at a temperature of 190 °C. Cooking was timed with 1 minute contact time on each side of the pancake. Then, the filloas were left to rest at room temperature for 1 minute. For the quantification of bioactive compounds (carotenoids and fatty acids), the filloas were lyophilized and stored in the absence of light at −20 °C. A triplicate of the feed was performed for each factor (blank, dye, HPW7 and HPW13), analysis group (microbiological and physicochemical), as well as for each study day, generating a total of 96 samples.
000 rpm for 3 minutes in an ice bath. The filloas were cooked on a 12 cm circular hotplate at a temperature of 190 °C. Cooking was timed with 1 minute contact time on each side of the pancake. Then, the filloas were left to rest at room temperature for 1 minute. For the quantification of bioactive compounds (carotenoids and fatty acids), the filloas were lyophilized and stored in the absence of light at −20 °C. A triplicate of the feed was performed for each factor (blank, dye, HPW7 and HPW13), analysis group (microbiological and physicochemical), as well as for each study day, generating a total of 96 samples.
Extraction of bioactive compounds
Standards used for the characterization and quantification of carotenoid and fatty acid compounds in the filloas are summarized in ESI Table S2.† MS-grade ultrapure water, ethanol, methanol, methyl tert-butyl ether (MTBE) and dimethylformamide (DMF) were purchased from Scharlab (Barcelona, Spain). Individual stock solutions of carotenoids were prepared in DMF according to the supplier's recommendations. Alpha-tocopherol and alpha-tocopherol acetate were used as an internal standard and a surrogate (100 mg L−1), respectively. Fatty acid standards were prepared in MTBE using nonadecanoic acid as an internal standard and lignoceric acid as a surrogate (2 mg mL−1). All solutions were stored in amber glass vials and protected from light at −20 °C.For the extraction, 1 g of the lyophilized sample was added with 36 μL of the surrogate solution and 2 mL of MTBE. The mixture was disrupted using an IKA T25 rotor–stator system at 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 minute in an ice bath, and then centrifuged at 3800g, separating the upper organic phase. The extraction was repeated four times until the enriched samples had lost their color. The MTBE extract was gently evaporated under a nitrogen stream and reconstituted according to the subsequent analyses.
000 rpm for 1 minute in an ice bath, and then centrifuged at 3800g, separating the upper organic phase. The extraction was repeated four times until the enriched samples had lost their color. The MTBE extract was gently evaporated under a nitrogen stream and reconstituted according to the subsequent analyses.
HPLC-DAD analysis (carotenoid characterization)
For the chromatographic analysis, the procedure of Castillo et al. 2020 was used, with slight modifications.15 Briefly, a Jasco AS-4100 model HPLC-DAD, incorporating a PU-4180 quaternary pump and a PDA MD-4010 diode array detector, was used. A Kinetex 5 μm C18-100 Å column (4.6 × 150 mm, 2.6 μm) maintained at a constant temperature of 30 °C was used for chromatographic separation. The mobile phase consisted of a 1% formic acid solution in water (A) and in methanol (B). Thus, with a constant flow rate of 1 mL min−1, a ratio of 20% of (A) to 80% of (B) was established for 5 min, and then progressively increased to 100% (B) in 10 min, and maintained for 25 min. For quantification, calibration standards were prepared at six concentration levels in DMF (0.5, 1, 5, 10, 15, and 20 mg L−1). Each level was injected at least in triplicate. A directly proportional relationship between the concentration and the chromatographic response was demonstrated, with coefficients of determination (R2) greater than 0.992 in all cases (ESI Fig. S2†). Sample extracts were diluted in 80 μl of DMF, after addition of 10 μL of the IS dilution to give a final concentration of 100 mg L−1, and filtered through 0.22 μm polytetrafluoroethylene (PTFE) filters before injection.GC-MS analysis (fatty acid characterization)
The analysis was performed using an Agilent 780A GC with an Agilent 7693 injector and coupled to an Agilent 5975C triple-axis inert mass quadrupole detector (Agilent Technologies, Palo Alto, CA, USA). Chromatographic separation was performed using a Zebron ZB column (30 × 0.25 mm × 0.25 μm) obtained from Phenomenex (CA, USA). Helium was used as a carrier gas at a constant column flow rate of 1 mL min−1. For the quantification of fatty acids, a calibration curve (ESI Fig. S3†) was prepared with the fatty acid standards at 9 concentration levels (0.2, 0.5, 1, 2, 2, 5, 10, 20, 50 and 100 mg L−1) in MTBE. The extract was diluted in 50 μl of MTBE containing 50 μL of the internal standard (nonadecanoic acid) to a final concentration of 3 mg L−1. A derivatization process using trimethyl sulfonium hydroxide (TMSH) was performed according to Rubio et al., 2018, with slight modifications.25 Briefly, dilutions of standards and samples were mixed with 50 μL of TMSH and allowed to react in the absence of light for 30 min. Selected Ion Monitoring (SIM) acquisition mode was implemented monitoring 2 or 3 ions for an unequivocal identification and quantification of the fatty acids.Stability study
To uncover the effects of the addition of the microalgae paste and artificial coloring on the preservation of the filloas, the four levels (ESI Fig. S1†) were analyzed in triplicate. Each set consisted of identical pieces of filloas with average values of 10.6 cm diameter and 157 ± 1 g mass. To ensure the suitability of the analyses, the wet microalgae paste was sterilized in an autoclave prior to the enrichment of the samples to prevent contamination of the culture media containing the microalgae. Autoclaving of the microalga paste is applied only in the stability study, and its microbiological profile is evaluated to ensure the absence of pathogenic microorganisms. After cooking and subsequent cooling to room temperature, the filloas were stored in food grade bags. Immediately afterwards, all samples were placed in trays and stored at a constant temperature of 20 °C. Four different storage times, measured in days, were used to monitor changes in the physical properties and microbial load, namely T0 (immediately after treatments), T3, T6 and T9.Microbiological analysis
Microorganism analysis
Texture analysis
Texture profile analysis of the H. pluvialis-enriched filloa samples, synthetic dye, and control group was determined using a TA-XT plus texture analyzer from Stable Micro Systems (Vienna Court, Godalming, UK), equipped with a 5 kg load cell, using a 1′′ spherical P/1S aluminium probe and a smooth HDP/TPB ring holder. A compression force analysis was performed on the samples, with a trigger force of 5 g and a speed of 4.0 mm s−1, as a pre-test and 1 mm s−1 as the test speed. The samples were spread evenly in a hollow holder (8 cm diameter and 0.9 cm high) and tested in the objective mode. The probe distance was set to 40 mm, and force was applied in the concentric central zone until rupture. The toughness and extensibility values were measured with an acquisition rate of 200 points per second, obtaining a maximum variation of ±12 g and ±1.9 mm, respectively. All analyses were performed in triplicate on each day of analysis; the results were expressed as the mean ± standard deviation (SD). The data obtained were generated and analyzed using Exponent Stable Micro System software, version 6.16.18.0.Color analysis
The color measurements were carried out using a portable colorimeter model CR400 (Konica Minolta Sensing, Chiyoda, Tokyo, Japan) with 10° and 8 mm aperture viewing angles. To account for the heterogeneous color of the filloa surface, the color was measured at 10 different points, distributing 5 ring measurements in the central zone and 5 in the outer zone. Each analysis was carried out in triplicate through the considered storage sampling times. The recorded values were generated using the standard space defined by the International Commission on Illumination (CIE) L*a*b* of 1976, where L* represents the lightness from black (0) to white (100), a* the variation in redness (green-red) and b* between blue and yellow shades. Measurements were conducted under the standard illuminant CIE D65. All data were analyzed using the Color Image Processing plug-in of MATLAB® software. For a qualitative study, the values obtained were converted to their corresponding values on a hexadecimal scale (HEX) and their hue was then generated for visual analysis.Physicochemical analysis
To quantify the variation in the physical integrity of the filloas, the internal thickness, diameter and external thickness were measured on different days of storage. A digital vernier was used for this purpose, taking each measurement in triplicate. To observe the deterioration qualitatively, the variation on the different study days was documented by using image acquisition. Water activity (aw) measurements of the filloas were obtained using an Aqualab water activity analyzer (4TE Decagon, Munich, Germany). The measurements were conducted at three different points of each sample.To determine the moisture content of the samples, 2 g were extracted from three areas of the feed at equidistant points along the radius of the meal. The samples were placed on metal determination plates inside the moisture analyzer (Adam Equipment, PMB 163). The moisture content was removed through a progressive temperature ramp-up to 110 °C, where it remained stable for an average time of 20 minutes. The test ends when the variation in the mass of the feed due to moisture, within 1 min, is less than 0.001 g. The moisture value is represented as the percentage ratio 1 − (mo·mf−1), where mo is the value of the initial mass and mf is the mass of the food after its moisture removal.
Nutritional value
Ash, fat, and protein contents were evaluated according to the official Association of Official Analytical Chemists (AOAC) food analysis methods.30 The macro-Kjeldahl method was used to estimate the crude protein content (N × 6.25); crude fat was extracted using a Soxhlet apparatus using petroleum ether as a solvent; the ash content was assessed by incineration of the filloa samples at 550 ± 10 °C. The total carbohydrate content (g per 100 gDW) was determined by difference as follows: 100 − (g protein + g fat + g ash). Energy (kcal per 100 gDW) was calculated according to the Regulation (EU) no. 1169/201131 as follows: 4 (g protein + g carbohydrate) + 9 (g fat).Statistical analysis
All data were expressed as mean ± standard deviation, and tests were run in triplicate. For microbial data, after confirming the homoscedasticity of the samples, an analysis of variance (ANOVA) was performed using a mixed effects model with a Dunnett's test to classify differences according to the control group. For physicochemical parameters (texture, color, pH, water activity and physical integrity), a general linear ANOVA was carried out and Dunnett's method was also applied for classification. All analyses were performed using Minitab, LLC. 20.3. All statistical operations were performed at a significance level of 5%.Results and discussion
Nutritional composition
The nutritional composition of the filloa samples is shown in Table 1. The incorporation of synthetic dyes did not lead to any nutritional modification of the food. The fat content increased with the proportion of H. pluvialis paste, showing a statistically significant variation (p < 0.05) when compared to the control group. This can be explained by the composition of H. pluvialis, with up to 37% of lipids, mainly essential fatty acids.18 The ash and protein contents did not vary between samples. The carbohydrate content decreased in the supplemented samples due to their lower content of flour. On the other hand, the caloric value (kcal per 100 g) was higher in samples containing the microalga paste. Fat intake of about 35% of total energy, with a concomitant reduction in carbohydrate intake, could reduce the risk of dietary diseases.32 Thus, the results obtained show an important attractiveness of H. pluvialis as a food additive, providing a nutritional contribution, increasing the content of fatty acids mainly from the omega-3 and 6 families, as well as a decrease of the carbohydrate content without modifying the protein content and providing a higher caloric content.| Sample | Fat (g per 100 g) | Ash (g per 100 g) | Proteins (g per 100 g) | Carbohydrates (g per 100 g) | Energy (kcal per 100g) |
|---|---|---|---|---|---|
| Blank: the control group; dye: the synthetic colorant; HPW7 and HPW13: addition of wet H. pluvialis at concentrations of 7% and 13% (gmicroalgaDW gflour−1), respectively. *: significant effect. | |||||
| Blank | 4.39 ± 0.3 | 4.63 ± 0.36 | 8.7 ± 0.2 | 82.3 ± 0.9 | 403 ± 7.7 |
| Dye | 4.11 ± 0.12 | 4.48 ± 0.36 | 8.1 ± 0.5 | 83.3 ± 0.9 | 402± 6.7 |
| HPW7 | 5.08 ± 0.48 | 4.59 ± 0.29 | 8.7 ± 0.1 | 81.6 ± 0.9 | 407± 8.3 |
| HPW13 | 6.51 ± 0.3* | 4.59 ± 0.33 | 9.1 ± 0.3 | 79.5 ± 0.9* | 414± 7.5* |
| Dunnett's test p-value (n = 12) | 0.009 | >0.05 | >0.05 | 0.048 | 0.021 |
Bioactive compounds
| Concentration (μg g−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | AME-4 | AME-3 | AME-5 | AME-2 | β-Carotene | AME-1 | Lutein | Cantha. | Asta. | Total |
| Blank: the control sample; dye: the synthetic dye; HPW7 and HPW13: addition of wet H. pluvialis at concentrations of 7% and 13% (gmicroalgaDW gflour−1), respectively. Cantha: canthaxanthin. Asta: astaxanthin. AME: astaxanthin monoesters. DL: detection limit. *: significant effect. | ||||||||||
| Blank | <DL | <DL | <DL | <DL | <DL | <DL | 2.5 ± 0.1 | 1.3 ± 0.1 | <DL | 3.9 ± 0.1 |
| Dye | <DL | <DL | <DL | <DL | <DL | <DL | 2.3 ± 0.2 | 1.2 ± 0.1 | <DL | 3.5 ± 0.2 |
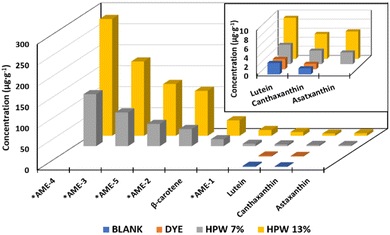
| HPW7 | 123.8 ± 0.8* | 80 ± 3* | 52.9 ± 0.5* | 40 ± 2* | 17 ± 1* | 5.9 ± 0.3* | 4.3 ± 0.1* | 3.0 ± 0.2* | 2.6 ± 0.1* | 330 ± 3* |
| HPW13 | 317 ± 2* | 172 ± 12* | 131 ± 15* | 105 ± 5* | 36 ± 5* | 14 ± 1* | 8.9 ± 0.5* | 5.5 ± 0.1* | 5.8 ± 0.6* | 798 ± 12* |
| Dunnett's test p-value (n = 12) | <0.001 | <0.002 | 0.001 | <0.001 | 0.004 | 0.001 | <0.001 | <0.001 | 0.001 | <0.001 |
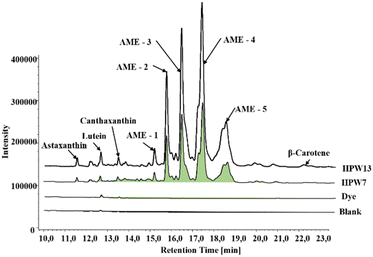
The ability of H. pluvialis to store high amounts of astaxanthin is well known, with 5% in free form and 95% esterified as astaxanthin monoesters (AME).18Fig. 2 shows a typical chromatogram of the samples tested, showing 5 intense peaks identified as AME in the two enriched samples. This profile agrees with that previously characterized, identifying the peaks as astaxanthin esters of the fatty acids C18:4 (AME1), C18:3 (AME2), C18:2 (AME3), C18:1 (AME4) and C16:0 (AME5).15 Thus, the enriched samples show the PUFA > MUFA > SFA ratio with the omega-9,6 and 3 families.
Small concentrations of lutein and canthaxanthin were present in the control sample, attributed to the content per se in the egg yolk. These values do not show significant differences when the artificial dye is added, in contrast to the two supplemented samples, in which the concentration of lutein and canthaxanthin increased 2 to 4 times. A significant contribution of β-carotene from the microalga paste is also evident at concentrations up to 36 μg g−1.
The carotenoid profile obtained here, although outstanding, is complex to compare with other similar studies addressing the incorporation of bioactive compounds from H. pluvialis into food matrices. This is because only a single tracer, astaxanthin, is reported. In turn, this is generated by the singular focus towards the isolation of a single compound (free astaxanthin), which is usually added to food matrices in the form of powder enriched in this carotenoid derived from H. pluvialis. Some foods such as cookies or bread have been enriched in concentrations of 0.4 to 15% of pure astaxanthin.20,33 However, the synergistic activity of astaxanthin is known when combined with other carotenoids such as lutein, canthaxanthin, as well as carotenoids of lower polarity such as β-carotene.34,35 In addition, the esterified form of astaxanthin has shown greater stability and bioavailability compared to its free structure and has also shown increased solubility when bound to amphipathic fatty chains.17,36
| Concentration (mg g−1) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | C6:0 | C10:0 | C16:0 | C14:0 | C17:0 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C20:4 | C20:5 | Total |
| Blank: the control sample; dye: the synthetic dye; HPW7 and HPW13: addition of wet H. pluvialis at concentrations of 7% and 13% (gmicroalgaDW gflour−1), respectively. *: significant effect. | |||||||||||||
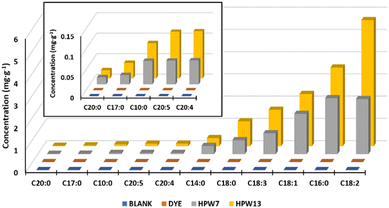
| Blank | 1.7 ± 0.68 | 0.71 ± 0.04 | 21.2 ± 0.17 | 3 ± 1.0 | 0.22 ± 0.01 | 4.9 ± 0.19 | 11.3 ± 0.47 | 15.1 ± 0.9 | 1.6 ± 0.1 | 0.11 ± 0.01 | 0.48 ± 0.01 | 0.43 ± 0.02 | 61 ± 3 |
| Dye | 1.5 ± 0.64 | 0.72 ± 0.01 | 21.21 ± 0.05 | 3.5 ± 0.4 | 0.23 ± 0.01 | 4.9 ± 0.10 | 11.6 ± 0.20 | 15.1 ± 0.2 | 1.7 ± 0.1 | 0.11 ± 0.01 | 0.48 ± 0.01 | 0.43 ± 0.01 | 61.0 ± 0.6 |
| HPW7 | 1.3 ± 0.35 | 0.77 ± 0.08 | 22.7 ± 0.34 | 4 ± 1.87 | 0.25 ± 0.01* | 5.5 ± 0.41* | 13.1 ± 0.89* | 17.6 ± 0.8* | 2.5 ± 0.1* | 0.13 ± 0.01* | 0.52 ± 0.02* | 0.47 ± 0.01* | 69 ± 4* |
| HPW13 | 1.5 ± 0.47 | 0.76 ± 0.06 | 24.7 ± 0.21* | 4 ± 1.12 | 0.26 ± 0.01* | 6.0 ± 0.25* | 13.6 ± 0.44* | 20.8 ± 0.8* | 3.2 ± 0.2* | 0.13 ± 0.01* | 0.60 ± 0.04* | 0.55 ± 0.01* | 76 ± 2* |
| Dunnett's test p-value (n = 12) | >0.05 | >0.05 | 0.001 | >0.05 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.005 |
This lipid profile is closely related to the astaxanthin concentration in the microalga. During the culture phase of H. pluvialis, nutrient starvation conditions are induced which trigger both carotenogenesis (astaxanthin formation) and lipid deposition. In turn, traditional approaches to microalga production tend to focus on a single product, with very limited viability, being maintained only by high-value products such as astaxanthin.37 In response to this, the application of wet paste that simultaneously binds high-value carotenoids as bioactive lipid chains exemplified here, from a single microalga feedstock, is shown to be the best option.
Moreover, this multi-extractive approach provides an additional synergy, as astaxanthin-rich red blood cells, as well as fatty acids such as oleic acid, have shown higher bioactivity and appear to be more suitable for human consumption in nutraceuticals or pharmaceuticals.38 These compounds show a symbiotic behavior, modulating lipid degradation, thanks to the antioxidant capacity of carotenoids, while maintaining greater stability and bioactivity in compounds such as astaxanthin when in esterified form.39 This is a clear advantage of using microalga in a paste form (as a whole), as opposed to isolating a single compound by extraction.
Stability study
| L* | a* | b* | ||
|---|---|---|---|---|
| Blank: the control sample; dye: the synthetic dye; HPW7 and HPW13: addition of wet H. pluvialis at concentrations of 7% and 13% (gmicroalgaDW gflour−1), respectively. *: significant effect. | ||||
| Day | 0 | 53.6 ± 0.4 | 19.2 ± 0.2 | 22.4 ± 0.3 |
| 3 | 53.0 ± 0.3 | 19.3 ± 0.2 | 23.9 ± 0.1 | |
| 6 | 51.2 ± 0.3* | 19.2 ± 0.1 | 22.2 ± 0.5 | |
| 9 | 48.6 ± 0.3* | 19.1 ± 0.1 | 22.8 ± 0.3 | |
| p-Value (n = 120) | Dunnett's test | <0.001 | >0.05 | >0.05 |
| Type | Blank | 68.8 ± 0.3 | 2.4 ± 0.3 | 29.1 ± 0.4 |
| Dye | 50.9 ± 0.3* | 30.2 ± 0.2* | 24.9 ± 0.2* | |
| HPW7 | 46.7 ± 0.3* | 22.6 ± 0.3* | 18.8 ± 0.4* | |
| HPW13 | 40.0 ± 0.3* | 21.8 ± 0.3* | 16.3 ± 0.5* | |
| p-Value (n = 120) | Dunnett's test | >0.001 | >0.001 | >0.001 |
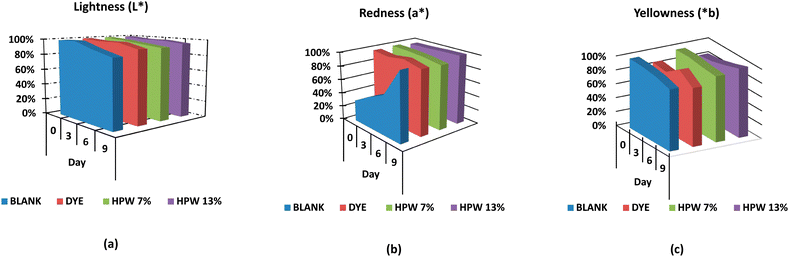
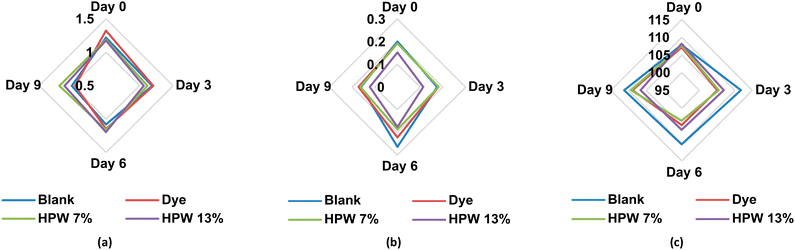
The blank underwent greater darkening in contrast to the other samples, being critical from the sixth day, as shown in Fig. 4a. The dyed sample behaved similarly to the HPW13 sample. In turn, HPW7 maintained the brightness with only a difference of less than 2% concerning the dyed sample.
Although the a* and b* values did not demonstrate significant modifications with storage time, these parameters were represented (Fig. 4b and c) for the different levels of addition of the filloas to evaluate their tendency. Therefore, a balanced profile of the parameter a* for all levels of addition is highlighted in contrast to the control, which acquires high reddening from the 6th day of analysis onwards. HPW7 and HPW13 samples showed a lower variability of the a* and b values in contrast to the dye addition.
The comparative analysis with other studies that address the stability in the coloration provided by H. pluvialis in food is complex, due to the scarce information, being the main focus, the coloration provided by the microalgae but not the behavior of the color as a function of time in the food. An investigation evaluating the coloring capacity of dried H. pluvialis in food emulsions shows, analogous to this study, a significant loss in the brightness of the enriched food sample. In turn, it also exhibits significant variations of the a* and b* parameters in the sample.40 This may be due to the pre-processing performed on the algae prior to its addition to the food matrix. A negative effect on the stability of the carotenoids in the drying process has been demonstrated, as well as the above-mentioned increased instability due to the removal of lipid chains in the search for pure astaxanthin. The application of the wet paste, without drying or subsequent pre-treatment, may therefore result in a longer duration of color in the food. Likewise, as already mentioned, the addition of this drying step involves additional costs that make it economically incompatible at industrial scales.41 Moreover, the industrial approach to a single compound, such as astaxanthin for the generation of color in food, loses value compared to the possibility of exploiting the microalgae in its wet paste form, which simultaneously binds other high-value carotenoids as bioactive lipid chains, as proposed here.
| Toughness (g) | Extensibility (mm) | Diameter (mm) | Internal thickness (mm) | External thickness (mm) | ||
|---|---|---|---|---|---|---|
| Blank: the control group; dye: the synthetic colorant; HPW7 and HPW13: addition of wet H. pluvialis at concentrations of 7% and 13% (gmicroalgaDW gflour−1), respectively. *: significant effect. | ||||||
| Day | 0 | 364 ± 14 | 21.6 ± 0.4 | 107.5 ± 0.1 | 1.23 ± 0.03 | 0.24 ± 0.01 |
| 3 | 352 ± 23 | 17.1 ± 0.6* | 107.3 ± 0.6 | 1.15 ± 0.05 | 0.16 ± 0.08* | |
| 6 | 278 ± 24* | 13.7 ± 0.7* | 105.7 ± 0.7 | 1.14 ± 0.05 | 0.21 ± 0.03 | |
| 9 | 132 ± 25* | 10.0 ± 0.7* | 108.9 ± 0.6 | 1.07 ± 0.05 | 0.15 ± 0.09* | |
| p-Value (n = 12) | Dunnett's test | <0.003 | <0.002 | <0.200 | <1.000 | >0.001 |
| Type | Blank | 215 ± 14 | 13.7 ± 0.4 | 110 ± 3 | 1.12 ± 0.03 | 0.25 ± 0.01 |
| Dye | 287 ± 24 * | 14.8 ± 0.7 | 106 ± 1* | 1.15 ± 0.04 | 0.20 ± 0.02* | |
| HPW7 | 307 ± 25* | 16.1 ± 0.7* | 106 ± 1* | 1.17 ± 0.04 | 0.18 ± 0.02* | |
| HPW13 | 319 ± 24 * | 16.8 ± 0.7* | 106 ± 1* | 1.14 ± 0.04 | 0.14 ± 0.02* | |
| p-Value (n = 12) | Dunnett's test | <0.015 | <0.004 | >0.001 | <1.000 | >0.008 |
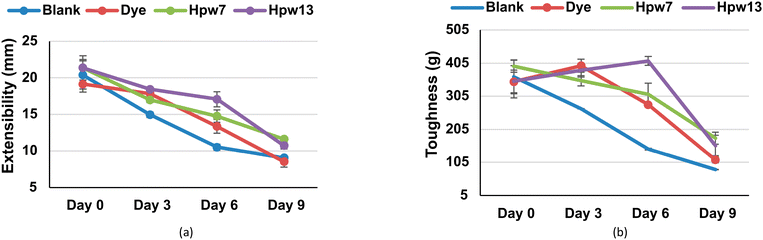
In relation to the storage time, significant modifications are generated mainly in the extensibility (day 3), toughness (day 6) and external thickness (day 9). As the days go by, the filloas increase rigidity, decreasing their elongation capacity. Similarly, they lose strength, being more susceptible to breakage. All these parameters are significantly modulated by the addition of microalgae paste, which, unlike dye, can maintain the extensibility in percentages higher than 38% and 20% relative to the blank for the levels HPW7 and HPW13, respectively. At the same time, an inverse relationship (Fig. 5a) is shown between the addition of the microalgae paste and the external thickness of the filloas. In contrast, the control group shows an overall growth of the external thickness, presenting a maximum on the sixth day of storage, decreasing in the form blank > dye > HPW7 > HPW13. This can be explained by the fact that the thickness revealed a labile structure, fragmenting over the days, and giving a different contour that increased radially. Therefore, a thinner and more durable consistency could be associated with higher structural preservation of the food. This behavior is assumed to be due to the contribution of carotenoids by the microalgae, which are known to influence the rheological properties of foods.45 Both the internal thickness and diameter of the filloas were not significantly altered by storage time. In contrast, less variability is shown in these parameters (Fig. 5b and c) when the concentration of microalgae in the filloas is increased.
Regarding the toughness of the filloas, a significant decrease is evident after the sixth day of storage (Fig. 6), reaching a decrease of 55% for the blank throughout the 9 days of analysis. Both dye and HPW7 and HPW13 show significant effects on this property, improving as follows: HPW13 > HPW7 > dye. The stabilizing effect by the dye is assumable due to concentrations of E202 (potassium sorbate) used as a preservative in producing this type of dye. The action of E202 in modifying starch films is known to improve their elongation at break when the concentration of sorbate is increased.46
| APC | B. cereus | pH | Moisture (%) | Water activity | ||
|---|---|---|---|---|---|---|
| Blank: the control group; dye: the synthetic colorant; HPW7 and HPW13: addition of wet H. pluvialis at concentrations of 7% and 13% (gmicroalgaDW gflour−1), respectively. *: significant effect. | ||||||
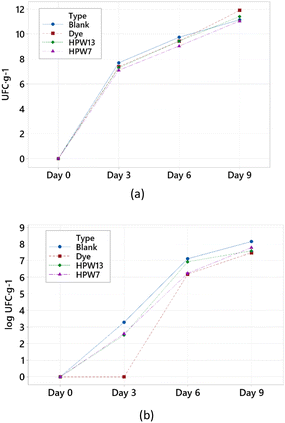
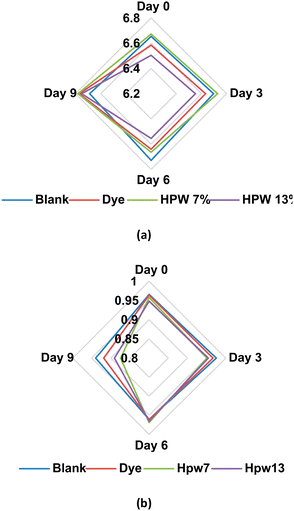
| Day | 0 | N/D | 0.0 ± 0.2 | 6.60 ± 0.01 | 50.7 ± 0.3 | 0.959 ± 0.004 |
| 3 | 7.4 ± 0.3* | 2.1 ± 0.2* | 6.65 ± 0.02* | 48.7 ± 0.3 | 0.962 ± 0.007 | |
| 6 | 9.4 ± 0.5* | 6.6 ± 0.2* | 6.65 ± 0.01* | 49.8 ± 0.1 | 0.964 ± 0.005 | |
| 9 | 11.4 ± 0.2* | 7.8 ± 0.3* | 6.75 ± 0.02* | 52.2 ± 0.2 | 0.906 ± 0.007* | |
| p-Value (n = 12) | Dunnett's test | <0.001 | <0.001 | <0.050 | >1.000 | <0.015 |
| Type | Blank | 7.1 ± 0.1 | 4.6 ± 0.2 | 6.69 ± 0.02 | 50.3 ± 0.2 | 0.961 ± 0.004 |
| Dye | 7.2 ± 0.2 | 3.4 ± 0.2* | 6.66 ± 0.03 | 50.1 ± 0.5 | 0.953 ± 0.005 | |
| HPW7 | 6.8 ± 0.1* | 4.1 ± 0.2 | 6.71 ± 0.02 | 50.2 ± 0.1 | 0.938 ± 0.007* | |
| HPW13 | 7.0 ± 0.1 | 4.3 ± 0.3 | 6.59 ± 0.02* | 50.8 ± 0.2 | 0.940 ± 0.007* | |
| p-Value (n = 12) | Dunnett's test | >0.015 | <0.001 | <0.001 | >0.900 | <0.001 |
Conclusions
The ability of the microalgae H. pluvialis as a multifunctional food ingredient for the improvement of the color, nutritional profile, and functional properties of foods, in contrast to a single-purpose synthetic additive, was demonstrated. The addition of H. pluvialis wet paste generated an attractive and homogeneous mixture, which improved the physical profile of the food (filloa). In contrast to the unique coloring effect of the dye in the food, a minimum level of addition of the microalgae wet paste (7 gmicroalga DW 100 gflour−1) provided a total free astaxanthin content of 2.6 μg g−1 and an esterified astaxanthin content of 303 μg g−1, as well as an overall carotenoid contribution of 330 μg g−1; this increase was proportional to a higher level of addition. Furthermore, the significant enrichment of omega-6 and omega-3 essential fatty acids by adding H. pluvialis in the filloas, especially linoleic, palmitic, and oleic acids, increased the original lipid content by 20%. In addition to the high bioactive contribution, the microalgae paste, through a global action, generated a long-lasting coloring of greater luminosity and an important modulation of the physicochemical properties, obtaining a filloa of greater extensibility and integrity than its synthetic alternative up to 6 days after storage. On the other hand, the content of stabilizers and preservatives present in the synthetic dye showed a high inhibitory effect on the growth of B. cereus in the food, but was not significant in the case of total aerobic mesophilic microorganisms (TAM). At the same time, the addition of the minimum level of the microalgae paste generated an inhibitory action on TAM, as well as a significant decrease in water activity in the filloas, thus hindering potential bacterial growth. In addition, the incorporation of wet microalgae biomass without drying and pretreatment steps, using its pool of bioactive compounds, offers a promising avenue for its use on the industrial scale.Author contributions
Conceptualization, L.B., M.L. and C.G.-J.; methodology, T.C.F., S.A.H., P.R., and F.A.F.; software, A.C.; formal analysis, A.C.; investigation, A.C. and T.C.F.; resources, S.P., M.L., C.G.-J., and L.B.; writing—original draft preparation, A.C.; writing—review and editing, A.C., T.C.F., M.L., C.G.-J., and L.B.; supervision, T.C.F., F.A.F., P.R., and S.A.H.; project administration, S.A.H., P.R., T.C.F., and L.B.; funding acquisition, L.B., C.G.-J., and M.L. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was funded by project ED431 2020/06 (Galician Competitive Research Groups Xunta de Galicia). All these programmes are co-funded by FEDER (EU). The authors are also grateful to the Foundation for Science and Technology (FCT; Portugal) for financial support through national funds FCT/MCTES (PIDDAC) to CIMO (UIDB/00690/2020 and UIDP/00690/2020) and SusTEC (LA/P/0007/2021). Castillo A. acknowledges the support of the European Grouping of Territorial Cooperation Galicia-Norte Portugal (GNP, EGTC) under the IACOBUS Program and the MCI of Spain for his contract part of the grant DIN2021-011976 funded by the MCIN/AEI/10.13039/501100011033. L. Barros and S. Heleno acknowledge the national funding by FCT, P. I., through the institutional scientific employment program-contract for their contracts, and Filipa A. Fernandes for the PhD grant (SFRH/BD/145467/2019).References
- M. Paakki, I. Aaltojärvi, M. Sandell and A. Hopia, Int. J. Gastron. Food Sci., 2019, 16, 100131 CrossRef.
- J. H. Han, Innovations in Food Packaging: Second Edition, 2014, pp. 213–255 Search PubMed.
- Grand View Research , Food Additives Market Size, Share & Trends Analysis Report, San Francisco, United States, 2022.
- N. Martins, C. L. Roriz, P. Morales, L. Barros and I. C. F. R. Ferreira, Trends Food Sci. Technol., 2016, 52, 1–15 CrossRef CAS.
- L. J. Stevens, J. R. Burgess, M. A. Stochelski and T. Kuczek, Clin. Pediatr., 2014, 53, 133–140 CrossRef PubMed.
- M. Oplatowska-Stachowiak and C. T. Elliott, Crit. Rev. Food Sci. Nutr., 2016, 57, 524–548 CrossRef PubMed.
- E. Guerra, M. Llompart and C. Garcia-Jares, J. Chromatogr. A, 2017, 1529, 29–38 CrossRef CAS PubMed.
- M. L. Wells, P. Potin, J. S. Craigie, J. A. Raven, S. S. Merchant, K. E. Helliwell, A. G. Smith, M. E. Camire and S. H. Brawley, J. Appl. Phycol., 2017, 29, 949 CrossRef CAS PubMed.
- J. Matos, C. Cardoso, N. M. Bandarra and C. Afonso, Food Funct., 2017, 8, 2672–2685 RSC.
- B. F. Lucas, M. G. de Morais, T. D. Santos and J. A. V. Costa, LWT–Food Sci. Technol., 2018, 90, 270–276 CrossRef CAS.
- M. Fradique, A. P. Batista, M. C. Nunes, L. Gouveia, N. M. Bandarra and A. Raymundo, J. Sci. Food Agric., 2010, 90, 1656–1664 CrossRef CAS PubMed.
- A. Niccolai, M. Venturi, V. Galli, N. Pini, L. Rodolfi, N. Biondi, M. D’Ottavio, A. P. Batista, A. Raymundo, L. Granchi and M. R. Tredici, Sci. Rep., 2019, 9, 19433–19445 CrossRef PubMed.
- W. Meng, T. Mu and G.-V. Marco, Future Foods, 2022, 183–201 Search PubMed.
- Y. Torres-Tiji, F. J. Fields and S. P. Mayfield, Biotechnol. Adv., 2020, 41, 107536 CrossRef CAS PubMed.
- A. Castillo, S. Pereira, A. Otero, S. Fiol, C. Garcia-Jares and M. Lores, RSC Adv., 2020, 10, 27995–28006 RSC.
- K. Jomova and M. Valko, Eur. J. Med. Chem., 2013, 70, 102–110 CrossRef CAS PubMed.
- A. Castillo, S. Pereira, A. Otero, C. Garcia-Jares and M. Lores, J. Appl. Phycol., 2022, 36, 1–17 Search PubMed.
- M. M. R. Shah, Y. Liang, J. J. Cheng and M. Daroch, Front. Plant Sci., 2016, 7, 531 Search PubMed.
- T. Zhou, X. Wang, Y. Ju, C. Shi and G. Kan, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 394, 022007 Search PubMed.
- A. K. M. M. Hossain, M. A. Brennan, S. L. Mason, X. Guo, X. A. Zeng and C. S. Brennan, Foods, 2017, 6, 1–10 CrossRef PubMed.
- F. Ahmed, Y. Li, K. Fanning, M. Netzel and P. M. Schenk, Food Res. Int., 2015, 74, 231–236 CrossRef CAS PubMed.
- S. Pereira and A. Otero, Algal Res., 2020, 51, 102027 CrossRef.
- A. D. Bettge, Cereal Foods World, 2014, 59, 26–29 CrossRef.
- D. Turck, J. Castenmiller, S. de Henauw, K. I. Hirsch-Ernst, J. Kearney, A. Maciuk, I. Mangelsdorf, H. J. McArdle, A. Naska, C. Pelaez, K. Pentieva, A. Siani, F. Thies, S. Tsabouri, M. Vinceti, F. Cubadda, K. H. Engel, T. Frenzel, M. Heinonen, R. Marchelli, M. Neuhäuser-Berthold, M. Poulsen, Y. Sanz, J. R. Schlatter, H. van Loveren, R. Ackerl, W. Gelbmann, H. Steinkellner and H. K. Knutsen, EFSA J., 2020, 18, 5993–60002 Search PubMed.
- L. Rubio, J. P. Lamas, M. Lores and C. Garcia-Jares, Food Anal. Methods, 2018, 11, 3235–3242 CrossRef.
- ISO , Microbiology of the food chain – Horizontal method for the enum Colony count at 30 degrees C by the pour plate technique, 2013.
- ISO , ISO 4832 Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of coliforms – Colony-count technique., 2006.
- ISO , ISO 21527-1/2:2008 – Microbiology of food and animal feeding stuffs—Horizontal method for the enumeration of yeasts and moulds (1st ed.). Switzerland: International Standardization Organization., 2008.
- ISO , ISO 6888-1:1999- Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of coagulase-positive staphylococci (Staphylococcus aureus and other species) – Part 1: Technique using Baird-Parker agar medium. International Organization for StandardizatioN, 1999.
- AOAC International, Official methods of analysis of AOAC International, AOAC International, Maryland, USA, 20th edn., 2016 Search PubMed.
- European Union, Regulation (EU) No 1169/2011 of the European Parliament and of the 634 Council of 25 October 2011. Official Journal of the European Union, 2011.
- M. Dehghan, A. Mente, X. Zhang, S. Swaminathan, W. Li, V. Mohan, R. Iqbal, R. Kumar, E. Wentzel-Viljoen, A. Rosengren, L. I. Amma, A. Avezum, J. Chifamba, R. Diaz, R. Khatib, S. Lear, P. Lopez-Jaramillo, X. Liu, R. Gupta, N. Mohammadifard, N. Gao, A. Oguz, A. S. Ramli, P. Seron, Y. Sun, A. Szuba, L. Tsolekile, A. Wielgosz, R. Yusuf, A.H. Yusufali, K.K. Teo, S. Rangarajan, G. Dagenais, S.I. Bangdiwala, S. Islam, S. S. Anand and S. Yusuf, Lancet, 2017, 390, 2050–2062 CrossRef CAS PubMed.
- Y. Ohi, T. Namiki, M. Katatae, H. Tsukahara and A. Kitamura, J. Jpn. Soc. Food Sci. Technol., 2009, 56, 579–584 CrossRef CAS.
- W. Stahl, A. Junghans, B. De Boer, E. S. Driomina, K. Briviba and H. Sies, FEBS Lett., 1998, 427, 305–308 CrossRef CAS PubMed.
- J. Liang, Y. X. Tian, F. Yang, J. P. Zhang and L. H. Skibsted, Food Chem., 2009, 115, 1437–1442 CrossRef CAS.
- Q. Zhou, J. Xu, L. Yang, C. Gu and C. Xue, J. Sci. Food Agric., 2019, 99, 3662–3671 CrossRef CAS PubMed.
- M. M. R. Shah, Y. Liang, J. J. Cheng and M. Daroch, Front. Plant Sci., 2016, 7, 531 Search PubMed.
- M. C. Cerón, M. C. García-Malea, J. Rivas, F. G. Acien, J. M. Fernandez, E. del Río, M. G. Guerrero and E. Molina, Appl. Microbiol. Biotechnol., 2007, 74, 1112–1119 CrossRef PubMed.
- K. A. Keene, R. M. Ruddy and M. J. Fhaner, ACS Omega, 2019, 4, 983–991 CrossRef CAS PubMed.
- L. Gouveia, A. Raymundo, A. P. Batista, I. Sousa and J. Empis, Eur. Food Res. Technol., 2006, 222, 362–367 CrossRef CAS.
- L. M. Wendt, C. Kinchin, B. D. Wahlen, R. Davis, T. A. Dempster and H. Gerken, Biotechnol. Biofuels, 2019, 12, 1–14 CrossRef PubMed.
- H. Dizlek, A. L. Girard and J. M. Awika, LWT–Food Sci. Technol., 2022, 160, 113320 CrossRef CAS.
- B. Akshata, D. Indrani and P. Prabhasankar, J. Food Process. Preserv., 2019, 43, e14129 CAS.
- E. Argüello García, L. Córdova Téllez, J. Martínez Herrera, O. Sánchez Sánchez, P. Pérez Herrera and J. M. Zaldívar Cruz, J. Food Sci. Technol., 2020, 57, 3502 CrossRef PubMed.
- E. Domian and M. Szczepaniak, J. Food Eng., 2020, 274, 109827 CrossRef CAS.
- H. Barzegar, M. H. Azizi, M. Barzegar and Z. Hamidi-Esfahani, Carbohydr. Polym., 2014, 110, 26–31 CrossRef CAS PubMed.
- A. R. Rao, A. H. Reddy and S. M. Aradhya, Curr. Trends Biotechnol. Pharm., 2010, 4, 807–817 Search PubMed.
- A. H. Rather, S. Singh and S. Choudhary, J. Drug Delivery Ther., 2021, 11, 28–30 CrossRef CAS.
- V. Hlordzi, F. K. A. Kuebutornye, G. Afriyie, E. D. Abarike, Y. Lu, S. Chi and M. A. Anokyewaa, Aquac. Rep., 2020, 18, 100503 CrossRef.
- D. Rodrigo, C. M. Rosell and A. Martinez, Foods, 2021, 10, 302 CrossRef CAS PubMed.
- M. Raevuori, Eur. J. Appl. Microbiol. Biotechnol., 1976, 2, 205–213 CrossRef CAS.
- M. del Torre, M. della Corte and M. L. Stecchini, Int. J. Food Microbiol., 2001, 63, 199–207 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo01028g |
| This journal is © The Royal Society of Chemistry 2023 |