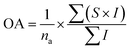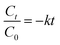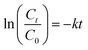Effect of different storage conditions on the quality attributes of sweet lime juice subjected to pulsed light and thermal pasteurization
Received
6th February 2023
, Accepted 15th June 2023
First published on 4th July 2023
Abstract
The primary issue of the sweet lime juice processing industry is its limited stability. The aim of this study is to examine the shelf life of pulsed light (PL) treated sweet lime juice at 4, 15, and 25 °C while packed in two different packaging materials (coextruded ethylene vinyl alcohol copolymer [EVOH] and multi-layered polyethylene terephthalate [ML PET] films). The PL-treated (3000 J cm−2) juice was analyzed for microbial count, enzyme activity, bioactive compound degradation, and sensory attributes for up to 46 days. Different packaging materials and storage temperatures had a significant effect (p < 0.05) on the microbial, enzyme inactivation, and biochemical attributes but had no significant influence (p > 0.05) on pH, acidity, and soluble solids in the samples. The juice treated under equivalent thermal conditions (95 °C/5 min) and PL-treated juice possessed sensory scores of 5.8 and 7.2, respectively. The PL-treated juice preserved 23% more antioxidants, 12.6% more phenolics, and 35.3% more vitamin C than the thermally pasteurized beverage after 46 days of storage (at 4 °C). The juice stored in ML-PET had a better shelf life and nutrient retention than that in the EVOH pouch. Interestingly, the sensory score and vitamin C profile were above the allowable limit even after 46 days of storage at 4 °C. However, the microbial count (>6 log cfu mL−1) restricted the shelf life up to 46, 30, and 13 days, at 4, 15, and 25 °C, respectively for the juice packed in the ML-PET pouch. To conclude, PL can be a great non-thermal technology to extend the shelf life of sweet lime juice from 6 to 46 days during refrigerated storage (4 °C).
Sustainability spotlight
This research examines the shelf life of PL-treated sweet lime juice at 4, 15, and 25 °C and in various packaging materials (coextruded ethylene vinyl alcohol copolymer [EVOH] and multi-layered polyethylene terephthalate [ML PET] films). Microbiological count and enzymatic activity were analyzed on untreated, PL-treated, and thermal-treated juices. Batch-processed PL and thermal sample treatment intensities were assessed as lethality, or the lowest intensity necessary to inactivate natural or inoculated microbiota by 5 log10 cycles, and juice spoiling enzyme activity by 90%. The physicochemical and nutritional attributes of the untreated, PL-treated, and thermal-treated juice samples were also investigated. The study hopes to satisfy all stakeholders, including the manufacturer, retailer, and consumers by providing the shelf life of PL and thermal pasteurized sweet lime juice. Further, by exploring new juice processing technologies while keeping sustainability in mind, we can reduce the adverse impact of food production and processing on the environment and promote food sustainability.
|
1. Introduction
Fruit juices contribute to human nutrition and wellness. Consumers want fruit juices that are convenient, fresh-like, fairly priced, functional, healthy, and long-lasting.1 Sweet lime juice has been chosen as a research material. The mild palatable flavor, unique aroma, and color make the juice more refreshing, and it is highly preferred among other juices. Fresh sweet lime juice at room temperature contains various disease-causing and spoilage microorganisms. Due to fruits' sugar content and enzyme activity, their shelf life might be shortened by microbiological, chemical, and enzymatic changes if juice production is not managed appropriately. Thermal processing is commonly used to avoid fruit juice degradation, however the harmful effects of applied temperatures on juice nutrition have encouraged research into non-thermal food processing alternatives. Non-thermal procedures may preserve the nutritional and functional characteristics of juice, inactivate microorganisms, and increase shelf life. Fruit juices stored at 4 °C have a longer shelf life.2
Pulsed light (PL) may be used to non-thermally pasteurize fruit juices using short bursts of light pulses (100–1100 nm) to inactivate bacteria and enzymes that cause spoilage.3–6 The shelf life of pulsed light treated fruit juices depends on the juice type, initial microbial load, pulse intensity, processing time, and storage conditions. Pulsed light treatment may increase the shelf life of fruit juices by weeks or months. Thermal and PL treatments may be useful for ensuring microbiological safety and enzymatic stability in juices, but their long-term storage effects must be explored. Stability relates to the microbiological, enzymatic, physicochemical, and sensory qualities of juice throughout its storage period. The effect of PL on fruit juice shelf life isn't well studied. Palgan et al.7 studied the shelf life of PL-treated apple-cranberry juice blends. Similarly, Basak et al.8 attempted shelf-life extension of apple ber, carambola, and black table grape juice blends by PL treatment. However, the shelf life of pulsed light-treated juices, notably sweet lime juice, is under-studied.
This research examines the shelf life of PL-treated sweet lime juice at 4, 15, and 25 °C and in various packaging materials (coextruded ethylene vinyl alcohol copolymer [EVOH] and multi-layered polyethylene terephthalate [ML PET] films). Microbiological count and enzymatic activity were analyzed on untreated, PL-treated, and thermal-treated juices. Batch-processed PL and thermal sample treatment intensities were assessed as lethality, or the lowest intensity necessary to inactivate natural or inoculated microbiota by 5 log10 cycles, and juice spoiling enzyme activity by 90%. The physicochemical and nutritional attributes of the untreated, PL-treated, and thermal-treated juice samples were also investigated. By providing the shelf life of PL and thermal pasteurized sweet lime juice, the study hopes to satisfy all stakeholders, including the manufacturer, retailer, and consumers.
2. Materials and methods
2.1. Preparation of the sample
Greenish to light yellow colored sweet lime (Citrus limetta var Phule mosambi) was selected for juice extraction. The fruits were decontaminated using 100 ppm sodium hypochlorite before being peeled and put through a centrifugal juicer (HR 1863/20 Philips India). The light yellowish colored juice had a total soluble solid (TSS) content of more than 11 °Bx with an acidity ranging between 2.2 and 2.4% w/w equivalent citric acid. The pH of the resultant juice was 3.5 ± 0.01.
2.2. Experimental design and selection of processing conditions
The PL intensity selected for the treatment was the minimum intensity (3000 J cm−2) required to achieve 5 log cycle reduction in natural microbiota and complete inactivation of the spoilage enzymes such as pectin methyl esterase (PME), polyphenol oxidase (PPO), and peroxidase (POD). This indicates that the intensity below 3000 J cm−2 cannot guarantee complete microbial safety and enzyme stability in the sweet lime juice. Shaik and Chakraborty5 reported that among the natural microorganisms (aerobic mesophiles [AM] and yeasts and molds [YM]), and inoculated microorganisms (E. coli, S. cerevisiae, L. monocytogenes), the YM group in the sweet lime juice was most resistant to PL treatment. Moreover, PPO was the most resistant spoilage enzyme among PPO, POD, and PME. Eventually, a PL treatment of 3000 J cm−2 was sufficient to reduce the YM count by 5 log cycles and inactivate the PPO by 99%. Therefore, the juice was treated at 3000 J cm−2 and stored in two different packaging materials at 3 different storage temperatures (4, 15, and 25 °C). Similarly, the effect of thermal processing on the microbial, enzymatic inactivation, and biochemical attributes of sweet lime juice has been studied and 95 °C/5 min are the conditions at which the microbial safety (5 log reduction in S. cerevisiae), enzymatic stability (99.9% inactivation in PPO enzyme), and nutritional quality (90% retention in vitamin C) were achieved. Therefore, the storage studies were conducted under these conditions. The samples were aseptically transferred to sterile EVOH and ML pouches after the PL and thermal treatments. The untreated samples were also packed accordingly in EVOH and ML pouches. The two separate packaging materials employed for the storage research were multilayered (ML) polyethylene terephthalate (PET) film (12 μm/oriented nylon 15 μm/aluminum foil 12 μm/polypropylene 70 μm) and coextruded ethylene vinyl alcohol (EVOH) films (polypropylene 28 μm/nylon-6 13 μm/EVOH 8 μm/nylon-6 17 μm/polypropylene 34 μm). The multi-layer PET film and EVOH films had a thickness of 109 μm and 100 μm, respectively. The specifications of the packaging materials were provided by the manufacturer. The films' mechanical and high-barrier properties were considered.9 Coextruded EVOH films are the most preferred fruit packaging material. This film's hydrophilic ethyl vinyl alcohol monomer blocks oxygen, while the nylon/polypropylene layer enhances the sealability and moisture barrier.10 The ML-PET film blocks oxygen and moisture better.11 The surface of the pouch was exposed to UV light for 24 h before usage. In these pouches, 200 mL of juice samples were heat sealed and kept in an incubator at 4.0 ± 0.5 °C, 15.0 ± 0.5 °C, and 25.0 ± 0.5 °C with 85% relative humidity (RH). Prior to the storage, the incubator was decontaminated with ethanol and 100 ppm hypochlorite solution before being dried by air. Up to the 47th day of storage, the juice samples were examined for microbiological quality and enzyme stability.
2.3. Pulsed light and thermal treatments
Treatment of sweet lime juice was conducted using pulsed light equipment (X-1100 system, Xenon Corporation, Wilmington, MA, USA), which works in batch mode. The PL equipment consists of a capacitor, air blower (which prevents overheating), lamp supporting system, and treatment chamber (Fig. 1). Radiometers or optical sensors measure fluence intensity at the same height as the sample. 200 mL juice was poured horizontally 2.4 cm beneath the lamp's surface into a top-open glass cylinder (3.5 cm × 37.5 cm; 1 Hz frequency, 400 μs pulse width; PL conditions 3000 J cm−2, average 10.0 ± 0.01 W cm−2). The maximum temperature increase was 24.1 ± 0.2 °C. Further, for thermal treatment, the juice was packaged in 200 mL translucent multi-layer PET and EVOH films which had a thickness of 109 μm and 100 μm, respectively. The samples were treated at 95.0 ± 0.5 °C for 5 min in a thermostatic water bath (Lab-lineTM, USA). Two different treatments were implemented to achieve microbial and enzymatic stability in the juice. The non-thermal pasteurization was achieved by pulsed light (PL) treatment at 3000 J cm−2. A separate thermal treatment was conducted in a batch mode when the juice was packed inside the pouch. The thermal pasteurization conditions were 95 °C/5 min. A K-type thermocouple was used to detect the sample core temperature while maintaining a dummy pouch in the same location. The sample center took 137 seconds to heat up to 95 °C, then 25 seconds to cool down to 4 °C. Each of the thermal and PL pasteurized juices were packed in two different packaging materials viz. EVOH and ML-PET. Each pouch was stored at 3 different storage temperatures. Besides, untreated samples were also packed in both packaging materials and stored at 3 different temperatures. Thus, there were 3 × 2 × 3 = 18 pouches of juice on day zero of storage considering all the treatments (untreated, PL and thermal), packaging materials (EVOH and ML-PET), and storage temperatures (4, 15, and 25 °C). All these samples were stored at the respective temperatures and analyzed after a specific time interval of up to 47 days. Maintaining aseptic conditions while performing thermal pasteurization in a thermostatic water bath is difficult. Hence, it is prepacked to maintain sterile conditions during treatment. Meanwhile, for pulsed light, the treatment is carried out in an aseptic environment. We have compared package thermal treatment with direct PL treatment of the juice. A direct thermal pasteurization may have different treatment time to achieve equivalent lethality, thus yielding a different dataset. For shelf-life studies, the thermal treatment was chosen since it caused a 5 log reduction in aerobic mesophiles (AM), yeast, and molds (YM). Additionally, no indications of PME, POD, or PPO activity were seen immediately after the treatment, indicating complete inactivation.
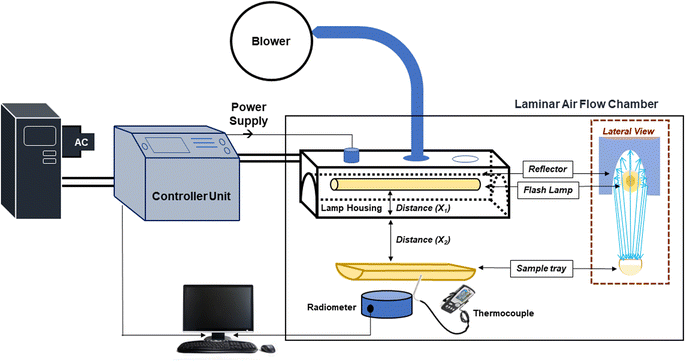 |
| | Fig. 1 Schematic representation of the pulsed light system used for the study. | |
2.4. Determination of quality characteristics
2.4.1. Natural microbiota enumeration.
Aerobic mesophiles (AM) and yeast and molds (YM) were quantified in untreated, PL-treated, and thermal treated juice samples during storage. Plate count agar (HIMEDIA® M091-500G) and yeast & mold agars (HIMEDIA® M424-500G) from HiMedia laboratories were used. The enumeration of aerobic mesophiles (AM) and yeast and molds (YM) was performed as per the protocol followed.5 The serial dilution pour plate method was followed.12 Suitable dilutions were made from the inocula (sample) and one mL of diluted inoculum was transferred into a sterile agar plate followed by liquid (≤45 °C) agar, and vigorously mixed. AM plates were incubated at 37 °C for 24 h, while YM plates were incubated at 30 °C for 48 h. The microbial detection limit was 10 cfu mL−1 of juice.
2.4.2. Enzyme activity.
The extraction and quantification of POD and PPO and PME extraction and analysis were conducted as reported by Shaik and Chakraborty.5 The enzyme's residual activity is given relative to the untreated sample (assuming 100% activity).
2.4.3. Measurement of pH, total soluble solids, titratable acidity, and color profile.
The total soluble solids (TSS), pH, titratable acidity (TA), and color profile of the juice sample were calculated using the methods described in Shaik and Chakraborty.5
2.4.4. Estimation of total phenolics, antioxidant capacity, and vitamin C.
Total phenolic content, antioxidant capacity, and vitamin C were determined using the spectrophotometric technique reported by Shaik & Chakraborty.5 Total phenolic content (TPC) was measured in gallic acid equivalents per liter (g GAE per L). Antioxidant capacity (AOX) was quantified as g GAEAC per L of sample. L-Ascorbic acid was used as a reference compound to estimate vitamin C as g AA per L.
2.5. Sensory analysis
The sensory profile was analyzed for the microbially safe juices during storage. The study was reviewed, approved by the Institutional Review Board (IRB) at the Institute of Chemical Technology, Mumbai and informed consent was obtained from each subject prior to their participation in the study. A panel of 25 semi-trained people (16 males and 9 females aged 23 to 35) from the Institute of Chemical Technology, Mumbai evaluated aroma, taste, color, consistency, mouthfeel, and aftertaste. The panel was trained for 12 days (1.5 h per day) till evaluation findings became repetitive.8 50 mL juice was served in a 100 mL sterile transparent glass container (coded with alpha-numeric digits) for sensory evaluation. Low-salt crackers and lukewarm water eliminated taste bias between two samples. The panelists rated the juice sample from 1 (extreme dislike) to 9 (extreme like). In addition to the hedonic score (9-point scale), they assessed significance (I) to each sensory trait from 1 to 5, meaning not important to extremely important. The overall acceptability (OA) of the individual juice sample was calculated using eqn (1)| | 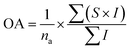 | (1) |
where na is the number of attributes, S is the hedonic score (1 to 9 scale), and I is the importance score (scale of 1 to 5).
2.6. Kinetic data modelling
The bioactive compound degradation was fitted and described by either a zero-order model or a first-order model in the thermal and pulsed light-treated sweet lime juices. Both zero-order kinetic (eqn (2)) and first-order kinetic (eqn (3)) models were used to fit the changes in total phenolic compounds, antioxidant capacity, and ascorbic acid that occurred in the juice during storage.| | 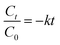 | (2) |
| | 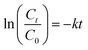 | (3) |
where C0 and Ct represent the concentration of the bioactive attribute analyzed at day ‘0’ after treatment and at day ‘t’ of storage, respectively; k is the rate constant (mol L−1 s−1 for zero-order and per day for first order). Based on adjusted R2 > 0.9, the best-fitting model from these two models was selected for each attribute.
2.7. Principal component analysis
The datasets of six attributes in sweet lime juice, including changes in TPC, AOX, Vit-C, ΔE*, BI, and OA, after storage at different temperatures were subjected to principal component analysis (PCA) so that the variability could be explained by a smaller number of components. For this, Origin Pro 10.0 (Origin Software, USA) was employed with a multivariate analysis of variance (MANOVA) methodology. Any two responses were compared using Pearson's correlation coefficients.
2.8. Statistical analysis
Three treatments and three replications were used to analyze each sample, yielding nine data points for each set of treatment conditions of each processing technique. All the results were expressed as a mean of nine parallel readings along with a standard error except for sensory analysis. Using one-way analysis of variance (ANOVA) and the Tukey's HSD test using the SPSS programme (IBM, SPSS v16, USA), the 95% confidence interval was used to test for significant changes in mean values.
3. Results and discussion
3.1. Changes in quality characteristics during storage
3.1.1. Natural microbiota enumeration.
Microbial stability is the key to fruit juice safety during storage. The untreated sweet lime juice had AM and YM count of 5.4 ± 0.2 and 5.1 ± 0.2 respectively. The untreated sample had AM count <6.0 log10 cfu mL−1 on day 2 at 25 °C (Table 1). At 15 and 4 °C, AM and YM counts in untreated juice sample were below 6.0 log10 cfu mL−1 for 5 and 6 days, respectively. Untreated juice packed in either EVOH or ML-PET showed AM and YM populations of >6.0 log10 cfu mL−1 on days 3, 6, and 7 when stored at 25, 15, and 4 °C, respectively. Those spoiled samples (AM and YM populations >6.0 log10 cfu mL−1) bulged due to gas production in the course of fermentation. In PL-treated samples, the AM and YM populations were below the detection limit (1 log10 cfu mL−1) from day 0 to day 35 when stored at 4 °C. However, AM and YM populations were > 1.5 log10 cfu mL−1 after 5 days at 25 °C (Fig. 2 and 3). The AM and YM counts on the 47th day were >6 log cfu mL−1, but they were less than 6 log cfu mL−1 on day 46. Therefore, the shelf life of PL-treated sweet lime juice packed in ML film was 46 days at 4 °C. AM and YM in PL-treated samples were affected by packing material after 35 days at 4 °C. The AM and YM counts were 5.6 and 4.2 log cfu mL−1 at 15 °C for 30 days in EVOH pouches. The samples packed in ML film possessed a lower microbial load than EVOH samples. ML film's increased barrier qualities (water vapor permeability [WVP] of 0.037 g mm2 m−2 h−1 kPa−1) may have limited the oxygen migration needed for microbial growth. Studies have shown a similar pattern for high-pressure processed sugarcane juice that is stored in EVOH and ML-PET packaging at both ambient and refrigerated temperatures.13,14 This is reflected in the AM and YM counts in the PL-treated sweet lime juice packed in the EVOH and ML-PET pouches. The oxygen transmission rates for EVOH and ML-PET are 0.62 and 0.54 cm3 (m2 per day·per atm), respectively. The oxygen barrier properties of EVOH are compromised when exposed to high-moisture foods like juice, and the oxygen permeability of coextruded EVOH increases at RH above 75–80%.15 The type of microbial growth that can occur in the juice is influenced by the oxygen barrier properties of the package, as most molds and yeasts that grow in citrus juice are aerobic, meaning that higher levels of oxygen inside the juice can lead to slightly higher levels of microbial growth.16
Table 1 Changes in quality attributes of untreated sweet lime juice packed in two different packaging materials and stored at different temperatures
| Attributes |
Untreated at day 0 |
Packaging material/storage temperature |
| EVOH/4 °C |
ML/4 °C |
EVOH/15 °C |
ML/15 °C |
EVOH/25 °C |
ML/25 °C |
| Day 4 |
Day 6 |
Day 4 |
Day 6 |
Day 3 |
Day 5 |
Day 3 |
Day 5 |
Day 2 |
Day 2 |
| AM (log cfu mL−1) |
5.4 ± 0.2 |
5.7 ± 0.1 |
5.9 ± 0.3 |
5.5 ± 0.2 |
5.8 ± 0.2 |
5.6 ± 0.3 |
5.9 ± 0.4 |
5.6 ± 0.3 |
5.8 ± 0.1 |
5.7 ± 0.2 |
5.7 ± 0.1 |
| YM (log cfu mL−1) |
5.1 ± 0.2 |
5.3 ± 0.1 |
5.6 ± 0.2 |
5.3 ± 0.2 |
5.7 ± 0.1 |
5.5 ± 0.2 |
5.7 ± 0.2 |
5.5 ± 0.3 |
5.8 ± 0.1 |
5.4 ± 0.2 |
5.2 ± 0.4 |
| PPO activity (%) |
100 |
74.3 ± 1.1 |
69.8 ± 1.2 |
75.9 ± 1.4 |
70.7 ± 1.1 |
63.9 ± 0.9 |
44.1 ± 1.1 |
65.6 ± 1.3 |
48.1 ± 1.1 |
38.3 ± 1.5 |
41.4 ± 1.2 |
| POD activity (%) |
100 |
72 ± 1.2 |
68.3 ± 1.3 |
74.2 ± 1.2 |
69.8 ± 1.2 |
62.7 ± 1.2 |
43.8 ± 1.4 |
63.6 ± 1.2 |
47.5 ± 1.3 |
37.4 ± 1.1 |
40.2 ± 1.5 |
| PME activity (%) |
100 |
71.4 ± 1.3 |
67.4 ± 1.1 |
73.8 ± 1.4 |
67.1 ± 1.1 |
61.6 ± 1.3 |
41.3 ± 1.2 |
62.8 ± 1.1 |
42.8 ± 1.2 |
34.6 ± 1.1 |
36.3 ± 0.9 |
| Color change (ΔE*) |
|
8.65 ± 0.2 |
9.2 ± 0.1 |
8.3 ± 0.1 |
8.9 ± 0.2 |
8.86 ± 0.2 |
9.3 ± 0.1 |
8.5 ± 0.2 |
9.0 ± 0.1 |
13.2 ± 0.3 |
11.7 ± 0.1 |
| Browning index (BI) |
60.0 ± 1.1 |
60.2 ± 1.2 |
60.2 ± 1.4 |
60.1 ± 1.4 |
60.1 ± 1.5 |
61.5 ± 1.3 |
61.9 ± 1.4 |
61.4 ± 1.5 |
61.8 ± 1.6 |
61.7 ± 1.0 |
61.7 ± 1.7 |
| Total phenolics (g GAE per L) |
25.7 ± 0.1 |
24.4 ± 0.3 |
23.9 ± 0.4 |
24.8 ± 0.4 |
24.4 ± 0.5 |
22.3 ± 0.3 |
22.7 ± 0.5 |
23.3 ± 0.3 |
22.9 ± 0.5 |
21.8 ± 0.2 |
21.6 ± 0.6 |
| Antioxidant activity (g GAEAC per L) |
22.4 ± 0.3 |
20.3 ± 0.5 |
18.2 ± 0.3 |
20.7 ± 0.6 |
21.3 ± 0.4 |
19.4 ± 0.2 |
18.7 ± 0.3 |
19.8 ± 0.2 |
19.3 ± 0.1 |
18.1 ± 0.3 |
18.8 ± 0.5 |
| Vitamin C (g L−1) |
2.76 ± 0.2 |
2.66 ± 0.3 |
2.59 ± 0.4 |
2.71 ± 0.3 |
2.65 ± 0.4 |
2.58 ± 0.2 |
2.49 ± 0.1 |
2.64 ± 0.6 |
2.57 ± 0.2 |
2.25 ± 0.5 |
2.41 ± 0.1 |
| pH |
3.5 ± 0.05 |
3.52 ± 0.07 |
3.53 ± 0.04 |
3.53 ± 0.06 |
3.54 ± 0.03 |
3.5 ± 0.04 |
3.48 ± 0.05 |
3.48 ± 0.04 |
3.53 ± 0.04 |
3.52 ± 0.04 |
3.53 ± 0.03 |
| TSS |
11.8 ± 0.1 |
11.85 ± 0.1 |
11.89 ± 0.1 |
11.83 ± 0.1 |
11.86 ± 0.1 |
11.85 ± 0.1 |
11.9 ± 0.1 |
11.86 ± 0.1 |
11.91 ± 0.1 |
11.92 ± 0.1 |
11.92 ± 0.1 |
| TA (% citric acid) |
2.2 ± 0.1 |
2.25 ± 0.1 |
2.3 ± 0.1 |
2.22 ± 0.1 |
2.3 ± 0.1 |
2.3 ± 0.1 |
2.34 ± 0.1 |
2.25 ± 0.1 |
2.33 ± 0.1 |
2.28 ± 0.1 |
2.37 ± 0.1 |
| Overall acceptability (OA) |
8.3 ± 0.1 |
6.9 ± 0.08 |
6.7 ± 0.06 |
7.3 ± 0.2 |
6.9 ± 0.09 |
6.3 ± 0.1 |
5.8 ± 0.1 |
6.5 ± 0.08 |
6.0 ± 0.1 |
4.3 ± 0.2 |
4.7 ± 0.1 |
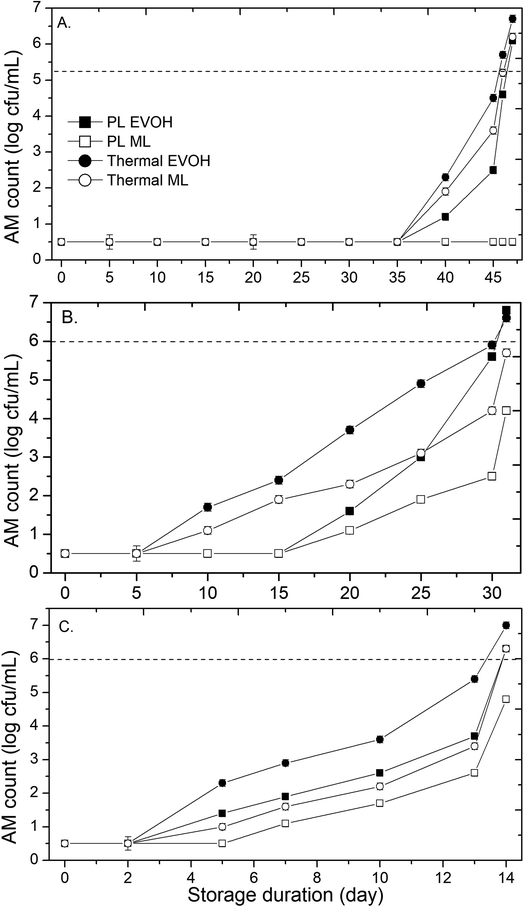 |
| | Fig. 2 Effect of different storage temperatures (A) 4 °C, (B) 15 °C, and (C) 25 °C and packaging materials (EVOH and ML) on the aerobic mesophiles of PL and thermal treated sweet lime juice. | |
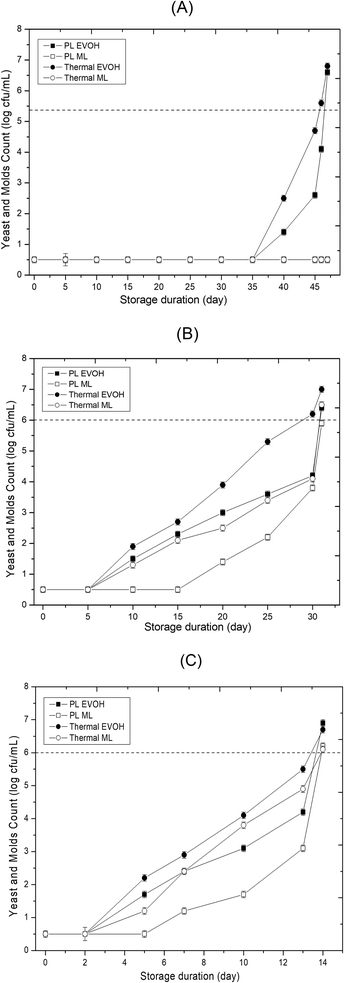 |
| | Fig. 3 Effect of different storage temperatures (A) 4 °C, (B) 15 °C, and (C) 25 °C and packaging materials (EVOH and ML) on the yeasts and molds of PL and thermal treated sweet lime juice. | |
Further, the AM and YM count for thermal treated juice remained below the detection limit for 35 days at 4 °C, regardless of packaging material. At 15 and 25 °C, the microbial population of below detection limit (<1 log cfu mL−1) was only for the initial 5 days in thermal treated samples irrespective of packaging material used. As storage temperature rises, the influence of packing material becomes more noticeable. AM populations in EVOH and ML pouches were 1.6 and 1.1 log cfu mL−1 in PL-treated samples, and 3.7 and 2.3 log cfu mL−1 in thermally treated samples on day 20 at 15 °C. YM load had a similar tendency (Fig. 3).
The photochemical, photothermal, and photophysical mechanisms contribute to PL inactivation.17,18 UV-C is used to irradiate liquid foods or chopped fruits in most studies. For instance, after 3 days, untreated apple juice had AM count >1 log cfu mL−1 while the UV-C treated apple juice didn't increase YM, AM, or lactic acid bacteria for 5 days. A 4.1 kJ m−2 UV-C exposure decreased the fresh-cut watermelon bacteria population by >1 log cycle in 8 days.19 Cao et al.20 found no YM population increase in PL-treated blueberries for 7 days at 4 °C and 3 days at 25 °C. Alginate-coated and PL-treated cantaloupes had 28 and 24 day refrigerated shelf lives. Repeated PL processing of 0.9 J cm−2 every 48 h delayed AM and YM to the shelf life limit of 6 log10 cfu mL−1. In the literature, there are studies depicting a wide range of shelf life for PL treated fruit juices. For instance, Unluturk & Atilgan21 determined the shelf-life of white grape juice treated with UV-C irradiation. The shelf-life was 14 days because the AM population reached 6 log10 cfu mL−1 after day 14. Ferrario et al.17 examined yeast growth in PL-treated apple juice (71.6 J cm−2). After 15 days, S. cerevisiae increased by 2.4 log10 cfu mL−1, whereas A. acidoterrestris spores remained steady and 15 day shelf life was determined. PL (71.6 J cm−2) stabilized orange, apple, and strawberry liquids at 5 °C for 8–10 days. When stored at 5 °C in glass bottles, PL treated (71.6 J cm−2) apple, orange, and strawberry juices were microbiologically safe for 8 to 10 days.22 The PL + US-treated mulberry juice has a shelf-life of 120 days at 25 °C and 180 days at 5 °C in a screw-top plastic container.23 According to Preetha et al.24 a maximum log reduction of 5.2 in S. cerevisiae was achieved using the pulsed light technology when a fluence of 19.2 J cm−2 was applied to tender coconut water. Further, Kaya et al.25 reported that in verjuice beverage PL could inactivate 0.96 ± 0.27 log cfu mL−1 in S. cerevisiae even after delivering a dose of 34 J cm−2 and being placed 5 mm away from the lamp. Preetha et al.26 reported a maximum log reduction of 5.54 cfu mL−1 of APC populations observed in tender coconut water followed by pineapple and orange juice with 5.38 and 5.24 log reductions by treating them with higher energy of 729 J cm−2. The inactivation of microbes depended on the properties of light absorption by the fruit juices. In the current study, PL-treated and thermal-processed juice survived 46 days, whereas untreated juice lasted 6 days at 4 °C. Due to delayed metabolic responses at colder temperatures, 4 °C may prolong shelf life.27
3.1.2. Enzyme activity.
Enzyme activity affects the sensory quality and acceptance of juices. All enzymes (PPO, POD, and PME) were inactivated at the outset of storage in PL and thermal treated samples. The 25th and 30th day revival in PPO and POD activity for the PL-treated juice packed in the EVOH pouch shows reversible denaturation. PME activity in the juice was not detectable till 47 days of storage at 4 °C, but activity increased as the storage temperature rose. PPO (Fig. 4), POD (Fig. 5), and PME (Fig. 6) activity remained the same for 20 days in pulsed light and thermal-treated samples at 4 °C. PPO, POD, and PME enzyme activity increased with time at 15 and 25 °C, with greater activity at 25 °C. In the untreated sample, PPO, POD, and PME activity decreased with storage time and temperature (Table 1). This is due to the shift in temperature, from ambient to refrigerated storage, which causes the enzyme to lose activity. Once cold adaptation is established, substrate interactions modify the enzyme conformation.18 The decrease in system entropy at low temperatures stabilizes the enzyme, explaining a revival in enzyme activity during storage at 4 °C.28 47 days after PL treatment, PPO activity was 7.2%. PPO activity did not recover in the thermal samples during storage. PL treatment may have modified chemical or structural alterations in enzyme conformation. Untreated samples had 68.3% POD activity after 6 days. POD activity was insignificant for the first 25 days of storage, but increased to 6.1% on the 47th day at 4 °C. Throughout storage, the thermal treated samples showed no POD activity. PPO and POD cause phenolic breakdown and browning. In our research, PL and thermal treatments completely inactivated PPO, POD and PME. Despite inactivation, POD and PPO activity increased to some extent during storage days. Browning index values rose for PL and thermal treated samples due to POD and PPO activity during storage (Fig. 4 and 5). The main mechanism for enzyme inactivation in PLT is a combination of individual or collective effects of various phenomena such as protein unfolding with or without aggregation, the dissociation of prosthetic or non-protein groups, oxidation of proteins, loss of α-helix content, disulfide or peptide bond breakage, and site-sensitive fragmentation.27,29,30 In order to prevent unwanted browning and cloud instability, these quality damaging enzymes must be inactivated.31
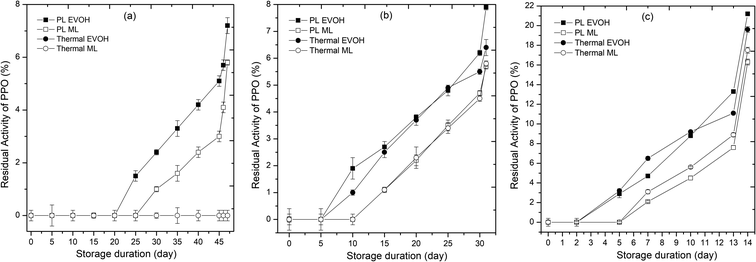 |
| | Fig. 4 Effect of different storage temperatures (A) 4 °C, (B) 15 °C, and (C) 25 °C and packaging materials (EVOH and ML) on the polyphenoloxidase of PL and thermal treated sweet lime juice. | |
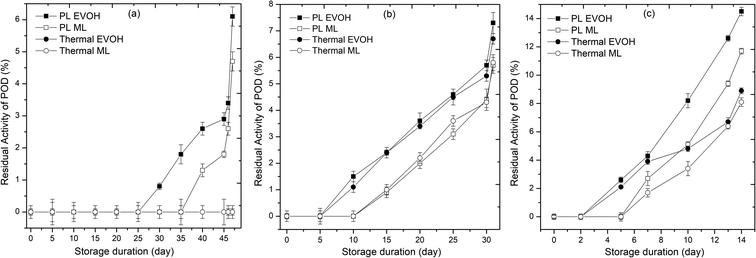 |
| | Fig. 5 Effect of different storage temperatures (A) 4 °C, (B) 15 °C, and (C) 25 °C and packaging materials (EVOH and ML) on the peroxidase of PL and thermal treated sweet lime juice. | |
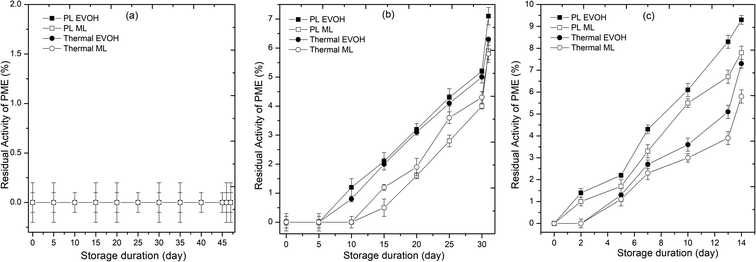 |
| | Fig. 6 Effect of different storage temperatures (A) 4 °C, (B) 15 °C, and (C) 25 °C and packaging materials (EVOH and ML) on the pectinmethylesterase of PL and thermal treated sweet lime juice. | |
PL treatment generated an enzyme conformational change, which was reversed during storage. This reversible conformational shift, however, was found to result in an additional rise throughout storage time. The enzyme's proximity has been discovered to favour a specific conformation, which is one of the important elements affecting the enzyme's stability. PPO photoreaction with surrounding chemicals affects enzyme aggregation or unfolding.32
Enzymes may be inactivated by irreversible active site changes. Wang et al.33 discovered decreased β-sheet composition and increased β-turns and random coils. PL unfolds and aggregates enzymes. Packaging material influenced enzyme activity at all temperatures. The ML-stored juice demonstrated lower enzyme activity than the EVOH-stored juice. ML film blocked oxygen and moisture, improving enzyme stability.34 The activity of enzymes such as PPO and POD in juice is affected by the amount of oxygen present. At storage temperatures of 4 and 15 °C, the enzyme activity remained below 10% throughout the storage period, as observed in studies of PL-treated mixed fruit beverage stored in EVOH pouches at 4 °C.8 However, at 25 °C, PPO and POD activity increased to a maximum of 21% and 15%, respectively. In all cases, juice packed in ML-PET showed lower enzyme activity than juice packed in EVOH pouches, possibly due to the compromised oxygen transmission rate in ML-PET compared to coextruded EVOH films. The oxygen transmission rates for EVOH and ML-PET are 0.62 and 0.54 cm3 per m2 per day·per atm, respectively. The slow diffusion of oxygen inside the juice promotes the action of oxidoreductases, resulting in slightly higher residual enzyme activity compared to ML-PET packed juices. This has a direct impact on the total phenolic content, ascorbic acid degradation, and browning index of the juice. Higher enzyme activity in the juice leads to a higher browning index and lower ascorbic acid content.18 Overall, PL ensures enzymatic stability (<10% residual activity) in refrigerated juices till the end of the microbiological shelf life on the 46th day.
3.1.3. pH, titratable acidity, and total soluble solids.
Untreated juice pH was 3.5 ± 0.05 and the changes during storage weren't significant (p > 0.05) in EVOH and ML pouches (Table 1). On day 6 at 4 °C, the ML pouch sample pH was 3.54 ± 0.03. The control sample's titratable acidity (TA) followed the trend for pH (Table 1). TA levels did not rise significantly (p > 0.05) with storage time for any sample. Untreated sample TSS was 11.8 °Brix. Maximum TSS of the untreated juice was 11.92 °Brix on day 2 at 25 °C in EVOH and ML pouches (Table 1). Untreated sample TSS variations may be attributed to the enzymatic or biochemical breakdown of complex carbohydrates during storage. Different packaging materials and storage temperatures had no significant influence on pH, TA, and TSS in all samples. The ranges of pH, TSS and TA values for pulsed light and thermal treated samples during the corresponding storage period at each temperature (15 °C/25 °C) were 3.48–3.54, 11.8–11.9 and 2.2–2.3, respectively. The physicochemical parameters of the untreated juice are summarized in Table 1. PL and thermal treatment intensities used in this study may not be enough to dissociate ions. Kwaw et al.23 observed a similar effect at 14 J cm−2 for PL-treated mulberry juice. Gooseberry juice12 showed slight changes in acidity and TSS with PL treatment. Light pulses could not disrupt covalent bonds, which are needed for decomposition processes that change pH, TA, and TSS.8 Thus, the treated samples exhibited similar pH, TSS, and TA levels to the control. Due to the hydrolysis of complex sugars into simple sugars, the microbes start growing utilizing the simple sugars keeping pH stable. Similarly, the processing temperature, together with the infrared and ultraviolet spectra, could not dissociate any of the sugar molecules into soluble fragments in the juice.12 The storage temperature up to 25 °C and water vapor permeability of 0.042 and 0.037 g mm2 m−2 h−1 kPa−1 for EVOH and ML films did not alter these parameters considerably.
3.1.4. Vitamin C content.
Vitamin C is a common fruit quality indicator. The untreated, pulsed light treated, and thermal treated samples had 2.76 g L−1, 2.22 g L−1, and 1.64 g L−1 ascorbic acid (AA) respectively on day 0. AA content in all samples dropped with storage time (Fig. 7). After 46 days of storage at 4 °C, 73.4% of 0th day AA was preserved in PL-treated and EVOH packed juice, whereas 25.2% and 32.5% of it had deteriorated after 30 and 13 days, respectively. The AA in the PL treated sample packed in the ML-PET pouch degraded by 19.8% and 18.9% at 4 °C and 15 °C after 46 days, and 27.9% at 25 °C after 2 days (Fig. 7; Table 1), while the AA in the thermal treated sample packed in EVOH stored at 25 °C degraded quickly. AA degraded by 39% after 13 days at 25 °C. The vitamin C degradation followed first-order kinetics (Table 2). The rate constant (k) at 4 °C ranged from 7.27 to 9.97 × 10−3 per day, and 4.65 to 6.65 × 10−3 per day for thermally treated and pulsed light-treated samples, respectively. Meanwhile, at 15 °C, the corresponding values were 8.22 to 12.78 × 10−3 per day and 7.0 to 9.64 × 10−3 per day respectively. Likewise, at 25 °C, the values were 38.60 to 55.05 × 10−3 per day and 23.0 to 33.43 × 10−3 per day respectively. Among all the k values, the highest value was obtained for the thermally treated EVOH sample stored at 25 °C. This indicates that thermal treatment had a higher impact on degradation of vitamin C at 25 °C. Between EVOH and ML-PET films, there was a significant difference in the k values for vitamin C (p < 0.05). Due to the high oxygen and moisture barriers found in ML-PET films, a higher retention of vitamin C is seen in these materials.15 This reduces oxygen diffusion thus preventing aerobic oxidation.16 A higher k value is obtained for the EVOH thermal treated sample because of its poor moisture resistance, which results in dissolved oxygen that causes the oxidation reaction. The oxygen barrier properties make the PL/ML-PET the most stable sample. ML film's improved oxygen barrier may cause reduced aerobic AA oxidation into dehydro-ascorbic acid. Only anaerobic AA degradation with a modest response rate was feasible.35 The PL-treated juice preserves ascorbic acid better than thermal and untreated samples. Minimal oxygen transfer may have caused aerobic oxidation during refrigeration. Metal-catalyzed aerobic oxidation of ascorbic acid by dissolved oxygen in juices may have reduced vitamin C during storage. Temperature and dissolved oxygen concentration affect juice ascorbic acid breakdown during storage.
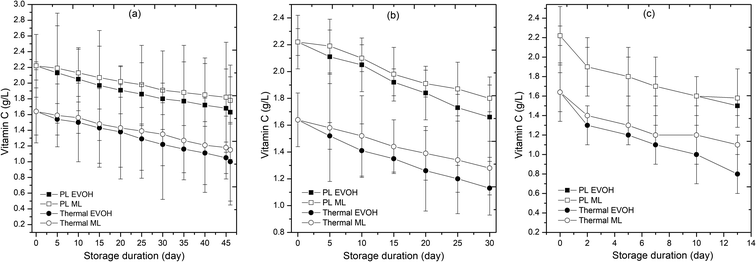 |
| | Fig. 7 Effect of different storage temperatures (A) 4 °C, (B) 15 °C, and (C) 25 °C and packaging materials (EVOH and ML) on the vitamin C of PL and thermal treated sweet lime juice. | |
Table 2 Estimated parameters from first-order kinetic model fitting for various bioactive compounds in PL and thermal treated sweet lime juice samples during storage in different packaging materials
| Treatment |
Bioactive compounds |
Packaging material |
Model fitting parameters from the first-order kinetic model for sweet lime juice during storage |
| Rate constant k (10−3) per day ± CI |
Adjusted R2 |
Reduced chi-square |
| Storage temperature |
| 4 °C |
15 °C |
25 °C |
| Thermal |
TPC |
EVOH |
6.2 ± 0.004 |
9.22 ± 0.002 |
18.96 ± 0.002 |
0.98 |
0.0001 |
| ML-PET |
4.11 ± 0.002 |
5.29 ± 0.002 |
14.45 ± 0.003 |
0.96 |
0.0002 |
| AOX |
EVOH |
7.97 ± 0.005 |
11.17 ± 0.004 |
24.97 ± 0.003 |
0.93 |
0.0002 |
| ML-PET |
5.0 ± 0.003 |
8.96 ± 0.003 |
20.39 ± 0.004 |
0.95 |
0.0003 |
| Vit-C |
EVOH |
9.97 ± 0.004 |
12.78 ± 0.002 |
55.05 ± 0.005 |
0.96 |
0.0003 |
| ML-PET |
7.27 ± 0.003 |
8.22 ± 0.001 |
38.60 ± 0.004 |
0.97 |
0.0004 |
| Pulsed light |
TPC |
EVOH |
4.46 ± 0.005 |
7.46 ± 0.003 |
15.32 ± 0.006 |
0.98 |
0.0003 |
| ML-PET |
3.79 ± 0.004 |
5.92 ± 0.002 |
12.64 ± 0.003 |
0.97 |
0.0005 |
| AOX |
EVOH |
5.41 ± 0.004 |
8.26 ± 0.005 |
17.77 ± 0.005 |
0.95 |
0.0006 |
| ML-PET |
3.6 ± 0.004 |
5.59 ± 0.004 |
13.02 ± 0.004 |
0.94 |
0.0004 |
| Vit-C |
EVOH |
6.65 ± 0.003 |
9.64 ± 0.003 |
33.43 ± 0.005 |
0.98 |
0.0005 |
| ML-PET |
4.65 ± 0.002 |
7.0 ± 0.002 |
23.00 ± 0.003 |
0.96 |
0.0004 |
3.1.5. Total phenolics.
The presence of phenolic compounds is observed as an indication of the nutritional significance of sweet lime juice. PL retains 95.7% of total phenolics, while thermal pasteurization degrades 15% of the initial phenolics. During storage, thermal-treated samples lost more phenolics than PL-treated ones. This may be because the photochemical reactions induced by UV radiation are less powerful than the high temperatures of thermal treatments. During refrigerated storage till the 25th day, the total phenolic content (TPC) in the PL treated juice packed in the EVOH pouch reduced from 24.6 ± 0.5 to 22.4 ± 0.4 g GAE per L, which was significant. Similarly, thermal treated juices had a TPC of 19.3 ± 0.6 g GAE per L on the 25th day. At the 46th day, PL and thermal treated fruit juices had 80.4% and 70.6% residual TPC, respectively (Fig. 8). The effect of packaging material on the degradation of TPC followed a similar trend to that of vitamin C. TPC degradation rates in EVOH and ML films differed significantly (p < 0.05) at any storage temperature. The degradation kinetics of TPC followed first-order kinetics with the k values at 4 °C varying from 4.11 to 6.2 × 10−3 per day and 3.79 to 4.46 × 10−3 per day for the thermally and PL-treated samples (Table 2). Meanwhile, at 15 °C, the corresponding values were 5.29 to 9.22 × 10−3 per day and 5.92 to 7.46 × 10−3 per day respectively. Likewise, at 25 °C, the values were 14.45 to 18.96 × 10−3 per day and 12.64 to 15.32 × 10−3 per day respectively. Between EVOH and ML-PET films, there was a significant difference in the k values for TPC (p < 0.05). The higher k value was for the thermally treated EVOH sample. This indicates that thermal treatment had a greater impact on phenolic degradation than PL. Consequently, the PL/ML-PET sample has high retention of phenolics in sweet lime juice. The enhanced oxygen barrier of ML film may have increased TPC retention in ML pouches. Oxygen passage via EVOH film may degrade phenolics during storage. Like vitamin C, total phenolic degradation in juice during storage is less studied. Basak et al.8 observed a significant decrease in TPC during storage of a mixed fruit beverage at 4 °C. The reduction in phenolics is due to UV-induced photochemical processes.36 Caminiti et al.37 found that 3.3 J cm−2 reduced TPC by 0.8% in carrot and apple juice. The browning and haze formation of phenolic compounds might indicate quality problems. The authors noticed that the loss in TPC was mainly during storage and not after PL and thermal treatments. Negligible POD and PPO activity in the juice during storage helped stabilize phenolics.
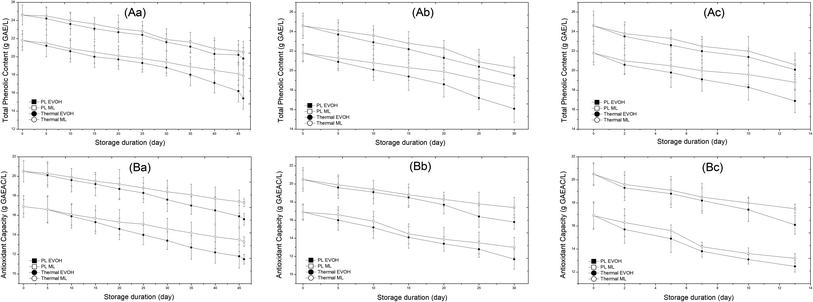 |
| | Fig. 8 Effect of different storage temperatures (A) 4 °C, (B) 15 °C, and (C) 25 °C and packaging materials (EVOH and ML) on the total phenolic content (A) and antioxidant capacity (B) of PL and thermal treated sweet lime juice. | |
3.1.6. Antioxidant capacity.
Untreated fruit juice had antioxidant capacity of 22.4 ± 1.2 g GAEAC per L. Untreated samples lost 18.7% antioxidant capacity (AOX) after 6 days at 4 °C. After thermal treatment, AOX dropped by 24.5%. After PL exposure, 91.5% of AOX was retained. After 46 days, the PL-treated sample in the ML pouch had 17.3 ± 1.4 g GAEAC per L AOX, which is more than that of the thermal treated juice (16.9 ± 0.7 g GAEAC per L) on day 0 and nearly equal to the untreated juice after 6 days (18.2 ± 0.4 g GAEAC per L) at 4 °C. The change in the AOX capacity of the juice during storage is illustrated in Fig. 8. The PL-treated samples retained more AOX than the thermal treated ones. A possible reason could be that the rise in sample temperature after PL treatment is less than for the thermal treated sample. The rise in temperature in PL treatment is due to the absorption of the IR portion of PL. White light increases temperature and also the absorption of the light by the juice which is not transparent. Further, operating the PL's xenon lamp for a longer period may also cause some heat to escape out to the surroundings and nearby sample. Similar to TPC and vitamin C, packaging material had a significant effect on the degradation of antioxidants. On day 46, the PL treated samples packed in EVOH and ML-PET and stored at 4 °C had 15.6 ± 1.1 g GAEAC per L and 17.3 ± 1.3 g GAEAC per L antioxidant capacity. The thermal treated samples had 11.5 ± 0.8 and 13.3 ± 1.2 g GAEAC per L after 46 days at 4 °C. Hypotheses suggest that storage temperature increased antioxidant degradation. Higher humidity makes antioxidants more susceptible to degradation and increases the rate at which organic acids dissolve, both of which are detrimental to quality.38 PL treatment degrades AOX through infrared photothermal action and UV-C photo oxidation of phenolic structures.18 Fruit juices have minimal AOX due to diminished TPC and vitamin C after storage. Furthermore, following 72 °C/26 s thermal treatment of apple-cranberry juice, 91.6% antioxidant retention was recorded.7 Mena et al.39 also obtained losses of 10% and 20% in TEAC and DPPH values, respectively, of pomegranate juice due to pasteurization (65 °C/30 s). The decrease in antioxidant capacity values could be due to partial loss of ascorbic acid and total flavanones. Elez-Martĩnez et al.40 observed a significant decrease in the DPPH value (26.1%) after thermal treatment (90 °C/1 min) of orange juice. The loss of AOX during storage followed first-order kinetics. The values of the rate constant (k) at 4 °C varied from 5.0 to 7.97 × 10−3 per day and 3.6 to 5.41 × 10−3 per day for thermally treated and PL-treated samples. Meanwhile, at 15 °C, the corresponding values were 8.96 to 11.17 × 10−3 per day and 5.59 to 8.26 × 10−3 per day respectively. Likewise, at 25 °C, the values were 20.39 to 24.97 × 10−3 per day and 13.02 to 17.77 × 10−3 per day respectively. The lower k value of the PL/ML-PET sample indicates the stability and retention of antioxidant capacity during storage.39 The k value is higher than TPC which indicates the thermal sensitivity of AOX.40 At 4 °C, the lower k value of 3.6 × 10−3 per day for the PL/ML-PET sample indicates the stability and retention of AOX. However, AOX content was more effectively retained in PL treatments than in sweet lime juice that had undergone thermal treatment.
3.1.7. Color and browning index.
Besides the nutritional content of juice, color is a major component in customer choice. Untreated fresh juice showed L*, a*, and b* values of 64.3, −4.05, and 11.2, and a browning index (BI) of 60.0 ± 1.1. Untreated juice packed in the EVOH pouch and stored at 4 °C had a total color change (ΔE*) of 8.65 ± 0.2 while the corresponding value for the sample stored in the ML pouch was 8.3 ± 0.1 on the 4th day. Similarly, the same untreated juice when packed in EVOH and ML PET pouches and stored at 15 °C had ΔE* of 8.86 ± 0.2 and 8.5 ± 0.2 on the 3rd day itself. This indicates that the rise in storage temperature had a significant effect on the rate of color change. The PL and thermal treated samples when stored at 4 °C showed a decreasing trend in the total color change. For instance, on the 5th day the PL treated sample stored in the EVOH pouch at 4 °C had ΔE* of 7.2, while on the 46th day it was 3.8. The corresponding values for thermal treated samples are 6.8 and 5.3, respectively. The ΔE* decreasing trend was similar for 15 °C stored samples. However, the samples stored at 25 °C showed an increasing ΔE* trend throughout storage days.
Color coordinates (L*a*b*) changed intensely for PL and thermal samples held at different temperatures in two pouches. For all samples, L* and b* values declined with time and storage temperature while an increase in a* values was noticed. However, thermal treated juices brown from Maillard browning, ascorbic acid breakdown, and polyphenol degradation.41 Ascorbic acid (R = 0.879) and total phenolics (R = 0.915) loss corresponded well with thermal treated juice BI values. The non-thermal treatment preserved ascorbic acid and total phenolics in the PL-treated juice, which reduced browning. The sample's overall color change value (ΔE*) represented all three-color alterations. PL and heat treatments yielded 7.6 and 8.9 initial ΔE* values. Due to colorful compounds, intense heat treatment (95 °C/5 min) raised ΔE*.42 The change in color (ΔE*) rose with storage time and temperature for all samples irrespective of packaging material (Fig. 9). The thermal treated sample packed in the EVOH pouch after 13 days at 25 °C had the highest ΔE* value (9.9). ΔE* for the PL-treated sample stored at 4 °C in EVOH film for 46 days is 4.1. The corresponding value when the juice sample is stored in the ML pouch is 3.7. Therefore, it is evident that the packaging material has a significant effect on the storage life.
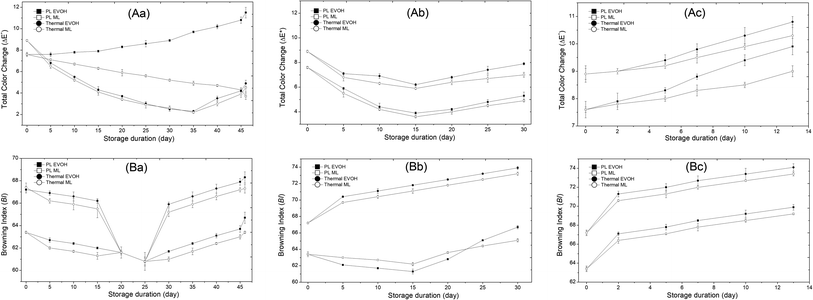 |
| | Fig. 9 Effect of different storage temperatures (A) 4 °C, (B) 15 °C, and (C) 25 °C and packaging materials (EVOH and ML) on the total color change (A) and browning index (B) of PL and thermal treated sweet lime juice. | |
The browning index (BI) measures brown color development in the juice during processing and storage.16 All samples' browning indices (BI) rose over time. The BI for the PL treated EVOH pouch sample on the 46th day was 64.7 while for the ML-PET pouch it was 63.4. The thermal treated sample in EVOH film had the greatest BI value (68.3) after 46 days at 4 °C.
ML-PET film exhibited a greater barrier property (WVP 0.037 g mm2 m−2 kPa−1 and thickness 109 mm) than EVOH film (WVP 0.042 g mm2 m−2 kPa−1 and thickness 100 mm). The rate of rise in ΔE* was highest for the thermal sample packed in EVOH (Fig. 9) and lowest for the PL sample packed in the ML pouch. Browning may be caused by residual PPO activity. But the PPO activity was less than 10% in both samples throughout the storage period irrespective of the temperature of storage. These samples may exhibit browning from non-enzymatic browning or ascorbic acid degradation.43 Shelf life is subject to storage temperature. Storage temperature increased BI. Quality deterioration and enzyme activity during storage may be the reason.
The thermal treated samples showed enhanced BI from Maillard browning. The PL and thermal treated samples showed L* and b* decreases and a* increases during storage. Thermal treated samples increased a* values more. Juice browning is mostly non-enzymatic due to complete inactivation of enzymes during treatment. But, for PL, there is reactivation of enzymes after a specific period of time which results in a browning reaction. Ascorbic acid (R = 0.879) and total phenolic (R = 0.915) loss correlated well with juice BI values. Non-thermal treatment decreased browning because PL-treated juice retained ascorbic acid and total phenolics. The juice's shelf life is seldom included in PL research on color. To conclude, juices typically preserve AOX, TPC, and color after PL treatment.
3.1.8. Sensory profile.
The customer accepts sweet lime juice based on its first sensory appearance; thus, its sensory assessment is crucial. The overall acceptability (OA) of fresh juice was 8.3 on a scale of 1 to 9. A lower limit of 5 was deemed to be undesirable on a 9-point hedonic scale used to measure overall sample acceptability (OA).8 The control sample's flavor was unacceptably bad the day after being stored at 25 °C, with OA values of 4.3 and 4.7 in EVOH and ML-PET pouches, respectively. For three days, the control sample stayed constant at 15 °C (OA = 6.3, 6.5 for EVOH and ML). After six days at 4 °C, EVOH and ML samples had scores of 6.7 and 6.9 (Fig. 10). Day 0 results for PL and thermally treated samples packed in EVOH were 8.1 and 7.8, respectively. A cooked taste and browning following a prolonged thermal treatment at 95 °C may account for the initial lower sensory score of 7.8. In Fig. 10, the OA scores of the treated samples are compared against the packaging materials and storage temperature. Samples and packaging materials lost value with time in a consistent manner. For every sample that was deemed microbially safe (AM and YM count <6 log cfu mL−1), the OA was greater than 5. Taste, color, appearance, flavor, and aftertaste were all impacted by colour degradation, browning, and bioactive loss during storage. Thermal sample OA decreased during time relative to PL. Higher temperatures depleted sensory scores faster. Ketones, aldehydes, and other intermediates may generate off-flavor and discoloration at 25 °C because metabolic and biological processes accelerate.16,44 At 25 °C, enzymatic and non-enzymatic browning rates rise, decreasing the sample's appearance and color score. The sample had no off-odor from the packaging material, and EVOH and ML films had identical sensory ratings (p > 0.05). However, extensive heat treatment at 95 °C may alter the sample's metabolic profile, lowering its sensory score after storage.43
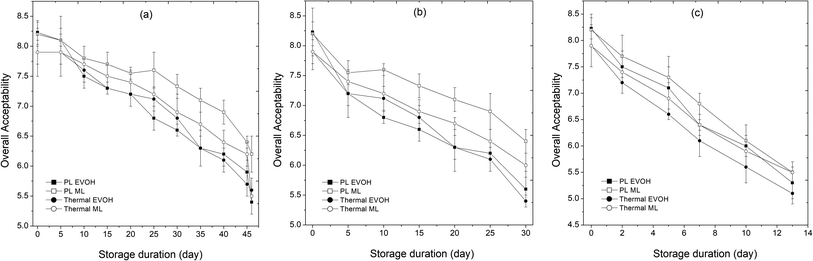 |
| | Fig. 10 Effect of different storage temperatures (A) 4 °C, (B) 15 °C, and (C) 25 °C and packaging materials (EVOH and ML) on the sensory profile of PL and thermal treated sweet lime juice. | |
3.2. Shelf life of the treated juice
The untreated sweet lime juice lasted 6 days at 4 °C, but the PL processed juice lasted 46 days. The juice's microbial population determined its shelf life. The microbial count in the juice was regarded as highly unacceptable for consumption at or above 6 log10 cfu mL−1, which was deemed to be an indicator of microbial spoilage.45 Ferrario et al.17 examined yeast growth in PL-treated apple juice (71.6 J cm−2). After 15 days, S. cerevisiae increased by 2.4 log10 cfu mL−1, whereas A. acidoterrestris spores remained steady and a 15 day shelf life was determined. PL (71.6 J cm−2) stabilized orange, apple, and strawberry liquids at 5 °C for 8–10 days.22 The AM-based threshold was >7 log cfu mL−1, whereas the sensory criterion was 25% rejection probability limit. In this study, sensory and vitamin C profiles were at allowed limits even after 46 days. But microbiological counts exceeding 6 log cfu mL−1 reduced shelf life to 46 days at 4 °C. At its expiry, the EU Association of Juices and Nectars from Fruits and Vegetables recommended 20 mg 100 mL−1 vitamin C in orange juice.46 PL-treated juice had 1.78 ± 0.5 g L−1 vitamin C after 46 days. Thermal-treated juice has 1.64 ± 0.3 g L−1 vitamin C. To summarize, PL extended sweet lime juice's shelf life to 46 days.
3.3. Principal component analysis
During the storage period, the quality attributes such as TPC, AOX, Vit-C, ΔE*, BI, and OA are affected by their interrelationship with each other. Two principal components were recognized from these six dependent variables using multivariate analysis such that the most variability could be defined. The two new variables were principal components 1 and 2 (PC1 and PC2). In an orthogonally located bi-rotated space of PC1 and PC2, the related data for each quality attribute on each sampling day during storage at a given temperature of 4, 15, and 25 °C are shown in Fig. 11. PC1 and PC2 could account for more than 85%, 95%, and 97% of the data set's variability at 4, 15, and 25 °C respectively. PC1 is accomplished by explaining 70%, 73%, and 91% variability while 15%, 22%, and 6% variability was described by PC2 at 4, 15, and 25 °C respectively. The eigenvalues for PC1 are more than 5 which specifies that the determined variability is due to PC1. From the loading plot in Fig. 11(A), it is clear that vitamin C, TPC, AOX, BI, and OA are on the positive side of the PC1. The total color change (ΔE*) is on the negative side of PC1. This indicates that the increase in ΔE* considerably changes the acceptability of the sample (Fig. 11(A)). The correlation values of these attributes are presented in Table 3. The data set for TPC, AOX, and vitamin C showed more than 95% correlation; as a result, they are in the same coordinate. It is stated that phenolic compounds have the capability to nullify free radicals, and in addition, have AOX activity. The antioxidant activity of vitamin C is also included in the sample's total AOX capacity. Similarly, there is a 95% correlation between ΔE* and BI. During the storage period, the change in L* and a* significantly changes the color which is reflected in BI values. The OA data depends on a variety of factors, including taste, flavor, color, and nutritional profile, rather than just color variation and nutritional profile. Hence, the correlation among the attributes with OA was in between 51% and 97% (Table 3). At 25 °C, the strongest negative association between ΔE* and OA (r = −0.97, p < 0.05) indicates that the color change was the most vital consideration for defining its OA. This could explain why more change shift in juice total color change (ΔE* = 9.9) towards brown or darker color resulted in a lower OA score (5.3 out of 9). These results are in line with the literature.47–49
 |
| | Fig. 11 Loading and scoring plots of two principal components (PC1 and PC2) in a rotated space originating from principal component analysis (PCA) of different quality attributes of sweet lime juice affected during storage at (A) 4 °C, (B) 15 °C and (C) 25 °C. In the plot, labels T and P represent thermal (95 °C/5 min) and pulsed light treatment (3000 J cm−2), respectively; E and M stand for EVOH and ML film, respectively; and different numbers indicate the respective sampling days where 0 stands for day 0 and 46 is for day 46. Total phenolics; AOX: antioxidant capacity; vitamin C; total colour change; browning index; overall acceptability. | |
Table 3 Correlation matrix obtained after multivariate analysis of different quality attributes of sweet lime juice during storagea
| Storage temperature (°C) |
TPC (g GAE per L) |
AOX (g GAEAC per L) |
Vit-C (g L−1) |
TCC (ΔE*) |
BI (%) |
OA |
| 4 |
15 |
25 |
4 |
15 |
25 |
4 |
15 |
25 |
4 |
15 |
25 |
4 |
15 |
25 |
4 |
15 |
25 |
|
TPC: total phenolic content; AOX: antioxidant capacity; Vit-C: vitamin C; TCC: total colour change; BI: browning index; OA: overall acceptability.
|
| TPC (g GAE per L) |
1 |
1 |
1 |
0.978 |
0.958 |
0.968 |
0.953 |
0.931 |
0.977 |
0.518 |
−0.260 |
−0.975 |
−0.611 |
−0.831 |
−0.955 |
0.768 |
0.808 |
0.799 |
| AOX (g GAEAC per L) |
0.978 |
0.957 |
0.967 |
1.000 |
1.000 |
1.000 |
0.989 |
0.985 |
0.986 |
0.537 |
−0.387 |
−0.951 |
−0.650 |
−0.933 |
−0.954 |
0.666 |
0.676 |
0.685 |
| Vit-C (g L−1) |
0.953 |
0.930 |
0.977 |
0.989 |
0.985 |
0.986 |
1.000 |
1.000 |
1.000 |
0.573 |
−0.443 |
−0.950 |
−0.670 |
−0.948 |
−0.967 |
0.585 |
0.590 |
0.707 |
| TCC (ΔE*) |
−0.611 |
−0.831 |
−0.954 |
−0.649 |
−0.933 |
−0.954 |
−0.670 |
−0.948 |
−0.967 |
−0.207 |
0.589 |
0.912 |
1.000 |
1.000 |
1.000 |
−0.291 |
−0.417 |
−0.761 |
| BI (%) |
0.767 |
0.807 |
0.799 |
0.666 |
0.676 |
0.685 |
0.585 |
0.590 |
0.707 |
0.097 |
0.213 |
−0.809 |
−0.291 |
−0.417 |
−0.761 |
1.000 |
1.000 |
1.000 |
| OA |
1 |
1 |
1 |
0.978 |
0.958 |
0.968 |
0.953 |
0.931 |
0.977 |
0.518 |
−0.260 |
−0.975 |
−0.611 |
−0.831 |
−0.955 |
0.768 |
0.808 |
0.799 |
4. Conclusion
PL processing (3000 J cm−2) enhanced the storage life of sweet lime juice significantly. Throughout storage at 4 °C, the thermal treated samples showed reduced antioxidant capacity, total phenolics, and vitamin C compared to the PL-treated samples. The thermal treated juices have elevated browning indices, compromising taste. Both PL and thermal treated juices maintained their pH, titratable acidity, and total soluble solids throughout storage. Interestingly, the sensory acceptability and vitamin C content were above the minimum limit (OA of 5 out of 9 and 20 mg mL−1 vitamin C in the juice) after 46 days of storage. However, the microbiological count (>6 log cfu mL−1) limited shelf life. At the end of 46 days, when compared to day 0, the PL-treated juice retained 83.3% phenolics, 84.3% antioxidant capacity, and 80% vitamin C. The degradation of these bioactive parameters followed first-order kinetics with adjusted R2 > 0.9. Priority research should focus on bioactive degradation, enzyme stability, or inactivation mechanisms in sweet lime juice during storage.
Author contributions
Lubna Shaik: methodology, formal analysis, investigation, validation, resources, writing – original draft preparation, reviewing, and editing. Snehasis Chakraborty: conceptualization, supervision, writing – reviewing and editing, visualization.
Conflicts of interest
The authors have declared no conflicts of interest for this article.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Lubna Shaik is grateful to the All-India Council for Technical Education (AICTE) for providing a scholarship through the National Doctoral Fellowship (NDF) 2019–20 scheme.
References
- A. A. Vilas-Boas, D. Magalhães, D. A. Campos, S. Porretta, G. Dellapina, G. Poli and M. Pintado, Innovative processing technologies to develop a new segment of functional citrus-based beverages: current and future trends, Foods, 2022, 11(23), 3859, DOI:10.3390/foods11233859.
- J. Mandha, H. Shumoy, A. O. Matemu and K. Raes, Characterisation of fruit juices and effect of pasteurisation and storage conditions on their microbial, physicochemical, and nutritional quality, Food Biosci., 2022, 102335, DOI:10.1016/j.fbio.2022.102335.
- N. E. Nyamende, G. O. Sigge, Z. A. Belay, R. R. Mphahlele, A. B. Oyenihi, A. Mditshwa and O. J. Caleb, Advances in non-thermal technologies for whole and minimally processed apple fruit – a review, Food Biosci., 2022, 102170, DOI:10.1016/j.fbio.2022.102170.
- M. N. Salazar-Zúñiga, E. Lugo-Cervantes, J. Rodríguez-Campos, R. Sanchez-Vega, M. J. Rodríguez-Roque and C. G. Valdivia-Nájar, Pulsed light processing in the preservation of juices and fresh-cut fruits: a review, Food Bioprocess Technol., 2022, 1–16, DOI:10.1007/s11947-022-02891-4.
- L. Shaik and S. Chakraborty, Effect of pH and total fluence on microbial and enzyme inactivation in sweet lime (Citrus limetta) juice during pulsed light treatment, J. Food Process. Preserv., 2022, 46(8), e16749, DOI:10.1111/jfpp.16749.
- R. Huang and H. Chen, Sanitation of tomatoes based on a combined approach of washing process and pulsed light in conjunction with selected disinfectants, Food Res. Int., 2019, 116, 778–785, DOI:10.1016/j.foodres.2018.09.011.
- I. Palgan, I. M. Caminiti, A. Muñoz, F. Noci, P. Whyte, D. J. Morgan, D. A. Cronin and J. G. Lyng, Combined effect of selected non-thermal technologies on Escherichia coli and Pichia fermentans inactivation in an apple and cranberry juice blend and on product shelf life, Int. J. Food Microbiol., 2011, 151(1), 1–6, DOI:10.1016/j.ijfoodmicro.2011.07.019.
- S. Basak, S. Mahale and S. Chakraborty, Changes in quality attributes of pulsed light and thermal treated mixed fruit beverages during refrigerated storage (4 °C) condition, Innovative Food Sci. Emerging Technol., 2022, 78, 103025, DOI:10.1016/j.ifset.2022.103025.
- F. Salehi, Edible coating of fruits and vegetables using natural gums: a review, Int. J. Fruit Sci., 2020, 20(2), S570–S589, DOI:10.1080/15538362.2020.1746730.
- C. Maes, W. Luyten, G. Herremans, R. Peeters, R. Carleer and M. Buntinx, Recent updates on the barrier properties of ethylene vinyl alcohol copolymer (EVOH): a review, Polym. Rev., 2018, 58(2), 209–246, DOI:10.1080/15583724.2017.1394323.
- D. Wu, M. Zhang, B. Bhandari and Z. Guo, Combined effects of microporous packaging and nano-chitosan coating on quality and shelf-life of fresh-cut eggplant, Food Biosci., 2021, 43, 101302, DOI:10.1016/j.fbio.2021.101302.
- S. Chakraborty, S. Ghag, P. P. Bhalerao and J. S. Gokhale, The potential of pulsed light treatment to produce enzymatically stable Indian gooseberry (Emblica officinalis Gaertn.) juice with maximal retention in total phenolics and vitamin C, J. Food Process. Preserv., 2020, 44(12), e14932, DOI:10.1111/jfpp.14932.
- S. Pandraju and P. S. Rao, High-pressure processing of sugarcane juice (Saccharum officinarum) for shelf-life extension during ambient storage, Sugar Technol., 2020, 22, 340–353, DOI:10.1007/s12355-019-00769-y.
- P. Sreedevi, L. E. Jayachandran and P. Srinivasa Rao, Application of high-pressure processing for extending the shelf life of sugarcane juice under refrigerated conditions, J. Food Process. Preserv., 2020, 44(12), e14957, DOI:10.1111/jfpp.14957.
- K. K. Mokwena and J. Tang, Ethylene vinyl alcohol: a review of barrier properties for packaging shelf stable foods, Crit. Rev. Food Sci. Nutr., 2012, 52(7), 640–650, DOI:10.1080/10408398.2010.504903.
- M. R. Manikantan, R. Pandiselvam, T. Arumuganathan, C. Indurani and N. Varadharaju, Low-density polyethylene based nanocomposite packaging films for the preservation of sugarcane juice, J. Food Sci. Technol., 2022, 1–8, DOI:10.1007/s13197-021-05174-6.
- M. Ferrario, S. Guerrero and S. Alzamora, Study of pulsed light-induced damage on saccharomyces cerevisiae in apple juice by flow cytometry and transmission electron microscopy, Food Bioprocess Technol., 2014, 7, 1001–1011, DOI:10.1007/s11947-013-1121-9.
- L. Shaik and S. Chakraborty, Nonthermal pasteurization of pineapple juice: a review on the potential of achieving microbial safety and enzymatic stability, Compr. Rev. Food Sci. Food Saf., 2022, 21(6), 4716–4737, DOI:10.1111/1541-4337.13042.
- J. M. Fonseca and J. W. Rushing, Effect of ultraviolet-C light on quality and microbial population of fresh-cut watermelon, Postharvest Biol. Technol., 2006, 40(3), 256–261, DOI:10.1016/j.postharvbio.2006.02.003.
- X. Cao, R. Huang and H. Chen, Evaluation of pulsed light treatments on inactivation of Salmonella on blueberries and its impact on shelf-life and quality attributes, Int. J. Food Microbiol., 2017, 260, 17–26, DOI:10.1016/j.ijfoodmicro.2017.08.012.
- S. Unluturk and M. R. Atilgan, Microbial safety and shelf life of UV-C treated freshly squeezed white grape juice, J. Food Sci., 2015, 80(8), M1831–M1841, DOI:10.1111/1750-3841.12952.
- M. Ferrario, S. M. Alzamora and S. Guerrero, Study of pulsed light inactivation and growth dynamics during storage of Escherichia coli ATCC 35218, Listeria innocua ATCC 33090, Salmonella Enteritidis MA 44 and Saccharomyces cerevisiae KE 162 and native flora in apple, orange and strawberry juices, Int. J. Food Sci. Technol., 2015, 50(11), 2498–2507, DOI:10.1111/ijfs.12918.
- E. Kwaw, W. Tchabo, Y. Ma, M. T. Apaliya, A. S. Sackey, B. K. Mintah and S. Ma, Effect of storage on quality attributes of lactic-acid-fermented mulberry juice subjected to combined pulsed light and ultrasonic pasteurization treatment, J. Food Meas. Charact., 2018, 12(3), 1763–1771, DOI:10.1007/s11694-018-9791-7.
- P. Preetha, A. P. Venugopal, N. Varadharaju and Z. J. Kennedy, Inactivation of Escherichia coli in tender coconut (Cocos nucifera L.) water by pulsed light treatment, Int. J. Curr. Microbiol. Appl. Sci., 2017, 6(7), 1453–1461, DOI:10.20546/ijcmas.2017.607.174.
- Z. Kaya, S. Unluturk, O. Martin-Belloso and R. Soliva-Fortuny, Effectiveness of pulsed light treatments assisted by mild heat on Saccharomyces cerevisiae inactivation in verjuice and evaluation of its quality during storage, Innovative Food Sci. Emerging Technol., 2020, 66, 102517, DOI:10.1016/j.ifset.2020.102517.
- P. Preetha, N. Varadharaju, G. Jeevarathinam, J. Deepa, A. P. Mohan Kumar, M. Balakrishnan, P. Rajkumar and R. Pandiselvam, Optimization of continuous flow pulsed light system process parameters for microbial inactivation in tender coconut water, pineapple and orange juice, J. Food Process Eng., 2023, 46(3), e14254, DOI:10.1111/jfpe.14254.
- L. Manzocco, A. Rumignani and C. Lagazio, Emotional response to fruit salads with different visual quality, Food Qual Prefer, 2013, 28(1), 17–22, DOI:10.1016/j.foodqual.2012.08.014.
- Y. Han, J. H. Cheng and D. W. Sun, Activities and conformation changes of food enzymes induced by cold plasma: a review, Crit. Rev. Food Sci. Nutr., 2019, 59(5), 794–811, DOI:10.1080/10408398.2018.1555131.
- J. A. Pellicer and V. M. Gómez-López, Pulsed light inactivation of horseradish peroxidase and associated structural changes, Food Chem., 2017, 237, 632–637, DOI:10.1016/j.foodchem.2017.05.151.
- J. A. Pellicer, P. Navarro and V. M. Gómez-López, Pulsed light inactivation of polygalacturonase, Food Chem., 2019, 271, 109–113, DOI:10.1016/j.foodchem.2018.07.194.
- D. Cautela, D. Castaldo and B. Laratta, Thermal inactivation of pectin methylesterase in pineapple juice, J. Food Meas. Charact., 2018, 12, 2795–2800, DOI:10.1007/s11694-018-9894-1.
- C. C. Sew, H. M. Ghazali, O. Martín-Belloso and M. A. Noranizan, Effects of combining ultraviolet and mild heat treatments on enzymatic activities and total phenolic contents in pineapple juice, Innovative Food Sci. Emerging Technol., 2014, 26, 511–516, DOI:10.1016/j.ifset.2014.05.008.
- B. Wang, Y. Zhang, C. Venkitasamy, B. Wu, Z. Pan and H. Ma, Effect of pulsed light on activity and structural changes of horseradish peroxidase, Food Chem., 2017, 234, 20–25, DOI:10.1016/j.foodchem.2017.04.149.
- S. Dhawan, C. Varney, G. V. Barbosa-Cánovas, J. Tang, F. Selim and S. S. Sablani, Pressure-assisted thermal sterilization effects on gas barrier, morphological, and free volume properties of multilayer EVOH films, J. Food Eng., 2014, 128, 40–45, DOI:10.1016/j.jfoodeng.2013.12.012.
-
J. F. Gregory III, Vitamins, in Food Chemistry, ed. O. R. Fennema, Marcel Dekker, New York, 1996, pp. 531–616 Search PubMed.
- T. Koutchma, Advances in ultraviolet light technology for non-thermal processing of liquid foods, Food Bioprocess Technol., 2009, 2(2), 138–155, DOI:10.1007/s11947-008-0178-3.
- I. M. Caminiti, F. Noci, D. J. Morgan, D. A. Cronin and J. G. Lyng, The effect of pulsed electric fields, ultraviolet light or high intensity light pulses in combination with manothermosonication on selected physico-chemical and sensory attributes of an orange and carrot juice blend, Food Bioprod. Process., 2012, 90(3), 442–448, DOI:10.1016/j.fbp.2011.11.006.
- S. C. Lourenço, M. Moldão-Martins and V. D. Alves, Antioxidants of natural plant origins: from sources to food industry applications, Molecules, 2019, 24(22), 4132, DOI:10.3390/molecules24224132.
- P. Mena, N. Martĩ, D. Saura, M. Valero and C. Garcıa-Viguera, Combinatory effect of thermal treatment and blending on the quality of pomegranate juices, Food Bioprocess Technol., 2013, 6, 3186–3199, DOI:10.1007/s11947-012-0961-z.
- P. Elez-Martĩnez, R. C. Soliva-Fortuny and O. Martın-Belloso, Comparative study on shelf life of orange juice processed by high intensity pulsed electric fields or heat treatment, Eur. Food Res. Technol., 2006, 222, 321–329, DOI:10.1007/s00217-005-0073-3.
- H. W. Yeom, C. B. Streaker, Y. Zhang, Y. Sun, H. Zhang, Q. Mai, B. Zhang, H. Li and Z. Deng, The degradation rules of anthocyanins from eggplant peel and antioxidant capacity in fortified model food system during the thermal treatments, Food Biosci., 2020, 38, 100701, DOI:10.1016/j.fbio.2020.100701.
- Q. H. Zhang and D. B. Min, Effects of pulsed electric fields on the quality of orange juice and comparison with heat pasteurization, J. Agric. Food Chem., 2000, 48(10), 4597–4605, DOI:10.1021/jf000306p.
- G. A. González-Tejedor, G. B. Martínez-Hernández, A. Garre, J. A. Egea, P. S. Fernández and F. Artés-Hernández, Quality changes and shelf-life prediction of a fresh fruit and vegetable purple smoothie, Food Bioprocess Technol., 2017, 10(10), 1892–1904, DOI:10.1007/s11947-017-1965-5.
- D. Nath, R. Santhosh, K. Pal and P. Sarkar, Nanoclay-based active food packaging systems: a review, Food Packag. Shelf Life, 2022, 31, 100803, DOI:10.1016/j.fpsl.2021.100803.
-
Health Protection Agency, 2009, Guidelines for Assessing the Microbiological Safety of Ready To-Eat Foods Placed on the Market, retrieved from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/fil/363146/Guidelines_for_assessing_the_microbiological_safety_of_ready-to_eat_foods_on_the_market.pdf, accessed on 19th Dec 2022 Search PubMed.
- A. Jacob, M. Rajavel, I. P. Sudagar, R. Pandiselvam and P. Rajkumar, Effect of pulsed light treatment on sugars and microbial load in mature coconut water, J. Pharm. Innov., 2022, 9, 887–890 Search PubMed . https://www.thepharmajournal.com.
- B. K. Tiwari, K. Muthukumarappan, C. P. O'donnell and P. J. Cullen, Effects of sonication on the kinetics of orange juice quality parameters, J. Agric. Food Chem., 2008, 56(7), 2423–2428, DOI:10.1021/jf073503y.
- M. Igual, E. García-Martínez, M. M. Camacho and N. Martínez-Navarrete, Changes in flavonoid content of grapefruit juice caused by thermal treatment and storage, Innovative Food Sci. Emerging Technol., 2011, 12(2), 153–162, DOI:10.1016/j.ifset.2010.12.010.
- M. Abid, S. Jabbar, T. A. O. Wu, M. M. Hashim, B. Hu, M. Saeeduddin and X. Zeng, Qualitative assessment of sonicated apple juice during storage, J. Food Process. Preserv., 2015, 39(6), 1299–1308, DOI:10.1111/jfpp.12348.
|
| This journal is © The Royal Society of Chemistry 2023 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*