Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thermal and nonthermal processing of an underutilized fruit Emblica officinalis (Amla): a sustainable approach
Rishika
Tewari
 a,
Vivek
Kumar
a,
Vivek
Kumar
 *a and
H. K.
Sharma
b
*a and
H. K.
Sharma
b
aDepartment of Food Technology, Harcourt Butler Technical University, Kanpur 208002, Uttar Pradesh, India. E-mail: viveksachan99@rediffmail.com
bDepartment of Chemical Engineering, NIT Agartala (Tripura), India
First published on 24th July 2023
Abstract
Indian gooseberry or amla (Emblica officinalis) belongs to the Euphorbiaceae family and is undoubtedly the chief medicinal plant of the Indian Ayurvedic medicine system. The unique array of biologically active compounds in amla endows it with ethnomedical and pharmacological value. Almost every part of the amla tree can be utilized in various medicinal formulations for the treatment of several ailments including cancer, dyslipidemia, diabetes, digestive issues and neurological disorders. However, the post-harvest losses of this fruit are as high as 30–40%, and thus the production of value-added products of amla fruit is profitable to both consumers and farmers, as it helps in capturing the benefits throughout the year. Therefore, in this comprehensive review, we provide a better understanding on the quality of amla based on its various bioactive components. Also, we present the thermal and non-thermal processing, packaging and storage, value-added product and health aspects of the amla fruit in detail.
Sustainability spotlightAmla (Emblica officinalis) is a unique fruit due to the presence of numerous bioactive compounds, which offer a wide range of therapeutic values. However, because of its perishable nature, it is necessary to develop sustainable techniques to process amla fruit and extend its shelf life with the maximum retention of its bioactive compounds. Recently, minimal processing techniques have gained popularity because of their numerous advantages in terms of operation and energy consumption. Therefore, the other uses of amla (Emblica officinalis) and its sustainable technological development need are to be comprehensively reviewed and made available to the scientific fraternity and society. Therefore, herein, we review all the recent scientific findings to address the above-mentioned problems and their relation with the United Nations sustainability development goals: good health and well-being (SDG 3) and responsible consumption and production (SDG 12). |
1. Introduction
Emblica officinalis, a member of the genus Phyllanthus (Family Euphorbiaceae), is an underutilized fruit, which is known as Indian gooseberry, emblic myrobalan, malacca and amla. This fruit is often utilized in Ayurveda and Unani medicine and cultivated throughout India, thriving well in tropical and subtropical regions. Amla is a non-climacteric seasonal fruit, which grows abundantly in various regions of the world including Sri Lanka, Malaysia, China, Pakistan, Bhutan, Bangladesh, Thailand, Vietnam, Iran, Iraq, Japan, Panama, Puerto Rico and Florida. India ranks first in the world for the production of this fruit,1 which was 1.2 million tonnes in 2021–2022, with Madhya Pradesh occupying the first rank in its production, followed by Uttar Pradesh and Tamil Nadu.2This fleshy yellow greenish fruit has an astringent flavor and is eaten raw, roasted or in a pickle form in various regions of India. It is the major ingredient for ayurvedic medicinal preparations and health foods such as chyawanprash and triphala. It is regarded as a general tonic that is beneficial for enhancing the mental and physical health of all age groups. Its fruit is hard and round in shape and is divided into 6–7 segments. Also, its color varies from pale green, when unripe to light yellow after ripening. The size of the fruit can be as large as plum and as small as a marble. Its surface is shiny with a translucent and thin skin. Besides its salty taste, amla possesses five tastes out of six given by nature. It is sour on the first bite but becomes sweet after mixing with saliva. Amla is also referred to as amritphal and tridoshic, which has the potential to cure all three doshas of our body according to the Ayurveda medicinal system, making it a divine fruit.3
Amla is a well-known fruit possessing an excellent nutritional profile, which makes it a super fruit. It is a reservoir of several phytochemicals including polyphenols, tannins, ascorbic acid and other alkaloids.4 Various investigations have revealed the presence of flavonoids such as quercetin and kaempferol.5 Different spectral studies have also confirmed the presence of alkaloids such as phyllantine and phyllantidine. It also contains various micro and macro elements such as calcium, magnesium, selenium, boron. The presence of these phytochemicals makes amla fruit useful in ayurvedic preparations and contributes to various defensive actions against bacteria and viruses. These actions include free radical scavenging, metal chelation, and singlet oxygen quenching. The therapeutic uses of amla fruit include the treatment of diseases such as diabetes, inflammation, various skin disorders, liver ailments, neurological issues, digestive dysfunctions, cancer, and immunological disorders.6 Muzaffar et al.7 gave insight into the biochemical composition, post-harvest processing and therapeutic potential of Indian gooseberry. Ahmad et al.8 reviewed the therapeutic benefits of Phyllanthus emblica, whereas Gul et al.9 reviewed the nutraceutical and functional benefits of amla. Recently, there has been a surge in the consumption of amla-based products, and therefore more focus is needed on the nutritional value of these product and minimal processing techniques. Herein, we review the varietal compositional changes with a comprehensive understanding of the different processing techniques and changes in the functional constituents of amla.
2. Varieties and characteristics of amla fruit
Different varieties of amla fruits are cultivated in various parts of India. NA-6, NA-7, NA-8, NA-9, NA-10, Banarasi, Chakaiya, Krishna, Francis (or Hathijhool), Kanchan, Gujarat-1, Gujarat-2, Goma Aishwarya, Neelam, Desi, Bansi red, and Laxmi-52 are some of the common varieties.10 Some characteristic features of the different varieties of amla fruit are presented in Table 1. Generally, amla varieties are categorized into three groups depending on their maturity stages. Banarasi, NA-9, NA-10 and Krishna are early-maturing varieties available from mid-October to mid-November. Median maturing varieties include Francis, Kanchan, NA-6 and NA-7, which have fruiting season from mid-November to mid-December. Chakaiya and Balwant fall in the late-maturing category with a short season of availability starting from mid-December to mid-January.| Name of variety | Size and color | Other characteristic features |
|---|---|---|
| Banarasi | Large in size and lobed | Thin and semi-translucent skin and bears less fruits per tree than other varieties. Poor keeping quality |
| Chakaiya | Small to medium in size and whitish-green in color | Moderate keeping quality, no premature dropping of fruits, and most preferred for pickles and shreds |
| Kanchan | Small-size fruits and yellowish-green in color | This variety developed through unintentional breeding of Chakaiya and has moderate ascorbic acid concentration |
| Francis/Hathijhool | Big in size, flattened and whitish green in color | Low in ascorbic acid content but rich in iron. Highly susceptible to necrosis |
| NA-6 | Medium size fruits, oval-round in shape and light green in color | This variety developed through unintentional breeding of Chakaiya and suitable for making jam and preserves due to its appreciable keeping quality |
| NA-7 | Medium to large in size with conical apex, smooth yellow to greenish in color | Selected seedling emerging from open pollinated strain of Francis, contains moderate amount of ascorbic acid and free from necrosis |
| NA-8 | Smaller in shape with rough light green colored thick skin | This variety developed through unintentional breeding of Chakaiya, susceptible to necrosis, and affordable keeping quality |
| NA-9 | Large oblong shape and flattened at the tips | This variety developed through unintentional breeding of Banarasi variety with high ascorbic acid content, ideal for making preserves, candy and jam |
| NA-10 | Average size, round, flattened, rough and light yellowish in color with pink tinge | Also known as Agra bold, developed through unintentional breeding of Banarasi and mildly vulnerable to necrosis |
| Lakshmi-52 | Large in size and light pink in color at early stages but color diminishes at maturity stages | This variety developed through unintentional breeding of Francis, bears larger number of fruits per tree and superior potential for processing into various value-based products including syrups, murabbas, and preserves |
| Goma Aishwarya | Medium in size | Bears average fruits per tree, yield per tree is lesser than Lakshmi-52, contains approximately 45–47% juice and suited for export purposes and processing |
| Desi | Small in size, round in shape with tiny dots of white color | Has largest and erect tree among all varieties with moderate bearing capability, moderate ascorbic acid content and usually used as rootstock for growing other varieties |
| Bansi red | Medium-sized fruit, slightly triangular to oval in shape | Has whitish spots on surface, flesh is fibrous and used for medicinal purposes |
Various attempts have been made by researchers to study the physical and morphological traits of different varieties of amla fruit. Proper knowledge of its physical traits is indispensable when designing processing equipment.4 Anand Aonla-II has an average vertical diameter of 38.80 mm and horizontal diameter of 33.28 mm. The weight of its fruit and pulp is 31.80 g and 28.21 g, respectively.11 The physical characteristics of six varieties of amla, namely, Balwant, NA-7, NA-9, Chakaiya, NA-10 and Hathijhool, revealed non-significant differences in the surface hardness of their fruit. The fruit of the NA-7 variety was bigger in size and surface area. NA-10 had highest fruit volume, whereas Hathijhool had the lowest. The rolling resistance of Chakaiya fruit was the maximum and Hathijhool had the minimum. Irrespective of the variety, the rolling resistance was found to be higher on a canvass surface and lower on stainless steel.4 The fruits of the NA-7, NA-10, Banarasi, Chakaiya and Wild varieties were observed for their morphological variations. The weight of both the fruit and pulp and the pulp thickness were the best in Banarasi cultivar.12 NA-7 had the highest volume (21.91 mL) and density (1.21 g cm−3) among the cultivars. The average length and diameter of the wild variety (2.72 cm and 3.00 cm) were lower than that of the Chakaiya variety12 (3.8 cm and 3.0 cm), respectively. However, the juice yield of the wild variety (48.3%) was higher than that of the Chakaiya variety (41.9%). Significant variations were found in the physical dimensions of the fruit with respect to different altitudes.14 The fruit weight ranged from 3.39 to 8.86 g and the fruit diameter varied from 1.78 to 2.34 cm. The fruit length was observed to be highest in Khola, which is located at an altitude of 919 meters above sea level (m asl). Paukhal located at 1263 m asl had the lowest fruit length. The specific gravity was maximum (1.31) in Dangchaura located at 692 m asl, whereas Chamoli seed source (1.01) had the lowest specific gravity located at 1062 m asl among amla fruits of 28 different locations. The pulp: stone ratio varied from 1.93 (Paukhal) to 9.82 (Chauki) among the populations. A significant (p < 0.05) positive relationship between the fruit weight versus malic acid, citric acid, tartaric acid and sugar content was observed. The variation in the different properties of the fruit was attributed to different climatic factors of their growth locations. Also, the variations were more evident in the fruits grown at a higher altitude, which was due to the differences in solar radiation.14
3. Composition of amla fruit
3.1 Proximate composition
The proximate composition of fruits includes moisture, fat, protein, fiber, ash, and total carbohydrate content. Various external factors including the environment affect their nutritional composition. The proximate composition of amla fruit reported by various researchers is summarized in Table 2. The moisture content of amla fruits reported in various studies ranged from 81.00 to 82.80%. A higher moisture exposes fruit to higher chances of spoilage, which can be avoided with proper storage conditions. The protein content of fresh amla fruits varied from 0.5 to 3.2%. Prominent amino acids reported are glutamic acid, proline, aspartic acid, alanine, cysteine and lysine.15 The total amino acid content in the fresh amla fruit has been reported to be 4.45 mg g−1, in which glutamic acid and proline constituted 29.6% and 14.6% of the total amino acids.16 Amla fruit is approximately 2.7 times richer in amino acids than an apple. Its protein composition varies with maturation level and cultivars. A lower amount of fats (0.1–0.5%) has been reported in amla fruits in different studies (Table 2). Lipids such as myristic acid, lauric acid, pentadecanoic acid, palmitoleic, and hexadecenoic acid have been reported in fresh amla fruit. Omega-6 fatty acids such as α- and γ-linolenic acids, which are essential fatty acids, have also been identified in fresh amla fruits.17 Amla fruit is an important source of carbohydrates (7.6–14.1%) (Table 2). Other carbohydrates present in this fruit are starch and pectin, where pectin is an important structural carbohydrate. Sugars and other carbohydrates play an important role in deciding the flavor of amla fruit. Fibers promote the peristalsis movement of the intestine. Thus, a higher content of fiber present in amla fruit (0.3–5.1%) is beneficial for digestive ailments. However, this is disadvantageous when the aim is to produce pulp and pulp-based products.| Parameters | Reported values | References |
|---|---|---|
| Proximate composition (%) | ||
| Moisture | 81.00–82.80 | Ganachari et al., 2005,30 Singh et al., 2011,31 Yadav et al., 2020,32 Hussain et al., 2021 (ref. 33) |
| Protein | 0.50–3.20 | Ganachari et al., 2005,30 Singh et al., 2011,31 Yadav et al., 2020,32 Hussain et al., 2021 (ref. 33) |
| Fat | 0.10–0.46 | Ganachari et al., 2005,30 Singh et al., 2011,31 Saxena and Chaturvedi, 2015,34 Tewari et al., 2019,4 Yadav et al., 2020 (ref. 32) |
| Carbohydrate | 7.60–14.10 | Ganachari et al., 2005,30 Singh et al., 2011,31 Saxena and Chaturvedi, 2015,34 Yadav et al., 2020,32 Hussain et al., 2021 (ref. 33) |
| Fiber | 3.0–5.10 | Ganachari et al., 2005,30 Singh et al., 2011,31 Saxena and Chaturvedi, 2015,34 Yadav et al., 2020 (ref. 32) |
| Ash | 0.50–2.30 | Ganachari et al., 2005,30 Singh et al., 2011,31 Yadav et al., 2020,32 Hussain et al., 2021 (ref. 33) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Physicochemical composition | ||
| Ascorbic acid (mg/100 g) | 498.81–720.00 | Tewari et al., 2019,4 Kavita et al., 2013,18 Tewari et al., 2021,19 Goyal et al., 2008,22 Pareek et al., 2011,23 Singh et al., 2011 (ref. 31) |
| Total phenolic content (mg GAE per g) | 24.61–31.12 | Sarangam and Chakraborty, 2015,35 Tewari et al., 2019,4 Tewari et al., 2021 (ref. 19) |
| Naringin (mg/100 g) | 3.00 | Tewari et al., 2019 (ref. 4) |
| Acidity (% citric acid) | 2.24–2.34 | Goyal et al., 2008,22 Pareek et al., 2011 (ref. 23) |
| Total sugar (%) | 3.11–3.31 | Goyal et al., 2008,22 Pareek et al., 2011 (ref. 23) |
| Reducing sugar (%) | 2.37–2.45 | Goyal et al., 2008,22 Pareek et al., 2011 (ref. 23) |
| Non reducing sugar (%) | 0.74–0.79 | Goyal et al., 2008,22 Pareek et al., 2011 (ref. 23) |
| Tannin (%) | 0.50–1.95 | Goyal et al., 2008,22 Pareek et al., 2011,23 Gorya and Bajwa, 2015,36 Kulkarni et al., 2017 (ref. 11) |
| TSS (°Brix) | 12.70–14.00 | Goyal et al., 2008,22 Pareek et al., 2011,23 Kulkarni et al., 2017 (ref. 11) |
| pH | 1.97–2.88 | Goyal et al., 2008,22 Pareek et al., 2011,23 Parveen and Khatkar, 2015,28 Kulkarni et al., 2017 (ref. 11) |
| Pectin (%) | 0.54–0.55 | Goyal et al., 2008,22 Pareek et al., 2011 (ref. 23) |
| Malic acid (%) | 1.43–3.67 | Naithani et al., 2020 (ref. 14) |
| Citric acid (%) | 1.37–3.61 | Naithani et al., 2020 (ref. 14) |
| Tartaric acid (%) | 3.21–8.46 | Naithani et al., 2020 (ref. 14) |
| Vitamin B1 (mcg) | 28.00 | Kavita et al., 2013 (ref. 18) |
| Vitamin B3 (mg/100 g) | 0.20–0.40 | Singh et al., 2011,31 Kavita et al., 2013 (ref. 18) |
| Vitamin E (mg/100 g) | 0.16 | Judprasong et al., 2013 (ref. 37) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Mineral composition (mg/100 g) | ||
| Potassium | 116.00–282.00 | Barthakur and Arnold, 1991,16 Waheed and Fatima, 2013 (ref. 29) |
| Calcium | 15.00–50.00 | Barthakur and Arnold, 1991,16 Singh et al., 2011,31 Kavita et al., 2013,18 Parveen and Khatkar, 2015 (ref. 28) |
| Phosphorous | 21.00–28.20 | Pareek et al., 2017,1 Kavita et al., 2013,18 Barthakur and Arnold, 1991 (ref. 16) |
| Magnesium | 11.80 | Barthakur and Arnold, 1991 (ref. 16) |
| Sulphur | 16.60 | Barthakur and Arnold, 1991 (ref. 16) |
| Iron | 1.00–6.93 | Barthakur and Arnold, 1991,16 Kavita et al., 2013,18 Parveen and Khatkar, 2015,28 Filipiak-Szok et al., 2015 (ref. 27) |
| Zinc | 1.60–17.90 | Barthakur and Arnold, 1991,16 Filipiak-Szok et al., 2015,27 Waheed and Fatima, 2013 (ref. 29) |
| Boron | 0.22 | Barthakur and Arnold, 1991 (ref. 16) |
| Copper | 0.28–2.73 | Barthakur and Arnold, 1991,16 Filipiak-Szok et al., 2015 (ref. 27) |
| Sodium | 4.20–7.36 | Barthakur and Arnold, 1991,16 Waheed and Fatima, 2013 (ref. 29) |
| Chloride | 35.50 | Barthakur and Arnold, 1991 (ref. 16) |
| Selenium | 0.05–2.23 | Barthakur and Arnold, 1991,16 Filipiak-Szok et al., 2015,27 Waheed and Fatima, 2013 (ref. 29) |
| Cobalt | 0.27 | Waheed and Fatima, 2013 (ref. 29) |
3.2 Physicochemical composition
Amla fruit possesses a rich nutritional profile containing different constituents. It is a significant source of ascorbic acid, the second richest after Barbados cherry. A wide variation in the concentration of ascorbic acid in amla fruit can be found in the literature (Table 2). Ascorbic acid is a water-soluble vitamin with an extraordinary antioxidant property. This vitamin is vital for immunity, collagen synthesis, blood vessels, bones and eyes. The lowest reported value is 191.13 mg/100 g,14 while 720 mg/100 g is the highest reported value.18 The total phenolic content reported in amla fruit varies from 24.61 to 31.12 mg gallic acid equivalent per gram (mg GAE per g) (Table 2). Phenolic acids are secondary metabolites, which have redox properties. Gallic acid and ellagic acids are the predominant phenolic compounds found in amla fruit. Chakaiya cultivar has been reported to contain 31.12 mg GAE per g polyphenols.19 The amount of phenolics differs widely with geographical locations and extraction methods. Naringin is one of the flavonoids present in amla fruit. The amount of naringin present depends on the maturity stage. The decrease in naringin content during complete maturation may be due to the higher expression of rate-limiting enzymes in the bio-synthesis of flavonoids. Generally, the naringin content declines as maturity progresses.20 The highest flavonoids in citrus fruits have been reported during the middle stages of development and the decrease during complete maturation may be probably due to the higher expression of chalcone isomerase and Chalcone synthase-1 (CHS-1), the rate-limiting enzymes in the biosynthesis of flavonoid.21 The naringin content of 3.00 mg/100 g was reported in fresh amla fruit.19 Naringin is the primary component responsible for bitterness in citrus fruits. Titratable acidity or simply acidity is one of the important indicators of fruits given that it measures the total amount of organic acids present. Upon ripening, the acidity declines and total sugars increase due to the hydrolysis of starch. The acidity (% citric acid) of 2.24–3.11% has been reported in fresh amla fruits in various studies.22,23 Monosaccharides such as D-glucose and D-fructose have been detected in the ethanolic extract of amla fruit. Residues of xylosyl, arabinose, mannosyl, galactosyl and glucosyl were also found in trace amounts.24 Carbohydrates such as raffinose, glucose, galactose, and fructose have also been reported in amla fruit.25The tannins present in amla fruits are known to retard the oxidation of ascorbic acid. Tannins with a content of 0.55% were reported in a study by Goyal et al.22 The total soluble solids are the measure of soluble solids present in fruits. It is also an indicator of the level of sugar, soluble proteins, and other organic constituents. The TSS of fresh amla fruits ranged from 12.10 to 14.00 °B (Table 2). TSS is a significant quality parameter, which has a great effect on the taste of fruit. Fruits of different varieties present a greater variation in ascorbic acid and other organic acids. Organic acids are responsible for the flavor of fruits and play an important role in their growth. Citric acid and malic acid ranged between 1.37–3.61% and 1.43–3.78%, respectively, in 28 varieties of amla fruits.14 The pH of amla fruit is generally in between 1.97 and 2.88, as found in various studies, which reveals its highly acidic nature (Table 2). Amla fruit also contains a hetero-polysaccharide, i.e., pectin, which is a structural polysaccharide present in its cell walls and intercellular layers. Pectin has been found to be useful in lowering serum cholesterol levels.26 Various studies have reported a content of pectin in the range of 0.54–0.55% in amla fruit.22,23 Water-soluble vitamins such as B2 and B3 and fat-soluble vitamin E have also been reported in amla fruit (Table 2).
3.3 Mineral composition
Amla fruit is also rich in minerals and contains an appreciable content of iron, calcium and phosphorous. The elemental analysis of amla fruit by X-ray fluorescence, ICP-MS, and ion chromatography revealed the presence of macro- and micro-elements.27 Reports suggest the presence of 6.93 mg/100 g iron, 3.39 mg/100 g zinc and 2.73 mg/100 g copper (Table 2). A higher bioavailability of iron can be achieved by increasing the intake of ascorbic acid-rich fruits. Thus, the ascorbic acid-rich amla fruit helps in preventing a reduction in ferric to ferrous ion, making it available for uptake by mucosal cells. Macro elements such as calcium and potassium were also reported in the nutritional analysis of amla fruits by Parveen and Khatkar.28 The calcium content ranged from 2.03 to 50.00 mg/100 g in different studies on amla fruits. Amla fruit also contains the essential micro element boron (0.22 mg/100 g) (Table 2). Instrumental neutron activation analysis (INAA) of amla fruits also revealed the presence of minerals such as cobalt and sodium.29 Potassium was found to be a major element (116 mg/100 g), whereas the sodium content was 7.36 mg/100 g in amla fruits. The presence of an adequate amount of sodium in amla helps in regulating the water content in the body, whereas the potassium in amla fruit maintains the pH level of the blood. Cobalt with a concentration of 0.27 mg/100 g present in amla fruits ensures the proper absorption of vitamin B12, thus helping to combat anemia. Another important micro elements found in amla fruit is selenium, which ranged from 0.24 to 0.58 mg/100 g. Selenium is an important cofactor of various enzymes such as glutathione peroxidase and other reductase enzymes.4. Volatile constituents of amla fruit
Amla fruit also contains a variety of volatile compounds, which are mainly essential oils known for their antimicrobial activity. These compounds are a complex mixture of alcohols, esters, terpenes and hydrocarbons (Table 3). Hydrocarbons such as nonacosane (3.54%), undecane (7.55%), decane (6.82%), tetracosane (16.20%) and butyl cyclohexane (2.97%) have been found in amla fruit.38 Hydro distillation and supercritical fluid extraction analysis of amla fruits of Guangdong Province, China revealed the presence of compounds such as hydrocarbons, terpene, terpenoid and carboxylic acid. Among them, the predominant compounds were tetracosane (16.20%), 1-octene-3-ol (13.57%) and β-caryophyllene (13.57%).39 Forty-two compounds constituting 96.13% of essential oil were identified during GC/MS analysis of amla fruit grown in Egypt.38 The main identified components were esters (33.26%) and hydrocarbons (30.29%). Among them, the major compounds detected were methyl salicylate (14.28%) and undecane (7.55%) (Table 3). Various compounds were detected in amla fruit cultivated in Sichuan, China.40 The major compounds were α-furfural (17.93%), 2-chloro-bicyclooct-5-ene-2-carbonitrile (7.69%), salicylic acid, methyl ester (7.25%), trans-2-decenal (5.05%), and hexahydrofarnesyl acetone (5.03%), accounting for 97.42% of the total area of the peaks. Volatile compounds are responsible for the flavor and taste of amla fruits. These compounds play a significant role in determining the principal sensory identity and acceptability by consumers. The concentration of volatile compounds is affected during processing. Generally, an increase in temperature during processing leads to a decline in volatile compounds, while volatile compounds also decrease at chilling temperatures, and therefore both chilling and non-chilling temperatures can cause a reduction in volatile compounds.41| Class | Compounds | Reported values (%) | References |
|---|---|---|---|
| Hydrocarbon | Nonacosane | 3.54 | El-Amir et al., 2014 (ref. 38) |
| Undecane | 3.10, 7.55 | Liu et al., 2009,39 El-Amir et al., 2014 (ref. 38) | |
| Decane | 6.82 | El-Amir et al., 2014 (ref. 38) | |
| Tetracosane | 16.20 | Liu et al., 2009 (ref. 39) | |
| Butyl cyclohexene | 2.97 | El-Amir et al., 2014 (ref. 38) | |
| Alcohol | 1-Octen-3-ol | 13.57 | Liu et al., 2009 (ref. 39) |
| Coahuilensol | 4.57 | El-Amir et al., 2014 (ref. 38) | |
| Ketone | Acetophenone | 4.16 | El-Amir et al., 2014 (ref. 38) |
| Aldehyde | Benzaldehyde | 11.98 | El-Amir et al., 2014,38 Wang et al., 2009 (ref. 40) |
| Cumin aldehyde | 4.64 | ||
| trans-2-Decanal | 5.05 | ||
| Ester | 2-Methyl butyl acetate | 8.60 | El-Amir et al., 2014 (ref. 38) |
| Ethyl benzoate | 8.03 | ||
| Methyl salicylate | 14.28, 7.25 | El-Amir et al., 2014,38 Wang et al., 2009 (ref. 40) | |
| Carboxylic acid | Palmitic acid | 8.51 | Liu et al., 2009 (ref. 39) |
| Furan | α-Furfural | 17.93 | Wang et al., 2009 (ref. 40) |
| Terpene | β-Elemene | 9.68 | Liu et al., 2009 (ref. 39) |
| β-Caryophyllene | 13.57 | Liu et al., 2009 (ref. 39) | |
| Hexahydrofarnesyl acetone | 5.03 | Wang et al., 2009 (ref. 40) | |
| Terpenoid | Thymol | 5.94 | Liu et al., 2009 (ref. 39) |
| Nerol | 2.96 | Liu et al., 2009 (ref. 39) | |
| Methyl eugenol | 3.30 | Liu et al., 2009 (ref. 39) | |
| Camphor | 9.70 | Liu et al., 2009 (ref. 39) |
5. Astringency in amla and effect of processing
Astringency is an oral sensory attribute, which has a huge influence on the choice, acceptability and consumption of fruits. Amla fruit is very astringent in nature, which limits its palatability despite being laden with strong antioxidant activity. Amla fruit exhibits different tastes such as sweet, sour, bitter and astringent. The presence of sucrose, fructose and their derivatives such as sugar alcohol and some amino acids, amines and some non-carbohydrates are the reason for the sweetness in fruits. Sourness in fruits comes from organic acids such as malic acid, citric acid, oxalic acid, and succinic acid tartaric acid. Astringency is a tactile feeling in the mouth, which is mainly due to the presence of tannins and other polyphenolic compounds such as catechin, epicatechin, cyanidin, proanthocyanidins, methyl gallate, ellagic acid and punicalagin. Generally, the feeling of astringency is experienced together with sourness and bitterness. Actually, it is a sensation in the oral cavity, which induces drying, roughing and puckering of the mouth epithelium.42 The reason for this astringent feeling has been established by various researchers over time.43,44 Reports have explained that astringency occurs because of the interaction of tannins and salivary proteins, which are rich in proline amino acid, leading to precipitation. This causes the loss of lubrication in the mouth, leading to contraction of epithelial tissues in the tongue, which generates a dry perception in the mouth. This process is known as astringency or convergence. Jobstl et al.45 reported the mechanism of astringency occurring in the oral cavity after consuming amla fruit, which is illustrated in a simplified form in Fig. 1. Tannins in amla fruit are released in the oral cavity. The proline-rich protein (PRP) present in the saliva binds to multidentate tannins and other polyphenols. At low concentration of tannins, compression of the loosely coiled PRP takes place. However, a higher concentration of tannins initiates cross-linking and other intermolecular interactions. These interactions cause the protein structure to aggregate and precipitate. Thus, precipitation induces the loss of lubrication, and consequently astringency. Amla fruit is rich in tannins with the raw fruit containing nearly 2.25 g/100 g and sundried powder containing 1.85 g/100 g.19 Catechin, epicatechin, cyanidin, proanthocyanidins, methyl gallate, ellagic acid, punicalagin are main tannins responsible for the astringency in amla. However, besides this, tannins have various other benefits. Tannins are considered significant in curbing free radicals, and thus assist in resisting the senescence of tissues. A moderate concentration of tannins can enhance the flavor of fruits. However, not much research has been regarding removing or lowering the astringency of fruits. No work has been reported with the direct aim to remove the astringency in amla fruit to date. However, it has been well established in the literature that some processing operations such as blanching,46,47 pretreatment,19 drying,19,48 and addition of chemicals such as potassium metabisulphite (KMS)49 result in the loss of tannins (17.5–22.00%) in amla fruit. Consequently, this loss of tannins can substantially lessen its astringency. Unit operations such as blanching and pasteurization can help lower the tannin concentration. This reduction can lead to higher acceptability of amla as a table fruit together with its use in various foods and beverages. Microfiltration has emerged as a promising technique, which can remove the low molecular weight compounds causing astringency. Pre-treatment of fruit juices with enzymes such as tannase and cellulase has been reported to increase the efficiency of the microfiltration process.50 Ares et al.51 carried out a study to reduce the astringency of two Uruguayan native plant extracts by adding sucrose, sucralose, polydextrose and milk. Sucrose and milk were found to be the most effective inhibitors of astringency and bitterness.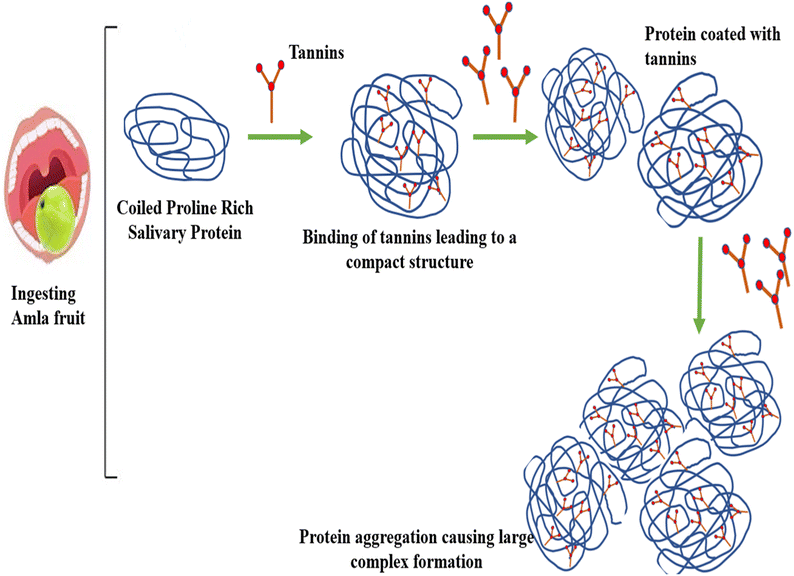 | ||
| Fig. 1 The possible mechanism of astringency occurring in the oral cavity after eating the amla fruit. | ||
6. Processing of amla fruits
The highly perishable amla fruit needs to be processed as its astringent and blunt taste limits its usage in raw form. Thus, post-harvest management of amla has become indispensable as its fruits have a shelf life of 5–6 days.1 However, post-harvest losses occur primarily due to mechanical, physical or pathological factors. Furthermore, chilling injury and white specks are the primary physiological disorders of amla fruit. Chilling injury leads to splitting of the fruit peel, which can be prevented by controlling the temperature at 12 °C for storage.52 White specks can be prevented by preserving fruits in a solution of potassium metabisulphite and sodium chloride.53 Another prominent disorder is pink spots, which occur due to boron deficiency. This can be controlled by spraying fruits with 0.6% borax solution.52 Amla fruit is also highly susceptible to post-harvest fungal pathogens including anthracnose, rust and fruit rot. Spraying of 0.5% sulphur has proven to be effective against rust.54 Also, 0.1% carbendazim has been proven to be useful in getting rid of anthracnose (causative organism: Colletotrichum gloeosporioides). Anthracnose fungal disease produces fruits with sunken, small, brown lesions on their surface.55 Phoma rot disease (causative organism: Phoma exigua) is characterized by small lemon-colored lesions, which then become enlarged and covers the whole fruit.56 Losses during storage due to rodents such as house rats and soft fur field rats have been reported in amla fruit. Deterioration due to above-mentioned factors lowers the marketability and acceptability among consumers. Thus, to curtail the post-harvest losses, which are nearly 30–40%,22 the processing of amla fruit becomes desirable and more significant compared to other fruits. Processing of amla fruit reported in the literature can be categorized in two major parts, which are discussed in the subsequent section.6.1 Thermal processing
Thermal processing is a very popular commercial cost-effective method for the processing of fruits and vegetables, which reduces microbial and enzymatic activity. Thermal treatment also increases the bioavailability of nutrients and improves the taste and texture of the product; however, it also has some negative aspects such as the loss of nutritional, functional and sensory quality of foods. The various thermal treatments on amla and its products are summarized in Table 4.| Amla products | Processing condition | Quality attributes | References |
|---|---|---|---|
| a SO2: sulphur dioxide, AA: ascorbic acid, TPC: total phenolic content, NEB: non-enzymatic browning, KMS: potassium metabisulphite, TSS: total soluble solids, RH: relative humidity, TA: titratable acidity, VI: vacuum impregnation, POD: peroxidase enzyme, DPPH: 2,2-diphenyl-1-picrylhydrazyl, Ea: activation energy, GAE: gallic acid equivalent, RT: room temperature, and RR: rehydration ratio. | |||
| Juice | Pasteurization | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Decrease in AA (60%) & TPC but increase in gallic acid and NEB • Decrease in AA (60%) & TPC but increase in gallic acid and NEB |
Bhattacherjee et al., 2011 (ref. 69) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Temperature: 75–95 °C • Temperature: 75–95 °C |
|||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • SO2: 500 ppm • SO2: 500 ppm |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Best treatment obtained at 80 °C having shelf-life of up to 9 months • Best treatment obtained at 80 °C having shelf-life of up to 9 months |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Storage at temperature 18–36 °C relative humidity: 40–80% & storage for 9 months • Storage at temperature 18–36 °C relative humidity: 40–80% & storage for 9 months |
|||
| Thermal pasteurization: 100 °C | During storage | Lather et al., 2015 (ref. 57) | |
| Chemical preservation | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Chemical: KMS at 1.5 g L−1, minimum change in TSS, no major change in sugars, maximum retention of ascorbic acid • Chemical: KMS at 1.5 g L−1, minimum change in TSS, no major change in sugars, maximum retention of ascorbic acid |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • KMS at 1.0 and 1.5 g L−1 • KMS at 1.0 and 1.5 g L−1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Pasteurization: maximum organoleptic score, lesser AA • Pasteurization: maximum organoleptic score, lesser AA |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Sodium benzoate at 1.0 and 1.5 g L−1 • Sodium benzoate at 1.0 and 1.5 g L−1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Best treatment obtained in KMS at 1.5 g L−1 having shelf-life up to 6 months • Best treatment obtained in KMS at 1.5 g L−1 having shelf-life up to 6 months |
||
| Thermal pasteurization: 90 °C/1 min | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • No changes in AA, TPC, TA, TSS of KMS-treated samples • No changes in AA, TPC, TA, TSS of KMS-treated samples |
Kumari and Khatkar, 2019 (ref. 58) | |
| Chemical preservation: KMS 500 ppm | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Significant changes in AA, TPC, TSS and TA of pasteurized sample • Significant changes in AA, TPC, TSS and TA of pasteurized sample |
||
| Storage at | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Decline in AA, TA during storage • Decline in AA, TA during storage |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • 20–35 °C at 30–70% RH • 20–35 °C at 30–70% RH |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Higher NEB at room temp than refrigerated • Higher NEB at room temp than refrigerated |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Refrigerated temperature (4 °C) • Refrigerated temperature (4 °C) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Best treatment obtained at 90 °C/1 min having shelf-life up to 180 days at 4 °C • Best treatment obtained at 90 °C/1 min having shelf-life up to 180 days at 4 °C |
||
| Pasteurization | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • AA in juice decreased during storage, 1.55% decrease in TPC during 12 months of storage • AA in juice decreased during storage, 1.55% decrease in TPC during 12 months of storage |
Bhattacherjee et al., 2014 (ref. 64) | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Temperature at 90 °C/2 min & preserved with 500 ppm SO2 • Temperature at 90 °C/2 min & preserved with 500 ppm SO2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Good quality spray dried powder from amla juice stored up to 5 months at RT • Good quality spray dried powder from amla juice stored up to 5 months at RT |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Juice stored for 0, 2, 4, 5, 6, 9 and 12 months at RT • Juice stored for 0, 2, 4, 5, 6, 9 and 12 months at RT |
|||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Spray drying of stored juice at inlet temp: 190 °C, feeding rate 16 rpm and 2% maltodextrin • Spray drying of stored juice at inlet temp: 190 °C, feeding rate 16 rpm and 2% maltodextrin |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Significant reduction of AA in powder, no major change in TPC, reduction in color values • Significant reduction of AA in powder, no major change in TPC, reduction in color values |
||
| Spray drying | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Decrease in DPPH, moisture content, hygroscopicity with increase in maltodextrin • Decrease in DPPH, moisture content, hygroscopicity with increase in maltodextrin |
Mishra et al., 2014 (ref. 62) | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Inlet temp: 125 °C, 150 °C, 175 °C, 200 °C • Inlet temp: 125 °C, 150 °C, 175 °C, 200 °C |
|||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Maltodextrin: 3%, 5%, 7%, 9% • Maltodextrin: 3%, 5%, 7%, 9% |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Decrease in density, smaller particle size and higher L* values and reduced TPC at higher temperature • Decrease in density, smaller particle size and higher L* values and reduced TPC at higher temperature |
||
| Fruit | Blanching | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Inactivation of POD, higher L* and b* of blanched samples • Inactivation of POD, higher L* and b* of blanched samples |
Chinprahast et al., 2012 (ref. 59) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • 70–90 °C/20–60 s • 70–90 °C/20–60 s |
|||
| VI | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • VI at 13.5 kPa and 50 °Brix samples had lower moisture and AA, increased color stability, higher carbohydrate and TSS • VI at 13.5 kPa and 50 °Brix samples had lower moisture and AA, increased color stability, higher carbohydrate and TSS |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Pressure: 6.8–13.5 kPa and with osmotic solution of sucrose (mixed with honey) of 30–50 °Brix • Pressure: 6.8–13.5 kPa and with osmotic solution of sucrose (mixed with honey) of 30–50 °Brix |
|||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Steeping in water: 30 days • Steeping in water: 30 days |
Decrease in | Bhattacherjee et al., 2013 (ref. 60) | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • TSS from 6.7 to 2.6 °Brix, TA from 1.52 to 0.48%, AA from 292.0 to 36.5 mg/100 g and TPC from 1.32 to 0.76% • TSS from 6.7 to 2.6 °Brix, TA from 1.52 to 0.48%, AA from 292.0 to 36.5 mg/100 g and TPC from 1.32 to 0.76% |
|||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Juice of steeped fruit withdrawn at 0, 5, 10, 15, 20 and 30 days and pasteurized at 90 °C and preserved with 500 ppm SO2 • Juice of steeped fruit withdrawn at 0, 5, 10, 15, 20 and 30 days and pasteurized at 90 °C and preserved with 500 ppm SO2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Best results for fruits steeped in water up to 10 days • Best results for fruits steeped in water up to 10 days |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • The resulted juice having shelf-life of 4 months • The resulted juice having shelf-life of 4 months |
|||
| Flakes | Drying | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • AA retention (vacuum): 64–94% & 93–96% for LPSSD • AA retention (vacuum): 64–94% & 93–96% for LPSSD |
Methakhup et al., 2005 (ref. 70) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Vacuum: 65 °C and 75 °C, absolute pressures: 7, 10 and 13 kPa • Vacuum: 65 °C and 75 °C, absolute pressures: 7, 10 and 13 kPa |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Decrease in L* and increase in a* than control • Decrease in L* and increase in a* than control |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Low-pressure superheated steam dryer (LPSSD) with absolute pressures: 7, 10 and 13 kPa, temp: 65 and 75 °C • Low-pressure superheated steam dryer (LPSSD) with absolute pressures: 7, 10 and 13 kPa, temp: 65 and 75 °C |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Vacuum drying: shorter drying time • Vacuum drying: shorter drying time |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Vacuum system 75 °C, absolute pressure of 7 kPa most suitable • Vacuum system 75 °C, absolute pressure of 7 kPa most suitable |
|||
| Shreds | Blanching (80 °C/3 min) | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • L* value decreased with drying time • L* value decreased with drying time |
Gupta et al., 2011 (ref. 71) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Hot water • Hot water |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Sharp increase in a* and b* value • Sharp increase in a* and b* value |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Salt solution • Salt solution |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Least changes in color in KMS blanching • Least changes in color in KMS blanching |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • 0.3% KMS • 0.3% KMS |
|||
| Drying | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Color degradation: control > salt > hot water > KMS • Color degradation: control > salt > hot water > KMS |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • 60 °C ± 3 °C in a cabinet tray dryer, 1.2 m s−1 airflow • 60 °C ± 3 °C in a cabinet tray dryer, 1.2 m s−1 airflow |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Color change followed zero order kinetics • Color change followed zero order kinetics |
||
| Blanching | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Reduced drying time in blanching • Reduced drying time in blanching |
Gupta et al., 2014 (ref. 63) | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • 0.3%KMS • 0.3%KMS |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • AA loss: 69% (blanched), 27.78% (unblanched) • AA loss: 69% (blanched), 27.78% (unblanched) |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • 80 °C/3 min (1 • 80 °C/3 min (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 product–solution ratio) 5 product–solution ratio) |
|||
| Cabinet tray drying | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Ea (blanched): 43.98 kJ mol−1 • Ea (blanched): 43.98 kJ mol−1 |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Air speed: 1.2 m s−1 • Air speed: 1.2 m s−1 |
|||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • 50–60 °C • 50–60 °C |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Ea (unblanched): 47.21 kJ mol−1 • Ea (unblanched): 47.21 kJ mol−1 |
||
| Cabinet drying | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Higher AA & TPC at 50 °C • Higher AA & TPC at 50 °C |
Kumari and Khatkar, 2018 (ref. 65) | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Five varieties (Banarasi, Chakaiya, Desi, Kanchan and NA-7) • Five varieties (Banarasi, Chakaiya, Desi, Kanchan and NA-7) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Lower AA & TPC at 40 °C • Lower AA & TPC at 40 °C |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • 40 °C, 50 °C, 60 °C, 30% RH • 40 °C, 50 °C, 60 °C, 30% RH |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Newton model best to describe drying behaviour • Newton model best to describe drying behaviour |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • No significant difference between pH, TSS • No significant difference between pH, TSS |
|||
| Slices | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Fluidized bed drying 60 °C, 70 °C, and 80 °C (115 m min−1 air velocity) • Fluidized bed drying 60 °C, 70 °C, and 80 °C (115 m min−1 air velocity) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Higher AA in fluidized bed drying • Higher AA in fluidized bed drying |
Murthy and Joshi, 2007 (ref. 61) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Sun drying • Sun drying |
|||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Tray drying: 50m min−1 and 70 °C • Tray drying: 50m min−1 and 70 °C |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Drying period: sun drying, 660 min & fluidized bed drying, 120 min, tray drying, 270 min • Drying period: sun drying, 660 min & fluidized bed drying, 120 min, tray drying, 270 min |
||
| Gratings | Cabinet tray dryer | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Salt pretreatment: better nutrients, color, AA: 79.51–84.89%, TPC: 176.5–220.3 mg GAE per g db • Salt pretreatment: better nutrients, color, AA: 79.51–84.89%, TPC: 176.5–220.3 mg GAE per g db |
Sonkar et al., 2020 (ref. 66) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • 55 °C ± 2 °C/8 h • 55 °C ± 2 °C/8 h |
|||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Krishna, Kanchan, NA-7 and Chakaiya • Krishna, Kanchan, NA-7 and Chakaiya |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Ambient storage better. NA-7 cultivar amla, pretreated with 1% salt-dried for 8 h is the most suitable • Ambient storage better. NA-7 cultivar amla, pretreated with 1% salt-dried for 8 h is the most suitable |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Salt pretreatment (1%) • Salt pretreatment (1%) |
|||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Storage: ambient, refrigerated, accelerated • Storage: ambient, refrigerated, accelerated |
|||
| Segments | Solar tunnel drying, sun drying | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Solar tunnel was better than sun drying • Solar tunnel was better than sun drying |
Priyanka and Carolinrathinakumari, 2020 (ref. 67) |
| Pretreated | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • 2% NaCl • 2% NaCl |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Solar tunnel had highest AA retention, lower moisture content, 20–30% less drying time, highest RR, lowest water activity • Solar tunnel had highest AA retention, lower moisture content, 20–30% less drying time, highest RR, lowest water activity |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • 0.1% KMS • 0.1% KMS |
|||
| Pretreatment: 360 W/8.5 min | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Retention of AA (99.33%), TPC (94.45%), naringin (97.11%), DPPH activity (96.61%) in the freeze drying • Retention of AA (99.33%), TPC (94.45%), naringin (97.11%), DPPH activity (96.61%) in the freeze drying |
Tewari et al., 2021 (ref. 19) | |
| Drying | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Sun (30–38 °C/7 h), tray (60 °C, 1.15 m s−1) • Sun (30–38 °C/7 h), tray (60 °C, 1.15 m s−1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Drying time: 260, 290, 525 and 690 min in vacuum, tray, sun and freeze drying • Drying time: 260, 290, 525 and 690 min in vacuum, tray, sun and freeze drying |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Vacuum (60 °C), freeze (−40 °C) • Vacuum (60 °C), freeze (−40 °C) |
|||
| Candy | Solar tunnel greenhouse dryer | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Color and taste of candy better in solar dryer • Color and taste of candy better in solar dryer |
Patil and Gawande, 2018 (ref. 72) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • 40° inclination angle • 40° inclination angle |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Modified Page model best to describe drying behavior • Modified Page model best to describe drying behavior |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Temperature: 55–60 °C • Temperature: 55–60 °C |
|||
| Open sun drying | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Payback age of 17 months • Payback age of 17 months |
||
| Powder | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Spray drying: 200 °C inlet, 150 °C outlet temperature • Spray drying: 200 °C inlet, 150 °C outlet temperature |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Powder yield in sun drying: 10.11%, tunnel drying: 8.78%, vacuum drying: 12.48%, spray drying: 4.90% and freeze drying: 2.23% • Powder yield in sun drying: 10.11%, tunnel drying: 8.78%, vacuum drying: 12.48%, spray drying: 4.90% and freeze drying: 2.23% |
Mishra et al., 2009 (ref. 13) |
| 40% aspiration speed, 5% maltodextrin | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Freeze drying • Freeze drying |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Highest AA & minerals in freeze drying and lowest in sun drying • Highest AA & minerals in freeze drying and lowest in sun drying |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Sun drying • Sun drying |
|||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Vacuum drying: 50 °C • Vacuum drying: 50 °C |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Freeze-dried lighter in color and sun-dried darkest in color • Freeze-dried lighter in color and sun-dried darkest in color |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Tunnel drying: 70 °C • Tunnel drying: 70 °C |
|||
Pasteurization and treatment of KMS (1.5 g L−1) have been reported to be effective in preserving the quality of juice up to 6 months.57 Pasteurization (90 °C/1 min) and preservation with 500 ppm KMS (without heat treatment) of amla juice stored at (a) 20–35 °C and RH 30–70% and (b) refrigerated at a temperature of 4 °C showed better retention of nutrients and other phytochemicals in a pasteurized sample.58 Blanched amla (90 °C/60 s) impregnated at 13.5 kPa with osmotic solution containing honey (50 °B) resulted in a product that had color stability, remarkably less moisture and higher carbohydrate and TSS content.59 A continuous decrease in ascorbic acid, polyphenols and TSS has been reported with prolonged steeping. Steeping in water for up to 10 days could retain the maximum nutrients in juice for up to 4 months of storage.60
In the case of amla fruit, sun and solar drying are the most common method for its processing. Besides, tray/cabinet or vacuum drying has also been exploited to some extent. Murthy and Joshi61 attempted to examine the fate of ascorbic acid during fluidized bed drying. Fluidized bed drying took the shortest time to dry slices at 80 °C with an air velocity of 115 m min−1 and it retained more ascorbic acid than sun drying. A comparative study on the physicochemical properties of two varieties (Wild and Chakaiya) of amla dried by different drying techniques showed that the powder yield was highest in vacuum drying (12.48%) and lowest in freeze drying (2.23%).13 The freeze-dried samples had the maximum retention of ascorbic acid and minerals, while the sundried samples showed the maximum loss. Mishra et al.62 showed that an appreciable quality of spray-dried powder from amla juice can be prepared at an inlet temperature of 175 °C and 7% maltodextrin. Amla shreds, blanched in 0.3% KMS solution at 80 °C for 3 min reduced the drying time. Vitamin C content of blanched dried shreds decreased by 69.36% whereas unbalanced dried reduced up to 27.78%.63 The pasteurized amla juice of cv Chakaiya was spray dried at an inlet drying temperature of 190 °C and feeding rate of 16 rpm 2% maltodextrin. High-quality amla powder could be prepared by spray-drying with a shelf life of 5 months at room temperature.64
The drying kinetics revealed that the maximum ascorbic acid and total phenolics were retained at 50 °C, while the lowest values were observed at 40 °C.65 A similar study was performed on 4 different cultivars of amla, wherein the effect of salt pretreatment on the drying of amla grating was evaluated. Fruits of Krishna, Kanchan, NA-7 and Chakaiya cultivars were grated and mixed with 1% common salt, followed by cabinet tray drying at 55 °C for 8 h. Salt pretreatment increased the retention of the nutrients and color of the gratings. The retention of ascorbic acid and polyphenols was 79.51–84.89% and 65.12–75.57%, respectively, in the salt-pretreated samples compared to the untreated sample. Among them, the salt-pretreated NA-7 variety was the best to produce dry amla powder. The need for blanching was prevented due to salt pretreatment, which was proven to be effective to prepare dry amla powder with a higher concentration of nutrients.66
Pre-treated, amla segments with 2% NaCl and 0.1 KMS followed by solar tunnel drying resulted in 20–30% reduction in drying time compared to sun drying. The NaCl-treated solar tunnel dried samples had the lowest water activity. The overall quality of the solar tunnel-dried samples was better than that of the sun-dried samples.67 Amla pretreated in a microwave (360 W) and dipped in water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio) for 8.5 min was subjected to four different drying techniques.19 The results exhibited that freeze drying took the longest for drying (690 min), while vacuum drying took the least time (260 min). The quality of segments was significantly affected due to drying and reported a loss of 5.54–19.88% and 2.89–12.81% in total phenolic and naringin, respectively. SEM revealed the non-shrunken, porous structure of freeze dried sample. Raaf et al.68 revealed the crystalline amorphous structure of amla and confirmed the presence of polyphenols, pectin and ascorbic acid.
10 ratio) for 8.5 min was subjected to four different drying techniques.19 The results exhibited that freeze drying took the longest for drying (690 min), while vacuum drying took the least time (260 min). The quality of segments was significantly affected due to drying and reported a loss of 5.54–19.88% and 2.89–12.81% in total phenolic and naringin, respectively. SEM revealed the non-shrunken, porous structure of freeze dried sample. Raaf et al.68 revealed the crystalline amorphous structure of amla and confirmed the presence of polyphenols, pectin and ascorbic acid.
6.2 Non-thermal processing
Generally, the non-thermal techniques used for the processing of foods are microwave, ultrasonication, ohmic, radiofrequency, high-pressure processing and pulsed electric field. Alternatively, there is a lack of research on the non-thermal processing of amla. The effects of non-thermal treatment on the quality of different products of amla fruit are summarized in Table 5. The high-electric field (HEF) technique has been employed for increasing the shelf life of amla fruit.73 The effect of an alternating current (AC) and direct current (DC) with an HEF strength of 430 kV m−1 was observed on amla fruits. Keeping quality of fruits in closed and open polyethylene pouches was studied at 4 °C, 20 °C and 35 °C for 15 days. The application of an AC HEF was better for the shelf-life extension and retention of nutrients compared to DC HEF. Ohmic heating can be a promising technique for the processing of amla pulp. The quality characteristics of ohmic-heated amla pulp were examined by Singh et al.49 Amla pulp was heated at 90 °C for 1 min at 11, 13, 15 and 17 V cm−1 voltage gradients. The retention of vitamin C, tannins and color was higher at 17 V cm−1 than that at other gradients and showed better quality attributes than other pulp samples.| Amla product | Processing condition | Quality attributes | Reference |
|---|---|---|---|
| a AC: alternating current, DC: direct current, AA: ascorbic acid, TA: titratable acidity, TN: tannins, PEF: pulsed electric field, DPPH: 2,2-diphenyl-1-picrylhydrazyl free radical scavenging activity, TSS: total soluble solids, BI: browning index, TPC: total phenolic content, MWCO: molecular weight cut off, NF: nanofiltration, UF: ultrafiltration, TAC: total antioxidant capacity, PPO: polyphenol oxidase enzyme, and POD: peroxidase enzyme. | |||
| Whole fruit | High electric field (HEF) | AC HEF better than DC HEF, physiological loss of mass less in AC HEF, no adverse effect on AA | Bajgai et al., 2006 (ref. 73) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Field strength 430 kV m−1 for 2 h • Field strength 430 kV m−1 for 2 h |
Shelf-life 25 days at 4 °C | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Storage at 4 °C, 20 °C and 35 °C • Storage at 4 °C, 20 °C and 35 °C |
|||
| Pulp | Ohmic treatment | Best results at 17 V cm−1, higher TA and TN, 7.14–17.13% loss of vitamin C | Singh et al., 2013 (ref. 49) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • 11, 13, 15, 17 V cm−1 at 90 °C/1 min • 11, 13, 15, 17 V cm−1 at 90 °C/1 min |
|||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • KMS 0.2% • KMS 0.2% |
|||
| Juice | PEF | 93.12% DPPH inhibition, no significant changes in pH, TSS, BI and conductivity | Bansal et al., 2013 (ref. 74) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Field strength 24 kV cm−1, treatment time 500 μs monopolar rectangular pulse of 1 μs width • Field strength 24 kV cm−1, treatment time 500 μs monopolar rectangular pulse of 1 μs width |
Shelf-life 6 weeks at 4 °C | ||
| Juice | PEF | 63% retention of vit C, 68.5% retention of TPC, lower BI, 5.1 log cycle reduction of Zygosaccharomyces bailii | Bansal et al., 2015 (ref. 75) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • 26 kV cm−1, 1 Hz frequency for 500 μs and monopolar rectangular pulse of 1 μs width • 26 kV cm−1, 1 Hz frequency for 500 μs and monopolar rectangular pulse of 1 μs width |
Shelf-life 40 days at 4 °C | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Juice inoculated with Zygosaccharomyces bailii • Juice inoculated with Zygosaccharomyces bailii |
|||
| Juice | Nano and ultrafiltration | 71.4% recovery rate in NF, improved color and clarity, retained maximum AA | Dawale and Jawade et al., 2016 (ref. 76) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Polyamide membrane with MWCO of 15 kDa, pore diameter 59 Å, 300 psi pressure for NF and 150 psi for UF • Polyamide membrane with MWCO of 15 kDa, pore diameter 59 Å, 300 psi pressure for NF and 150 psi for UF |
|||
| Juice | Thermal assisted high-pressure processing (THPP) | 85% AA retention, increased L*, TAC and TPC increased to 20 & 28% respectively | Raj et al., 2019 (ref. 78) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • 200–500 MPa, 1 s to 20 min and 30–60 °C • 200–500 MPa, 1 s to 20 min and 30–60 °C |
|||
| Juice | Pulsed light | Complete inactivation of PPO &POD at 2.9 kV/5 min, 3012 J cm−2 at 10.04 W cm−2, retention of TPC, antioxidants and vitamin C: 45%, 54, and 61% respectively | Chakraborty et al., 2020 (ref. 79) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Intensity: 1504–3012 J cm−2 • Intensity: 1504–3012 J cm−2 |
|||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Voltage: 2.7, 2.8 and 2.9 kV • Voltage: 2.7, 2.8 and 2.9 kV |
|||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Treatment time: 3, 4, and 5 min • Treatment time: 3, 4, and 5 min |
|||
| Juice | Ultrasonication | PPO inactivation rate 90.72%, POD inactivation rate 73.18%, optimal process conditions achieved at 70% pulsed at 5 s ON and 5 s off for 7 min 30 s. Increase in AA of sonicated amla juice as well as antioxidant activity | Aslam et al., 2023 (ref. 80) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Power: 455 W and frequency of 20 kHz • Power: 455 W and frequency of 20 kHz |
|||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Amplitude: 20–70% • Amplitude: 20–70% |
|||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Exposure time: 5–15 min • Exposure time: 5–15 min |
|||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) • Pulse duration (4–6 s ON/10 s) • Pulse duration (4–6 s ON/10 s) |
|||
The effect of a pulsed electric field (PEF) was studied on amla juice. The juice was PEF treated using a static chamber at 24 kV cm−1 for 500 μs with a pulse width of 1 μs. Another juice sample was thermally treated at 90 °C/60 s and both samples were stored at 4 °C for 6 weeks.74 PEF proved to be a better technique for processing the juice than the conventional technique, which substantially reduced the non-enzymatic browning (NEB) and the retention of antioxidants was superior to the conventional treatment. In another study, Bansal et al.75 examined the effect of PEF on amla juice inoculated with Zygosaccharomyces bailii. PEF treatment at 26 kV cm−1 for 500 μs successfully inactivated Z. bailii with a 5.1 log cycle reduction. The treated juice had a high retention of vitamin C (63%) and antioxidant capacity (88.9%). Although thermal treatment caused a 4.9 log cycle reduction in Z. bailii, it resulted in a significant reduction in vitamin C and antioxidant capacity. Both PEF and thermal treatments did not cause any changes in pH and soluble solids of amla juice. An examination of the morphology using electron microscopy depicted the leakage of cellular debris due to the breakage of cells of Z. bailii during PEF treatment.
Purification and concentration of amla juice were carried out using a spiral wound membrane module with a molecular weight cut-off of 15 kDa.76 Browning of fruit juices after processing due to polyphenol oxidase and peroxidase activity is one of the critical issues in the food industry.77 A novel technique known as thermal-assisted high-pressure processing (THHP) was employed for the processing of amla juice. The juice processed using THHP was nutritionally superior to the thermally treated samples. The extractability of phenolics and other antioxidants increased due to the high pressure together with the good retention of ascorbic acid and color upon THHP treatment.78
The pulsed light (PL) technique can be utilized as substitute for conventional pasteurization.79 A PL intensity of 1504–3012 J cm−2 was applied to amla juice. Deactivation of the PPO (polyphenol oxidase) and POD (peroxidase) enzymes was achieved at 2.9 kV (3012 J cm−2 at 10.04 W cm−2), helping to preserve the total phenolics, antioxidants and vitamin C content with a retention of 45%, 54%, and 61%, respectively. Thermal treatment (95 °C/30 min) of mechanically extracted amla juice also inactivated the enzymes but browning was higher than that in the PL treated juice. Ultrasonication (US) uses high energy, low frequency sound waves and has attracted significant attention in recent times as a non-thermal technique for the processing of foods. A probe-type ultrasonication system was found to be effective for the inactivation of the PPO and POD enzymes in fresh amla juice.80 Ultrasonic pre-treated slices (frequency of 40 kHz for 10 min) were found to have improved mass transfer dynamics during osmotic dehydration81 but it affected the color and vitamin C content of the slices.82 The in-depth and systematic application of non-thermal techniques can be undertaken to determine the best methodology for treatment and processing.
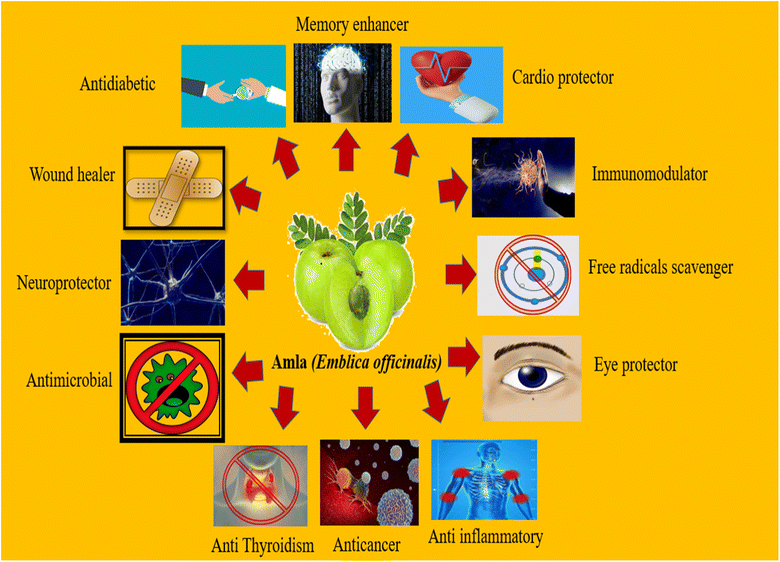
7. Health aspects of amla fruit
The consumption of fruits on a regular basis reduces the risk of various diseases due to the fact that fruits contain potent phytochemicals. These bioactive compounds are responsible for counter attacking the toxins in the body after the consumption of fruits. Emblica officinalis, amla has been used traditionally for ages to combat various diseases including lifestyle-related disorders such as diabetes, hypertension, hyperlipidemia and obesity. Amla is a powerful rejuvenator and helps in delaying and repairing tissue cells. Amla fruit is useful in the management of numerous diseases including cancer. Fig. 2 shows different pharmacological effects of amla fruit including antidiabetic,83,84 anti-inflammatory,85,86 cardioprotective,87,88 immunomodulator,89 memory enhancer,90 wound healer,91 neuroprotector,92,93 free radical scavenger,94,95 eye protector,96 antithyroidism,97 antimicrobial,98 and most important anticancer.99,100 Amla can also be helpful in treating a plethora of diseases and disorders including hyperacidity, constipation, colitis, haemorrhoids, dyspepsia, gastritis, ophthalmic issues, anaemia, cough, asthma, osteoporosis, premature greying of hair, weakness and fatigue, nerve debility and liver complaints.101 The clastogenicity and mutagenicity caused by certain metals are also inhibited by fruit extracts. Research on amla has demonstrated that it can decrease cyclo phosphamide-induced DNA damage in mouse bone marrow cells. Furthermore, it has been shown to decrease the cytochrome (Cyt) P450 levels, increase the glutathione levels and antioxidant enzymes (glutathione peroxidase (GPx), glutathione reductase) and boost the detoxifying enzyme glutathione-S-transferase (GST).102The various phytochemicals present in amla fruit and their biological actions are summarized in Table 6. The unique array of polyphenols including flavonoids and tannins imparts anti-cancerous property to amla. Polyphenols act on multiple metabolic pathways in different forms and regulates bodily mechanisms related to carcinogenesis.103 Various studies on amla revealed that 97.67% of the phenolics in amla are in the free form and the major constituents of the total phenolics are gallic acid, ellagic acid, tannins, quercetin, anthocyanidin and rutin. Gallic acid and methyl gallate have been reported to have antioxidant, antidiabetic and antiproliferative activities (Table 6). An extract of amla exhibited an inhibitory effect against enzymes involved in diabetes (α-amylase, α-glucosidase, and dipeptidyl peptidase-4). The inhibitory potential of the extract was effective in the control of type II diabetes mellitus.95 Ellagic acid has been proven to be an anticancer compound.83In vivo studies of amla extract showed that it has antitumor activity against cancer cell lines including A549 (lung), HepG2 (hepatocytes), MDB-MB-231 (breast, triple negative), HeLa (cervical), SK-OV3 (ovarian), and SW620 (colorectal).104
| Phytochemicals | Examples | Biological activity | References |
|---|---|---|---|
| Hydrolysable tannins | Emblicanin A and B | Antioxidant | Madhuri et al., 2011 (ref. 111) |
| Punigluconin | Antioxidant | Bhattacharya et al., 1999 (ref. 112) | |
| Pedunculagin | Antioxidant | Chang et al., 1995,113 Bhattacharya et al., 1999 (ref. 112) | |
| Chebulagic acid | Antioxidant, anti-inflammatory | Chen and Li, 2006,107 Reddy et al., 2009 (ref. 108) | |
| Corilagin | Antioxidant, anti-atherosclerotic | Singh et al., 2011,31 Srinivasan et al., 2018 (ref. 83) | |
| Geraniin | Antioxidant, anti-atherosclerotic | Kumaran and Karunakaran, 2006,114 Kim et al., 2010,115 Tewari et al., 2021 (ref. 19) | |
| Phenolic compounds | Gallic acid, methyl gallate | Antioxidant, anti-proliferative, antidiabetic, cardioprotective, antibacterial, anti-inflammatory | Singh et al., 2011,31 Srinivasan et al., 2018,83 Tewari et al., 2021 (ref. 19) |
| Ellagic acid | Antioxidant, anti-inflammatory, anti-proliferative, antidiabetic, anticancer | Singh et al., 2011,31 Srinivasan et al., 2018 (ref. 83) | |
| Vitamins | Ascorbic acid | Antioxidant, antidiabetic | Chauhan et al., 2005,116 Khan, 2009,117 Tewari et al., 2019 (ref. 4) |
| Flavonoids | Quercetin | Antioxidant, protein kinase inhibitor, antibacterial, anti-inflammatory, immunomodulatory | Baliga and Dsouza, 2011,118 Madhuri et al., 2011,111 Tewari et al., 2021 (ref. 19) |
| Kaempferol | Antioxidant, neuroprotective, anticancer, anti-osteoporotic | Jung et al., 2008,119 Charoenteeraboon et al., 2010,120 Tewari et al., 2021 (ref. 19) | |
| Organic acids | Citric acid | Antimicrobial, neuroprotective | Kulkarni and Ghurghure, 2018,109 Hassan et al., 2020 (ref. 110) |
| Malic acid | Antimicrobial, neuroprotective | Kulkarni and Ghurghure, 2018,109 Hassan et al., 2020 (ref. 110) | |
| Shikimic acid | Antimicrobial, neuroprotective | Kulkarni and Ghurghure, 2018,109 Hassan et al., 2020 (ref. 110) |
As an excellent source of ascorbic acid, amla fruit is known to act as a potent antioxidant (Table 6). A report cited a significant reduction in LDL level, C-reactive protein and protection against heart disorders after long-term use of amla.105 Treatment with amla induced a significant increase in HDL levels, lowering the total cholesterol, LDL VLDL, and triglycerides.106 The tannins present in amla such as emblicanin-A and B, ellagic acid, gallic acid, and corilagin have protective action against various cardiac disorders.3 Emblicanin A and B, punigluconin, and pedunculagin have excellent antioxidant activities. Chebulagic acid has anti-inflammatory activity in addition to being an antioxidant.107,108 Organic acids of amla fruit are well known for their antimicrobial and neuroprotective effects.109,110
8. Value addition of amla fruits
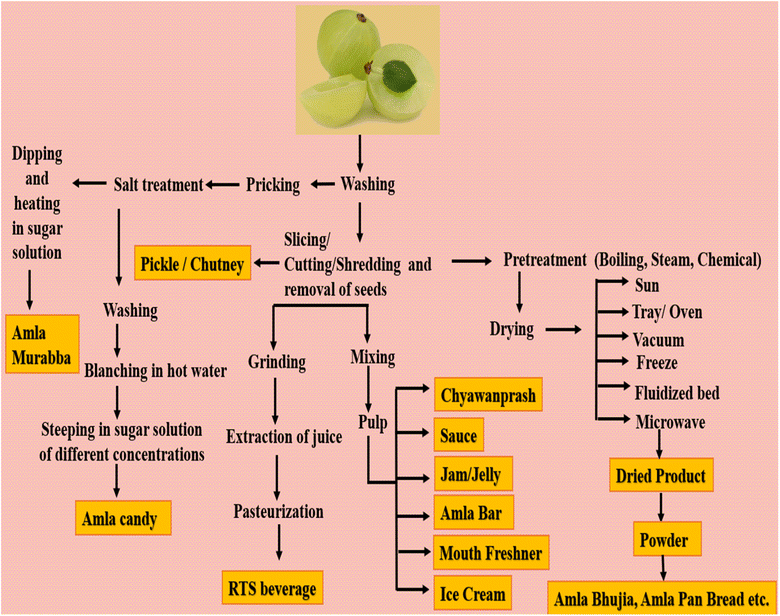
The shelf life of amla fruit is short, i.e., 5–6 days, and is highly susceptible to browning, bruises and necrosis. Furthermore, the post-harvest losses due to discoloration, brushing and microbial growth reduce its market potential. Thus, to overcome these losses, the fruit must be processed into value-added stable products with the aim of increasing its consumption and acceptance among consumers. Processing also adds value to the astringent and acidic nature of amla fruit together with an increase economic gains and provides high returns to farmers. Numerous amla-based products are already in the market. Presently, the increasing concern for health has expanded the market of value-added products from amla. Fig. 3 explains the processing of raw, fully mature amla fruit into various products.Amla murabba or preserve is one of the age-old products of its fruit. The preserve is made from steeping the fruit into heavy sugar solution until it becomes tender and transparent in color.121 Murabba is claimed to impart energy and strength to the body and is often advised by Vaidyas and Ayurvedacharyas to the patients. Usually, large size fruits are used for the preparation of preserve. Other popular products of amla are chyawanprash and triphala. Chyawanprash is believed to be a potent immunomodulator and many studies have claimed that it induces a significant boost in white blood cells in rodents. Amla is a rich source of ascorbic acid and its L-enantiomer, commonly referred as vitamin C, which is an essential micronutrient in different metabolic processes of the body. The importance of vitamin C has prompted fortification of this micronutrient in different food products. However, the important aspect to be investigated is the stability of this micronutrient in different popular products because of its high reactivity and instability. It is oxidized into DHA (dehydroascorbic acid) reversibly when exposed to heat, light, metal ions (transition) and alkaline pH.77,122 DHA is further irreversibly hydrolyzed to 2,3-diketogluconic acid. Therefore, the stability of ascorbic acid in amla-rich products such as chyawanprash and triphala needs to be critically studied. However, different studies have reported losses in ascorbic acid in chyawanprash due to the heat treatment of amla pulp. The degradation of ascorbic acid has also been reported during storage.123 Therefore, it is a challenge to render stability to ascorbic acid in amla-rich products to give benefits to the consumers. Bio-macromolecules can be used as carrier agents and micronutrients can be encapsulated by using different techniques such as spray drying, microfluidics, homogenization, complex coacervation, emulsification and spray chilling to protect them from the external environmental factors.
Triphala is an ayurvedic medicine made up of equal amounts of Terminalia chebula, Phyllanthus emblica, and Terminalia belerica. Polyphenols, vitamin C, and flavonoids are abundant in triphala, which impart therapeutic properties to it. Smaller-size fruits are utilized in making pickles and chutney. During the pickling process, astringency is removed through brining in potassium metabisulphite solution. Gajanana et al.124 proposed and standardized a recipe for amla syrup using amla juice, lime, ginger juice and sugar. Ready to serve (RTS) drink was prepared by mixing amla juice and bitter gourd in a ratio of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25. The addition of bitter gourd helped to remove the astringency of amla juice.125
25. The addition of bitter gourd helped to remove the astringency of amla juice.125
Fruit wine was prepared by fermenting amla using Saccharomyces cerevisiae.126 The alcohol content was 9.29% and compounds such as n-butanol, iso-butanol and n-propanol were detected in trace amounts. Amla candy, which falls in the category of intermediate moist foods, is also a popular product. Candies prepared from blanched amla retained more nutrients than lye-peeled amla.127 Dried amla shreds are also a valuable form of amla fruit. Shreds prepared from KMS blanched amla had superior quality.128 Amla bhujia was prepared from amla pulp, gram flour and spices. Sensory evaluation of amla bhujia had high acceptable scores.129 A fruit beverage containing amla juice, pomegranate, and musk melon was developed with the optimum proportion of 5%, 57%, and 38%, respectively.130 The functional and nutritional properties of ice cream were enhanced due to the addition of amla shreds, candy, pulp, preserve and powder. The inclusion of amla in processed form increased the phenolic and antioxidant properties but decreased the overrun in ice cream.35 A functional pan bread prepared from dried amla powder exhibited superior sensory and nutritional quality. Supplementing the bread with amla powder increased the loaf volume and bread volume.86 Amla mouth freshener, a substitute of pan masala, gutka and tobacco, was developed using amla fruit.131 The pulp of Desi and Banarasi cultivars of amla, carboxymethyl cellulose, areca nut, cardamom, sugar and milk powder was mixed in a proportion to prepare a nutritious and palatable amla mouth freshener, which was packed in HDPE bags at ambient temperature. The storage analysis proved that the content of ascorbic acid decreased, but the overall acceptability was not affected.132 A nutritious fruit bar was developed using amla and guava pulp,133 which had appreciable sensory score with high nutritional value. Amla candy can be prepared (cv Kanchan) using different spices and sugar syrup. Also, 68% sugar syrup and 10% spices have been recommended due to their good organoleptic properties and high shelf life.134
9. Packaging and storage of amla fruits
Packaging plays very important role in enhancing the shelf-life and maintaining the quality parameters. Firstly, fruits are sorted, and then packed for transportation. Generally, amla fruits are packed either in cloth sacks or jute gunny bags (capacity 50–100 kg) or corrugated boxes (capacity 20 kg). During their transportation, injuries are caused due to vibration and stacking. However, there is a lack of research on the effective packaging of amla. The use of corrugated fiber boxes (CFB) with paper liners has been suggested for the packing of amla fruit.135 Cloth sack storage without any liners suffered maximum spoilage (30.19%), whereas CFB with newspaper liners experienced the least spoilage (16%) and polythene liners had 17% spoilage after 13 days of storage. The lowest respiratory activity (81.1 mg CO2 per kg per h) with minimum loss and 11 days of extended shelf life in CFB under ambient conditions for the NA-7 cultivar of amla was reported.136 A perforated polythene bag was found to be useful in reducing the physiological loss with acceptable physiochemical quality of amla fruits. The fruits had an extended shelf life of 15 days at ambient temperature after treatment with 6% waxol and 4 × 105 μL cycocel chlormequat chloride (CCC)/L.137Dipping the fruits in different PGR (plant growth regulators) such as GA3 (gibberellic acid), NAA (naphthalene acetic acid) and chemicals such as CaCl2 have been found to be useful for extending the shelf life of fruits.138–140 However, there is a discrepancy in the temperature reported by various authors required for the proper storage of amla fruits. The quality and storage of amla fruits were significantly affected after treatment with CaCl2 and GA3. Treatment of amla fruits with calcium nitrate at 1.5% + perforated polythene bag and GA3:100 ppm + perforated polythene bag resulted in a reduction in physiological weight loss up to 16% and 16.34%, respectively.135 This treatment also decreased the respiratory activity (72–80 mg CO2 per kg per h). Treatment with GA3 yielded better retention of ascorbic acid and malic acid.141 Amla fruits cv Chakaiya were treated with different concentrations of GA3 (50 ppm and 100 ppm), naphthalene acetic acid, NAA (20 ppm and 30 ppm) and CaCl2 (1% and 1.5%), followed by air drying, and then stored at room temperature in 5 ply corrugated boxes lined with newspaper with 5% ventilation, which showed physiological weight loss (4.94%, 7.06% and 9.04%) during storage at 4, 8 and 12 days, respectively, in CaCl2 (1%) treated fruits.142
Different authors also reported various treatments useful to extend the shelf life of amla fruits. Treatment with borax can reduce pathological losses, whereas physiological weight can be reduced by treating with calcium nitrate. The study by Nath et al.143 demonstrated an extension in the shelf-life of amla fruit via three different treatments, including GA3 (50 ppm), borax (4%) and calcium nitrate (1%). It was found that up to 9 days of storage, the borax-treated sample showed zero pathological losses and reduced physiological weight loss was observed upon calcium nitrate treatment.
Goyal et al.22 reported a temperature of 0–2 °C for the storage of amla fruit, whereas other authors reported a temperature of 5–7 °C,144 12 °C (ref. 145) and 15 °C.146 Storing amla fruits at 12 °C proved to be effective and reduced the effects of chilling injury.52 Amla fruits impregnated in liquid and treated with 15% NaCl + 0.05% sodium benzoate + 0.1% EDTA and 15% NaCl + 0.05% sodium benzoate + 0.2 calcium lactate in association with EDTA or calcium lactate in liquid medium were found to have an extended shelf-life of up to two months at ambient temperature.147 Amla cut fruits having pretreatments with 0.05% sorbitol, 1% calcium chloride and 3% citric acid were packed in three different modified atmosphere packaging (MAP). MAP1 had O2 = 5%, CO2 = 10% & N2 = 85%, MAP2 had O2 = 10%, CO2 = 20% & N2 = 70%, whereas MAP3 consisted of O2 = 15%, CO2 = 30% & N2 = 55%. The fruits were stored at temperatures of 5 °C and 10 °C with a relative humidity of 90–95%. The samples packed in MAP1 remained fresh and retained the maximum polyphenols and antioxidant capacity with the least changes in firmness and puncture strength at both 5 °C and 10 °C. However, the weight loss increased with storage time.148
Presently, research is still lacking on understanding the post-harvest management of underutilized amla fruit. There is an urgent need for sustainable technology to realize a better shelf life and packaging of amla fruits. Newer techniques such as dynamic controlled atmosphere (DCA), and ultralow oxygen (ULO) storage need to be explored and introduced for the storage and reduction of post-harvest losses.
10. Recent application of nanoparticles from amla extract
In the past few years, there has been a significant increase in the use of plant-based nanomaterials because of their unique advantages such as eco-friendly nature, cost-effectiveness, low energy utilization, and simple and scalable approach to manufacture nanoparticles (NP). Furthermore, NP of different sizes and shapes can be produced from plant extracts. The size of nanoparticles typically varies between 1 and 100 nm, which determines the surface area, being an important characteristic that affects the distribution of nanoparticles in a system. NP with a size of <10 nm can easily pass through blood vessels, cleared by the kidneys and have less ligand-to-receptor interaction, whereas larger nanoparticles offer a higher surface area for reactions, hence imparting higher reactivity.Many reports have demonstrated that gold and silver nanoparticles from different plant sources exhibit strong antibacterial properties. Gold nanoparticles generated from Citrus maxima,149Carica papaya,150Ananas comosus151 and Punica granatum152 are some of the important plant sources that have been reported to show strong antibacterial properties. Numerous studies have also reported the antifungal, and most importantly anticancer properties of plants. Amla is one of these plants, and various attempts have been made to produce NP using its extract. Ankamwar et al.153 conducted a study, wherein silver and gold nanoparticles were produced from amla fruit extract. The extract acted as a reducing agent for the production of extracellular silver and gold nanoparticles. On treating the extract with an aqueous solution of silver sulphate and chloroauric acid solution, rapid reduction of the ions was observed. Transmetallation reaction resulted in the formation of highly stable silver and gold NP, which ranged in size from 10 to 20 and 15 to 25 nm, respectively. A similar approach was adopted by Mookriang et al.154 for the synthesis of NP using amla extract as the reducing agent. The time, temperature, and concentration of silver nitrate and amla extract were the factors influencing the synthesis of silver NPs. Treatment of silver nitrate dissolved in deionized water with amla fruit extract resulted in the formation of silver NP with an average diameter of 41.2 nm. In another study, the low-cost biogenic synthesis of silver NP using amla fruit extract was reported. The particle size ranged from 19.8 to 92.8 nm with an average diameter of 39 nm and the NP were characterized using FTIR, electron microscopy, X-ray diffraction and UV spectroscopy.155 The synthesized particles showed significant antimicrobial activity against the pathogen Acidovorax oryzae strain RS-2 of rice bacterial brown stripe at 20 μg mL−1. The study concluded that silver NP can be a good eco-friendly option to curb rice bacterial disease. A similar study by Meena et al.156 revealed the remarkable antibacterial activity of silver NP against E. coli. Silver NP were synthesized using amla fruit extract as the reducing and capping agent. The characterization of NP was done using XRD, TEM and UV spectroscopy, which revealed that their size was 20–25 nm. Also, the study revealed that the zone of inhibition increased on increasing the concentration of silver NP. As a miraculous plant and fruit, investigations are needed to compare the properties of nanoparticles from amla to that from other different plant sources for different uses and to prove their superiority or competitiveness.
11. Conclusion and future aspects
Amla fruit (Indian gooseberry), as rich source of antioxidants and phenolics, is an excellent fruit for maintain human health. Thus, it is necessary to extract the maximum benefits of this super berry by processing it into value-added functional products. Processing techniques such as blanching and drying result in the loss of bioactive compounds. Thus, to combat this loss, minimal processing methods need in depth exploration. Research must focus on making technology available to farmers at low cost. The utilization of bioactive compounds of amla fruit can enormously add value to the processed product. Post-harvest management of amla is also poor, which needs improvement. The medicinal property of amla is due to the presence of bioactive phytochemicals including ascorbic acid. Compounds such as gallic and ellagic acid, quercetin, kaempferol, vanillic acid, isocorilagin, and punigluconin have excellent antioxidant properties. In this case, many clinical trials have been conducted with the aim to reveal the therapeutic potential of amla fruit. However, evidence-based studies need to be the focus. The application of advanced techniques may be further helpful for the isolation of bioactive compounds from the fruit and value addition of this miraculous fruit.Author contributions
The author RT wrote the first draft of the manuscript. VK and HKS contributed as the principal authors to critically revise the manuscript and gave its final shape. All authors have read, critically reviewed, and approved the final manuscript.Conflicts of interest
The authors declare that there is no conflict of interests.Acknowledgements
The authors declare that they received no funding for this review article.References
-
S. Pareek, A. N. Shikov, O. N. Pozharitskaya, V. G. Makarov, G. A. González Aguilar, S. A. Ramalho and N. Narain, Indian Gooseberry (Emblica officinalis Gaertn.), in Fruit and Vegetable Phytochemicals, ed. E. M. Yahia, 2nd edn, 2017, pp. 1077–1106. DOI:10.1002/9781119158042.ch54
.
- NHB, National Horticulture Board, statistics accessed on 17 December 2022, https://agriexchange.apeda.gov.in/India%20Production/India_Productions.aspx?cat=fruit%26hscode=1039 Search PubMed.
- S. Dasaroju and K. M. Gottumukkala, Current trends in the research of Emblica officinalis (Amla): A pharmacological perspective, Int. J. Pharm. Sci. Rev. Res., 2014, 24(2), 150–159 CAS
.
- R. Tewari, V. Kumar and H. K. Sharma, Physical and chemical characteristics of different cultivars of Indian gooseberry (Emblica officinalis), J. Food Sci. Technol., 2019, 56(3), 1641–1648, DOI:10.1007/s13197-019-03595-y
.
- S. S. Patel and R. K. Goyal,
Emblica officinalis Gaertn: A comprehensive review on phytochemistry, pharmacology and ethnomedicinal uses, Res. J. Med. Plant, 2012, 6, 6–16, DOI:10.1080/13880209.2018.1492620
.
- S. A. Almatroodi, M. A. Alsahli, A. Almatroudi, K. Dev, S. Rafat, A. K. Verma and A. H. Rahmani, Amla (Emblica officinalis): Role in health management via controlling various biological activities, Gene Rep., 2020, 21, 100820, DOI:10.1016/j.genrep.2020.100820
.
- K. Muzaffar, S. A. Sofi, H. A. Makroo, D. Majid and B. N. Dar, Insight about the biochemical composition, postharvest processing, therapeutic potential of Indian gooseberry (amla), and its utilization in development of functional foods—A comprehensive review, J. Food Biochem., 2022, 46(11), e14132, DOI:10.1111/jfbc.14132
.
- B. Ahmad, N. Hafeez, A. Rauf, S. Bashir, H. Linfang, M. U. Rehman, M. S. Mubarak, M. S. Uddin, S. Bawazeer, M. A. Shariati and M. Daglia,
Phyllanthus emblica: A comprehensive review of its therapeutic benefits, S. Afr. J. Bot., 2021, 138, 278–310, DOI:10.1016/j.sajb.2020.12.028
.
- M. Gul, Z. W. Liu, R. Rabail, F. Faheem, N. Walayat, A. Nawaz, M. A. Shabbir, P. E. Munekata, J. M. Lorenzo and R. M. Aadil, Functional and Nutraceutical Significance of Amla (Phyllanthus emblica L.): A Review, Antioxidants, 2022, 11(5), 816, DOI:10.3390/antiox11050816
.
-
T. A. More, D. K. Samadia, O. P. Awasthi and S. S. Hiwale, in Varieties and hybrids of CIAH, Central Institute for Arid Horticulture, Bikaner, Rajasthan, India, 2008, p. 11 Search PubMed
.
- P. Kulkarani, H. Pandey, A. K. Sharma and D. C. Joshi, Physicochemical properties of aonla fruit and juice, Chem. Sci. Rev. Lett., 2017, 6(22), 1343–1347 CAS
.
- M. Gocher, R. Gochar, S. Rawat and D. K. Rana, Qualitative and quantities evaluation of Phyllanthus emblica L. fruits under valley condition of Garhwal Himalaya, J. Pharmacogn. Phytochem., 2020, 9(3), 1295–1299, DOI:10.22271/phyto.2020.v9.i3u.11488
.
- P. Mishra, V. Srivastava, D. Verma, O. P. Chauhan and G. K. Rai, Physico-chemical properties of Chakiya variety of Amla (Emblica officinalis) and effect of different dehydration methods on quality of powder, Afr. J. Food Sci., 2009, 3(10), 303–306 CAS
.
- D. C. Naithani, J. M. S. Rawat, B. Singh, V. P. Khanduri and M. K. Riyal, Determination of Physico-chemical Properties of Aonla (Emblica officinalis Gaertn) Fruits among Different Populations in Garhwal Himalaya, Int. J. Fruit Sci., 2020, 20(2), 1579–1589, DOI:10.1080/15538362.2020.1822264
.
- M. R. Hasan, M. N. Islam and M. R. Islam, Phytochemistry, pharmacological activities and traditional uses of Emblica officinalis: A review, Int. Curr. Pharm. J., 2016, 5(2), 14–21, DOI:10.3329/icpj.v5i2.26441
.
- N. N. Barthakur and N. P. Arnold, Chemical analysis of the emblic (Phyllanthus emblica L.) and its potential as a food source, Sci. Hortic., 1991, 47, 99–105, DOI:10.1016/0304-4238(91)90031-S
.
- M. Tilahun, L. Zhao, L. Sun, Y. Shen, L. Ma, T. R. Callaway, J. Xu and D. Bu, Fresh Phyllanthus Emblica (Amla) fruit supplementation enhances milk fatty acid profiles and the antioxidant capacities of milk and blood in dairy cows, Antioxidants, 2022, 11(3), 485, DOI:10.3390/antiox11030485
.
- M. B. Kavita, Amalaki (Indian Gooseberry): An ancient food supplement, Int. J. Res. Ayurveda Pharm., 2013, 4(11), 11–14, DOI:10.7897/2277-4343.04113
.
- R. Tewari, V. Kumar and H. K. Sharma, Pretreated Indian gooseberry (Emblica officinalis) segments: kinetic, quality and microstructural parameters, J. Inst. Eng.: Ser. A, 2021, 102, 523–534, DOI:10.1007/s40030-021-00538-9
.
- T. S. Chahal, J. Singh, P. P. S. Gill, S. K. Grewal and A. Sinha, Seasonal Variations in Biochemical and Antioxidant Properties in ‘Daisy’ Tangerine Fruits During Fruit Development, Erwerbs-Obstbau, 2023, 1–8, DOI:10.1007/s10341-023-00881-0
.
- R. K. Saini, A. Ranjit, K. Sharma, P. Prasad, X. Shang, K. G. M Gowda and Y. S. Keum, Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes, Antioxidants, 2022, 11(2), 239, DOI:10.3390/antiox11020239
.
- R. K. Goyal, R. T. Patil, A. R. P. Kingsly, H. Walia and P. Kumar, Status of postharvest technology of aonla in India – a review, Am. J. Food Technol., 2008, 3(1), 13–23, DOI:10.3923/ajft.2008.13.23
.
-
S. Pareek and L. Kitinoja, Aonla (Emblica officinalis Gaertn.), in Postharvest biology and technology of tropical and subtropical fruits, ed. E. M. Yahia, Woodhead Publishing, Cambridge, 2011, pp. 65–97, DOI:10.1533/9780857092762.65
.
- A. Bhattacharya, A. Chatterjee, S. Ghoshal and S. K. Bhattacharya, Antioxidant activity of active tannoid principle of Emblica officinalis (Amla), Indian J. Exp. Biol., 1999, 37(7), 676–680 CAS
.
- J. Kubola, S. Siriamornpun and N. Meeso, Phytochemicals, vitamin C and sugar content of Thai wild fruits, Food Chem., 2011, 126(3), 972–981, DOI:10.1016/j.foodchem.2010.11.104
.
- F. Brouns, E. Theuwissen, A. Adam, M. Bell, A. Berger and R. P. Mensink, Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women, Eur. J. Clin. Nutr., 2012, 66(5), 591–599, DOI:10.1038/ejcn.2011.208
.
- A. Filipiak-Szok, M. Kurzawa, M. Cichosz and E. Szłyk, Elemental analysis of medicinal herbs and dietary supplements, Anal. Lett., 2015, 48, 2626–2638, DOI:10.1080/00032719.2015.1041031
.
- K. Parveen and B. S. Khatkar, Physico-chemical properties and nutritional composition of aonla (Emblica officinalis) varieties, Int. Food Res. J., 2015, 22(6), 2358–2363 CAS
.
- S. Waheed and I. Fatima, Instrumental neutron activation analysis of Emblica officinalis, Terminalia belerica and Terminalia chebula for trace element efficacy and safety, Appl. Radiat. Isot., 2013, 77, 139–144, DOI:10.1016/j.apradiso.2013.03.007
.
- A. Ganachari, K. Thangavel and S. Eswaran, Post-Harvest Processing, Potential of Aonla (Emblica officinalis) fruit, Food Pack, 2005, 5(5), 34–35, DOI:10.3923/ajft.2008.13.23
.
- E. Singh, S. Sharma, A. Pareek, J. Dwivedi, S. Yadav and S. Sharma, Phytochemistry, traditional uses and cancer chemopreventive activity of Amla (Phyllanthus Emblica): the sustainer, J. Appl. Pharm. Sci., 2011, 2(1), 176–183 Search PubMed
.
- K. C. Yadav, R. Samikshya, D. Anish and D. S. Lila, Phytochemical, nutritional, antioxidant
activity and sensorial characteristics of amala (Phyllanthus Emblica L.) chutney, Asian Food Sci. J., 2020, 18(1), 43–52 Search PubMed
.
-
S. Z. Hussain, B. Naseer, T. Qadri, T. Fatima and T. A. Bhat, Anola (Emblica officinalis): morphology, taxonomy, composition and health benefits, in Fruits grown in highland regions of the Himalayas, Springer, Cham, Switzerland, 2021, pp. 193–206 Search PubMed
.
- G. Saxena and N. Chaturvedi, Proximate composition analysis of gooseberry fruit (Emblica officinalis Gaertn) and basil leaves (Occium basilium) and development of value added products by adding basil in gooseberry products, IIS Univ. J. Sci. Technol., 2015, 4(1), 19–22 Search PubMed
.
- S. Sarangam and P. Chakraborty, Comparative studies on quality analysis of freeze dried and cross flow dried amla powder, Int. J. Res. Eng. Technol., 2015, 4(4), 620–624, DOI:10.15623/ijret.2015.0404107
.
- R. K. Gorya and U. Bajwa, Enhancing the functional properties and nutritional quality of ice cream with processed amla (Indian gooseberry), J. Food Sci. Technol., 2015, 52, 7861–7871, DOI:10.1007/s13197-015-1877-1
.
- K. Judprasong, S. Charoenkiatkul, P. Thiyajai and M. Sukprasansap, Nutrients and bioactive compounds of Thai indigenous fruits, Food Chem., 2013, 140(3), 507–512, DOI:10.1016/j.foodchem.2013.01.057
.
- D. El-Amir, S. F. AbouZid, M. H. Hetta, A. A. Shahat and M. A. El-Shanawany, Composition of the essential oil of the fruits of Phyllanthus emblica cultivated in Egypt, J. Pharm., Chem. Biol. Sci., 2014, 2(3), 202–207 CAS
.
- X. Liu, M. Zhao, W. Luo, B. Yang and Y. Jiang, Identification of volatile components in Phyllanthus emblica L. and their antimicrobial activity, J. Med. Food, 2009, 12(2), 423–428, DOI:10.1089/jmf.2007.0679
.
- S. P. Wang, M. A. Yuan, S. H. Wang and F. Chen, Analysis of chemical composition of volatile oil of Phyllanthus emblica L. from Sichuan by GC-MS, West China J. Pharm. Sci., 2009, 3, 1–27 Search PubMed
.
- M. A. El Hadi, F. J. Zhang, F. F. Wu, C. H. Zhou and J. Tao, Advances in fruit aroma volatile research, Molecules, 2013, 18(7), 8200–8229, DOI:10.3390/molecules18078200
.
- R. Gawel, A. Oberholster and I. L. Francis, A “Mouth-feel Wheel”: Terminology for communicating the mouth-feel characteristics of red wine, Aust. J. Grape Wine Res., 2006, 6, 203–207, DOI:10.1111/j.1755-0238.2000.tb00180.x
.
- M. He, H. Tian, X. Luo, X. Qi and X. Chen, Molecular progress in research on fruit astringency, Molecules, 2015, 1434–1451, DOI:10.3390/molecules20011434
.
- S. Soares, E. Brandão, C. Guerreiro, S. Soares, N. Mateus and V. De Freitas, Tannins in food: Insights into the molecular perception of astringency and bitter taste, Molecules, 2020, 25, 2590, DOI:10.3390/molecules25112590
.
- E. Jobstl, J. O'Connell, J. P. A. Fairclough and M. P. Williamson, Molecular model for astringency produced by polyphenol/protein interactions, Biomacromolecules, 2004, 5, 942–949, DOI:10.1021/bm0345110
.
- P. Vijayanand, S. G. Kulkarni, P. Reena, M. Aksha and K. V. R. Ramana, Effect of processing on gooseberry fruits and quality changes in dehydrated gooseberry powder during storage, J. Food Sci. Technol., 2007, 44, 591–594, DOI:10.1007/s13197-010-0124-z
.
- V. K. Prajapati, P. K. Nema and S. S. Rathore, Effect of pretreatment and drying methods on quality of value-added dried aonla (Emblica officinalis Gaertn.) shreds, J. Food Sci. Technol., 2011, 48, 45–52, DOI:10.1007/s13197-010-0124-z
.
- S. Pareek and R. A. Kaushik, Effect of drying methods on quality of Indian gooseberry (Emblica officinalis Gaertn.) powder during storage, J. Sci. Ind. Res., 2012, 71(11), 727–732 CAS
.
- A. Singh, S. Santosh, M. Kulshreshtha, K. Chand, U. C. Lohani and N. C. Shahi, Quality characteristics of ohmic heated aonla (Emblica officinalis Geartn.) pulp, Indian J. Tradit. Knowl., 2013, 12(4), 670–676 Search PubMed
.
- I. Das, S. Sasmal and A. Arora, Effect of thermal and non-thermal processing on astringency reduction and nutrient retention in cashew apple fruit and its juice, J. Food Sci. Technol., 2021, 58(6), 2337–2348, DOI:10.1007/s13197-020-04744-4
.
- G. Ares, C. Barreiro, R. Deliza and A. Gámbaro, Alternatives to reduce the bitterness, astringency and characteristic flavour of antioxidant extracts, Food Res. Int., 2009, 42, 871–878, DOI:10.1016/j.foodres.2009.03.006
.
-
S. Pareek, Aonla (Emblica officinalis Gaertn.)-value added products, in Underutilized and underexploited horticultural crops, ed. Peter K. V., New India Publishing Agency, New Delhi, 2010, pp. 283–308 Search PubMed
.
- B. R. Premi, V. Sethi and D. B. Saxena, Studies on identification of white specks in cured aonla (Emblica officinalis Gaertn.) fruits, Food Chem., 1998, 61, 9–11, DOI:10.1016/S0308-8146(97)00121-0
.
- S. Gantait, M. Mahanta, S. Bera and S. K. Verma, Advances in biotechnology of Emblica officinalis Gaertn. syn. Phyllanthus emblica L.: a nutraceuticals-rich fruit tree with multifaceted ethnomedicinal uses, 3 Biotech, 2021, 11(2), 1–25, DOI:10.1007/s13205-020-02615-5
.
- P. Sengupta, S. Sen, K. Mukherjee and K. Acharya, Postharvest diseases of Indian gooseberry and their management: a review, Int. J. Fruit Sci., 2020, 20, 178–190, DOI:10.1080/15538362.2019.1608889
.
-
V. K. Wali, P. Bakshi, A. Jasrotia, B. Bhushan and M. Bakshi, Aonla, SKUAST-Jammu, India, 2015, p. 30 Search PubMed
.
- R. Lather, S. S. Sindhu and S. Kumar, Studies on preservation of aonla juice by preservatives and thermal processing, Int. J. Agric. Sci. Res., 2015, 5(5), 121–128 Search PubMed
.
- P. Kumari and B. S. Khatkar, Effect of processing treatment on nutritional properties and phytochemical contents of aonla (Emblica officinalis) juice, J. Food Sci. Technol., 2019, 56, 2010–2015, DOI:10.1007/s13197-019-03674-0
.
- N. Chinprahast, U. Siripatrawan, A. Leerahawong and K. Traiananwuttikul, Effects of blanching and vacuum impregnation on physicochemical and sensory properties of Indian gooseberry (Phyllanthus Emblica L.), J. Food Process. Preserv., 2013, 37, 57–65, DOI:10.1111/j.1745-4549.2011.00613.x
.
- A. Bhattacherjee, A. Dikshit, S. Kumar, D. K. Shukla and D. K. Tandon, Quality evaluation in storage of aonla (Emblica Officinalis Gaertn.) juice extracted from fruits preserved by steeping in water, Int. Food Res. J., 2013, 20, 1861–1865, DOI:10.1007/s13197-019-03674-0
.
- Z. V. P. Murthy and D. Joshi, Fluidized bed drying of aonla (Emblica officinalis), Drying Technol., 2007, 25, 883–889, DOI:10.1080/07373930701370290
.
- P. Mishra, S. Mishra and C. L. Mahanta, Effect of maltodextrin concentration and inlet temperature during spray drying on physicochemical and antioxidant properties of amla (Emblica officinalis) juice powder, Food Bioprod. Process., 2014, 92(3), 252–258, DOI:10.1016/j.fbp.2013.08.003
.
- R. K. Gupta, A. Sharma, P. Kumar, R. K. Vishwakarma and R. T. Patil, Effect of blanching on thin layer drying kinetics of aonla (Emblica officinalis) shreds, J. Food Sci. Technol., 2014, 51(7), 1294–1301, DOI:10.1007/s13197-012-0634-y
.
- A. Bhattacherjee, D. K. Tandon and A. Dikshit, Antioxidant activity and quality of spray dried aonla powder as affected by storage behavior of juice, J. Sci. Ind. Res., 2014, 73(9), 607–612 CAS
.
- P. Kumari and B. S. Khatkar, Nutritional composition and drying kinetics of aonla fruits, J. Food Sci. Technol., 2018, 55(5), 3135–3143, DOI:10.1007/s13197-018-3241-8
.
- N. Sonkar, D. Rajoriya, R. Chetana and K. Venkatesh Murthy, Effect of cultivars, pretreatment and drying on physicochemical properties of Amla (Emblica officinalis) gratings, J. Food Sci. Technol., 2020, 57(3), 980–992, DOI:10.1007/s13197-019-04131-8
.
- U. Priyanka and A. Carolinrathinakumari, Quality of solar tunnel dried amla segments, Int. J. Agric. Eng., 2020, 13(1), 74–79, DOI:10.1111/jfpe.12824
.
- A. Raaf, N. Suriaini, F. Djafar, Y. Syamsuddin and M. D. Supardan, Effect of drying temperature on the moisture loss, acidity and characteristics of Amla fruit, IOP Conf. Ser.: Earth Environ. Sci, 2021, 667, 012047 CrossRef
.
- A. K. Bhattacherjee, D. K. Tandon, A. Dikshit and S. Kumar, Effect of pasteurization temperature on quality of aonla juice during storage, J. Food Sci. Technol., 2011, 48, 269–273, DOI:10.1007/s13197-010-0171-5
.
- S. Methakhup, N. Chiewchan and S. Devahastin, Effects of drying methods and conditions on drying kinetics and quality of Indian gooseberry flake, LWT–Food Sci. Technol., 2005, 38, 579–587, DOI:10.1016/j.lwt.2004.08.012
.
- R. K. Gupta, P. Kumar, A. Sharma and R. T. Patil, Color kinetics of aonla shreds with amalgamated blanching during drying, Int. J. Food Prop., 2011, 14, 1232–1240, DOI:10.1080/10942911003637343
.
- R. C. Patil and R. R. Gawande, Drying characteristics of amla candy in solar, tunnel greenhouse dryer, J. Food Process Eng., 2018, 41(6), e12824, DOI:10.1111/jfpe.12824
.
- T. R. Bajgai, F. Hashinaga, S. Isobe, G. V. Raghavan and M. O. Ngadi, Application of high electric field (HEF) on the shelf-life extension of emblic fruit (Phyllanthus emblica L.), J. Food Eng., 2006, 74, 308–313, DOI:10.1016/j.jfoodeng.2005.03.023
.
- V. Bansal, M. L. Singla and C. Ghanshyam, Impact of nonthermal pulsed electric field on bioactive compounds and Browning activity in Emblica officinalis juice, Int. J. Nutr. Food Sci., 2013, 7, 897–901, DOI:10.5281/zenodo.1087750
.
- V. Bansal, A. Sharma, C. Ghanshyam, M. L. Singla and K. H. Kim, Influence of pulsed electric field and heat treatment on Emblica officinalis juice inoculated with Zygosaccharomyces bailii, Food Bioprod. Process., 2015, 95, 146–154, DOI:10.1016/j.fbp.2015.05.005
.
- S. A. Dawale and N. R. Jawade, Using nanofiltration and ultrafiltration for the concentration of Amla (Phyllanthus Emblica) Juice, Int. J. Adv. Des. Manuf. Technol., 2014, 8(1), 15–20, DOI:10.1016/j.desal.2006.03.423
.
- S. Bhat and H. K. Sharma, Combined effect of blanching and sonication on quality parameters of bottle gourd (Lagenaria siceraria) juice, Ultrason. Sonochem., 2016, 33, 182–189, DOI:10.1016/j.ultsonch.2016.04.014
.
- A. S. Raj, S. Chakraborty and P. S. Rao, Thermal assisted high-pressure processing of Indian gooseberry (Emblica officinalis L.) juice–Impact on colour and nutritional attributes, LWT–Food Sci. Technol., 2019, 99, 119–127, DOI:10.1016/j.lwt.2018.09.051
.
- S. Chakraborty, S. Ghag, P. P. Bhalerao and J. S. Gokhale, The potential of pulsed light treatment to produce enzymatically stable Indian gooseberry (Emblica officinalis Gaertn.) juice with maximal retention in total phenolics and vitamin C, J. Food Process. Preserv., 2020, 44, 12, DOI:10.1111/jfpp.14932
.
- R. Aslam, M. S. Alam, A. Ali, Y. Tao and S. Manickam, A chemometric approach to evaluate the effects of probe-type ultrasonication on the enzyme inactivation and quality attributes of fresh amla juice, Ultrason. Sonochem., 2023, 92, 106268, DOI:10.1016/j.ultsonch.2022.106268
.
- A. Singh, A. Mehta, A. P. Singh, P. K. Prabhakar and N. Kumar, Ultrasonic modulated osmotic dehydration of Aonla (Phyllanthus emblica L.) slices: an integrated modeling through ANN, GPR, and RSM, J. Food Process. Preserv., 2022, 46, 2, DOI:10.1111/jfpp.16247
.
- A. Mehta, A. Singh, A. P. Singh, P. K. Prabhakar and N. Kumar, Ultrasonic induced effect on mass transfer characteristics during osmotic dehydration of aonla (Phyllanthus emblica L.) slices: a mathematical modeling approach, J. Food Process Eng., 2021, 44, 12, DOI:10.1111/jfpe.13887
.
- P. Srinivasan, S. Vijayakumar, S. Kothandaraman and M. Palani, Anti-diabetic activity
of quercetin extracted from Phyllanthus Emblica L. fruit: in silico and in vivo approaches, J. Pharm. Anal., 2018, 8, 109–118, DOI:10.1016/j.jpha.2017.10.005
.
- P. Sharma, T. Joshi, S. Chandra and S. Tamta, In silico screening of potential antidiabetic phytochemicals from Phyllanthus Emblica against therapeutic targets of type 2 diabetes, J. Ethnopharmacol., 2020, 248, 112268, DOI:10.1016/j.jep.2019.112268
.
- M. Gupta, D. Banerjee and A. Mukherjee, Evaluation of analgesic, antipyretic and anti-inflammatory effects of methanol extract of traditional herbal medicine using rodents, J. Pharmacogn. Phytother., 2013, 5, 106–113, DOI:10.5897/JPP2013.0268
.
- N. Asmilia, A. Sutriana, D. Aliza and N. Sudril, Anti-inflammatory activity of ethanol extract from malacca leaves (Phyllanthus emblica) in carrageenan induced male mice, E3S Web Conf., 2020, 151, 01066, DOI:10.1051/e3sconf/202015101066
.
- S. Ojha, M. Golechha, S. Kumari and D. S. Arya, Protective effect of Emblica officinalis (amla) on isoproterenol-induced cardiotoxicity in rats, Toxicol. Ind. Health, 2012, 28, 399–411, DOI:10.1177/074823371141
.
- S. Rajak, S. K. Banerjee, S. Sood, A. K. Dinda, Y. K. Gupta, S. K. Gupta and S. K. Maulik,
Emblica officinalis causes myocardial adaptation and protects against oxidative stress in ischemic-reperfusion injury in rats, Phytother. Res., 2004, 18, 54–60, DOI:10.1002/ptr.1367
.
- P. Patel, H. S. Singh, A. Mishra and S. P. Ansari, Can Emblica officinalis and Tinospora cordifolia supplementation possess immunomodulatory and adaptogenic properties in murrah buffalo calves?, Indian J. Anim. Res., 2017, 51, 506–509, DOI:10.18805/ijar.v0iOF.7654
.
- S. K. Ali, A. R. Hamed, M. M. Soltan, U. M. Hegazy, E. E. Elgorashi, I. A. El-Garf and A. A. Hussein, In-vitro evaluation of selected Egyptian traditional herbal medicines for treatment of alzheimer disease, BMC Complementary Altern. Med., 2013, 13, 121, DOI:10.1186/1472-6882-13-121
.
- L. Chularojmontri, M. Suwatronnakorn and S. K. Wattanapitayakul,
Phyllanthus emblica L. enhances human umbilical vein endothelial wound healing and sprouting, J. Evidence-Based Complementary Altern. Med., 2013, 720–728, DOI:10.1155/2013/720728
.
- B. Shalini and J. Sharma, Beneficial effects of Emblica officinalis on fluoride-induced toxicity on brain biochemical indexes and learning-memory in rats, Toxicol. Int., 2015, 22, 35–39, DOI:10.4103/0971-6580.172254
.
- A. Justin Thenmozhi, M. Dhivyabharathi, T. R. William Raja, T. Manivasagam and M. M. Essa, Tannoid principles of Emblica officinalis renovate cognitive deficits and attenuate amyloid pathologies against aluminum chloride induced rat model of Alzheimer's disease, Nutr. Neurosci., 2016, 19, 269–278, DOI:10.1179/1476830515Y.0000000016
.
- S. N. Singh, A. Moses and A. David, Antimicrobial activity of Emblica officinalis extracts against selected bacterial pathogens, Int. J. Basic Appl. Res., 2019, 9, 325–330, DOI:10.22207/JPAM.13.4.11
.
- M. Majeed, S. Majeed, L. Mundkur, K. Nagabhushanam, S. Arumugam, K. Beede and F. Ali, Standardized Emblica officinalis fruit extract inhibited the activities of α-amylase, α-glucosidase, and dipeptidyl peptidase-4 and displayed antioxidant potential, J. Sci. Food Agric., 2020, 100, 509–516, DOI:10.1002/jsfa.10020
.
- N. R. Biswas, S. K. Gupta, G. K. Das, N. Kumar, P. K. Mongre, D. Haldar and S. Beri, Evaluation of Ophthacare® eye drops—a herbal formulation in the management of various ophthalmic disorders, Phytother. Res., 2001, 15, 618–620, DOI:10.1002/ptr.896
.
- S. Panda and A. Kar, Fruit extract of Emblica officinalis ameliorates hyperthyroidism and hepatic lipid peroxidation in mice, Pharmazie, 2003, 58(10), 753–755 CAS
.
- M. Dinesh, S. M. Roopan, C. I. Selvaraj and P. Arunachalam,
Phyllanthus emblica seed extract mediated synthesis of PdNPs against antibacterial, heamolytic and cytotoxic studies, J. Photochem. Photobiol., B, 2017, 167, 64–71, DOI:10.1016/j.jphotobiol.2016.12.012
.
- S. Mahata, A. Pandey, S. Shukla, A. Tyagi, S. A. Husain, B. C. Das and A. C. Bharti, Anticancer activity of Phyllanthus Emblica Linn. (Indian Gooseberry): Inhibition of transcription factor AP-1 and HPV gene expression in cervical cancer cells, Nutr. Cancer, 2013, 65, 88–97, DOI:10.1080/01635581.2013.785008
.
- C. Wiart, Note on the relevance of Emblica officinalis Gaertn. For the treatment and prevention of cancer, Eur. J. Cancer Prev., 2013, 22, 198, DOI:10.1155/2015/950890
.
- M. P. Kapoor, K. Suzuki, T. Derek, M. Ozeki and T. Okubo, Clinical evaluation of Emblica officinalis Gaertn (Amla) in healthy human subjects: Health benefits and safety results from a randomized, double-blind, crossover placebo-controlled study, Contemp. Clin. Trials Commun., 2019, 17, 100499, DOI:10.1016/j.conctc.2019.100499
.
- N. Sharma, P. Trikha, M. Athar and S. Raisuddin, Inhibitory effect of Emblica officinalis on the in vivo clastogenicity of benzo[a]pyrene and cyclophosphamide in mice, Hum. Exp. Toxicol., 2000, 19, 377–384, DOI:10.1191/096032700678815945
.
- M. Shynu, P. K. Gupta and M. Saini, Antineoplastic potential of medicinal plants, Recent Pat. Biotechnol., 2011, 5(2), 85–94, DOI:10.2174/87220811796365662
.
- C. Ngamkitidechakul, K. Jaijoy, P. Hansakul, N. Soonthornchareonnon and S. Sireeratawong, Antitumour effects of Phyllanthus Emblica L.: induction of cancer cell apoptosis and inhibition of in vivo tumour promotion and in vitro invasion of human cancer cells, Phytother. Res., 2010, 24, 1405–1413, DOI:10.1002/ptr.3127
.
- B. C. Variya, A. K. Bakrania and S. S. Patel,
Emblica officinalis (Amla): A review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms, Pharm. Res., 2016, 111, 180–200, DOI:10.1016/j.phrs.2016.06.013
.
- B. Gopa, J. Bhatt and K. G. Hemavathi, A comparative clinical study of hypolipidemic efficacy of Amla (Emblica officinalis) with 3-hydroxy-3-methylglutaryl-coenzyme-A reductase inhibitor simvastatin, Indian J. Pharmacol., 2012, 44, 238, DOI:10.4103/0253-7613.93857
.
- P. S. Chen and J. H. Li, Chemopreventive effect of punicalagin, a novel tannin component isolated from Terminalia catappa, on H-ras-transformed NIH3T3 cells, Toxicol. Lett., 2006, 163, 44–53, DOI:10.1016/j.toxlet.2005.09.026
.
- D. B. Reddy, T. C. M. Reddy, G. Jyotsna, S. Sharan, N. Priya, V. Lakshmipathi and P. Reddanna, Chebulagic acid, a COX–LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 cell line, J. Ethnopharmacol., 2009, 124, 506–512, DOI:10.1016/j.jep.2009.05.022
.
- K. V. Kulkarni and S. M. Ghurghure, Indian gooseberry (Emblica officinalis): Complete pharmacognosy review, Int. J. Chem. Stud., 2018, 2, 5–11 Search PubMed
.
- S. M. Hassan, S. S. Mughal, A. A. Aslam, M. Mushtaq, M. Munir, S. Pervez, N. Shabbir, A. R. Ayub and M. Farman,
Emblica officinalis (Amla): A prospective review on distinctive properties and therapeutic applications of Amla, Innovare J. Ayurvedic Sci., 2020, 8(3), 22–30 Search PubMed
.
- S. Madhuri, G. Pandey and K. S. Verma, Antioxidant, immunomodulatory and anticancer activities of Emblica officinalis: an overview, Int. Res. J. Pharm., 2011, 2(8), 38–42 CAS
.
- A. Bhattacharya, A. Chatterjee, S. Ghosal and S. K. Bhattacharya, Antioxidant activity of active tannoid principles of Emblica officinalis (Amla), Indian J. Exp. Biol., 1999, 37(7), 676–680 CAS
.
- J. H. Chang, J. H. Cho, H. H. Kim, K. P. Lee, M. W. Lee, S. S. Han and D. I. Lee, Antitumor activity of pedunculagin, one of the ellagitannin, Arch. Pharmacal Res., 1995, 18, 396–401, DOI:10.1007/BF02976342
.
- A. Kumaran and R. J. Karunakaran, Nitric oxide radical scavenging active components from Phyllanthus Emblica L, Plant Foods Hum. Nutr., 2006, 61, 1–5, DOI:10.1016/j.jpha.2017.10.005
.
- H. Y. Kim, T. Okubo, L. R. Juneja and T. Yokozawa, The protective role of Amla (Emblica officinalis Gaertn.) against fructose-induced metabolic syndrome in a rat model, Br. J. Nutr., 2010, 103, 502–512, DOI:10.1017/S0007114509991978
.
- O. P. Chauhan, S. Srivastava, P. Pandey and G. K. Rai, A study on the development of aonla blended sauce, Beverage & Food World, 2005, 32, 31–32 Search PubMed
.
- K. H. Khan, Roles of Emblica officinalis in medicine: a review, Bot. Res. Int., 2009, 2, 218–228 CAS
.
- M. S. Baliga and J. J. Dsouza, Amla (Emblica officinalis Gaertn), a wonder berry in the treatment and prevention of cancer, Eur. J. Cancer Prev., 2011, 20, 225–239, DOI:10.1097/CEJ.0b013e32834473f4
.
- S. Jung, K. Lee and S. Byun, Myricetin suppresses UVB-induced skin cancer by targeting Fyn, Cancer Res., 2008, 68, 6021–6029, DOI:10.1158/0008-5472.CAN-08-0899
.
- J. Charoenteeraboon, C. Ngamkitidechakul, N. Soonthornchareonnon, K. Jaijoy and S. Sireeratawong, Antioxidant activities of the standardized water extract from fruit of Phyllanthus emblica Linn, Songklanakarin J. Sci. Technol., 2010, 32(6), 599–604 CAS
.
- V. T. Kore, H. L. Devi and J. Kabir, Packaging, storage, and value addition of aonla, an underutilized fruit, in India, Fruits, 2013, 68, 255–266, DOI:10.1051/fruits/2013064
.
- X. Yin, C. Kaiwen, C. Hao, C. Xing, F. Shuai, S. Yuanda and L. Li, Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology, Antioxidants, 2022, 11(1), 153, DOI:10.3390/antiox11010153
.
- R. Sharma, N. Martins, K. Kuca, A. Chaudhary, A. Kabra, M. M. Rao and P. K. Prajapati, Chyawanprash: A Traditional Indian Bioactive Health Supplement, Biomolecules, 2019, 9(5), 161, DOI:10.3390/biom9050161
.
- K. Gajanana, A. K. Rokhade, P. B. Patil and M. S. Kulkarni, Standardisation of recipe for preparation of aonla squash, Beverage & Food World, 2007, 34, 55–56 Search PubMed
.
- A. Garg, Studies on processing and development of carbonated spiced RTS drink from bitter gourd and amla fruit, Process. Food Ind., 2010, 13, 23–26 Search PubMed
.
- V. P. Argade and V. V. Pande, Preparation and standardization of amla wine by using Saccharomyces cerevisiae yeast as a fermenter, Inventi Rapid: Planta Activa, 2015, 2, 1–5 Search PubMed
.
- D. K. Tandon, R. C. Yadav, S. Sood, S. Kumar and A. Dikshit, Effect of blanching and lye peeling on the quality of Aonla candy, Indian Food Packer, 2003, 57, 147–152 Search PubMed
.
- V. K. Prajapati, P. K. Nema and S. S. Rathore, Effect of pretreatment and drying methods on quality of value-added dried aonla (Emblica officinalis Gaertn) shreds, J. Food Sci. Technol., 2011, 48, 45–52, DOI:10.1007/s13197-010-0124-z
.
- S. Devi, E. Gupta and N. K. Maurya, Development of a value-added Amla product, Int. Arch. Appl. Sci. Technol., 2020, 11, 90–93, DOI:10.15515/iaast.0976-4828.11.2.9093
.
- P. P. Bhalerao, S. A. Mahale, R. Dhar and S. Chakraborty, Optimizing the formulation for a pomegranate-amla-muskmelon based mixed fruit beverage using sensory analysis and evaluating its thermal stability, LWT–Food Sci. Technol., 2020, 133, 109907 CrossRef CAS
.
- D. Alkandari, H. Sarfraz and J. S. Sidhu, Development of a functional food (pan bread) using amla fruit powder, J. Food Sci. Technol., 2019, 56, 2287–2295, DOI:10.1007/s13197-019-03718-5
.
- V. S. Barwal, V. Garg and R. Sharma, Development and quality evaluation of aonla mouth freshener, J. Food Sci. Technol., 2010, 47, 697–699, DOI:10.1007/s13197-010-0129-7
.
- M. K. Mahawar, K. Jalgaonkar, B. Bibwe, T. Kulkarni, B. Bhushan and V. S. Meena, Optimization of mixed aonla-guava fruit bar using response surface methodology, Nutr. Food Sci., 2018, 48, 621–630, DOI:10.1108/NFS-09-2017-0189
.
- S. Sohshangrit, V. M. Prasad and C. S. Nura, Standardization of Amla Candy (Emblica officinalis L.) Cv. Kanchan, Int. J. Plant Sci., 2022, 34(20), 608–614 Search PubMed
.
-
S. Singh, A. K. Singh and H. K. Joshi, Effect of size grading on quality and shelf life of aonla (Emblica officinalis Gaertn.) cv. NA-7, Proceedings of national seminar on commercialization of horticulture in non-traditional areas, Indian Society of Arid Horticulture, Central Institute for Arid Horticulture, Bikaner, India, 2005 Search PubMed
.
- V. Singh, P. Singh and A. K. Singh, Physicochemical composition and evaluation of aonla cultivars under Chhattisgarh conditions, Indian J. Hortic., 2009, 66, 267–270 Search PubMed
.
- S. S. Dhumal, A. R. Karale, V. K. Garande, B. T. Patil, S. D. Masalkarm and D. B. Kshirsagar, Shelflife of aonla fruits: Influenced by postharvest treatments and packaging materials, Indian J. Agric. Res., 2008, 42, 189–194 Search PubMed
.
- J. K. Singh, J. Prasad, H. K. Singh and A. Singh, Effect of micronutrients and plantgrowth regulators on plant growth and fruit drop in aonla (Emblica officinalis Gaertn.) fruits cv.Narendra Aonla-10, Plant Arch., 2008, 8(2), 911–913 Search PubMed
.
- S. Gangwar, H. S. Shukla, D. Katiyar and V. Pandey, Effect of calcium nitrate on physico-chemical changes and shelf-life of aonla (Emblica officinalis Gaertn.) fruits, HortFlora Res. Spectrum, 2012, 1(3), 253–258 Search PubMed
.
- R. Kumar, S. Lal and M. Kumar, Effect of postharvest packing materials and calcium on shelf life of guava, Agric. Res. J., 2014, 34, 127–130 Search PubMed
.
- H. Tarula, J. M. S. Rawat, K. K. Singh, V. Rawat and V. Singh, Effect of post-harvest treatments on quality and shelf life of aonla cultivars, HortFlora Res. Spectrum, 2015, 4, 17–21 Search PubMed
.
- R. Thokchom and G. Mandal, Effect of Various Post Harvest Treatments on Storage Behaviour of Aonla cv. Chakaiya, Int. J. Bio-Resour. Stress Manage., 2018, 9, 269–273, DOI:10.23910/IJBSM/2018.9.2.1849b
.
- V. Nath, I. S. Singh and S. Kumar, Evaluation of aonla cultivars for their shelf-life at ambient temperature, Narendra Deva J. Agric. Res., 2008, 7, 117 Search PubMed
.
-
R. K. Pathak, Status Report on Genetic Resources of Indian Gooseberry Aonla (Emblica officinalis Gaertn.) in South and South East Asia. IPGRI Office for South Asia, New Delhi, India, 2003 Search PubMed
.
-
S. Pareek and L. Kitinoja, Aonla (Emblica officinalis Gaertn.), in Postharvest biology and technology of tropical and subtropical fruits, ed. E. M. Yahia, Woodhead Publishing, Cambridge, 2011, pp. 65–97, DOI:10.1533/9780857092762.65
.
-
I. N. Gowda Doreyappa, D. V. Sudhakar Rao, R. B. Tiwari, S. Bhuvaneswari and C. K. Narayana, Postharvest management and value addition in aonla, IIHR, Tech. Bull., 2011, vol. 39, p. 15 Search PubMed
.
- B. L. Meena, R. A. Kaushik, D. K. Sarolia, R. K. Meena and D. Singh, Effect of postharvest treatments on keeping quality of Aonla fruits in liquid medium during storage, Int. J. Chem. Stud., 2018, 6(5), 921–924 CAS
.
- U. P. Singh, D. S. Bunkar, D. C. Rai, S. Singh and P. P. Singh, Cumulative effect of modified atmospheric packaging on the textural and chemical properties of aonla cut-fruits during storage, J. Pharmacogn. Phytochem., 2018, 7, 1754–1758 CAS
.
- J. Yu, D. Xu, H. N. Guan, C. Wang and L. K. Huang, Facile one-step green synthesis of gold nanoparticles using Citrus maxima aqueous extracts and its catalytic activity, Mater. Lett., 2016, 166, 110–112, DOI:10.1016/j.matlet.2015.12.031
.
- T. Muthukumar, B. Sambandam, A. Aravinthan, T. P. Sastry and J. H. Kim, Green synthesis of gold nanoparticles and their enhanced synergistic antitumor activity using HepG2 and MCF7 cells and its
antibacterial effects, Process Biochem., 2016, 51, 384–391, DOI:10.1016/j.procbio.2015.12.017
.
- M. R. Bindhuand and M. Umadevi, Antibacterial activities of green synthesized gold nanoparticles, Mater. Lett., 2014, 120, 122–125, DOI:10.1016/j.matlet.2014.01.108
.
- S. Lokina, R. Suresh, K. Giribabu, A. Stephen, R. Lakshmi Sundaram and V. Narayanan, Spectroscopic investigations, antimicrobial, and cytotoxic activity of green synthesized gold nanoparticles, Spectrochim. Acta, Part A, 2014, 129, 484–490, DOI:10.1016/j.saa.2014.03.10
.
- B. Ankamwar, C. Damle, A. Ahmad and M. Sastry, Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution, J. Nanosci. Nanotechnol., 2005, 5, 1665–1671, DOI:10.1166/jnn.2005.184
.
- S. Mookriang, A. Jimtaisong, N. Saewan, K. Kittigowittana, P. Rachtanapun, V. Pathawinthranond and T. Sarakornsri, Green synthesis of silver nanoparticles using a vitamin C rich Phyllanthus emblica extract, Adv. Mater. Res., 2013, 622–623, DOI:10.4028/www.scientific.net/AMR.622-623.864
.
- M. M. I. Masum, M. M. Siddiqa, K. A. Ali, Y. Zhang, Y. Abdallah, E. Ibrahim, W. Qiu, C. Yan and B. Li, Biogenic synthesis of silver nanoparticles using Phyllanthus emblica fruit extract and its inhibitory action against the pathogen Acidovoraxoryzae strain RS-2 of rice bacterial brown stripe, Front. Microbiol., 2009, 10, 820, DOI:10.3389/fmicb.2019.00820
.
- R. K. Meena, R. Meena, D. K. Arya, S. Jadoun, R. Hada and R. Kumari, Synthesis of silver nanoparticles by Phyllanthus emblica plant extract and their antibacterial activity, Mater. Sci. Res. India, 2020, 17, 136–145 CAS
.
| This journal is © The Royal Society of Chemistry 2023 |