Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improving endothelial health with food-derived H2S donors: an in vitro study with S-allyl cysteine and with a black-garlic extract enriched in sulfur-containing compounds†
Federica
Geddo
 a,
Giulia
Querio
a,
Giulia
Querio
 a,
Alberto
Asteggiano
bc,
Susanna
Antoniotti
a,
Alessandra
Porcu
d,
Andrea
Occhipinti
d,
Claudio
Medana
b and
Maria Pia
Gallo
a,
Alberto
Asteggiano
bc,
Susanna
Antoniotti
a,
Alessandra
Porcu
d,
Andrea
Occhipinti
d,
Claudio
Medana
b and
Maria Pia
Gallo
 *a
*a
aDepartment of Life Sciences and Systems Biology, University of Turin, Via Accademia Albertina 13, 10123 Turin, Italy. E-mail: mariapia.gallo@unito.it
bMolecular Biotechnology and Health Sciences Department, Università degli Studi di Torino, Via Pietro Giuria 5, 10125 Torino, Italy
cBiosfered S.r.l., Via Paolo Veronese 202, 10148 Torino, Italy
dAbel Nutraceuticals S.r.l., Via Paolo Veronese 202, 10148 Torino, Italy
First published on 14th April 2023
Abstract
A healthy vascular endothelium plays an essential role in modulating vascular tone by producing and releasing vasoactive factors such as nitric oxide (NO). Endothelial dysfunction (ED), the loss of the endothelium physiological functions, results in the inability to properly regulate vascular tone, leading to hypertension and other cardiovascular risk factors. Alongside NO, the gasotransmitter hydrogen sulfide (H2S) has emerged as a key molecule with vasodilatory and antioxidant activities. Since a reduction in H2S bioavailability is related to ED pathogenesis, natural H2S donors are very attractive. In particular, we focused on the sulfur-containing amino acid S-allyl cysteine (SAC), a bioactive metabolite, of which black garlic is particularly rich, with antioxidant activity and, among others, anti-diabetic and anti-hypertensive properties. In this study, we analyzed the protective effect of SAC against ED by evaluating reactive oxygen species level, H2S release, eNOS phosphorylation, and NO production (by fluorescence imaging and western blot analysis) in Bovine Aortic Endothelial cells (BAE-1). Furthermore, we chemically characterized a Black Garlic Extract (BGE) for its content in SAC and other sulfur-containing amino acids. BGE was used to carry out an analysis on H2S release on BAE-1 cells. Our results show that both SAC and BGE significantly increase H2S release. Moreover, SAC reduces ROS production and enhances eNOS phosphorylation and the consequent NO release in our cellular model. In this scenario, a natural extract enriched in SAC could represent a novel therapeutic approach to prevent the onset of ED-related diseases.
Introduction
The vascular endothelium is a monolayer of specialized cells that lines the inside of the circulatory system and is a key regulator of vascular homeostasis. In particular, the endothelial cells represent the interface between blood and both the vascular wall and the tissue interstitium, managing and mediating the effects of circulating molecules on the smooth muscle layer. Within this role, a healthy endothelium produces and releases itself vasoactive factors such as nitric oxide (NO) and other endothelium-dependent hyperpolarizing factors which maintain adequate vascular tone and blood fluidity, and promotes antioxidant and anti-inflammatory effects.1,2Endothelial dysfunction (ED) refers to a systemic condition characterized by the loss of these physiological functions caused by different risk factors, including dyslipidemia, diabetes mellitus, and aging.3 This turns out in the inability of the endothelium to properly regulate vascular tone, due to the reduction of NO production, which is associated with the impairment of vascular smooth muscle relaxation and hypertension.
Besides NO, the endogenous gasotransmitter hydrogen sulfide (H2S) is emerging as an essential molecule involved in the regulation of the cardiovascular system, by affecting vascular smooth muscle cells, endothelial cells, and perivascular nerves,4 in a strictly crosstalk with the NO-pathway. In mammalian species, H2S is mostly enzymatically produced by three enzymes in the cysteine biosynthesis pathway, including cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST), while only a small part is produced by non-enzymatic pathways.5 H2S main physiological effects on the vasculature includes vasorelaxation and antioxidant/anti-inflammatory activities,2 whose molecular mechanisms are related to H2S-dependent S-sulfhydration of proteins and redox reactions. Indeed, a reduction in H2S bioavailability is related to the pathogenesis of ED and leads to a series of cardiovascular disorders, such as atherosclerosis and hypertension.6 Moreover, H2S has recently emerged as an inhibitor of the sympathetic outflow, even if results on this point are still debated.7
In this scenario, H2S donors derived from natural sources could represent a novel therapeutic strategy for preventing the onset of ED-related diseases. Among these, garlic (Allium sativum) is well-known for its cardioprotective effects.8 In particular, S-allyl cysteine (SAC) is one of the most abundant bioactive compounds derived from aged garlic which is used as a dietary supplement and in traditional medicine; it is considered an H2S-producing agent9 and possesses high antioxidant and anti-inflammatory activities.10–12 Moreover, SAC exerts multiple healthy properties, such as anti-diabetic,13,14 anti-cancer,15 anti-hepatotoxic,16 anti-hypertensive,17 and neuroprotective effects.18
Since detailed investigations of the molecular pathways activated by SAC in endothelial cells are poor, the aim of this study was to evaluate on Bovine Aortic Endothelial cells (BAE-1) the effect of SAC on key elements taking part in vascular health: reactive oxygen species (ROS) intracellular level, H2S release, eNOS phosphorylation, and NO production.
Finally, a black garlic extract (BGE) with a high content of SAC was produced, and, together with the chemical characterization, the efficacy of this product was evaluated on BAE-1 cells by assessing H2S release in comparison to the treatment with the only standard purified molecule.
Materials and methods
Materials
Unless otherwise specified, materials were obtained from Sigma Aldrich (Sigma-Aldrich, Saint Louis, MO, USA). Plastic and reagents for cell cultures were from Euroclone (Euroclone s.p.a., Pero, Italy). Antibodies for immunoblotting experiments were obtained from BD (BD Biosciences, Franklin Lakes, New Jersey, USA) for monoclonal anti-eNOS (Code 610296), Thermo Fisher Scientific (Thermo Fisher Scientific, Waltham, MA, USA) for polyclonal anti-p-eNOS (Code 36-9100), and Sigma-Aldrich for monoclonal anti-β-actin (Code A5316); horseradish peroxidase-conjugated secondary antibodies were from Thermo Fisher Scientific (anti-mouse code: 31430; anti-rabbit code: SA00001-2). S-Allyl-cysteine (SAC) standard was purchased from TCI Europe chemicals (Zwijndrecht, Belgium); S-1-propenyl cysteine (S1PC), γ-Glutamyl-S-1-propenyl cysteine (GS1PC) and γ-Glutamyl-S-1-allyl cysteine (GSAC) were purchased from Medchemtronica (Sollentuna, Sweden). The Black Garlic Extract was produced by the company Biosfered S.r.l. (Turin, Italy) and commercialized as a food supplement ingredient with the brand name SalliCys®. Black garlic was prepared based on Choi et al.19 Unpeeled raw garlic heads were incubated in a thermohygrostatic chamber between 60 and 85 °C at high humidity. During the aging process, raw garlic developed a darker color. The aging was stopped when garlic turned completely black. The ground black garlic was extracted by hydroalcoholic solution at room temperature and the supernatant was collected. Ethanol was then removed by vacuum evaporation before use.Cell culture
Bovine aortic endothelial cells (BAE-1) were obtained from the European Collection of Authenticated Cell Cultures (ECACC, Salisbury, UK). Cells were maintained in DMEM supplemented with 10% heat-inactivated fetal calf serum (FCS), 50 ug ml−1 gentamicin, and 2 mM glutamine, in a humidified atmosphere of 5% CO2 in air. Cells were used at passage 2–6.To perform the experiments, FCS was not removed as previously done.20–22
For fluorescent microscopy experiments on live cells, BAE-1 were plated in DMEM + 10% FCS on uncoated glass bottom dishes, 35 mm diameter (Ibidi, Martinsried, Germany), at a density of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2 and maintained at 37 °C. After 24 h, cells were loaded with the appropriate probe and stimulated as planned.
000 cells per cm2 and maintained at 37 °C. After 24 h, cells were loaded with the appropriate probe and stimulated as planned.
Cell viability assay
Cellular viability was studied by means of crystal violet staining since MTT or similar assays could not be used due to chemical interference of reducing substances in the cellular medium (sulfhydryl-containing compounds such as SAC) with tetrazolium salts.23 BAE–1 cells were plated in 96−well plates in DMEM + 10% FCS at a density of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cell per cm2 (3300 cells per well). Two days later, the medium was replaced with DMEM + 10% FCS, either alone (control condition) or supplemented with SAC at different concentrations (10 μM, 100 μM, 500 μM, 1 mM, 10 mM), 6 wells for each condition. After 4 or 24 h, the effect on cell growth was estimated by staining with crystal violet: cells were fixed in 2.5% glutaraldehyde in PBS for 20 min, then stained with crystal violet (0.1% in 20% methanol); plates were let dry, then the dye was solubilized in acetic acid (10%, v/v) and absorbance read at 595 nm in a microplate reader (FilterMax F5™ Multi-Mode, Molecular Devices, Sunnyvale, CA, USA). Data were expressed as percentages of Abs referred to the control condition; percentage values were then summarized and expressed as mean ± s.e.m.
000 cell per cm2 (3300 cells per well). Two days later, the medium was replaced with DMEM + 10% FCS, either alone (control condition) or supplemented with SAC at different concentrations (10 μM, 100 μM, 500 μM, 1 mM, 10 mM), 6 wells for each condition. After 4 or 24 h, the effect on cell growth was estimated by staining with crystal violet: cells were fixed in 2.5% glutaraldehyde in PBS for 20 min, then stained with crystal violet (0.1% in 20% methanol); plates were let dry, then the dye was solubilized in acetic acid (10%, v/v) and absorbance read at 595 nm in a microplate reader (FilterMax F5™ Multi-Mode, Molecular Devices, Sunnyvale, CA, USA). Data were expressed as percentages of Abs referred to the control condition; percentage values were then summarized and expressed as mean ± s.e.m.
H2S release
BAE-1 cells, grown on glass bottom dishes, were loaded with 1 μM SF7-AM probe (Cayman Chemical, AnnArbor, Michigan, USA), for 30 min in the dark. After incubation with the probe, cells were washed two times with phosphate buffered saline (PBS), containing Ca2+ and Mg2+, and then treated with 100 μM sodium hydrosulphide (NaHS) as a positive control or 100 μM SAC, in PBS, for 30 min. Then living cells were washed two times with PBS and the fluorescence was acquired with a fluorescence inverted microscope (Olympus IX70) at 488 nm with a 50× Uplan FI oil-immersion objective. Fluorescence intensity was evaluated in 15 random fields for each condition through the definition of the Regions Of Interest (ROIs) using the software ImageJ (Rasband, W. S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA; https://imagej.nih.gov/ij/) and expressed in percentage as mean value of the resulting fluorescence for each condition compared to control of three independent experiments ± s.e.m.Reactive oxygen species (ROS)
BAE-1 cells, grown on glass bottom dishes, were pretreated with 20 μM menadione (as a positive control) for 1 h according to Warren et al.,24 or 100 μM SAC for 4 h or SAC in combination with menadione; the cells were loaded with 5 μM CellROX® green probe (Thermo Fisher Scientific) for the last 30 min in the dark. Then living cells were washed two times with PBS and the fluorescence was acquired with a fluorescence inverted microscope (Olympus IX70) at 488 nm with a 50× Uplan FI oil-immersion objective. Fluorescence intensity was evaluated in 15 random fields for each condition through the definition of the ROIs using the software ImageJ and expressed in percentage as the mean value of the resulting fluorescence for each condition compared to control of three independent experiments ± s.e.m.NO release
BAE-1 cells grown on glass bottom dishes were loaded with 5 μM DAR4M-AM probe (Calbiochem), for 30 min in the dark. After incubation with the probe, cells were washed two times with PBS, and then treated in PBS with 100 μM ATP as a positive control, for 5 min, or 100 μM SAC, for 30 min. Then living cells were washed two times with PBS and the fluorescence was acquired with a fluorescence inverted microscope (Olympus IX70) at 568 nm with a 50× Uplan FI oil-immersion objective. Fluorescence intensity was evaluated in 15 random fields for each condition through the definition of the ROIs using the software ImageJ and expressed in percentage as the mean value of the resulting fluorescence for each condition compared to control of three independent experiments ± s.e.m.Immunoblotting
BAE-1 cells were seeded on plastic dishes, 20 cm2 of the growth area, at a density of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2, in 10% FCS DMEM, and incubated at 37 °C for 48 h. Cells were then treated with 100 μM ATP for 5 min as a positive control or with 100 μM SAC, for 30 min or 4 h. Cells were lysed in 200 μL RIPA lysis buffer (ThermoFisher Scientific) containing phosphatase inhibitor cocktail (PhosSTOP, Roche, Mannheim, Germany), forced through a 1 mL syringe needle and centrifuged at 10
000 cells per cm2, in 10% FCS DMEM, and incubated at 37 °C for 48 h. Cells were then treated with 100 μM ATP for 5 min as a positive control or with 100 μM SAC, for 30 min or 4 h. Cells were lysed in 200 μL RIPA lysis buffer (ThermoFisher Scientific) containing phosphatase inhibitor cocktail (PhosSTOP, Roche, Mannheim, Germany), forced through a 1 mL syringe needle and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min at 4 °C. Proteins (20 μg per lane) were resolved on 8% SDS-PAGE, transferred to a polyvinylidene fluoride membrane (PVDF, Thermo Fisher Scientific) in cold transfer buffer (25 mM Tris pH 8.3, 192 mM glycine, 0.1% SDS, 20% methanol) and blocked for 1 h at 37 °C in TBST (10 mM Tris–HCl, 0.1 M NaCl, 0.1% Tween 20, pH 7.5) plus 5% non-fat dry milk. Blots were incubated overnight at 4 °C with primary antibodies (1
000 rpm for 5 min at 4 °C. Proteins (20 μg per lane) were resolved on 8% SDS-PAGE, transferred to a polyvinylidene fluoride membrane (PVDF, Thermo Fisher Scientific) in cold transfer buffer (25 mM Tris pH 8.3, 192 mM glycine, 0.1% SDS, 20% methanol) and blocked for 1 h at 37 °C in TBST (10 mM Tris–HCl, 0.1 M NaCl, 0.1% Tween 20, pH 7.5) plus 5% non-fat dry milk. Blots were incubated overnight at 4 °C with primary antibodies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 monoclonal anti-eNOS; 1
500 monoclonal anti-eNOS; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 polyclonal anti-p-eNOS; 1
250 polyclonal anti-p-eNOS; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 monoclonal anti-β actin). Membranes were then washed three times with TBST and incubated for 1 h at room temperature with secondary antibodies (anti-mouse, 1
2000 monoclonal anti-β actin). Membranes were then washed three times with TBST and incubated for 1 h at room temperature with secondary antibodies (anti-mouse, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, for monoclonal antibodies; anti-rabbit, 1
000, for monoclonal antibodies; anti-rabbit, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, for p-eNOS) followed by a second set of three washes with TBST. Bands were visualised by chemiluminescence with Western Lightning Plus-ECL (PerkinElmer, Waltham, MA, USA). Protein levels were determined using the software ImageJ; for each condition ratio of p-eNOS/eNOS was evaluated, then normalized toward positive control; results of n = 3 independent experiments were averaged and expressed in percentage as mean ± s.e.m. Aspecific staining of secondary antibodies was checked; comparison of β actin intensity ensured equal protein loading.
000, for p-eNOS) followed by a second set of three washes with TBST. Bands were visualised by chemiluminescence with Western Lightning Plus-ECL (PerkinElmer, Waltham, MA, USA). Protein levels were determined using the software ImageJ; for each condition ratio of p-eNOS/eNOS was evaluated, then normalized toward positive control; results of n = 3 independent experiments were averaged and expressed in percentage as mean ± s.e.m. Aspecific staining of secondary antibodies was checked; comparison of β actin intensity ensured equal protein loading.
HPLC-MRM quantitative analysis of sulfur-containing compounds
For the analysis, 100 μL of the liquid extract were weighed and mixed with 1 mL of the initial solvent of the chromatographic run (0.1% formic acid in acetonitrile/0.1% formic acid in water 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95). The resulting solution was then diluted 20 folds and filtered with a 0.22 μm PTFE syringe filter. The concentration levels for the calibration curve were prepared in the initial solvent of the chromatographic run as well. The quantitative analysis of SAC, S1PC, GSAC, G1SPC was performed with a HPLC-triple quadrupole MS instrument LCMS8045 by Shimadzu (Japan). An organic gradient of B (acetonitrile 0.1% formic acid) in A (water with 0.1% formic acid) was applied on a Luna C18(2) 150 × 3 mm, 3 μm, 100 Å HPLC column (Phenomenex). The gradient method was set as follows: 5% B for the first 2 minutes, 7 minutes 40% B; from the 8th to 9th minute B was kept at 100%, from 9.30 to 14.30 the column was re-conditioned (5% solvent B). Injection volume was 5 μL. Column oven was kept at 40 °C. The MS instrument source parameters were: Source: ESI, Nebulizing gas flow (N2): 3 L min−1, DL temperature: 250 °C, heat block temperature: 400 °C, drying gas flow (N2): 10 L min−1, capillary voltage: 4 kV, positive ion mode. The mass spectrometer was operated in MRM analysis mode using the following transitions: SAC and S1PC: 162.0 > 145.0 as quantitative ions and 162.0 > 73.05, 162 > 41.15 as qualitatives; GSAC and GS1PC: 290 > 162.1 as quantitative and 290 > 145, 290 > 250 as qualitative transitions. Dwell time was set to obtain a total cycle time of 300 ms.
95). The resulting solution was then diluted 20 folds and filtered with a 0.22 μm PTFE syringe filter. The concentration levels for the calibration curve were prepared in the initial solvent of the chromatographic run as well. The quantitative analysis of SAC, S1PC, GSAC, G1SPC was performed with a HPLC-triple quadrupole MS instrument LCMS8045 by Shimadzu (Japan). An organic gradient of B (acetonitrile 0.1% formic acid) in A (water with 0.1% formic acid) was applied on a Luna C18(2) 150 × 3 mm, 3 μm, 100 Å HPLC column (Phenomenex). The gradient method was set as follows: 5% B for the first 2 minutes, 7 minutes 40% B; from the 8th to 9th minute B was kept at 100%, from 9.30 to 14.30 the column was re-conditioned (5% solvent B). Injection volume was 5 μL. Column oven was kept at 40 °C. The MS instrument source parameters were: Source: ESI, Nebulizing gas flow (N2): 3 L min−1, DL temperature: 250 °C, heat block temperature: 400 °C, drying gas flow (N2): 10 L min−1, capillary voltage: 4 kV, positive ion mode. The mass spectrometer was operated in MRM analysis mode using the following transitions: SAC and S1PC: 162.0 > 145.0 as quantitative ions and 162.0 > 73.05, 162 > 41.15 as qualitatives; GSAC and GS1PC: 290 > 162.1 as quantitative and 290 > 145, 290 > 250 as qualitative transitions. Dwell time was set to obtain a total cycle time of 300 ms.
Statistical analysis
Data are presented as mean ± s.e.m. All data were analyzed with ANOVA followed by Bonferroni's multiple comparisons for post hoc tests. Differences with p < 0.05 were considered statistically significant.Results
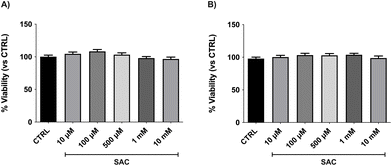
SAC does not affect BAE-1 endothelial cells viability
First aim of this study was to evaluate the effect of SAC on cellular viability. Cells were plated in 96-well plates and treated with different concentrations of SAC (10 μM, 100 μM, 500 μM, 1 mM, 10 mM). The staining with crystal violet was performed after 4 h or 24 h of treatment. As shown in Fig. 1, both the acute (4 h) and the chronic (24 h) exposure did not significantly affect cellular viability, even at the highest doses.SAC enhances hydrogen sulfide release acting as an organic donor
Given that SAC has been the subject of this research on endothelial cells because of its role as an H2S donor, a key point of the study was to verify the actual H2S increase in SAC-treated BAE-1 cells. These measurements were performed with the specific fluorescent H2S-probe SF7-AM with fluorescence microscopy. The SF7-AM loaded cells were treated with 100 μM NaHS as a positive control or 100 μM SAC for 30 min. As expected, a significant increase in fluorescence was observed in NaHS-treated cells; furthermore, this increase was significantly detected even in SAC-treated cells, in a comparable way to the positive control (Fig. 2). This result confirmed that SAC acts as an organic donor of H2S in our cellular model.SAC has an antioxidant activity on menadione-treated cells
As in our model SAC confirmed its noticeable property of H2S donor, following experiments were focused on verifying its capacity in inducing the well-known H2S-dependent cellular protective pathways. Among these, we started with the antioxidant mechanism.To evaluate the antioxidant activity of SAC, we performed experiments in fluorescence microscopy, using the CellROX® green fluorescent probe. BAE-1 cells were pretreated with 20 μM menadione (MEN, positive control of oxidative stress) for 1 h or 100 μM SAC for 4 h alone or in combination with MEN and then labeled with 5 μM CellROX® green probe for the last 30 min in the dark. As shown in Fig. 3, SAC did not increase the production of reactive oxygen species (ROS), while the stressor MEN induced an increment of fluorescence intensity as expected, meaning a rise of ROS. However, in cells treated with MEN in combination with SAC, fluorescence was reduced compared to the only MEN treatment. This result confirmed the SAC antioxidant activity in the presence of an oxidative stressor.
SAC improves Endothelial Nitric Oxide Synthase (eNOS) phosphorylation
Previous studies demonstrated that the counteracting effect of H2S on oxidative stress takes part in the modulation of nitric oxide release, in particular by improving eNOS coupling and phosphorylation.25 To assign this further protective mechanism to SAC, we performed western blot analysis testing if SAC impacted nitric oxide synthase phosphorylation. Cells were treated with 100 μM ATP for 5 min as a positive control or with 100 μM SAC, for 30 min or 4 h. As represented in Fig. 4, ATP enhanced the nitric oxide phosphorylation as expected. Interestingly, even the SAC treatment both for 30 min and 4 h increased the eNOS phosphorylation in a significant way compared to the control (untreated), thus suggesting a possible implication of SAC in the enhancement of NO release.SAC increases nitric oxide release
To demonstrate the involvement of SAC in increasing nitric oxide (NO) release following eNOS phosphorylation, experiments in fluorescence microscopy with the NO probe DAR4M-AM were performed. Cells were first incubated with 5 μM DAR4M-AM probe for 30 min in the dark and then treated with 100 μM ATP or 100 μM SAC, respectively for 5 and 30 min. As shown in Fig. 5, the increased fluorescence intensity after ATP stimulation, which was used as a positive control, was similar to SAC treatment. These results demonstrated the role of SAC in improving NO release in BAE-1 cells.HPLC-MRM method development and application for the quantitation of S-compounds in BGE and pilot process optimization
The previously observed biological effects of SAC could be translated to black garlic, where SAC and other sulfur-containing amino acids have been shown to be highly concentrated with respect to fresh garlic and represent the main antioxidant compounds.26 In this context, black garlic extract-based formulas represent appealing tools for a nutraceutical approach to the prevention of endothelial dysfunction. To extend our results on SAC in this more food-related field, we moved to the chemical characterization and biological testing on BAE-1 cells of a black garlic extract kindly provided by Biosfered S.r.l. (Turin, Italy). To characterize the S-compounds profile in the black garlic extract (BGE), we developed a fast, simple, and sensitive HPLC-MRM MS method.For each molecule we investigated three different MRM events; for the isomeric nature of those chemical compounds (SAC is an isomer of S1PC and GSAC is isomeric to GS1PC) the evaluation of the relative abundance of the fragments, together with the elution order is mandatory to avoid their misidentification. The highest-abundant fragment was chosen as the quantifier ion. Once developed and optimized, the method was validated according to ICH guidelines.
The described validated method was used to accurately dose the S-containing compounds SAC, S1PC, GSAC, and GS1PC for the in vitro experiments and to monitor their formation yield during the controlled garlic aging process. The fermentation method which starts with the garlic bulb and leads to the final BGE requires a strict monitoring of the S-compounds abundance profile to trace their formation kinetics and the best moment to stop the aging process. For this study, we developed a BGE extract with a standardized content of S-compounds comparable to the final food-grade ingredient administrable to the cell cultures by dilution with their growth media. The liquid extract showed this profile of S-compounds abundance: SAC: 2.95 mM, S1PC: 0.51 mM, GSAC: 2.8 mM, and GSPC: 0.42 mM (Fig. 6). For the purpose of our experiment, the cell medium solution was prepared to contain a standardized concentration of SAC (100 μM) in order to reflect the concentration of SAC used in the experiments with the standard pure molecule.
 | ||
| Fig. 6 Chromatographic separation of the S-compounds in the liquid sample of BGE used for cells treatments. For each peak, the compound name, as well as its structural formula, is presented. | ||
The black garlic extract enriched in SAC improves hydrogen sulfide levels in endothelial cells
First, to test the BGE effect on cellular viability, cells were plated in 96-well plates and treated with 100 μM SAC or 100 μM BGE for 1 h, followed by the crystal violet staining. As shown in Fig. 7A, 100 μM SAC does not affect cellular viability (as already demonstrated in Fig. 1) as well as the treatment with 100 μM BGE. Then, to verify if BGE could enhance H2S levels in a similar way to the standard, experiments with SF7-AM loaded cells were performed. Cells were treated with 100 μM NaHS or 100 μM SAC or 100 μM BGE. As previously described, there is a significant increase in fluorescence intensity when cells were stimulated with NaHS and SAC, but even with BGE treatment, the fluorescence is higher in a significant way in comparison to the control condition (Fig. 7B and C), thus suggesting that BGE enhances H2S levels in our cellular model.Discussion
This study is focused on two main goals: first, to demonstrate in BAE-1 cells that the sulfur-containing amino acid S-allyl cysteine (SAC) is an effective intracellular source of H2S and is able to improve nitric oxide release; second, to demonstrate the H2S donor capabilities on BAE-1 cells of the chemically characterized commercial black garlic extract (BGE) (SalliCys®). The ‘polar star’ of these items is the gasotransmitter H2S, which we showed was released from both SAC and BGE and to whom the protective mechanisms activated by SAC on endothelial cells are ascribed.The fundamental role of H2S in vascular physiology has been indeed clearly evidenced,27 and the beneficial effects of its supplementation to improve endothelial function have been proven both in experimental models and in clinical investigations.28
Several methods of H2S delivery and supplementation have been tested, the main classes of H2S pro-drugs being hydrolysis-based donors, plant-derived natural donors, and controlled-release donors.29 Among these molecules, plant-derived donors, mainly organic polysulfides from garlic, received great attention in the last decade for their potential use as nutraceuticals, in particular for the prevention of cardiovascular disorders. SAC belongs to the organic polysulfide family and has been studied for many years for its biological activities,30 particularly in counteracting endothelial dysfunction through H2S release.31
However, reports directly showing H2S intracellular increase after treatment with SAC are still lacking. To prove this H2S rise in BAE-1 cells, we performed fluorescence measurements with the H2S probe SF7-AM. Results are shown in Fig. 2 and highlight a significant increase in intracellular H2S levels in both NaHS (positive control) and SAC-treated cells. To our knowledge, this is the first evidence in a cellular model of the SAC-induced H2S rise. The H2S intracellular detection with fluorescent probes has been previously shown in several endothelial cell models, as in NaHS-stimulated32 or VEGF-stimulated HUVEC,33 and here we showed this rise after treatment of endothelial cells with the organic and natural H2S donor SAC. This evidence establishes a solid ground to support the subsequent experiments focused on the biological effects of SAC on BAE-1 cells. Among these, we first investigated and confirmed the antioxidant mechanism (Fig. 3), showing a significant reduction of menadione-induced intracellular ROS in BAE-1 cells preincubated with SAC. The antioxidant activity of SAC on endothelial cells was first highlighted by Ide et al.34 and is dependent on the multifaceted antioxidant role of H2S: it indeed reduces ROS levels by both direct mechanisms (ROS scavenging, increase in SOD activity) and indirect pathways (increase in non-enzymatic antioxidant systems, increase in Nrf2-dependent expression of antioxidant enzymes).35
In agreement with the results on ROS, we then underlined a positive modulatory role of SAC on eNOS activity and thus on the endothelial function. As shown in Fig. 4 and 5, SAC enhances the eNOS phosphorylation and the nitric oxide intracellular level in BAE-1. These data are aligned with previous studies highlighting the strict interconnection between H2S and NO, because of the multiple mechanisms by which H2S positively affects eNOS activity.27 Thus, we confirmed these mechanisms also in SAC-treated endothelial cells, strengthening the interest in the molecule as a natural compound improving nitric oxide-mediated vascular functions. The choice to test 100 μM SAC was grounded on the evidence that this concentration strictly corresponds to the plasmatic level of SAC (15,2 μg ml−1) detected in the plasma of rats subjected to oral administration of 25 mg kg−1 SAC.36 Moreover, this oral dosage corresponds to that employed in several in vivo studies on rats, aimed to highlight SAC-dependent cardioprotective effects;31 giving the high bioavailability of the molecule, this dosage could lead to the same plasmatic concentration of 15,2 μg ml−1. SAC pharmacokinetic has been indeed investigated by Lee et al.36 and by Amano et al. in rats;37 these studies showed a very high bioavailability of the molecule, that was respectively 96% (with 25 mg kg−1 SAC o.a.) and (5 mg kg−1 o.a.). We could speculate a similar bioavailability in humans, even if specific data are still missing. We then decided to go forward by achieving the in vitro characterisation of the functional effects of the BGE, in order to understand whether the addition of the matrix complexity was affecting results obtained with SAC. The choice of black garlic comes from its higher content in SAC, which, as reported by several studies, varies from three to six times with respect to fresh garlic.26 To fulfill this purpose, we first decided to quantitate the S-compounds present in the BGE to standardize their abundance in the aliquot which was administered to the cells. The knowledge of the accurate quantitation value is essential to calculate the dose–response relationship and accordingly, to formulate a proper dosage. The scientific literature reports several methods for the quantitation of S-compounds: an application for the S-allyl cysteine quantitative study has been developed with a HPLC-FLD and HPLC-UV detector; in this case, for the poor response of SAC with spectrophotometric detectors a derivatization step is strongly required.38–40 The use of a MS detector overcomes this limitation and allows the analysis to reach a higher sensitivity; an interesting HPLC-MS method was validated for the quantitation of SAC in heated garlic juice with similar conditions to ours;41 the paper presents an interesting comparison between UV and MS detector and highlights the sensitivity differences between the two techniques. For our method, the optimal chromatographic conditions were found using a Luna C18 (2) column with an organic gradient of 0.1% formic acid in ACN starting from 5% in 0.1% formic acid in water. An initial isocratic step with the initial solvent condition was revealed as a successful strategy for the separation of the hydrophilic isomeric compounds SAC and S1PC without co-elutions as well as the later-eluting GSAC and GS1PC in a total of 14 minutes. After quantifying the S-containing compounds SAC, S1PC, GSAC, and GS1PC in the BGE for their proper dosage on BAE-1 cells, we verified that 100 μM BGE didn't affect cell viability after 1 h of treatment. For times of exposure exceeding 1 h, cells were suffering from osmotic stress caused by the high total sugars content of the extract (382 g kg−1 – 38.2% w/w, spectrophotometer Anthrone assay data, not shown). Since the BGE doesn't reach the target cells (after oral assumption) in this form, as sugars follow different metabolic pathways after ingestion, the cytotoxicity (for >1 h exposure) and NO release of the whole extract were not representative. Successively, we demonstrated the H2S-releasing feature of BGE on cells. As shown in Fig. 7, BGE-treated cells displayed a significant increase in the H2S level with respect to control cells, similarly to NaHS and SAC-treated cells. This last experiment represents a smart and original approach to test plant-derived extracts as H2S-donors on in vitro cellular models; furthermore, it supports the forcefulness of BGE for further investigations and nutraceutical applications.
Conclusions
In conclusion, our study clearly highlights the beneficial roles of SAC on endothelial health, by demonstrating its features as an antioxidant, H2S donor, and NO availability enhancer; moreover, the results showing SAC-induced nitric oxide release and eNOS phosphorylation boost knowledges of its strict relation with the NO-pathway. In addition, by directly showing the intracellular H2S rise in SAC and BGE-treated endothelial cells, this study recommends an efficient and useful approach to characterize the vasoprotective H2S-dependent functions of bioactive molecules or extracts. Furthermore, the presented profiling of S-compounds paves the way to a more comprehensive characterization of the other compounds present in the BGE through untargeted MS-based metabolomic approaches. This global metabolomic profile of the ingredient, now in progress, will draw a bigger picture of the pool of the bioactive metabolites residing in this unknown yet promising food matrix.Conflicts of interest
A. O. and A. P. are employed by Abel Nutraceuticals S.r.l.; A. A. is a student of the Ph.D. Program in Chemical and Material Sciences of the University of Turin (XXXVI cycle) and in apprenticeship at Biosfered S.r.l. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.Acknowledgements
We thank Biosfered S.r.l. for kindly providing the Black Garlic Extract (SalliCys®).References
- S. Godo and H. Shimokawa, Endothelial Functions, Arterioscler. Thromb. Vasc. Biol., 2017, 37, e108–e114 CrossRef CAS PubMed.
- V. Ciccone, S. Genah and L. Morbidelli, Endothelium as a Source and Target of H2S to Improve Its Trophism and Function, Antioxidants, 2021, 10, 486 CrossRef CAS PubMed.
- P. Poredos, A. V. Poredos and I. Gregoric, Endothelial Dysfunction and Its Clinical Implications, Angiology, 2021, 72, 604–615 CrossRef CAS PubMed.
- R. Wang, C. Szabo, F. Ichinose, A. Ahmed, M. Whiteman and A. Papapetropoulos, The role of H2S bioavailability in endothelial dysfunction, Trends Pharmacol. Sci., 2015, 36, 568–578 CrossRef CAS PubMed.
- K. M. Casin and J. W. Calvert, Harnessing the Benefits of Endogenous Hydrogen Sulfide to Reduce Cardiovascular Disease, Antioxidants, 2021, 10, 383 CrossRef CAS PubMed.
- V. Citi, A. Martelli, E. Gorica, S. Brogi, L. Testai and V. Calderone, Role of hydrogen sulfide in endothelial dysfunction: Pathophysiology and therapeutic approaches, J. Adv. Res., 2021, 27, 99–113 CrossRef CAS PubMed.
- S. Huerta de la Cruz, G. J. Medina-Terol, J. A. Tapia-Martínez, D. L. Silva-Velasco, J. H. Beltran-Ornelas, A. Sánchez-López, M. Sancho and D. Centurión, Hydrogen sulfide as a neuromodulator of the vascular tone, Eur. J. Pharmacol., 2023, 940, 175455 CrossRef CAS PubMed.
- R. Varshney and M. J. Budoff, Garlic and Heart Disease, J. Nutr., 2016, 146, 416S–421S CrossRef CAS PubMed.
- A. Corvino, F. Frecentese, E. Magli, E. Perissutti, V. Santagada, A. Scognamiglio, G. Caliendo, F. Fiorino and B. Severino, Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases, Antioxidants, 2021, 10, 429 CrossRef CAS PubMed.
- A. Shang, S.-Y. Cao, X.-Y. Xu, R.-Y. Gan, G.-Y. Tang, H. Corke, V. Mavumengwana and H.-B. Li, Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.), Foods, 2019, 8, 246 CrossRef CAS PubMed.
- A. L. Colín-González, S. F. Ali, I. Túnez and A. Santamaría, On the antioxidant, neuroprotective and anti-inflammatory properties of S-allyl cysteine: An update, Neurochem. Int., 2015, 89, 83–91 CrossRef PubMed.
- A. L. Colín-González, R. A. Santana, C. A. Silva-Islas, M. E. Chánez-Cárdenas, A. Santamaría and P. D. Maldonado, The Antioxidant Mechanisms Underlying the Aged Garlic Extract- and S-Allylcysteine-Induced Protection, Oxid. Med. Cell. Longevity, 2012, 2012, 1–16 CrossRef PubMed.
- G. Saravanan and P. Ponmurugan, Ameliorative potential of S-allyl cysteine on oxidative stress in STZ induced diabetic rats, Chem.-Biol. Interact., 2011, 189, 100–106 CrossRef CAS PubMed.
- G. Saravanan and P. Ponmurugan, Beneficial Effect of S-allylcysteine (SAC) on Blood Glucose and Pancreatic Antioxidant System in Streptozotocin Diabetic Rats, Plant Foods Hum. Nutr., 2010, 65, 374–378 CrossRef CAS PubMed.
- M. Thomson and M. Ali, Garlic [Allium sativum]: A Review of its Potential Use as an Anti-Cancer Agent, Curr. Cancer Drug Targets, 2003, 3, 67–81 CrossRef CAS PubMed.
- S. Kodai, S. Takemura, Y. Minamiyama, S. Hai, S. Yamamoto, S. Kubo, Y. Yoshida, E. Niki, S. Okada, K. Hirohashi and S. Suehiro, S -allyl cysteine prevents CCl4 -induced acute liver injury in rats, Free Radic. Res., 2007, 41, 489–497 CrossRef CAS PubMed.
- J.-M. Kim, N. Chang, W.-K. Kim and H. S. Chun, Dietary S-Allyl- L -cysteine Reduces Mortality with Decreased Incidence of Stroke and Behavioral Changes in Stroke-Prone Spontaneously Hypertensive Rats, Biosci. Biotechnol. Biochem., 2006, 70, 1969–1971 CrossRef CAS PubMed.
- Y. Kosuge, Neuroprotective mechanisms of S-allyl-L-cysteine in neurological disease (Review), Exp. Ther. Med., 2020, 19, 1565–1569 Search PubMed.
- I. S. Choi, H. S. Cha and Y. S. Lee, Physicochemical and antioxidant properties of black garlic, Molecules, 2014, 19, 16811–16823 CrossRef PubMed.
- G. Querio, S. Antoniotti, F. Geddo, R. Levi and M. P. Gallo, Trimethylamine N-Oxide (TMAO) Impairs Purinergic Induced Intracellular Calcium Increase and Nitric Oxide Release in Endothelial Cells, Int. J. Mol. Sci., 2022, 23, 3982 CrossRef CAS PubMed.
- G. Querio, S. Antoniotti, F. Foglietta, C. M. Bertea, R. Canaparo, M. P. Gallo and R. Levi, Chamazulene Attenuates ROS Levels in Bovine Aortic Endothelial Cells Exposed to High Glucose Concentrations and Hydrogen Peroxide, Front. Physiol., 2018, 9, 246 CrossRef PubMed.
- S. Fornero, E. Bassino, R. Ramella, C. Gallina, S. K. Mahata, B. Tota, R. Levi, G. Alloatti and M. P. Gallo, Obligatory Role for Endothelial Heparan Sulphate Proteoglycans and Caveolae Internalization in Catestatin-Dependent eNOS Activation, BioMed Res. Int., 2014, 2014, 1–10 CrossRef PubMed.
- E. Scarcello, A. Lambremont, R. Vanbever, P. J. Jacques and D. Lison, Mind your assays: Misleading cytotoxicity with the WST-1 assay in the presence of manganese, PLoS One, 2020, 15, e0231634 CrossRef CAS PubMed.
- M. C. Warren, E. A. Bump, D. Medeiros and S. J. Braunhut, Oxidative stress–induced apoptosis of endothelial cells, Free Radicals Biol. Med., 2000, 29, 537–547 CrossRef CAS PubMed.
- A. L. King, D. J. Polhemus, S. Bhushan, H. Otsuka, K. Kondo, C. K. Nicholson, J. M. Bradley, K. N. Islam, J. W. Calvert, Y.-X. Tao, T. R. Dugas, E. E. Kelley, J. W. Elrod, P. L. Huang, R. Wang and D. J. Lefer, Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 3182–3187 CrossRef CAS PubMed.
- T. Ahmed and C.-K. Wang, Black Garlic and Its Bioactive Compounds on Human Health Diseases: A Review, Molecules, 2021, 26, 5028 CrossRef CAS PubMed.
- G. K. Kolluru, R. E. Shackelford, X. Shen, P. Dominic and C. G. Kevil, Sulfide regulation of cardiovascular function in health and disease, Nat. Rev. Cardiol., 2023, 20, 109–125 CrossRef CAS PubMed.
- E. Magli, E. Perissutti, V. Santagada, G. Caliendo, A. Corvino, G. Esposito, G. Esposito, F. Fiorino, M. Migliaccio, A. Scognamiglio, B. Severino, R. Sparaco and F. Frecentese, H2S Donors and Their Use in Medicinal Chemistry, Biomolecules, 2021, 11, 1899 CrossRef CAS PubMed.
- Y. Zheng, X. Ji, K. Ji and B. Wang, Hydrogen sulfide prodrugs—a review, Acta Pharm. Sin. B, 2015, 5, 367–377 CrossRef PubMed.
- B. Yudhistira, F. Punthi, J. Lin, A. S. Sulaimana, C. Chang and C. Hsieh, S–Allyl cysteine in garlic (Allium sativum): Formation, biofunction, and resistance to food processing for value–added product development, Compr. Rev. Food Sci. Food Saf., 2022, 21, 2665–2687 CrossRef CAS PubMed.
- J. M. Alves-Silva, M. Zuzarte, H. Girão and L. Salgueiro, Natural Products in Cardiovascular Diseases: The Potential of Plants from the Allioideae Subfamily (Ex-Alliaceae Family) and Their Sulphur-Containing Compounds, Plants, 2022, 11, 1920 CrossRef CAS PubMed.
- L. Wu, L. Chen, M. Kou, Y. Dong, W. Deng, L. Ge, H. Bao, Q. Chen and D. Li, The ratiometric fluorescent probes for monitoring the reactive inorganic sulfur species (RISS) signal in the living cell, Spectrochim. Acta, Part A, 2020, 231, 118141 CrossRef CAS PubMed.
- V. S. Lin, A. R. Lippert and C. J. Chang, Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H 2 O 2 -dependent H 2 S production, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 7131–7135 CrossRef CAS PubMed.
- N. Ide and B. H. Lau, Garlic compounds protect vascular endothelial cells from oxidized low density lipoprotein-induced injury, J. Pharm. Pharmacol., 1997, 49, 908–911 CrossRef CAS PubMed.
- J. Liu, F. M. Mesfin, C. E. Hunter, K. R. Olson, W. C. Shelley, J. P. Brokaw, K. Manohar and T. A. Markel, Recent Development of the Molecular and Cellular Mechanisms of Hydrogen Sulfide Gasotransmitter, Antioxidants, 2022, 11, 1788 CrossRef CAS PubMed.
- S. Lee, N. I. Chang, M. Yoo, J. H. Choi and D. Shin, Development and Validation of S-Allyl-L-Cysteine in Rat Plasma Using a Mixed-Mode Reversed-Phase and Cation-Exchange LC-ESI-MS/MS Method: Application to Pharmacokinetic Studies, J. Chromatogr. Sci., 2015, 53, 54–59 CAS.
- H. Amano, D. Kazamori and K. Itoh, Pharmacokinetics of S-Allyl-L-cysteine in Rats Is Characterized by High Oral Absorption and Extensive Renal Reabsorption, J. Nutr., 2016, 146, S456–S459 CrossRef PubMed.
- S. E. Bae, S. Y. Cho, Y. D. Won, S. H. Lee and H. J. Park, A comparative study of the different analytical methods for analysis of S-allyl cysteine in black garlic by HPLC, LWT – Food Sci. Technol., 2012, 46, 532–535 CrossRef CAS.
- S. Hirata, M. Abdelrahman, N. Yamauchi and M. Shigyo, Characteristics of chemical components in genetic resources of garlic Allium sativum collected from all over the world, Genet. Resour. Crop Evol., 2016, 63, 35–45 CrossRef CAS.
- C. Malaphong, A. Tangwanitchakul, S. Boriboon and N. Tangtreamjitmun, A simple and rapid HPLC method for determination of S-allyl-L-cystein and its use in quality control of black garlic samples, LWT, 2022, 160, 113290 CrossRef CAS.
- S. Lee, M. Yoo, S. Kim and D. Shin, Identification and quantification of S-allyl-l-cysteine in heated garlic juice by HPLC with ultraviolet and mass spectrometry detection, LWT – Food Sci. Technol., 2014, 57, 516–521 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo00412k |
| This journal is © The Royal Society of Chemistry 2023 |