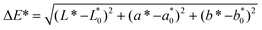Open Access Article
Open Access ArticleBread melanoidins as potential new sustainable bakery ingredients: a study using fat and fat-free bakery food models†
Noelia
Díaz-Morales‡
 ,
Mónica
Cavia-Saiz
,
Mónica
Cavia-Saiz
 ,
Ma Dolores
Rivero-Perez
,
Inmaculada
Gómez
,
Ma Dolores
Rivero-Perez
,
Inmaculada
Gómez
 ,
Gonzalo
Salazar-Mardones
,
Gonzalo
Salazar-Mardones
 ,
Isabel
Jaime
,
María L.
González-SanJosé
and
Pilar
Muñiz
,
Isabel
Jaime
,
María L.
González-SanJosé
and
Pilar
Muñiz
 *
*
Department of Biotechnology and Food Science, Faculty of Sciences, University of Burgos, Plaza Misael Bañuelos, 09001, Burgos, Spain. E-mail: pmuniz@ubu.es; ndiaz@ubu.es; monicacs@ubu.es; drivero@ubu.es; igbastida@ubu.es; gsalazar@ubu.es; ijaime@ubu.es; marglez@ubu.es; pmuniz@ubu.es; Fax: +34-947258831; Tel: +34-947258800, ext. 8210
First published on 27th January 2023
Abstract
Melanoidins isolated from bakery by-products are proposed as new sustainable ingredients for bakery products. The colour, odour profile, texture, water activity, and antioxidant capacity of two bakery food models, fat and fat-free, enriched with 2% and 4% soft bread and common bread melanoidins, were analysed. The colour of the bakery food models with melanoidins was darker than that of the respective control; the fat-free models with melanoidins showed higher values of hardness than the control, while no significant effect was observed in the fat models; the water activity did not change compared to the control; the odour profile was significantly modified with different effects depending on the type of melanoidin quantity added and the food model (fat or fat-free); and the antioxidant capacity increased proportionally to the quantity of melanoidin added. In general, melanoidins from soft bread exhibited a higher effect than the melanoidins from common bread. The melanoidins isolated from both fat and fat-free bakery food models did not show cytotoxicity nor did they modify the levels of reactive oxygen species in Caco-2 cells. Therefore, the results seem to indicate the favourable potential of bread melanoidins as new sustainable ingredients for bakery products.
1. Introduction
Food waste is a global problem, and bread is one of the most commonly wasted food products in the countries of the developed world. Bread waste is produced during the manufacturing stage due to processing factors, faults during the operation, rejection during the quality control, and improper handling during the storage/packing, among others.1 Furthermore, to respond to the consumer demands associated with specific bakery products such as soft bread, bakery companies remove the crusts from loaves, losing up to 40% of the bread2 and generating a large amount of waste that needs adequate management.The crusts of bakery products contain melanoidins, brown-coloured pigments of a high molecular weight, which have a high value, both for their technological properties and for their effect on health.3,4 Melanoidins are formed in the last stages of the Maillard reaction (MR), which takes place during the cooking, thermal processing, and storage of foods, and show a high degree of complexity with an unfamiliar structure.5 Melanoidins’ structure and composition change depending on the food; for instance, in bread, melanoidins are formed by gluten proteins and starch cross-linked by the Maillard reaction products.6 Melanoidins have drawn a lot of attention owing to their positive effects on the texture and flavour of many food products and, therefore, they are currently used as functional food ingredients in the food industry.
From a technological point of view, melanoidins have a great impact on the sensory quality of food products and contribute to achieving pleasant attributes for consumers, since they provide sensory properties such as colour, texture, and aroma.7,8 From a health point of view, melanoidins have biological properties with positive health effects, such as chemopreventive, antioxidant, and antimicrobial activities.5,9,10 European diets include a substantial melanoidin intake of around 10–12 g per day;11,12 bakery products are significant contributors to this intake. In fact, average amounts of melanoidins from bread and biscuit consumption have been estimated to be 1.8–15 g and 3.2–8.5 g per day, respectively.11
The promising use of crust melanoidins obtained from soft and common bread as food ingredients for bread has recently been demonstrated. These melanoidins have high antioxidant capacity, antimicrobial activities on spoilage microbiota and food pathogens, a positive volatile profile, no cytotoxic effects, and no undesirable compounds (furfural and 5-hydroxy-methyl-furfural).13,14 In this regard, it has been suggested that the addition of melanoidins to bakery foods can improve their sensory characteristics.
Based on the previous considerations, the present study has been conducted to revalue the by-products obtained from bakery companies, by isolating melanoidins from the industrial crust residues and using them for reincorporation in other bakery products. In this sense, the present study evaluated the effect of the incorporation of melanoidins in two models of bakery food matrices (with fat and fat-free), since it is hypothesized that they will have a favourable influence on the technological (colour, texture, and aroma) and functional (antioxidant) characteristics of the products.
2. Materials and methods
2.1. Melanoidin separation
The crusts of soft and common bread were used in this study. A Spanish bakery company (Cerealto Foods, Spain) kindly supplied the samples of soft bread crust obtained from processing crustless soft white bread. The common bread was subjected to expansion in a bakery pilot plant, as indicated in a previous study,13 where the process used to obtain melanoidins from the bread crusts is also indicated. Briefly, the crust samples were ground in a coffee mill (Taurus Minimoka GR20, Taurus Group, Oliana, Spain) and sieved to a particle size <1 mm and melanoidins were extracted by in vitro digestion using Pronase E according to the method described by Roncero-Ramos et al. (2013).15 After digestion, the soluble fraction was freeze-dried (FreeZone 12 L Console Freeze Dry System with a drying chamber, Labconco, MO, USA) to obtain the isolated melanoidins as powder products. Melanoidins from the bakery model matrices were also obtained by the same procedure.2.2. Expansion of the bakery food models supplemented with bread-melanoidins
Two models of bakery food matrices (fat and fat-free) were prepared according to the recipe described in the AACC method.16 Control formulations (FC and FFC) were subjected to expansion according to the following recipe: wheat flour (12.5 g); sucrose (finely granulated, 5.25 g); and water (6.25 mL). In addition to this, all-purpose vegetable shortening (5 g) was added to the fat control cookies (FC). Isolated melanoidins from common and soft bread crusts were added at 2% or 4% according to the formulation. Thus, ten formulations were subjected to expansion (5 formulations with fat and 5 formulations without fat). The fat cookie formulations were: without melanoidins (FC); with 2% common bread melanoidins (F2-CBM) added; with 4% common bread melanoidins (F4-CBM) added; with 2% soft bread melanoidins (F2-SBM) added; and with 4% soft bread melanoidins (F4-SBM) added. The fat-free cookie formulations were: without melanoidins (FFC); with 2% common bread melanoidins added (FF2-CBM); with 4% common bread melanoidins added (FF4-CBM); with 2% soft bread melanoidins (FF2-SBM) added; and with 4% soft bread melanoidins added (FF4-SBM). The ingredients were mixed and kneaded (KitchenAid Ultra Power KSM90, USA) and the dough was divided into equal portions, with stainless cookie moulds 3 cm in diameter and 0.5 cm thick. Cookies were baked in an electric convection oven (Berto's, Padova, Italy) at 200 ± 5 °C for 15 minutes. Five cookies were obtained for each formulation.2.3. Colour analysis
The colour of the bakery food models was evaluated directly on the two surfaces of each sample (top and bottom), measuring the CIELab parameters such as lightness (L*), redness (a*), and yellowness (b*). The colour readings were taken at three randomly selected non-overlapping points on the surface of each sample. A Konica Minolta CM-2600d (Konica Minolta Business Technologies Inc., Tokyo, Japan) with the standard illuminant D65 and the standard observer angle 10° (International Commission on Illumination, 2004) was used.The colour of the isolated powder melanoidins was measured similarly after placing the powders in white paper capsules with an approximate area of 3 cm2.
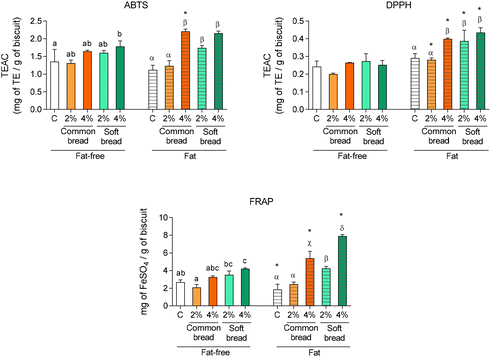
The total colour difference among the products and the control was calculated from the CIELab* parameters according to the following formula:
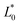 ,
, 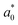 , and
, and 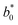 are the values of the control sample, respectively.
are the values of the control sample, respectively.
Furthermore, the absorbance values at 345 and 420 nm of the melanoidin solutions were also considered, being the complementary indexes of the colour. Melanoidins extracted from the bakery food matrices were dissolved in Milli-Q water at a concentration of 10 mg mL−1 to prepare an initial solution, which was then diluted at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to obtain the working solution of the samples.13 A spectrophotometer (U-2000 Hitachi, Ltd, Hubbardston, MA, USA) and 1 cm-path length cuvettes were used. Measurements were performed in triplicate (three different working solutions).
3 to obtain the working solution of the samples.13 A spectrophotometer (U-2000 Hitachi, Ltd, Hubbardston, MA, USA) and 1 cm-path length cuvettes were used. Measurements were performed in triplicate (three different working solutions).
2.4. Texture analysis
A puncture test was performed using a TA.XTPlus Texture Analyzer (Stable Microsystems Ltd, Surrey, UK) using the following conditions: test mode, puncture; pre-test speed, 1 mm s−1; test speed, 0.5 mm s−1; post-test speed, 10 mm s−1: trigger force, 5 g. A cylindrical flat-end puncture probe P/2 (2 mm diameter) was used. Each sample was centred on the base plate and the puncture probe penetrated 2 mm into the sample. The texture measurement of the samples involved plotting force (N) versus time (s) and two parameters were calculated: (1) area under the curve (as N s) up to 2 mm of puncturing; and (2) the maximum force (N) as an index for the crust hardness. The texture parameters of the bakery food matrices were measured in triplicate (three different pieces). Texture Expert software version 1.2 was used for data analysis.2.5. Water activity analysis
Water activity values were measured using an AquaLAB CX-2 (Decagon Devices Inc., Pullman, WA, USA). Duplicate measurements from the ground bakery food matrices were carried out.2.6. Odour profile analysed using an electronic nose
The odour profile of the bakery food models was determined in quintuplicate using an α-FOX 4000 electronic nose (AlfaMOS, Toulouse, France) equipped with 18 metal oxide sensors, and a static headspace system (HS100 CTC-Combi-Pal, CTC Analytics AG, Zwingen, Switzerland). Briefly, the analytical protocol was as follows: glass vials (10 mL) containing 1 g of each ground sample, sealed with Teflon septum taps, were incubated for 10 minutes at 50 °C, and then volatiles were injected into the headspace (2 mL s−1 of air), and the sensor responses were collected during 120 s. The response intensity was calculated as the change in the resistance of each sensor with respect to the resistance at time zero. For this work, the maximum values of the response intensity were considered.2.7. Antioxidant capacity analysis
Quencher methods, ABTS (2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid), DPPH (2,2-diphenyl-1-picrylhydrazyl) and FRAP (ferric reducing power assay), were performed following the procedure described by Del Pino-García et al. (2015)17 with slight modifications. Then, 20 mg of the bakery food model and 6 mL of the ABTS solution; 80 mg of the bakery food model and 3 mL of the DPPH solution; and 10 mg of the bakery food model and 6 mL of the TPTZ solution were used. After incubation with continuous shaking for 10 minutes and centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500g for 3 minutes, the absorbance of the supernatants (reactive solutions) was measured at the respective wavelength. The antioxidant capacity was measured in quintuplicate.
500g for 3 minutes, the absorbance of the supernatants (reactive solutions) was measured at the respective wavelength. The antioxidant capacity was measured in quintuplicate.
2.8. Cell studies
Human colon adenocarcinoma Caco-2 (ATCC® HTB-37™) cells were purchased from the American Type Culture Collection (ATCC, Barcelona, Spain). The cells were cultured as a monolayer in Eagle's Minimum Essential Medium (MEM) supplemented with 20% v/v heat inactivated fetal bovine serum (FBS), 1% (v/v) non-essential amino acids, 1% (v/v) L-glutamine, 1% (v/v) sodium pyruvate, and 1% (v/v) penicillin/streptomycin. The cell cultures were incubated at 37 °C and 90% humidity under a 5% CO2 atmosphere.2.9. Statistical analysis
Two-way analysis of variance (ANOVA) was performed, and statistical comparisons of the different treatments were performed using Sidak's multiple comparison post hoc test. When comparisons were carried out against the control (this is the case for cell viability assays), Dunnett's multiple comparison test was conducted. Values of p < 0.05 were considered statistically significant. All statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA). For the statistical analysis of the odour profile data, one-way analysis of variance (ANOVA) of the response of the sensors was performed using Tukey's multiple comparison post hoc test and the SPSS Statistics version 24 (IBM Corp., New York, NY, USA) package.3. Results and discussion
The main characteristics of the melanoidins used in this study, such as the absence of cytotoxicity, antimicrobial and antioxidant properties, colour, and elemental composition, were shown in previous studies.13,14 According to these studies, it was established that the most suitable concentrations of melanoidins for incorporation in the studied bakery food models were 2% and 4%.3.1. Effect of bread-melanoidin addition on the quality properties of the bakery food models
The surface colour, texture, and the odour profile are the main features of baking products that are considered as being preferential by consumers; therefore, they are very important for determining the quality of these products. For that reason, this study aimed at analyzing these parameters, together with others directly correlated with them, such as water activity that influences the texture, or antioxidant capacity with a direct effect on odour by delaying rancidity.The effect of common and soft bread melanoidins on the CIELab* colour parameters (Table 1) pointed out that in the case of fat-free bakery matrices, the incorporation of 2% or 4% of common or soft bread melanoidins did not significantly change (p > 0.05) the values of the L*, a* and b* parameters. However, the values of these parameters in the case of fat bakery food models were significantly modified (p < 0.05). Fat matrices supplemented with melanoidins showed significantly lower values of L*, which decreased proportionally with the increase in the quantity of the added melanoidins, leading to darker or browner samples. These changes were statistically significant with soft bread melanoidins, which also increased the a* value (redness). However, component b* (yellowness) remained constant or decreased. Passos et al. (2017)7 also observed a decrease in the b* component in cookies after the incorporation of high amounts of coffee melanoidins. The soft bread melanoidins used are rich in melanoidins with a high molecular weight and are relatively dark in colour, corresponding to the intense absorbance at 420 nm (Diaz-Morales et al., 2021).13 Hence, they are probably responsible for the colour changes observed in the fat bakery food models.
| Fat-free bakery food models | Fat bakery food models | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Common bread melanoidins | Soft bread melanoidins | Control | Common bread melanoidins | Soft bread melanoidins | ||||||
| 2% | 4% | 2% | 4% | 2% | 4% | 2% | 4% | ||||
| Different Latin letters indicate significant differences among the formulations of bakery food models without fat (p < 0.05). Different Greek letters indicate significant differences among the formulations of bakery food models with fat (p < 0.05). # Indicates significant differences between the fat-free and fat models in the same formulation (p < 0.05). Comparisons were made by two-way ANOVA, followed by Sidak's multiple comparison test. | |||||||||||
| Top surface | L* | 67 ± 6a | 70 ± 2a | 66 ± 1a | 62 ± 7a | 62 ± 7a | 58 ± 9χ# | 57 ± 12βχ# | 51 ± 5βχ# | 49 ± 8αβ# | 41 ± 6α# |
| a* | 6 ± 3ab | 4 ± 1a | 6 ± 3ab | 8 ± 3b | 8 ± 3ab | 9 ± 3α# | 8 ± 4α | 13 ± 2β# | 13 ± 1β# | 13 ± 1β# | |
| b* | 24 ± 2a | 24 ± 1a | 25 ± 3a | 25 ± 3a | 26 ± 3a | 30 ± 2β# | 28 ± 2αβ# | 31 ± 2β# | 28 ± 4β | 24 ± 5α | |
| ΔE* | 5 ± 2ab | 4 ± 1a | 9 ± 2c | 7 ± 4bc | 7 ± 2α | 12 ± 4β# | 6 ± 1α# | 19 ± 4δ# | |||
| Bottom surface | L* | 59 ± 6a | 58 ± 5a | 56 ± 3a | 59 ± 6a | 52 ± 5a | 55 ± 8β | 52 ± 8β | 37 ± 9α# | 42 ± 7α# | 34 ± 5α# |
| a* | 10 ± 4a | 11 ± 4a | 14 ± 1a | 11 ± 3a | 13 ± 1a | 10 ± 5α | 13 ± 5αβ | 14 ± 2β | 14 ± 2β | 13 ± 2αβ | |
| b* | 30 ± 3a | 31 ± 3a | 32 ± 1a | 31 ± 2a | 32 ± 1a | 30 ± 3χδ | 32 ± 2δ | 23 ± 9αβ# | 26 ± 5βχ# | 19 ± 6α# | |
| ΔE* | 6 ± 3a | 6 ± 1a | 5 ± 2a | 7 ± 3a | 10 ± 4α | 21 ± 9β# | 12 ± 6α | 24 ± 4β | |||
Fat exerts a positive effect on the colour of the bakery products and the absence or reduction of fat renders less coloured or dark products.22,23 This fact explains the difference between the values of the CIELab* parameters of both model matrices (fat and fat-free) and could also explain why the added melanoidins significantly modified the colour parameters of only the fat matrices.
The parameter “difference in the colour” (ΔE*) allows estimation of the global colour changes between the two samples. Thus, comparing the food bakery models with their respective controls, a notable global effect of the addition of melanoidins on the colour of the bakery matrices was detected. For all the cases, significant colour differences were observed (ΔE* values higher than 1 and statistically different from 0). However, in general, values over 5 (the limit value for visual colour discrimination) were detected in the fat model matrices or when soft bread melanoidins were used (Table 1).
From the increase in colour (darkness) observed mainly upon the addition of soft bread melanoidins, it is possible to hypothesize that they could be a good alternative to achieve the desirable toasted colour by applying a low-intensity baking process (low temperature and/or short time) with the corresponding energy reduction and the decrease in the risk of undesirable compounds formed during heating, such as acrylamide.
The incorporation of melanoidins involved some significant changes in the texture of the studied bakery food models, which were mainly dependent on the types of melanoidins added and the presence/absence of fat (Fig. 1A). The obtained results agree with those reported by the other authors, who also pointed out the changes in hardness due to the different Maillard products.7,24
The fat-free model matrices added with common bread melanoidins (2% and 4%) showed significant increases in the hardness, whereas no changes were observed in the fat model matrices. Fat provides lubricity to the dough, reduces the formation of the gluten network (“shortening effect”), and is involved in the transfer of heat and expansion during the baking process.25 All of these effects could explain why, in the presence of fat, the effect of the added melanoidins was not notable.
In contrast with the previous results, the addition of soft bread melanoidins did not involve significant texture changes. The different behaviors of both melanoidins could be due to their different characteristics; some of them were revealed in previous papers.13,14 A large number of studies on melanoidins, isolated from real foods and model systems, reported the huge variety of their structures and activities, due to the conjunction of factors (ingredients or reactive compounds, heating conditions, and so on) that influence their formation.12
Furthermore, from the fact that melanoidins are hygroscopic substances, their effect on the hardness of the fat-free matrices could be correlated with their possible effect on water activity. So, although the addition of melanoidins did not involve significant differences in the water activity of the studied matrices (Fig. 1B), the data showed an increasing tendency when common bread melanoidins were added. Regardless, the obtained water activity values ranged within the interval of aw of 0.16–0.27, which are typical values for bakery products such as cookies (mean value around 0.2).26
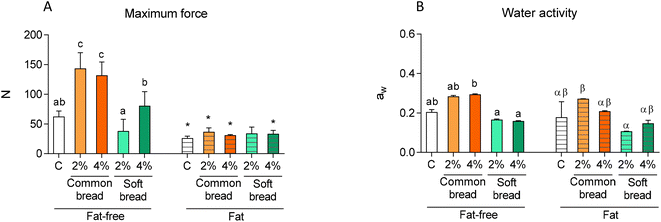
The incorporation of melanoidins involved some significant changes in the odour profile (e-nose sensor response) of the studied bakery food models, which were mainly dependent on the types of melanoidins added and the presence/absence of fat (Fig. 2). The data from 4 of 18 sensors were not included in the analysis, due to their low responses (response intensity lower than 1).
The addition of common bread melanoidins, at both 2% and 4%, modified the odour profile of the bakery food models, either with fat or without fat. In general, the incorporation of common bread melanoidins produced significant increases in the maximum response intensity for about half of the sensors, in both types of matrices (fat and fat-free), and then the odour profile was noticeably modified both qualitatively and quantitatively. Conversely, when soft bread melanoidins were added, the odour profiles were quite like those of the control, mainly in the case of fat matrices. In the case of fat-free matrices, the data showed significant quantitative differences (lower intensity response) for most of the sensors.
Considering that the higher the quantity of volatiles in the matrices the higher the response of the sensors, the obtained results can be explained by the different volatile compositions of both melanoidins. A previous study14 noted that common bread melanoidins are richer in volatile components, and that they showed more volatiles and were in higher quantity compared to soft bread melanoidins. This previous work revealed that while the volatile profile of common bread melanoidins included, among others, superior alcohols, pyrazines, and some lactones, neither of these compounds were detected in soft bread melanoidins. Furthermore, soft bread melanoidins did not show some acids such as butanoic, isovaleric, and octanoic; all of these volatile compounds can interact with sensors and modify their response. The volatile composition of common bread melanoidins could also explain the increase in sensor responses with higher concentrations of these melanoidins, which was not observed in the case of soft bread melanoidins.
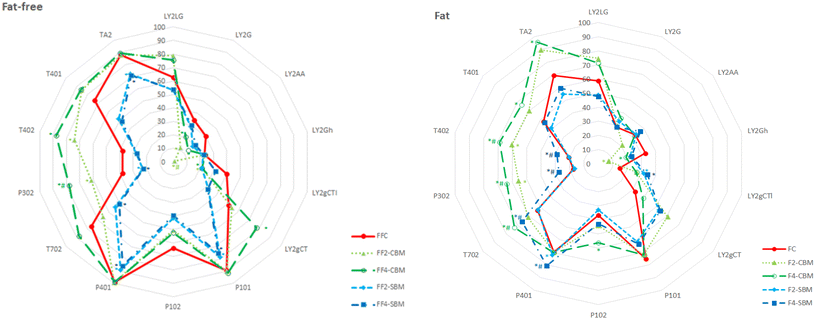
The incorporation of melanoidins involved some significant changes in the antioxidant capacity of the studied bakery food models, which were dependent on the types of melanoidins added and the presence/absence of fat (Fig. 3). In general, the addition of melanoidins into fat bakery food models involved the most noticeable changes, showing significant and concentration-dependent increases in the antioxidant activity. The results indicate the influence of fat on the antioxidant capacity of the bakery food models, possibly by the liposolubility of melanoidins. Wagner et al. (2002)31 previously described similar results – increases in the antioxidant activity of melanoidins in a fat medium.
In summary, it seems to be possible to assert that incorporation of bakery melanoidins into bakery products contributes to increasing their antioxidant capacity and corroborates the idea that melanoidins could be used as potential functional food ingredients to increase the shelf-life of bakery products, noted previously by other authors.5,6
3.2. Characteristics of the melanoidins formed in the bakery food models enriched with bread melanoidins
From the fact that melanoidin formation depends on, among other factors, the ingredients used to make the bakery products, the hypothesis that melanoidins as an ingredient of the dough probably influence the properties of the new melanoidins formed during the baking process was checked. To check this, the properties of the melanoidins isolated from the studied bakery food models (with and without melanoidins) were compared. To our knowledge, no previous studies have focused on this fact.Due to the importance of food safety and security, one of the properties evaluated and compared based on the new melanoidins was their cytotoxicity. This fact was under consideration because although melanoidins added in the expansion process of the bakery food models did not show cytotoxicity,14 the new melanoidins generated in the model matrices could have developed a more complex structure and incorporated cytotoxic compounds that might change the non-toxic characteristics of the initial melanoidins.
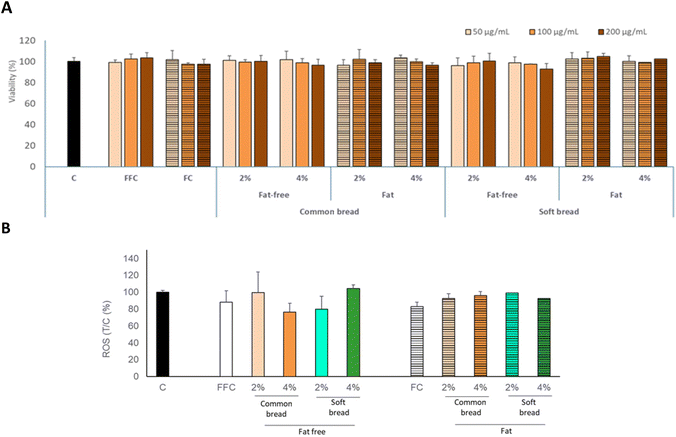
No cytotoxicity (decrease of cell viability) was observed at the studied concentrations for Caco-2 cells (Fig. 4). These data agree with the results previously reported, indicating no cytotoxic effect of the different melanoidins isolated from the bakery products.12,32,33 Furthermore, the new melanoidins did not exhibit prooxidant activity, as there was no increase in the intracellular ROS levels in the presence of melanoidins (Fig. 4). These results agree with those previously reported upon working with melanoidins from bakery and other foods.34–36 Altogether, the cell viability and intracellular ROS levels indicate that the harmless effect of the initial melanoidins, similar to the new melanoidins from the control model matrices, remains in the new melanoidins formed in the bakery food models supplemented with melanoidins.
The new melanoidins formed in the fat bakery food models were, in general, darker than those of the control model matrices (Table 2), as can be seen from the data for powder melanoidins (L* and ΔE*) and from the melanoidin solutions (365 nm and 420 nm). The effect of melanoidins on colour was concentration dependent, and variable with the types of melanoidins added. The darkest melanoidins were those isolated from the model matrix with 4% soft bread melanoidins added. Fat-free bakery food models showed similar results, but with fewer differences in the L* values. These results could be because the initial SBM were darker compared to the CBM, as observed in previous studies.13 Regardless, altogether, the colour results pointed out the contribution of the added melanoidins to the colour of the newly formed melanoidins.
| Formulation | L* | a* | b* | ΔE* | 365 nm (UA) | 420 nm (UA) | ABTS (TEAC) | FRAP, mg g−1 | |
|---|---|---|---|---|---|---|---|---|---|
| Different Latin letters indicate significant differences among the fat-free model matrices (p < 0.05). Different Greek letters indicate significant differences among the matrices with fat (p < 0.05). Comparisons were made by two-way ANOVA, followed by Sidak's multiple comparison test. FF: fat-free; F: fat; CBM: common bread melanoidins; SBM: soft bread melanoidins; UA: the unit of absorbance – the antioxidant total capacity evaluated using ABTS and FRAP. TEAC (trolox equivalent antioxidant capacity): mg of Trolox per g melanoidin. | |||||||||
| Fat-free model matrices | FFC | 69 ± 0.6bc | 2 ± 1b | 13 ± 0.4a | — | 0.123 ± 0.05a | 0.039 ± 0.001a | 2 ± 0.1a | 4 ± 0.3b |
| FF2-CBM | 64 ± 0.3ab | 1 ± 0.1a | 14 ± 0.2ab | 5 ± 0.3c | 0.179 ± 0.01c | 0.048 ± 0.001b | 3 ± 0.3b | 6 ± 1.0c | |
| FF2-SBM | 69 ± 0.1bc | 4 ± 0.5d | 16 ± 0.2cd | 3 ± 0.2a | 0.161 ± 0.01b | 0.053 ± 0.005c | 3 ± 0.2ab | 5 ± 0.4ab | |
| FF4-CBM | 73 ± 0.1c | 3 ± 0.1c | 15 ± 0.1bc | 4 ± 0.1b | 0.120 ± 0.03a | 0.027 ± 0.002a | 2 ± 0.4ab | 4 ± 0.1a | |
| FF4-SBM | 64 ± 0.2a | 4 ± 0.8d | 18 ± 0.3d | 7 ± 0.4d | 0.272 ± 0.03d | 0.081 ± 0.001d | 3 ± 0.2b | 7 ± 0.8c | |
| Fat model matrices | FFC | 68 ± 0.2δ | 3 ± 0.4α | 16 ± 1.4α | — | 0.253 ± 0.05α | 0.110 ± 0.05β | 2 ± 0.1β | 5 ± 0.4α |
| F2-CBM | 69 ± 0.3ε | 3 ± 0.1α | 15 ± 0.1α | 2 ± 0.3α | 0.196 ± 0.08α | 0.070 ± 0.04α | 2 ± 0.1α | 5 ± 0.5α | |
| F2-SBM | 65 ± 0.4β | 5 ± 0.1β | 21 ± 0.2β | 5 ± 0.2β | 0.357 ± 0.12χ | 0.122 ± 0.06χ | 4 ± 0.1β | 7 ± 0.7β | |
| F4-CBM | 62 ± 0.2γ | 6 ± 0.1γ | 22 ± 0.2β | 9 ± 0.2χ | 0.468 ± 0.02δ | 0.177 ± 0.02δ | 3 ± 0.2β | 7 ± 0.7β | |
| F4-SBM | 57 ± 0.3α | 7 ± 0.1δ | 22 ± 0.2β | 13 ± 0.3δ | 0.786 ± 0.06ε | 0.299 ± 0.03ε | 5 ± 0.4χ | 11 ± 1.4χ | |
The comparison of the colour results of the new melanoidins and the bakery food models seemed to indicate a direct correlation between them, and then reinforced the idea of a direct impact of melanoidins on the colour of the bakery food models.
The new melanoidins formed in each studied bakery food model showed values of antioxidant activities similar to those obtained previously.13 These results corroborate the crucial role of melanoidins in preventing oxidative damage due to their antioxidant capacity, including the metal ion chelation capacity and radical-scavenging activity.5,10,31
The data from ABTS and FRAP indicated some significant differences (Table 2). However, in general, the results do not allow the assertion that the addition of melanoidins involved significant modification of the antioxidant properties of the new melanoidins formed. Only the melanoidins isolated from the bakery food model with 4% SBM had higher values of antioxidant capacity compared to those isolated from the control matrices, regardless of the presence or absence of fat and the method of analysis to evaluate the antioxidant capacity. In the other cases of this study, the addition of melanoidins did not involve significant changes or these were discordant between the matrices or the doses of melanoidins added. The obtained results seem to have a correlation with the higher antioxidant activity of SBM compared to CBM.14
4. Concluding remarks
Melanoidins isolated from different bakery by-products, especially those isolated from the crusts removed from soft bread, are a good candidate for use as “new” ingredients for bakery foods because they significantly improve properties such as colour, texture, and antioxidant capacity, which are directly correlated with the acceptance of consumers and with the global quality of these types of products. Furthermore, the melanoidins formed in the bakery products with the melanoidins added did not show cell cytotoxicity and maintained their antioxidant properties.Therefore, the isolation of melanoidins from bakery by-products can be an alternative to reuse and revalue these by-products, contributing to the circular economy and the sustainable production of food.
Abbreviations
| CBM | Melanoidins isolated from common bread |
| SBM | Melanoidins isolated from soft bread |
Author contributions
Noelia Díaz-Morales: methodology, investigation, and writing – review and editing. Mónica Cavia-Saiz: methodology, investigation, validation, and writing – review and editing. M. Dolores Rivero-Pérez: methodology and writing – review and editing. Inmaculada Gómez Bastida: methodology, validation, and writing – review and editing. Gonzalo Salazar-Mardones: methodology, investigation, resources, and writing – review and editing. Isabel Jaime Moreno: supervision and writing – review and editing. Maria L. González-SanJosé: conceptualization, supervision, writing – original draft, and writing – review and editing. Pilar Muñiz: conceptualization, supervision, project administration, funding acquisition, resources, writing – original draft, and writing – review and editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors acknowledge financial support from the autonomous government of Castilla y León and FEDER (JCyL/FEDER) BU243P18.References
- M. Verni, A. Minisci, S. Convertino, L. Nionelli and C. G. Rizzello, Wasted bread as substrate for the cultivation of starters for the food industry, Front. Microbiol., 2020, 28, 293 CrossRef PubMed.
- V. Narisetty, R. Cox, N. Willoughby, E. Aktas, B. Tiwari, A. S. Matharu, K. Salonitis and V. Kumar, Recycling bread waste into chemical building blocks using a circular biorefining approach, Sustainable Energy Fuels, 2021, 5, 4842–4849 RSC.
- C. Delgado-Andrade and V. Flogliano, Dietary advanced glycosylation end-products (dAGEs) and melanoidins formed through the Maillard reaction: Physiological consequences of their intake, Annu. Rev. Food Sci. Technol., 2018, 25, 271–291 CrossRef PubMed.
- S. Perez-Burillo, S. Rajakaruna, S. Pastoriza, O. Paliy and J. A. Rufian-Henares, Bioactivity of food melanoidins is mediated by gut microbiota, Food Chem., 2020, 316, 126309 CrossRef CAS PubMed.
- M. Mesías and C. Delgado-Andrade, Melanoidins as a potential functional food ingredient, Curr. Opin. Food Sci., 2017, 14, 37–42 CrossRef.
- M. Nooshkam, M. Varidi and D. K. Verma, Functional and biological properties of Maillard conjugates and their potential application in medical and food: A review, Food Res. Int., 2020, 131, e109003 CrossRef PubMed.
- C. P. Passos, K. Kukurová, E. Basil, P. A. R. Fernandes, A. Neto, F. M. Nunes, M. Murkovic, Z. Ciesarová and M. A. Coimbra, Instant coffee as a source of antioxidant-rich and sugar-free coloured compounds for use in bakery: Application in biscuits, Food Chem., 2017, 231, 114–121 CrossRef CAS PubMed.
- M. Starowicz and H. Zieliński, How Maillard reaction influences sensorial properties (color, flavor and texture) of food products?, Food Rev. Int., 2019, 35, 707–725 CrossRef CAS.
- N. Tamanna and N. Mahmood, Food processing and Maillard reaction products effect on human health and nutrition, J. Food Chem., 2015, 526762 Search PubMed.
- H. Y. Wang, H. Qian and W. R. Yao, Melanoidins produced by the Maillard reaction: Structure and biological activity, Food Chem., 2011, 128, 573–584 CrossRef CAS.
- V. Fogliano and F. J. Morales, Estimation of dietary intake of melanoidins from coffee and bread, Food Funct., 2011, 2, 117–123 RSC.
- S. Pastoriza and J. A. Rufián-Henares, Contribution of melanoidins to the antioxidant capacity of the Spanish diet, Food Chem., 2014, 164, 438–445 CrossRef CAS PubMed.
- N. Diaz-Morales, M. Cavia-Saiz, G. Salazar, M. D. Rivero-Perez and P. Muñiz, Cytotoxicity study of bakery product melanoidins on intestinal and endothelial cell lines, Food Chem., 2021, 343, 128405 CrossRef CAS PubMed.
- N. Diaz-Morales, M. Ortega-Heras, A. M. Diez-Maté, M. L. Gonzalez-SanJose and P. Muñiz, Antimicrobial properties and volatile profile of bread and biscuits melanoidins, Food Chem., 2022, 373, 131648 CrossRef CAS PubMed.
- I. Roncero-Ramos, C. Delgado-Andrade, A. Haro, B. Ruiz-Roca, F. J. Morales and M. P. Navarro, Effects of dietary bread crust Maillard reaction products on calcium and bone metabolism in rats, Amino Acids, 2013, 44, 1409–1418 CrossRef CAS PubMed.
- AACC International, AACC International approved methods of analysis, Methods 10-54.01, American Association of Cereal Chemists (AACC) International, St. Paul, MN, 11th edn, 1999 Search PubMed.
- R. Del Pino-García, J. García-Lomillo, M. D. Rivero-Perez, M. L. Gonzalez-SanJosé and P. Muñiz, Adaptation and validation of quick, easy, new, cheap, and reproducible (quencher) antioxidant capacity assays in model products obtained from residual wine pomace, J. Agric. Food Chem., 2015, 63, 6922–6931 CrossRef PubMed.
- P. R. Twentyman and M. Luscombe, A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity, Br. J. Cancer, 1987, 56, 279–285 CrossRef CAS PubMed.
- D. Figueroa, M. Asaduzzaman and F. Young, Real time monitoring and quantification of reactive oxygen species in breast cancer cell line MCF-7 by 2′,7′-dichlorofluorescin diacetate (DCFDA) assay, J. Pharmacol. Toxicol. Methods, 2018, 94, 26–33 CrossRef CAS PubMed.
- E. Purlis, Browning development in bakery products – A review, J. Food Eng., 2010, 99, 239–249 CrossRef CAS.
- R. Pino and M. L. González-Sanjosé, (EU COST action 919) European Commission: Luxembourg Office for Official, in Melanoidins in food and health, ed. G. Vegarud and F. J. Morales, Publications of the European Communities, 2003, vol. 4, pp. 120–124 Search PubMed.
- B. Wekwete and K. P. Navder, Effects of avocado fruit puree and oatrim as fat replacers on the physical, textural and sensory properties of oatmeal cookies, J. Food Qual., 2007, 31, 131–141 CrossRef.
- A. Zbikowska, M. Kowalska and J. Pieniowska, Assessment of shortcrust biscuits with reduced fat content of microcrystalline cellulose and psyllium as fat replacements, J. Food Process. Preserv., 2018, 42, 1–10 CAS.
- P. Zhou, M. Guo, D. Liu, X. Liu and T. P. Labuza, Maillard-reaction-induced modification and aggregation of proteins and hardening of texture in protein bar model systems, J. Food Sci., 2013, 78, 437–444 CrossRef PubMed.
- B. Pareyt and J. A. Delcour, The role of wheat flour constituents, sugar, and fat in low moisture cereal-based products: A review on sugar-snap cookies, Crit. Rev. Food Sci. Nutr., 2008, 48, 824–839 CrossRef CAS PubMed.
- A. Piga, P. Catzeddu, S. Farris, T. Roggio, A. Sanguinetti and E. Scano, Texture evolution of “Amaretti” cookies during storage, Eur. Food Res. Technol., 2005, 221, 387–391 CrossRef CAS.
- M. Ortega-Heras and M. L. González-Sanjosé, (EU: COST action 919) European Commission: Luxembourg Office for Official, in Melanoidins in food and health, ed. G. Vegarud and F. J. Morales, Publications of the European Communities, 2003, vol. 4, pp. 35–40 Search PubMed.
- L. Paravisini and E. Guichard, in Flavour: From food to perception, ed. C. Guichard, M. Salles and A. Morzel, John Wiley & Sons Ltd, 2016, pp. 208–234 Search PubMed.
- C. Delgado-Andrade and F. J. Morales, Unraveling the contribution of melanoidins to the antioxidant activity of coffee brews, J. Agric. Food Chem., 2005, 53, 1403–1407 CrossRef CAS PubMed.
- F. J. Morales, V. Somoza and V. Fogliano, Physiological relevance of dietary melanoidins, Amino Acids, 2012, 42, 1097–1109 CrossRef CAS PubMed.
- K. H. Wagner, S. Derkits, M. Herr, W. Schuh and I. Elmadfa, Antioxidant potential of melanoidins isolated from a roasted glucose-glycine model, Food Chem., 2002, 783, 375–382 CrossRef.
- S. Gonzalez-Mateo, M. L. Gonzalez-Sanjose and P. Muñiz, Presence of Maillard products in Spanish muffins and evaluation of colour and antioxidant potential, Food Chem. Toxicol., 2009, 47, 2798–2805 CrossRef CAS PubMed.
- M. A. Martin, S. Ramos, R. Mateos, J. A. Rufian-Henares, F. J. Morales, L. Bravo and L. Goya, Biscuit melanoidins of different molecular masses protect human HepG2cells against oxidative stress, J. Agric. Food Chem., 2009, 57, 7250–7258 CrossRef CAS PubMed.
- R. Del Pino-Garcia, M. L. Gonzalez-SanJose, M. D. Rivero-Perez and P. Muñiz, Influence of the degree of roasting on the antioxidant capacity and genoprotective effect of instant coffee: contribution of the melanoidin fraction, J. Agric. Food Chem., 2012, 60, 10530–10539 CrossRef CAS PubMed.
- S. Pastoriza, I. Seiquer, M. Mesías, J. A. Rufián-Henares and C. Delgado-Andrade, Evaluation of the availability and antioxidant capacity of Maillard compounds present in bread crust: studies in Caco-2 cells, Foods, 2017, 6, 5 CrossRef PubMed.
- S. Tores de la Cruz, A. Iriondo-DeHond, T. Herrera, Y. Lopez-Tofiño, C. Galvez-Robleño, M. Prodanov, F. Velazquez-Escobar, R. Abalo and M. D. D. Castillo, An assessment of the bioactivity of coffee silverskin melanoidins, Foods, 2019, 12, 68 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo03909e |
| ‡ Present address: Institute of Biomedical Research of Salamanca (IBSAL), 37007 Salamanca, Spain. E-mail: noeliadm@usal.es |
| This journal is © The Royal Society of Chemistry 2023 |