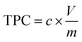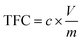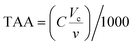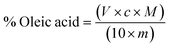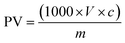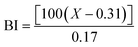Open Access Article
Open Access ArticleFortification of vegetable fat with natural antioxidants recovered by bergamot pomace for use as an ingredient for the production of biscuits
Antonio
Gattuso
 ab,
Amalia
Piscopo
ab,
Amalia
Piscopo
 a,
Simone
Santacaterina
a,
Elisa
Imeneo
a,
Alessandra
De Bruno
a,
Simone
Santacaterina
a,
Elisa
Imeneo
a,
Alessandra
De Bruno
 *c and
Marco
Poiana
a
*c and
Marco
Poiana
a
aDepartment of AGRARIA, University Mediterranea of Reggio Calabria, 89124 Reggio Calabria, Italy
bExperimental Station for the Industry of the Essential Oils and Citrus Products, SSEA, 89127 Reggio Calabria, Italy
cDepartment of Human Sciences and Promotion of the Quality of Life, San Raffaele University, Rome, Italy. E-mail: alessandra.debruno@uniroma5.it
First published on 13th October 2023
Abstract
Modern consumers are increasingly interested in eating healthy food and paying attention to the reduction of synthetic preservatives and the increased use of natural preservatives. For this reason, the aim of this work was to assess the effect of the addition of natural antioxidants extracted from Bergamot Pomace on the fortification of vegetable fat to improve its functional and qualitative characteristics. Furthermore, vegetable fat of neo formulation was used as an ingredient for the formulation of baked products (biscuits). The main physicochemical, microbiological, sensorial and antioxidant properties were evaluated for the different samples (fats and biscuits) to define the possible application of antioxidant compounds recycled from citrus waste. The effect of the fortification was demonstrated by the good results obtained regarding the enhancement of the oxidative stability; indeed, the addition of antioxidants to the biscuits protected them against the oxidation that could happen during the baking process. The antioxidant and preservative effects of the flavonoids resulted in an increase of the oxidative stability of the biscuits with a potential extension of shelf life.
Sustainability spotlightThe agro-food industry produces a large amount of waste, which could represent a raw material due to its high content of bioactive and beneficial compounds. In this work, bergamot pomace was valorised through bioactive compound (polyphenol) recovery. The extract obtained represents a functional and sustainable ingredient for use in the food industry. Specifically, it was used for the fortification of a vegetable fat that can replace and promote the use of new fats to replace common animal fats and all the problems associated with them (emissions, land and water consumption, etc.). Through this approach it is possible to reduce waste with positive impacts on human health and the environment by promoting sustainable food production. |
1. Introduction
In the last few decades, plant-based milk products have been constantly growing in response to the increase in diseases related to animal milk consumption. Several studies have reported allergies,1,2 lactose intolerance,3,4 and hypercholesterolemia due to the high amount of fats5 and antibiotic residues6 associated with milk consumption. Furthermore, animal production has a negative impact on the environment concerning all factors linked to livestock, e.g., through the utilization of resources such as land and water, eutrophication, and gas emissions resulting in pollution and climate change. For these reasons, the demand for milk replacements has increased and consumers of a vegan diet have influenced the market trend in support of such sustainable food productions.7 In fact, this social situation has encouraged researchers to come up with novel solutions in milk and milk dairy products, and to study differences among alternatives like coconut milk, which has a good taste and low calories, almond milk, soymilk which is rich in protein, or rice milk. The preferred alternative with the best nutritional intake in the human diet is soymilk.8 Considering these positive contributions and in light of increasing consumer demand, there has been a surge in research and development of technology to increase and improve the range of plant-based foods. For example, plant-based milks are widely used to produce analogue milk products such as yogurt, ice-cream or cheese.Nevertheless, these products that are rich in polyunsaturated fats and micronutrients, possess high oxidation9 and microbial growth, and for this reason, food industries need to employ strategies such as adding antioxidants, preservatives and stabilizers to improve the final products for consumers.
Modern consumers are increasingly interested in the consumption of healthy foods and are paying more and more attention to the reduction of synthetic preservatives and the increased use of natural preservatives/antioxidants.10 In addition, it is important that these natural antioxidants be obtained with green, environmentally friendly and natural strategies. This is done for health reasons, and because it is ethical, sustainable and provides different functions: antibacterial,11,12 insecticidal and anti-fungal activities,13 antioxidants14,15 and preservatives.16,17
A great source of natural antioxidants is represented by food processing wastes and by-products. Food wastes are an extraordinary source of antioxidant compounds, that if appropriately recovered can be recycled back into the production process. By means of the recovery of these fractions, the sustainability of the entire process can be increased by improving wastewater management. In fact, bioactive compounds that are present in these extracted fractions could reduce the biodegradability of waste. This is an environmental, economic and social challenge when trying to find a solution through responsible practices to reduce the environmental impact. In this way, the recovery of agri-food waste avoids pollution, and the use of natural resources such as water, energy, land and much more that could be needed to produce nutrients supporting a circular economy in favor of waste instead of raw materials. In order to follow a model of a circular and environmentally friendly economy, it is essential to apply green extraction techniques. Advances in green solutions have been pursued in recent years considering ionic liquids, and supercritical and subcritical fluids,18 but also water and ethanol as good substitutes of organic solvents used in the past.19,20
For example, great interest has been attracted by Bergamot Pomace (BP), the waste resulting from the processing of bergamot. Bergamot (Citrus bergamia, Risso) is a citrus belonging to the Rutaceae family, cultivated mainly in the province of Reggio Calabria,21,22 and used mostly for the production of essential oils (claimed DPI since 1999 from the European Union), and juice. BP consists of skins, pulp and seeds, and is concretely an important source of bioactive molecules that are of considerable interest at the scientific level due to their beneficial effects on health.23 These recovered bioactive compounds that show antioxidant properties as demonstrated by De Bruno et al.,24 can be applied for the formulation/fortification of other food products or food ingredients. For example, they could be used to improve the qualitative aspects of plant-based foods. For this reason, the aim of this paper was to investigate the possible application of natural antioxidants recovered from bergamot processing waste for the fortification of vegetable fat for application as an ingredient for the formulation of baked products (biscuits). The fortification had a dual purpose: improving the sustainability of the entire citrus processing and creating new foods for consumer needs.
2. Materials and methods
2.1. Materials and chemicals
Bergamot Pomace (BP) was collected by a company located in Reggio Calabria (Italy). Sunflower oil, soy milk, wheat flour, sugar, salt, and chemical baking powder (disodium diphosphate, sodium hydrogen carbonate and cornstarch) were purchased from a local supermarket.2.2. Extraction of antioxidant compounds from bergamot pomace
The antioxidant extract (AE) was obtained following the method reported by Gattuso et al.24 Briefly, 200 g of powdered BP (powder was obtained by grinding of BP with a laboratory blender, 12% of moisture content) was mixed with 800 mL of an hydro-alcoholic mixture (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water; 1
water; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and kept under a heater and continuous stirring for 30 minutes at 70 °C.
1, v/v) and kept under a heater and continuous stirring for 30 minutes at 70 °C.
Afterward, the mixture was centrifuged (9000 rpm, 10 min, 4 °C) in a refrigerated centrifuge (NF 1200R, Nüve, Ankara, Turkey) and the liquid extract was concentrated with a Rotavapor to remove the ethanol (97 mbar of vacuum and at 25 °C).
2.3. Physicochemical and antioxidant evaluation of the AE
The AE was characterized for physicochemical and antioxidant characteristics; in particular, the analyses that have been carried out were as follows.pH was measured using a Crison pH-meter, basic model 20, according to the AOAC International Method (14.022).25
Color was measured using a Minolta CM-700d Spectrophotometer, with reference to the CIE L* a* b* coordinates (where L* represent the brightness; for a* positive values indicate redness, and negative values indicate greenness; and for b* positive values indicate yellow and negative values indicate blue) using a D65 illuminant.
Total Polyphenol Content (TPC) was determined by following the method reported by González-Molina et al.26 with appropriate modification. For the assay, 0.2 mL of diluted (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) AE were place in a volumetric flask (25 mL) with 5 mL of distilled water and 1 mL of Folin–Ciocalteu reagent. After 8 min, Na2CO3 (20%) was added and brought to volume with distilled water. The mixtures were incubated for two hours at room temperature in the dark. Gallic acid solution was used as a reference standard and absorbance was measured at 765 nm in a double-beam ultraviolet-visible spectrophotometer (PerkinElmer UV-Vis 2, Waltham, MA, USA).
20) AE were place in a volumetric flask (25 mL) with 5 mL of distilled water and 1 mL of Folin–Ciocalteu reagent. After 8 min, Na2CO3 (20%) was added and brought to volume with distilled water. The mixtures were incubated for two hours at room temperature in the dark. Gallic acid solution was used as a reference standard and absorbance was measured at 765 nm in a double-beam ultraviolet-visible spectrophotometer (PerkinElmer UV-Vis 2, Waltham, MA, USA).
The results are expressed as mg of gallic acid equivalents on 100 mL−1 of antioxidant extract (mg GAE 100 mL−1 AE).
Total Flavonoid Content (TFC) was determined following the method reported by Cerdá-Bernad et al.27 with slight modifications. Briefly, 0.300 mL of sample was mixed with 1 mL of water and 150 μL of sodium nitrite (5%, w/v) solution. After 6 min, 150 μL of aluminium trichloride (10%) solution was added, and 6 min after that, 2 mL of sodium hydroxide (1 M) solution was added. The volume was adjusted to 5 mL with water. A solution without sample, was used as a blank and the absorbance was measured at 510 nm. The results were expressed as mg of catechin equivalents 100 mL−1 of antioxidant extract (mg CE 100 mL−1 AE).
where c is the concentration of catechin obtained from the calibration curve, V is the volume of extract in mL, and m is the mass of extract in grams.
Total antioxidant activity (TAA) was analyzed in vitro through two different assays, DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)) following the methods reported by Mafrica et al.28 with appropriate modifications. For DPPH, the reaction was prepared by mixing in a cuvette 20 μL of diluted (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) AE with 2980 μL of 6 × 10−5 mmol L−1 of DPPH solution and left in the dark under continuous stirring for 30 min. The decrease in absorbance was measured at 515 nm. For the ABTS assay, 20 μL of diluted AE was added to the ABTS solution to achieve a final volume of 3 mL and left in the dark for 6 min in a cuvette. The absorbance was measured at 734 nm. The results of both assays were expressed as mmol Trolox per L of antioxidant extract.
20) AE with 2980 μL of 6 × 10−5 mmol L−1 of DPPH solution and left in the dark under continuous stirring for 30 min. The decrease in absorbance was measured at 515 nm. For the ABTS assay, 20 μL of diluted AE was added to the ABTS solution to achieve a final volume of 3 mL and left in the dark for 6 min in a cuvette. The absorbance was measured at 734 nm. The results of both assays were expressed as mmol Trolox per L of antioxidant extract.
where C is the concentration of the Trolox solution obtained from inhibition percentage, Vc is the total volume in the reaction cuvette (3 mL) and v is the volume of the sample.
Polyphenol profile and quantification with ultra-high performance liquid chromatography (UHPLC) was conducted applying the same conditions as described by Gattuso et al.23 using a UHPLC PLATINblue (Knauer, Berlin, Germany), equipped with a binary pump system using a Knauer blue orchid column C18 (1.8 μm, 100 × 2 mm) coupled with a PDA-1 (Photo Diode Array Detector) PLATINblue (Knauer, Germany). First, 5 μL of filtered sample was injected and the eluents were water (A) acidified with formic acid (pH 3.1) and acetonitrile (B). The gradient elution program consisted of 0–3 min, 95% A and 5% B; 3–15 min, 95–60% A and 5–40% B; 15–15.5 min, 60–0% A and 40–100% B; and finally returning to the initial conditions (column temperature: 30 °C). External standards (concentration between 1 and 100 mg L−1) were analyzed for quantification and Clarity 6.2 software was used. The results were expressed as mg mL−1 of AE.
2.4. Preparation of vegetable fats (VFs) and biscuits (Bs)
In the experimental procedure, VFs were used after their characterization, as ingredients in biscuit (B) formulations. For each VF sample, a biscuit sample was made. Biscuits were prepared in the laboratory of Food Technology of the Mediterranean University of Reggio Calabria (Italy). The ingredients of the biscuit's and their denominations are reported in Table 2 and the only variable is the VF used in their production. The dough was prepared using a mixer (Bimby TM31, Vorwerk, Wuppertal, Germany), mixing the ingredients (at about 3200 rpm) until a homogeneous dough is obtained. Then, the dough was rolled out with a rolling pin calibrated to obtain a homogeneous thickness of 3 mm. The baking process was carried out in an electric oven (Angelo Po Combistar FX, Carpi, Modena, Italy) at a temperature of 180 °C for 10 minutes (Fig. 1). Baked samples were subject to characterization.
2.5. Characterization of the physicochemical properties of the VFs
| Chroma (C): (a2 + b2)1/2 |
| Hue angle (h°): arctan(b*/a*) |
Total acidity was calculated according to Official and standards (AOCS) methods (Ca 5a 40; Cd 8–53; Ch 5–91) as % of oleic acid.29–31 It was calculated as follows:
Peroxide value (PV) was also determined to evaluate the antioxidant effect of AE addition. The analysis was performed according to the standard method of AOCS (965.33.12).32 PV was expressed as meq. O2 per kg and calculated using the following equation:
Oxidative stability was determined using an Accelerated Storage Test (OXITEST). VFs were submitted to high oxidative conditions in an OXITEST reactor. This test detects the time necessary to reach an end point of oxidation that corresponds to a detectable rancidity or a rapid change in the oxidation rate and allows the sample Induction Period (IP) to be obtained within a short time. The method recommended by the AOCS International Standard Procedure (Cd 12c–16) for the determination of oxidation stability of food, fats, and oils (AOAC)33 was followed. In order to determine the oxidative stability, samples were treated under conditions of accelerated oxidation, monitoring the oxygen uptake of the reactive constituents of the food samples in an oxidation test reactor (VELP Scientifica, Usmate Velate, MB, Italy). Briefly, 10 g of sample were distributed homogenously in a hermetically sealed titanium chamber; oxygen was purged into the chamber up to a pressure of 6 bar. The reactor temperature was set at 90 °C. These reaction working conditions allow the sample Induction Period (IP) to be obtained within a short time. The OXITEST allows the modification of absolute pressure inside the two chambers to be measured and, through the OXISoftTM Software (Version 10002948 Usmate Velate, MB, Italy), the IP is automatically generated, expressed as hours, by the graphical method.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (70
water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, v/v) and 5 mL of hexane for 10 min. Then, the hydro-alcoholic phase was separated from the lipid phase in a refrigerated centrifuge apparatus at 6000 rpm, 4 °C for 10 min. Hydro-alcoholic extracts (VFEs) were recovered, filtered through a 0.45 μm nylon filter of diameter 15 mm (Thermo Fischer Scientific, Waltham, MA, USA) and stored until evaluation of the phenolic compounds and antioxidant activity.
30, v/v) and 5 mL of hexane for 10 min. Then, the hydro-alcoholic phase was separated from the lipid phase in a refrigerated centrifuge apparatus at 6000 rpm, 4 °C for 10 min. Hydro-alcoholic extracts (VFEs) were recovered, filtered through a 0.45 μm nylon filter of diameter 15 mm (Thermo Fischer Scientific, Waltham, MA, USA) and stored until evaluation of the phenolic compounds and antioxidant activity.
In order to determine the total phenolic content (TPC), 0.300 mL of extract was mixed with 0.300 mL of Folin reagent, 0.250 mL of distilled water and, after 4 min, 2.4 mL of an aqueous solution of Na2CO3 (5%). The mixture was maintained in a 40 °C water bath for 20 min and TPC was determined at 750 nm. The results were expressed as mg of gallic acid equivalent on 100 g−1 of VF.
The total flavonoid content (TFC) was evaluated following the method described in Section 2.3. The results are expressed as mg of catechin equivalents 100 g−1 of vegetable fat (mg CE 100 g−1 VF).
Radical scavenging activity was tested with two different assays: DPPH and ABTS. In vitro assays were performed following the methods reported in Section 2.3. The results are expressed as mmol Trolox per kg of VF.
Identification and quantification of phenolic compounds in VFs was carried out as described in Section 2.3, and the results are expressed as mg 100 g−1 of sample.
2.6. Characterization of physicochemical and antioxidant properties of biscuits (Bs)
where
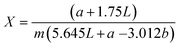 .
.
The doughs were subjected to stickiness tests. These tests were performed on 20 g of sample employing a Chen and Hoseney probe (A/DSC) (Stable Micro Systems Ltd., Godalming, UK). To evaluate the forces of insertion and withdrawal from the dough the following parameters were used: pre-test speed, 0.50 mm s−1; test speed, 0.50 mm s−1; post-test speed, 10.00 mm s−1; distance, 5.0 mm; trigger force, 5.0 g; data acquisition rate, 500 pps.
The entire biscuit was used to perform a TPA test using a 100 mm compression plate (P/100) probe (Stable Micro Systems Ltd., Godalming, UK) with the following parameters: pre-test speed, 1.00 mm s−1; test speed, 5.00 mm s−1; post-test speed, 5.00 mm s−1; distance: 20.0 mm, trigger force, 5.0 g; data acquisition rate, 400 pps. The test results expressed different textural characteristics, such as cohesiveness, gumminess, chewiness, springiness, and resilience.
A three-point bending test (TPB) was carried out using a three-point bend ring probe (HDP/3PB). The sample was placed on the two adjustable supports and the cutting probe was lowered until it touched the sample, imparting a force that was increased until the biscuit breaks. The maximum peak force was used to calculate the hardness value. For this test, the operative conditions were: pre-test speed, 1 mm s−1; test speed, 3 mm s−1; post-test speed, 10.00 mm s−1; distance, 7.5.0 mm; trigger force, 5.0 g; data acquisition rate, 400 pps.
2.7. Statistical elaboration
All the analysis were performed in triplicate (n = 3), and the experimental results were expressed as mean value ± standard deviation. The significant differences (p < 0.05) among mean values were determined by one-way analysis (ANOVA, Analysis of Variance), applying SPSS Software (Version 15.0, SPSS Inc., Chicago, IL, USA). A series of multiple comparisons, with Tukey's post hoc test, was performed to determine individual significant differences (p < 0.05).3. Results and discussion
3.1. Antioxidant characteristics of bergamot pomace extract (AE)
Citrus by-products and in particular bergamot pomace were characterized by a high antioxidant content, and for this reason can be reused for different aims, such as natural preservatives for food production. Indeed, in this study, the BP was used for extracting the antioxidant compounds to apply for the formulation of vegetable fat.For the extraction process, a procedure was followed that was described in our previous work23 using a hydroalcoholic mixture to obtain a liquid extract rich in phenolic compounds. The green solvents used were ethanol and water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v). Ethanol is environmentally friendly and allows a good extraction of polyphenols to be obtained.38 This extraction allows citrus waste to be converted into a source of value-added products to use in functional food as widely demonstrated in the literature.39 The liquid extract showed a low pH of 3.31. In general, many foods are chemically acidified to hinder the growth of microorganisms, which cause contamination and loss of foodstuffs. Because of the low pH value of the extract, it could be a good basic matrix that when added to food also has acidifying power.
50, v/v). Ethanol is environmentally friendly and allows a good extraction of polyphenols to be obtained.38 This extraction allows citrus waste to be converted into a source of value-added products to use in functional food as widely demonstrated in the literature.39 The liquid extract showed a low pH of 3.31. In general, many foods are chemically acidified to hinder the growth of microorganisms, which cause contamination and loss of foodstuffs. Because of the low pH value of the extract, it could be a good basic matrix that when added to food also has acidifying power.
With the aim to determine the aliquots of extract to be added to the vegetable fat, the extract was characterized spectrophotometrically for total polyphenol content (TPC) and total flavonoid content (TFC). Its radical scavenging activity was also evaluated. As reported in Table 3, the AE exhibited a high value of TPC of 776.24 mg GAE 100 mL−1 and TFC of 267.42 mg CE 100 mL−1. The total antioxidant activity level of the AE was investigated with ABTS and DPPH assays, which showed values of 795.06 and 992.2 mmol Trolox per L, respectively.
| Physicochemical | pH | 3.31 ± 0.05 |
| L* | 49.35 ± 0.14 | |
| a* | 0.57 ± 0.07 | |
| b* | 2.85 ± 0.14 | |
| Total antioxidant assays | TPC (mg GAE 100 mL−1) | 776.24 ± 16.05 |
| TFC (mg CE 100 mL−1) | 267.42 ± 7.04 | |
| ABTS (mmol Trolox per L) | 795.06 ± 115.98 | |
| DPPH (mmol Trolox per L) | 992.2 ± 124.08 | |
| Phenolic acids (mg mL−1) | p-Coumaric acid | 0.1 ± 0.02 |
| Ferulic acid | 0.05 ± 0.01 | |
| Flavanones (mg mL−1) | Eriocitrin | 0.06 ± 0.01 |
| Neoeriocitrin | 3.64 ± 0.29 | |
| Narirutin | 0.04 ± 0 | |
| Naringin | 3.21 ± 0.17 | |
| Neohesperidin | 1.83 ± 0.19 | |
| C-Glycosyl flavones (mg mL−1) | Melitidin | 0.73 ± 0.05 |
| Brutieridin | 1.48 ± 0.14 |
The main individual phenolic compounds evaluated through the chromatographic analysis (UHPLC-DAD) were reported in order of retention time (Table 3). They were (from the one with the highest concentration to the lowest) neoeriocitrin (3.64 ± 0.29 mg mL−1), naringin (3.21 ± 0.17 mg mL−1), neohesperidin (1.83 ± 0.19 mg mL−1), brutieridin (1.48 ± 0.14 mg mL−1), melitidin (0.73 ± 0.05 mg mL−1), p-coumaric acid (0.1 ± 0.02 mg mL−1), eriocitrin (0.06 ± 0.01 mg mL−1), ferulic acid (0.05 ± 0.01 mg mL−1), and narirutin (0.04 ± 0 mg mL−1). The major flavonoid compounds detected were in accordance with Nogata et al.40 and Bartella et al.41
3.2. Physicochemical and antioxidant characteristics of enriched vegetable fats (VFs)
The physicochemical characteristics of the different vegetable fat samples (enriched samples compared to control) were reported in Table 4. Water activity (aw), moisture content (MC%), L* and a* color parameters did not show significant differences (p > 0.05) among the different vegetable fats (VFs). In contrast, high significant differences were shown for b*; in fact, there was a positive increase of this character, which highlights the yellow color in the VF, probably due to the presence of carotenoids and other pigments abundantly present in citrus peel extract.42 It increased from 6.69 ± 0.41 in VFC to 7.23 ± 0.03 in VF4%. This was also found by Kneifel et al.,43 who observed changes in b* values ascribed to the carotene content in butter samples. The chroma (C*) value or saturation describes the vividness or colourfulness44 and for this character, a statistical difference was noted, with the highest values recorded in VF2% and VF4%, 7.17 ± 0.11 and 7.24 ± 0.03, respectively. The hue angle (h°) is specified as 0°/360° for red/magenta, 90° for yellow, 180° for green, and 270° for blue or purple, as well as intermediate hues between adjacent pairs of these fundamental colors. It specifies the relative proportions of redness and yellowness.45 The results of h° of the VF samples did not reveal any statistical differences, and the values are similar to those reported by Chudy et al.,46 who investigated color in various butter samples and obtained comparable h° results, showing a similarity between these and those studied in this work.| VFC | VF2% | VF4% | Sign. | |
|---|---|---|---|---|
| a Small letters show a significant difference by Tukey's post hoc test. ** Significance at p < 0.01; n.s. – not significant. | ||||
| a w | 0.981 ± 0.001 | 0.984 ± 0.002 | 0.985 ± 0.003 | n.s. |
| MC% | 21.13 ± 2.09 | 22.01 ± 3.75 | 23.22 ± 0.83 | n.s. |
| L* | 88.56 ± 3.25 | 88.78 ± 0.78 | 87.68 ± 1.09 | n.s. |
| a* | −0.15 ± 0.19 | −0.26 ± 0.03 | −0.23 ± 0.02 | n.s. |
| b* | 6.69 ± 0.41b | 7.16 ± 0.11a | 7.23 ± 0.03a | ** |
| C* | 6.69 ± 0.41b | 7.17 ± 0.11a | 7.24 ± 0.03a | ** |
| h° | 91.24 ± 1.84 | 92.13 ± 0.23 | 91.84 ± 0.17 | n.s. |
| TA (% oleic acid) | 6.87 ± 0.55 | 5.76 ± 0.52 | 7.44 ± 0.23 | n.s. |
| PV (meq. O2 per kg) | 4.09 ± 0.95 | 2.38 ± 0.04 | 3.25 ± 0.43 | n.s. |
| IP (hh:mm) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 ± 0.12c 37 ± 0.12c |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 ± 0.15b 36 ± 0.15b |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 08 ± 0.08a 08 ± 0.08a |
** |
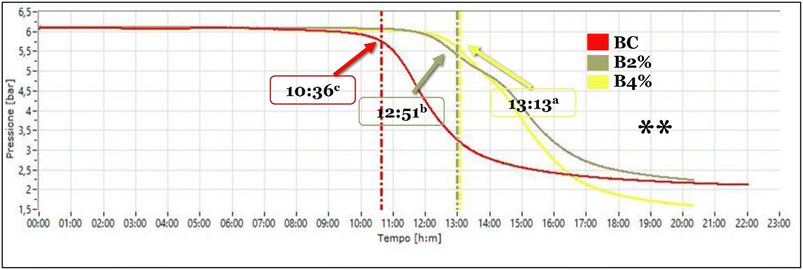
In Table 4, the results of total acidity (TA), peroxide value (PV), and induction period (IP) are also reported. The statistical analysis carried out on the samples showed that TA and PV did not show significant differences among the VFC, VF2%, and VF4% samples.
Regarding the evaluation of oxidation stability determined with the Oxitest system, the results were expressed as hours of induction period (IP), and the mean of the values is 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 for VFC, 12
37 for VFC, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 for VF2%, and 13
36 for VF2%, and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 08 for VF4% with statistical differences among the samples (p < 0.01). The results showed that the VFC had the shortest IP, followed by VF2%, and VF4% had the longest IP. This means that VF4% is more stable and could have a longer shelf life compared to the other two vegetable fats. In addition, the IP also increased in VF2% compared to the control, which confirmed the antioxidant effect of the added extract, in accordance with El-aal and Halaweish47 who found that orange peel extract improved the oxidative stability of soybean oil. Moreover, the impact of distinct plant extracts on the induction period (IP) was examined in fat samples and validated the aforementioned effect.48
08 for VF4% with statistical differences among the samples (p < 0.01). The results showed that the VFC had the shortest IP, followed by VF2%, and VF4% had the longest IP. This means that VF4% is more stable and could have a longer shelf life compared to the other two vegetable fats. In addition, the IP also increased in VF2% compared to the control, which confirmed the antioxidant effect of the added extract, in accordance with El-aal and Halaweish47 who found that orange peel extract improved the oxidative stability of soybean oil. Moreover, the impact of distinct plant extracts on the induction period (IP) was examined in fat samples and validated the aforementioned effect.48
For the purpose of verifying the antioxidant activity in VF samples, different total antioxidant assays were performed and the results are reported in Table 5. Specifically, VF2% and VF4% have significantly higher levels of TPC, ABTS, and DPPH antioxidant activity than VFC, indicating that the antioxidant capacity of the vegetable fats increased with the addition of the antioxidant extract. The increase in antioxidant capacity was more evident in the VF4% sample, which showed the highest values of TPC, ABTS and DPPH. Overall, these data suggested that the addition of the antioxidant extract to vegetable fat can significantly increase its antioxidant capacity, which may have important implications for food and nutrition industries seeking to improve the antioxidant content of their products. However, it is important to note that the specific antioxidant extract used can impact the results and should be carefully considered when interpreting the findings.
| VFC | VF2% | VF4% | Sign. | |
|---|---|---|---|---|
| a Small letters show a significant difference by Tukey's post hoc test. ** Significance at p < 0.01. | ||||
| TPC (mg GAE 100 g−1) | 75.82 ± 3.19c | 210.61 ± 8.89b | 286.31 ± 2.74a | ** |
| TFC (mg CE 100 g−1) | 19.88 ± 2.98c | 38.48 ± 0.7b | 55.75 ± 0.64a | ** |
| ABTS (mmol TE per kg) | 50.69 ± 2.11c | 115.45 ± 19.16b | 147.25 ± 6.19a | ** |
| DPPH (mmol TE per kg) | 2.69 ± 0.29c | 9.71 ± 0.38b | 14.65 ± 1.65a | ** |
In addition to the determination of total antioxidants, a more detailed analysis for the identification of the individual phenolic compounds has also been carried out. The phenolic compound concentrations detected showed a trend of increasing in relation to the quantities of added extract. In Table 6, the results of a comparison between VF2% and VF4% obtained by liquid chromatographic analysis performed with the UHPLC-DAD system were reported. The results exhibited that VF4% contained significantly higher levels of p-coumaric acid, neoeriocitrin, naringin and neohesperidin compared to VF2%. The differences in the levels of these compounds between VF2% and VF4% are statistically significant (p < 0.01) except for narirutin, melitidin and brutieridin (p < 0.05). Furthermore, the total phenolic content revealed by UHPLC was significantly higher in VF4% than in VF2%, with a respective content of 35.63 ± 3.1 and 19.67 ± 1.15 mg 100 g−1 of VF.
| mg 100 g−1 of VF | VF2%* | VF4%* | Sign. |
|---|---|---|---|
| a ** Significance at p < 0.01; * significance at p < 0.05. | |||
| p-Coumaric acid | 0.06 ± 0.02 | 0.18 ± 0.04 | ** |
| Neoeriocitrin | 6.15 ± 0.64 | 11.47 ± 1.22 | ** |
| Narirutin | 0.06 ± 0.01 | 0.1 ± 0.02 | * |
| Naringin | 6.08 ± 0.2 | 11.14 ± 1.33 | ** |
| Neohesperidin | 3.86 ± 0.69 | 6.33 ± 0.44 | ** |
| Melitidin | 1.05 ± 0.09 | 2.04 ± 0.51 | * |
| Brutieridin | 2.41 ± 0.28 | 4.36 ± 0.86 | * |
| Total | 19.67 ± 1.15 | 35.63 ± 3.1 | ** |
3.3. Physicochemical and antioxidant characteristics of biscuits
After the enriched vegetable fat preparation and characterization, these ingredients were applied for the formulation of a bakery product, specifically “biscuits”. The physicochemical characteristics of the biscuit samples (Table 7), such as moisture content, water activity and color of the samples, were studied. MC and aw are important factors affecting the quality and stability of food products, as they can affect their color, texture, and microbial growth. Regarding MC, the results suggested that there was no statistical difference for the three samples compared. Concerning the aw, its measurement is crucial in the bakery sector because it is associated with the stability and safety of food products over their storage duration.49 The results showed significant differences between the aw values, with B4% having the lowest mean value (0.333 ± 0.007) and BC having the highest value (0.488 ± 0.001). This effect could be due to the presence of phenolic compounds, which lowered the water activity of the bakery product by binding to water molecules and diminishing the free water levels.| BC | B2% | B4% | Sign. | |
|---|---|---|---|---|
| a Small letters show a significant difference by Tukey's post hoc test. ** Significance at p < 0.01; n.s. – not significant. | ||||
| MC% | 4.94 ± 0.21 | 5.53 ± 0.17 | 5.36 ± 0.11 | n.s. |
| a w | 0.488 ± 0.001a | 0.397 ± 0.011b | 0.333 ± 0.007c | ** |
| L* | 73.9 ± 2.85 | 72.09 ± 6.22 | 72.53 ± 5.91 | n.s. |
| a* | 4.87 ± 3.05 | 6.71 ± 4.05 | 6.26 ± 4.39 | n.s. |
| b* | 22.41 ± 2.72 | 23.19 ± 2.25 | 22.86 ± 2.81 | n.s. |
| C* | 23.05 ± 3.29 | 24.37 ± 3.07 | 23.97 ± 3.75 | n.s. |
| h° | 78.56 ± 6.07 | 74.81 ± 8.39 | 75.99 ± 8.84 | n.s. |
| BI | 40.84 ± 10.14 | 46.21 ± 12.56 | 44.92 ± 13.99 | n.s. |
As regards the rheological properties of biscuit dough, the results shown in Table 8 reported the changes due to the addition of enriched VFs. Statistical analysis showed differences among the samples with similar results for the Bs formulated with enriched VFs (B2% and B4%). The lower values of stickiness, work of adhesion and ratio of dough strength/cohesiveness for samples B2% and B4% were probably due to the presence of antioxidant compounds which could modify the surface by decreasing the hydrophilicity of the dough.
| Stickiness (g) | Work of adhesion (g s) | Dough strength/cohesiveness (mm) | |
|---|---|---|---|
| a Small letters show a significant difference by Tukey's post hoc test. ** Significance at p < 0.01; * significance at p < 0.05. | |||
| BC | 24.97 ± 3.52a | 0.78 ± 0.13a | 0.45 ± 0.07a |
| B2% | 18.26 ± 3.08b | 0.54 ± 0.08b | 0.4 ± 0.02b |
| B4% | 19.31 ± 4.12b | 0.59 ± 0.11b | 0.4 ± 0.03b |
| Sign. | ** | ** | * |
The results showed high statistical differences in springiness, cohesiveness, and resilience (p < 0.01), and statistical differences (p < 0.05) in gumminess (Table 9). Chewiness was not affected by the different formulations. These values were lower in B2% and B4% compared to BC, for retarding starch retrogradation due to the addition of polyphenols, in accordance with those reported by Li et al.50 This finding showed that there is a good potential of enriched VFs used as enhanced bakery ingredients in improving texture quality and the durability of the products.
| Springinessb | Cohesivenessb | Gumminessc | Chewinessc | Resilienceb | |
|---|---|---|---|---|---|
| a Small letters show a significant difference by Tukey's post hoc test. ** Significance at p < 0.01; * significance at p < 0.05; n.s. – not significant. b Dimensionless characteristic. c N. | |||||
| BC | 0.81 ± 0.06a | 0.82 ± 0.04a | 375.51 ± 54.24a | 305.09 ± 64.93 | 0.72 ± 0.05a |
| B2% | 0.71 ± 0.06a | 0.74 ± 0.05b | 317.71 ± 47.38b | 249.78 ± 39.84 | 0.62 ± 0.07b |
| B4% | 0.76 ± 0.04ab | 0.77 ± 0.03b | 341.8 ± 38.19ab | 260.8 ± 42.25 | 0.65 ± 0.04b |
| Sign. | ** | ** | * | n.s. | ** |
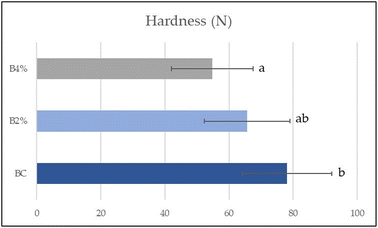
Three-point bending is a textural test commonly used for bakery goods such as biscuits, bread, crackers or other food products to evaluate mechanical properties. In this study, the hardness of the biscuits was evaluated (Fig. 2). This value decreased with the addition of phenolic compounds, with the lowest value for B4% (54.79 ± 12.74 N), the highest for BC (78.04 ± 13.97 N) and an intermediate value for B2%. The results can be explained by the added phytochemical extracts affecting the properties of wheat starch as reported by Zhu et al.51
The oxidation stability test showed different induction periods among B2%, B4% and BC (Fig. 3). Considering these differences, it is possible to hypothesize that these variations are probably due to the presence of antioxidants that carry out their function. In fact, it is well-known that antioxidants work by inhibiting or delaying the oxidation process, which can lead to rancidity and other negative changes in the quality of the products. There is also a correlation to the aw of biscuit samples. As reported by Conte et al.,52 water can act as an antioxidant at low levels of aw forming a barrier that protects sensitive sites from reacting with oxygen and also decreases the rate of free radical formation by increasing hydration of hydroperoxides and promoting recombination of free radicals. Additionally, it lowers metal catalytic activity. However, at higher aw values, water can have a pro-oxidant role acting as a plasticizing agent, promoting mobility and solubilization of the catalysts, and inducing matrix swelling, which exposes new reactive sites. This can lead to increased oxidation in the product.
Another important aspect evaluated for biscuits is the Maillard reaction products. These products were measured considering low molecular weight compounds without color that are formed early on (280 nm), intermediate molecular weight compounds with more advanced products (360 nm) and high molecular weight, colored compounds that are known as melanoidins (420 nm).53 The results reported in Table 10 show that there were no significant differences in MRP content among the samples, as the values for each wavelength were similar across all three samples. The MRP content in each sample ranged from 0.62 to 0.7 AU per g dw, suggesting that the addition of vegetable fats did not impact the formation of MRP in the biscuits.
| λ | BC | B2% | B4% | Sign. | |
|---|---|---|---|---|---|
| a n.s. – not significant. | |||||
| MRP | 280 nm | 0.69 ± 0.03 | 0.7 ± 0.02 | 0.62 ± 0.06 | n.s. |
| 360 nm | 0.15 ± 0.01 | 0.13 ± 0.01 | 0.12 ± 0.02 | n.s. | |
| 420 nm | 0.11 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.02 | n.s. | |
The Maillard reaction is a non-enzymatic browning reaction that occurs between amino acids and reducing sugars under heat treatment. This reaction is responsible for the formation of flavor and aroma compounds, as well as the characteristic brown color of baked goods, including biscuits. In moderate amounts, Maillard Reaction Products (MRPs) can contribute positively to the flavor and color of biscuits, enhancing their sensory properties. However, excessive formation of MRPs can lead to negative health effects, as some MRPs have been associated with the development of chronic diseases;54 they also result in a reduction of the nutritional value due to the loss of lysine and other amino acids through thermal degradation, as well as decreased protein bioavailability. For this reason, MRP quantity needs to be maintained equal to standard products, and it is important to limit excessive formation in order to reduce potential negative health effects.
Like for the vegetable fats, also for the biscuits the determination of the total antioxidant activity and the individual phenolic compounds was performed, and the results are shown in Table 11 and Fig. 4. The results indicated that samples B2% and B4% had higher TPC and antioxidant capacity values compared to the control one (BC). The differences between the samples were statistically significant, with B2% and B4% showing significantly higher values for all three characteristics. These findings demonstrated that the total phenolic content determined by the Folin–Ciocalteu method, and the antioxidant capacity in vitro analyzed with the ABTS and DPPH free radical scavenging methods, increased when the biscuits were formulated with vegetable fat containing phenolic compounds.
| BC | B2% | B4% | Sign. | |
|---|---|---|---|---|
| a Small letters show a significant difference by Tukey's post hoc test. ** Significance at p < 0.01; * significance at p < 0.05. | ||||
| TPC (mg GAE 100 g−1) | 14.25 ± 0.16c | 29.63 ± 1b | 32.96 ± 6.82a | ** |
| ABTS (mmol TE per kg) | 17.73 ± 2.09c | 35.24 ± 4.42b | 51.03 ± 11.32a | ** |
| DPPH (mmol TE per kg) | 156.58 ± 2.44b | 185.24 ± 3.77ab | 200.71 ± 27.17a | * |
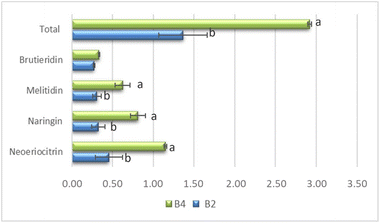
The persistence of phenolic compounds in the biscuits after baking is confirmed by the phenolic compounds identified with UHPLC. Not all the phenolic compounds present in the VFs were identified in the biscuits, probably due to the low quantity or even because in part they were damaged by the baking heat. The results reported in Fig. 4 show that there were statistical differences between the samples for the total content of flavonoids, melitidin, naringin and neoeriocitrin, but not for brutieridin that was found in a similar concentration in both biscuit samples.
4. Conclusions
This study provides information regarding the possible reuse of bergamot pomace, a by-product rich in antioxidant compounds, which are known for their beneficial effects on human health. These compounds have many useful properties in the food sector, for example as antioxidants, nutraceuticals and antimicrobials. The antioxidant extract obtained from bergamot pomace was used to formulate enriched vegetable fat. The enriched fat samples exhibited an increase of the antioxidant properties (TPC, TFC, ABTS and DPPH assays) showing statistical differences compared with control samples. The effect of the enrichment was confirmed by the antioxidant compounds identified with UHPLC and by a better oxidative stability compared to the control sample. Regarding the application of enriched vegetable fats as an ingredient for the formulation of biscuits, the results have highlighted that the physical characteristics of biscuits such as moisture, color and MRP have not undergone great variations compared with the control. One difference with a positive effect was revealed in aw, which decreased with the increase of extract in VF with well-known advantageous effects on the final products. The presence of antioxidant compounds in the enriched samples provided protection against heat treatment during the baking process and an increment of the oxidative stability of the biscuits, with a potential extension of the shelf life.Author contributions
Antonio Gattuso (A. G.) and Alessandra De Bruno (A. D. B.) and Marco Poiana (M. P.) conceived and designed the experiments. A. G., Elisa Imeneo (E. I.) and Simone Santacaterina (S. S.) performed the experiments; A. G. and A. D. B. wrote the original manuscript; M. P. and Amalia Piscopo (A. P.) validated and reviewed the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by PRIN 2017-GOOD-BY-WASTE. Obtain GOOD products exploit BY products reduce WASTE.References
- E. I. El-Agamy, Small Rumin. Res., 2007, 68(1–2), 64–72 CrossRef.
- R. G. Crittenden and L. Bennett, J. Am. Coll. Nutr., 2005, 24, 582S–591S CrossRef CAS PubMed.
- A. Szilagyi and N. Ishayek, Nutrients, 2018, 10(12), 1994 CrossRef PubMed.
- A. Shafi and Q. Husain, Avicenna J. Med. Biochem., 2022, 10(1), 71–81 CrossRef.
- D. M. Ney, J. Dairy Sci., 1991, 74(11), 4002–4012 CrossRef CAS PubMed.
- M. S. Rahman, M. M. Hassan and S. Chowdhury, Heliyon, 2021, 7(8), e07739 CrossRef CAS PubMed.
- S. Sethi, S. K. Tyagi and R. K. Anurag, J. Food Sci. Technol., 2016, 53(9), 3408–3423 CrossRef CAS PubMed.
- S. K. Vanga and V. Raghavan, J. Food Sci. Technol., 2018, 55(1), 10–20 CrossRef CAS PubMed.
- D. C. Valencia-Flores, M. Hernández-Herrero, B. Guamis and V. Ferragut, J. Food Sci., 2013, 78(2), E199–E205 CrossRef CAS PubMed.
- A. De Bruno, A. Gattuso, R. Romeo, S. Santacaterina and A. Piscopot, Appl. Sci., 2022, 12(21), 10965 CrossRef CAS.
- S. Martins, E. L. C. Amorim, T. J. S. Peixoto Sobrinho, A. M. Saraiva, M. N. C. Pisciottano, C. N. Aguilar, J. A. Teixeira and S. I. Mussatto, Ind. Crops Prod., 2013, 41, 306–311 CrossRef.
- A. P. A. Carvalho and C. A. Conte-Junior, Trends Food Sci. Technol., 2021, 111, 534–548 CrossRef CAS.
- I. D. Silva, L. E. Moraes Filho, V. F. Caetano, M. F. Andrade, F. Hallwass, A. M. Brito and G. M. Vinhas, Lebensm.-Wiss. Technol., 2021, 144, 111215 CrossRef CAS.
- E. D. N. S. Abeyrathne, K. Nam and D. U. Ahn, Antioxidants, 2021, 10, 1587 CrossRef CAS PubMed.
- A. De Bruno, R. Romeo, A. Gattuso, A. Piscopo and M. Poiana, Foods, 2021, 10, 2684 CrossRef CAS PubMed.
- A. Bensid, N. El Abed, A. Houicher, J. M. Regenstein and F. Özogul, Crit. Rev. Food Sci. Nutr., 2022, 62(11), 2985–3001 CrossRef CAS PubMed.
- N. F. Santos-Sánchez, R. Salas-Coronado, R. Valadez-Blanco, B. Hernández-Carlos and P. C. Guadarrama-Mendoza, Acta Sci. Pol. Technol. Aliment., 2017, 16(4), 361–370 Search PubMed.
- F. M. Perna, P. Vitale and V. Capriati, Green Sustainable Chem., 2020, 21, 27–33 CrossRef CAS.
- F. Chemat, M. Abert Vian, H. K. Ravi, B. Khadhraoui, S. Hilali, S. Perino and A. S. Fabiano Tixier, Molecules, 2019, 24, 3007 CrossRef CAS PubMed.
- M. Nutrizio, N. Maltar-Strmečki, F. Chemat, B. Duić and A. R. Jambrak, Food Eng. Rev., 2021, 13, 161–174 CrossRef CAS.
- E. Tsiokanos, N. Tsafantakis, A. Termentzi, N. Aligiannis, L. A. Skaltsounis and N. Fokialakis, Food Chem., 2021, 343, 128400 CrossRef CAS PubMed.
- A. Strano, G. Falcone, B. F. Nicolò, T. Stillitano, A. I. De Luca, F. S. Nesci and G. Gulisano, Agroecol. Sust. Food, 2017, 41, 1124–1145 Search PubMed.
- A. Gattuso, A. Piscopo, R. Romeo, A. De Bruno and M. Poiana, Agriculture, 2023, 13(5), 1095 CrossRef CAS.
- A. De Bruno, A. Gattuso, D. Ritorto, A. Piscopo and M. Poiana, Foods, 2023, 12, 488 CrossRef CAS PubMed.
- Association of Official Analytical Chemists, Method 14.022, in Hydrogen-ion Activity (pH) Method, ed. W. Horwitz, Association of Official Analytical Chemists, Washington, DC, USA, 13th edn, 1980, p. 213 Search PubMed.
- E. González-Molina, D. A. Moreno and C. García-Viguera, Food Chem., 2009, 115, 1364–1372 CrossRef.
- D. Cerdá-Bernad, J. Clemente-Villalba, E. Valero-Cases, J. J. Pastor and M. J. Frutos, Antioxidants, 2022, 11, 1650 CrossRef PubMed.
- R. Mafrica, A. De Bruno, D. Lanza and M. Poiana, Horticulturae, 2023, 9, 71 CrossRef.
- AOCS, Method Ca 5a 40, in Official Methods and Recommended Practices of the American Oil Chemists' Society, AOCS Press, Champaign, IL, USA, 6th edn, 2017 Search PubMed.
- AOCS, Method Cd 8–53, in Official Methods and Recommended Practices of the American Oil Chemists' Society, AOCS Press, Champaign, IL, USA, 6th edn, 2017 Search PubMed.
- AOCS, Method Ch 5-91, in Official Methods and Recommended Practices of the American Oil Chemists' Society, AOCS Press, Champaign, IL, USA, 1989 Search PubMed.
- AOAC, Peroxide value of oils and fats 965.33.12. Official methods of analysis of AOAC international, Maryland, USA, 17th edn, 2000 Search PubMed.
- AOCS, Method Cd 12c-16, in Official Methods and Recommended Practices of the American Oil Chemists' Society, AOCS Press, Champaign, IL, USA, 6th edn, 2017 Search PubMed.
- A. Baiano, G. Gambacorta, C. Terracone, M. A. Previtali, C. Lamacchia and E. La Notte, J. Food Sci., 2009, 4, 177–183 Search PubMed.
- D. Pathak, J. Majumdar, U. Raychaudhuri and R. Chakraborty, Int. Food Res. J., 2017, 24(1), 238–246 CAS.
- C. Delgado-Andrade, I. Seiquer, A. Haro, R. Castellano and M. P. Navarro, Food Chem., 2010, 122, 145–153 CrossRef CAS.
- M. Merlino, G. Tripodi, F. Cincotta, O. Prestia, A. Miller, A. Gattuso, A. Verzera and C. Condurso, Foods, 2022, 11, 2783 CrossRef CAS PubMed.
- E. Gil-Martín, T. Forbes-Hernández, A. Romero, D. Cianciosi, F. Giampieri and M. Battino, Food Chem., 2022, 378, 131918 CrossRef PubMed.
- M. A. Andrade, C. H. Barbosa, M. A. Shah, N. Ahmad, F. Vilarinho, K. Khwaldia, A. S. Silva and F. Ramos, Antioxidants, 2023, 12, 38 CrossRef CAS PubMed.
- Y. Nogata, K. Sakamoto, H. Shiratsuchi, T. Ishii, M. Yano and H. Ohta, Biosci., Biotechnol., Biochem., 2006, 70(1), 178–192 CrossRef CAS PubMed.
- L. Bartella, F. Mazzotti, I. R. Talarico, G. De Luca, I. Santoro, M. Prejanò, C. Riccioni, T. Marino and L. Di Donna, Antioxidants, 2022, 11, 1847 CrossRef CAS PubMed.
- A. Montero-Calderon, C. Cortes, A. Zulueta, A. Frigola and M. J. Esteve, Sci. Rep., 2019, 9(1), 16120 CrossRef PubMed.
- W. Kneifel, F. Ulberth and E. Schaffer, Le Lait, 1992, 72, 383–391 CrossRef.
- M. P. Mahajan and S. Bandyopadhyay, Color Res. Appl., 2020, 45, 325–335 CrossRef.
- N. Korley and P. H. Tuah, Food Sci. Technol., 2015, 16, 98–103 Search PubMed.
- S. M. Chudy, D. Cais-Sokolińska and J. Tomaszewska-Gras, Appl. Sci., 2022, 12, 11986 CrossRef CAS.
- H. A. Abd El-aal and F. T. Halaweish, Lucr. Stiint., Ser. Zooteh., 2010, 53(15), 233–240 Search PubMed.
- A. Gramza-Michalowska, J. Korczak and J. Regula, Asia Pac. J. Clin. Nutr., 2007, 16(Suppl 1), 85–88 CAS.
- M. Mathlouthi, Food Control, 2001, 12, 409–417 CrossRef.
- W. Li, C. Li, Z. Gu, Y. Qiu, L. Cheng, Y. Hong and Z. Li, Food Hydrocolloids, 2016, 52, 868–875 CrossRef CAS.
- F. Zhu, Y. Z. Cai, M. Sun and H. Corke, Food Chem., 2009, 112, 919–923 CrossRef CAS.
- P. Conte, S. Pulina, A. Del Caro, C. Fadda, P. P. Urgeghe, A. De Bruno, G. Difonzo, F. Caponio, R. Romeo and A. Piga, Foods, 2021, 10, 923 CrossRef CAS PubMed.
- G. Giovanelli and C. Cappa, Foods, 2021, 10, 417 CrossRef CAS PubMed.
- D. H. Bastos, É. Monaro, É. S. Siguemoto and M. Séfora, Food Industrial Processes Methods and Equipment, 2012, vol. 13, pp. 281–300 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |