Open Access Article
Open Access ArticleCarbon dots for food packaging applications
Deepika
,
Lokesh
Kumar
and
Kirtiraj K.
Gaikwad
 *
*
Department of Paper Technology, Indian Institute of Technology Roorkee, Roorkee-247667, Uttarakhand, India. E-mail: kirtiraj.gaikwad@pt.iitr.ac.in
First published on 13th December 2022
Abstract
Nanotechnology in food packaging has emerged as a viable commercial option. In particular, nanoparticles are being included in packaging materials to enhance the storage life and ensure the safety of food. Carbon dots offer several desired physical and chemical qualities, including their nanosize, numerous surface functional groups, nontoxicity, outstanding biocompatibility, excellent antibacterial and antioxidant activities, low cost, and ease of synthesis. Because of these reasons, carbon dots have the potential to be used in a wide variety of applications in the food packaging industry. Herein, the carbon dots for food packaging industries have been reviewed. The fabrication of carbon dots from microbes, plants, animals, and food items, the effect of carbon dots on engineering properties of polymeric films, and carbon dots as active antioxidant and antimicrobial agents are all discussed. In the end, challenges and future recommendations on carbon dots for food packaging are discussed.
1. Introduction
Food packaging has seen increased growth in recent years from users with hectic lifestyles who want food to be convenient and easy to consume.1 Over the past few years, marketing and distribution network involvement in package innovation and design has risen dramatically. Compared to the price of the spoiled products, food waste can cause a much higher economic penalty.2 In developed economies, consumer demand for pre-packaged food is still rising, and developing countries are also changing their lifestyle. Packaging helps to ensure the long-term viability of environmental resources by reducing product deterioration and waste and protecting products until they have fulfilled their purpose. An optimum level of packaging is essential to decrease waste across the distribution chain and reduce the overall cost of the product.3,4Biopolymer-based packaging is widely used due to its nontoxicity, good biocompatibility, and environmentally-friendly nature. Due to external factors, nearly a third of the food produced worldwide is wasted annually. Consequently, to resolve this issue, people have actively sought to retain good-quality food, use efficient techniques to increase the shelf-life, safety, and quality of the food, or embraced modern preservation and packaging technology. For food safety, one needs some functional or active materials that can slow the rate of food oxidation and stop microorganisms from growing in the food.5–7
An active packaging film is a thin, shelf-standing film with functional qualities that is used throughout food packaging. Active coatings directly applied over the food surface are generally used to decrease bacterial growth (antimicrobial coating), reduce the rate of food oxidation (antioxidant coating), and limit enzymatic browning, moisture loss, and respiration of food products. These active coatings work as primary packaging while also enhancing the shelf life of fresh vegetables and fruits. Food can be coated by either dipping into the film-forming solutions or spraying with the solution. The functional coating can act as an ultraviolet and gas barrier (gas exchange between the fresh product and surroundings).8,9
The application of carbon dots in biochemical sensing, photocatalysis, optical technology, and biological imaging has been widely explored. Because of their advantageous properties, such as low cost, simple synthesis, high water solubility, bioactivity, less hazardous, and light absorption, they are frequently used in medical applications.10 Nanomaterials based on carbon dots have gained popularity because of their superior electronic, optical, thermal, and mechanical characteristics (Fig. 1). There has been a rising demand for carbon dots (CDs) in food packaging, as carbon dots are considered to be active materials and can be used to form antioxidant or antimicrobial-based food packaging. Carbon dots (CDs) are synthesized from natural materials, and provide additional functions to food packaging, ultimately increasing the quality, freshness, and shelf life of food products.11 A review by Qu et al. (2018) gives an idea for using carbon dots for food safety and preservation. Carbon dots can be easily synthesized from waste materials or eco-friendly substances such as peels, leaves, honey, and fruits. The functionality (strength and barrier) of packaging is improved by the use of these active materials, which are cheap and have great physical and chemical properties.12 Researchers have recently used a variety of carbon dots from various sources to create active packaging materials that improve the shelf life of fresh produce.2 The study has shown that enoki-mushroom-based carbon dots incorporated into gelatin/carrageenan-based composite films enhance mechanical, antioxidant, and UV-blocking capabilities without compromising the film's transparency.3,8,13
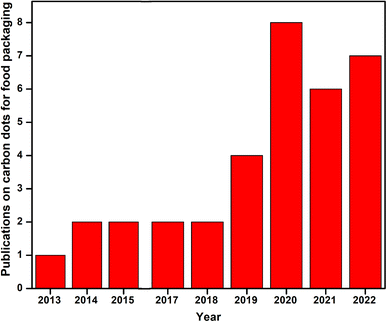 | ||
| Fig. 1 Publication trends in oxygen scavenging films used in food packaging from 2013 to 2022. Data were collected from Scopus using the keywords “carbon dot” packaging” and “carbon dot food”. | ||
This review provides information about carbon dots, the application of carbon dots in food packaging technology, and a brief introduction to the synthesis of carbon dots from microorganisms, plant, animal, and food items, followed by their engineering properties and applications as antioxidant, and antimicrobial agents. Furthermore, this review provides an overview of the ongoing studies and their application in food packaging. In the end, the challenges and future recommendations of carbon dots for food packaging applications are briefly discussed.
2. Carbon dots: a new type of carbon-based nanomaterial
In recent days nanotechnology has been a thriving area in every field. Carbon dots are a type of carbon nanomaterial that was discovered through the purification of singular-layer carbon nanotubes.14 Based on the dimensions, nanostructures can be classified into four categories: zero-dimensional (0-D), one-dimensional (1-D), two-dimensional (2-D), and three-dimensional (3-D) nanomaterials. Carbon dots are unique, discrete small particles and zero-dimensional semiconductors, smaller than 10 nm and completely spherical form of nanoparticles.15 CDs have high-quality physical and chemical properties, such as low cost of the source, good tensile strength, ultraviolet barrier, good photostability, low toxicity, good biocompatibility, antioxidant, antimicrobial, small size, good quantum yield, abundant availability, and photoluminescence, giving opportunities in lots of applications in various fields. On the surface of carbon dots, there are naturally occurring or active functionalized groups, such as hydroxyl (–OH), carboxyl (–COOH), and amino (–NH2), providing the chain formation reaction site or increasing the cross-linking properties in the chemical reaction.16,17CDs' surface functional groupings help enhance the water solubility, the adsorption effect, chemical reactivity, and derivatives of some polymeric, biological, or organic materials. CDs are commonly classified into carbon quantum dots (CQDs), graphene quantum dots (GQDs), graphene oxide quantum dots (GOQDs), and carbon nanodots (NCDs). CD formation depends on the surface adjustment of carbon nanoparticles with polymeric and organic molecules. The chemical composition or the interior structure of the carbon dots decides which kind of carbon source is the primary material and it depends on the synthesis method.18 Carbon dots extracted from non-biomass materials, such as polymer waste, liquid fuels, and battery discharge, are cytotoxic in nature and sometimes, after the addition of metal doping, change the properties of the whole matrix. These carbon dots are not suitable for food contact applications. Carbon dot surfaces can be altered by adding functional groups, such as amino, hydroxyl, carboxylic, and aldehyde groups, allowing them to interact with a wide range of molecules.19 CDs can also be synthesized from eco-friendly substances. Some environmentally-friendly sources such as animal-origin food (honey, meat, butter, kinds of seafood), fruits/vegetables, and plant-origin (waste paper, fruit juice, tea residue waste, plant extract, plant seed) are the most popular carbon dot sources for food grade applications, and are described in Fig. 2. These food grade carbon dots are synthesized without using any harmful, and non-food-grade chemicals.20,21
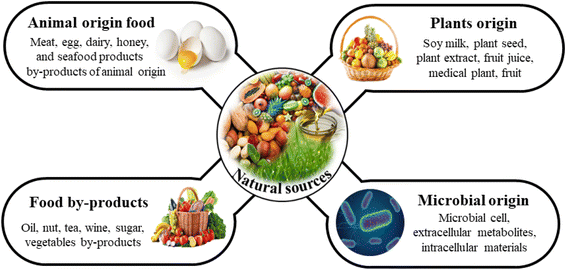 | ||
| Fig. 2 Various sources, including animal food, human origin, plant and microorganism origin materials used for the fabrication of carbon dots for active food packaging applications. | ||
3. Synthesis of carbon dots
Based on the source, CDs are synthesized in two ways. The first is the “top-down,” and the second is the “bottom-up” approach. The top-down is a physical approach in which bulk nanomaterial breaks down into carbon dots using chemical or electrochemical and physical techniques. The top-down approaches refer to chemical oxidation, arc discharge, laser ablation, and electrochemical methods.22 Another aspect of the bottom-up approach is the synthesis of carbon dots from smaller carbon units into ultra-small units by using some energy, such as hydrothermal treatment, ultrasonic treatment, thermal decomposition, pyrolysis, carbonization, and microwave synthesis.23 The surface properties can be optimized during the formation or synthesis process.The bottom-up approach is more compatible because of reagent requirements, low cost, efficiency, and compatibility with the chemistry green world. Fig. 3 explains the various processes for synthesizing carbon dots.20 For the synthesis of carbon dots, several synthetic (polymer waste, liquid fuels, batteries discharge) and natural compounds (biomass, micro-organism, plants) with carbon have been employed as precursors. In recent years, biomass has been extensively explored as a green source for generating food-grade carbon dots. Food-grade carbon dots can be synthesized from plants, food products, and microorganisms. It includes a wide range of organic substrates, including carbohydrates, proteins, alkaloids, and carotenoids. The hydrothermal and microwave methods are best suited for the synthesis of carbon dots. In the hydrothermal method, the degradation of biomass sources in the presence of water solvent at a certain temperature and pressure, and the range between water/sources is 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 75
1 to 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.24 The chemical, physical, and also functional carbon dot characteristics are enhanced through the doping process with heteroatoms such as phosphorus (P), nitrogen (N2), fluorine (F), sulfur (S), copper (Cu), magnesium (Mg), titanium (Ti) and different combinations of atoms. Based on the type of material, 2-types of doping may be performed in carbon dots, i.e., metal and non-metal doping, for example-nitrogen glucose-based carbon dots (NGCD) or N-functionalized carbon dots.25 Carbon dots have various properties, such as unique structure, nontoxicity, tiny size, drug delivery, diagnosis, bioimaging, bio-labeling, photodynamic therapy, easy synthesis, high surface charge, environmentally friendly, which ultimately make them suitable for food packaging applications.26
1.24 The chemical, physical, and also functional carbon dot characteristics are enhanced through the doping process with heteroatoms such as phosphorus (P), nitrogen (N2), fluorine (F), sulfur (S), copper (Cu), magnesium (Mg), titanium (Ti) and different combinations of atoms. Based on the type of material, 2-types of doping may be performed in carbon dots, i.e., metal and non-metal doping, for example-nitrogen glucose-based carbon dots (NGCD) or N-functionalized carbon dots.25 Carbon dots have various properties, such as unique structure, nontoxicity, tiny size, drug delivery, diagnosis, bioimaging, bio-labeling, photodynamic therapy, easy synthesis, high surface charge, environmentally friendly, which ultimately make them suitable for food packaging applications.26
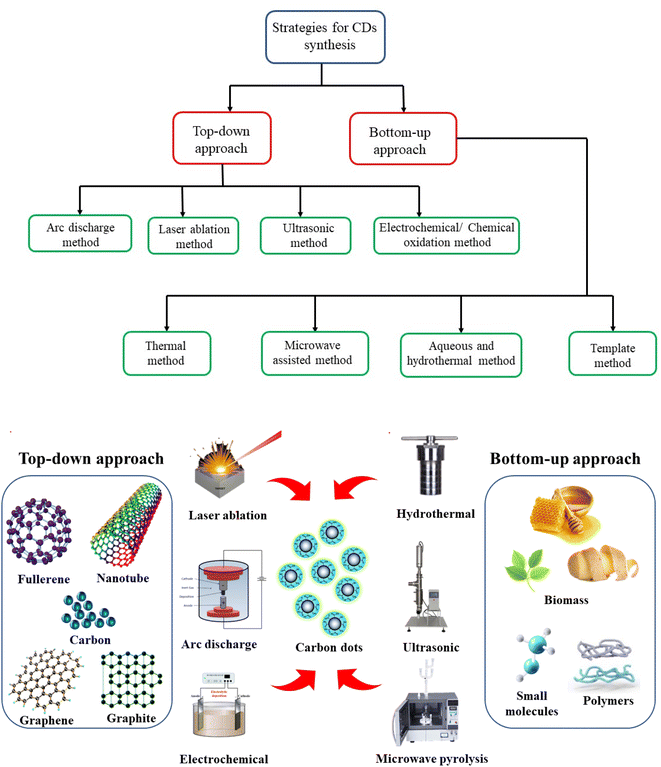 | ||
| Fig. 3 Potential synthetic techniques top-down and bottom-up approaches for the production of carbon dots from various sources for food packaging applications. | ||
4. Carbon dots for packaging applications
Carbon dots play the most crucial role and have a diverse range of potential applications in packaging. They can help develop new biodegradable, anti-ultraviolet, antibacterial, anti-oxidation, and biocompatible films for food packaging to retain the quality and freshness and increase the shelf-life of the food. The food packaging industry has higher requirements for carbon dots to extend product storage life and safety.12 The addition of carbon dots as active material enhances the physical, chemical, and mechanical properties of packaging without any effect on the remaining properties of the film and foods. It is aimed to develop carbon dots as a new biodegradable packaging material with antimicrobial and antioxidant properties, which can be used to preserve and enhance the shelf-life of food.27Table 1 presents the application of carbon dots in developing active and intelligent food packaging systems.| Biopolymer | Carbon source | Effect on polymer matrix | Ref. |
|---|---|---|---|
| Gelatin | Potato peel | The gelatin/carbon dots film exhibits UV-barrier, antioxidant, and antimicrobial properties | 29 |
| Gelatin/chitosan | Glucose | The composite film generates reactive oxygen species, exhibits high antibacterial and UV-blocking properties without altering mechanical properties and water vapor permeability | 30 |
| Cellulose | Glucose | The film showed antibacterial activities, enhanced water contact angle, and UV-blocking properties without reducing the transparency of the film | 25 |
| Pectin/gelatin | Turmeric | The film showed intense antioxidant, antimicrobial activity and improved the UV-blocking function without significantly affecting the transparency of the film | 31 |
| Chitosan | Silk sericin | The bio-nanocomposite film has enhanced flexibility, antimicrobial, UV shielding, biocompatibility, and antioxidant properties | 32 |
| Carboxymethyl cellulose | Chitosan | The developed film exhibits enhanced antioxidant, antimicrobial, and mechanical properties | 33 |
| Polyvinyl alcohol | 1,4 dihydroxyanthraquinone | The glyphosate film has carbon dots, and Cu2+ ions have good innovative sensing properties | 34 |
| Polyvinyl alcohol | Banana paste | The developed film protects against UV radiation, and increases the shelf life of fried meatballs | 35 |
| Gelatin/carrageenan | Enoki mushroom | The enoki-mushroom-based active antioxidant film prevents food oxidation. The film also exhibits high UV barrier and strength properties | 3 |
| Chitosan/polyvinyl alcohol | Waste acorn cups | The developed composite film has enhanced the UV absorption capacity | 36 |
| Polypropylene | Cysteine | The film has fluorescent, antioxidant properties, which can extend the shelf life of food products | 37 |
| Bacterial nanocellulose | Lactic acid | The developed composite film enhances stretchability and flexibility, and also increases antimicrobial, photoluminescent, and ultraviolet-blocking properties | 38 |
| Polyvinyl alcohol | Waste tea residue | The developed films are transparent, flexible, and showed high mechanical strength, and enhanced UV blocking capabilities | 13 |
| Chitosan | Banana | The developed film has good microbial stability, and heat treatment for improving the shelf life of soy milk | 39 |
| Starch | Soy protein isolate | The developed composite film reduces water sensitivity and enhanced mechanical properties | 40 |
| Zein | Zinc acetate dihydrate | The film with quantum dots was effective against S. aureus and E. coli, and showed antimicrobial properties | 41 |
| Bacterial cellulose | Postbiotics of L. acidophilus | The developed film has good UV-blocking, antioxidant, and antimicrobial properties against L. monocytogenes and E. coli | 38 |
| Polyvinyl alcohol/nano cellulose composite | Cyanobacteria | The developed composite film has good flexibility, UV, and infrared blocking tendency, and anti-counterfeiting properties | 42 |
| Polypyrrole–chitosan/polypyrrole – chitosan-poly ethylenediamine | Citric acid and D,L-cysteine | The developed composite film has antimicrobial activity against E. coli and S. aureus | 43 |
| Chitosan coating | Kelp (Laminaria japonica) | The developed coating decreases water mobility and effectively inhibits microorganisms which increases the quality as well as the shelf life of the food | 44 |
| Regenerated cellulose | Lactose | Nitrogen-doped films are highly transparent with enhanced mechanical properties | 45 |
| Collagen | Cotton cellulose | The composite thin films have high ionic strength and good UV barrier properties | 46 |
| Polyvinyl alcohol-cellulose nanofiber | The residue of radiata pine | The composite film has good UV barrier properties, mechanical properties, water resistance properties, and anti-counterfeiting | 47 |
| Poly methyl methacrylate | Carbon black pigment | The quantum carbon dots-based film has good UV-blocking properties | 48 |
| Nanocellulose- zinc oxide | Nanocellulose and 4,7,10-trioxa-1,13-tridecanediamine | Zinc oxide-based carbon dots composite film has good UV barrier properties and exhibits excellent thermal stability and photoluminescent stability | 49 |
| Polymethylmethacrylate | Cow milk | The cow milk-derived nanocomposite films have good antimicrobial and flexible properties | 50 |
Carbon dots have various applications and are used in food packaging that can enhance the properties of food products, such as quality and freshness.28 Research has shown that adding carbon dots increases the film's light-blocking property without affecting water vapor permeability (WVP), mechanical properties, and water contact angle (WCA). The composite film generates reactive oxygen species (ROS), which have a strong antibacterial effect on bacteria, exhibit high antifungal activity, and increases tensile strength and the elastic modulus of the film. This review sum-up up all recent research on carbon dots in food packaging as active packaging, intelligent-sensor packaging, anti-counterfeiting, the addition of nanoparticles in foods to increase shelf-life, and application in food preservation as a coating agent.
Carbon dots can be used in anti-counterfeiting applications for packaging. Anti-counterfeiting packaging is the practice of safe packaging that ensures product safety and prevents replicas. Carbon dots have photoluminescent properties that can help detect counterfeiting. For instance, carbon dots that emit a specific fluorescence signal could be embedded in the packaging substance and observed by flashing fluorescent lights. Carbon dots may be embedded in the interior of the polymer matrix, or they may be coated explicitly onto the surfaces of the polymer matrices. It is more challenging for counterfeiters to imitate expensive products. These labels are handy for expensive goods that might be produced locally.12 The application of carbon dots as additives, coating agents, antioxidant agents, and antimicrobial agents in food packaging as shown in Fig. 4 is self-explanatory because of some unique properties, such as good bio-compatibility, photoluminescence properties, and higher stability. As such, carbon dots are successfully applied to different fields such as active packaging, intelligent packaging, coating agent, and nano-level additives to enhance the food's shelf life. CDs have high functional properties, and thus they are more demanding in food packaging sectors.12
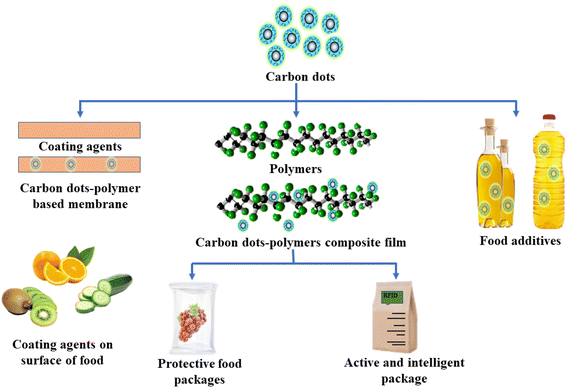 | ||
| Fig. 4 Potential applications of carbon dots as additives, coating agent, and active and intelligent agents for food packaging applications. | ||
5. Effect of carbon dots on engineering properties of the polymeric film
Mechanical properties (tensile strength, elongation at break, modulus of elasticity), barriers (water barrier, gas barrier, and UV barrier), and thermal stability effectiveness of polymer films are fundamental characteristics of packaging materials.51 This section highlights the thickness, mechanical properties, thermal properties, gas barrier, and morphological changes in polymer films. Table 2 describes the active and intelligent packaging system, which uses carbon dots made with natural resources as carbon sources and their effect on the composite matrix.| Polymeric film | Carbon source | Synthesis method | Thickness | Tensile strength | Modulus of elasticity | Elongation | Active properties | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control film | Active film | Control film | Active film | Control film | Active film | Control film | Active film | |||||
| Carboxymethyl cellulose/carbon quantum dots | Chitosan | Hydrothermal at 180 °C for 12 h | 58.3 μm | 61.3 μm | 30.7 MPa | 31.5 MPa | 1.3 GPa | 2.1 GPa | 6.4% | 7.9% | Antioxidant, antibacterial and antifungal activities | 33 |
| Silk sericin to silk sericin protein/chitosan | Silkworm cocoon | Hydrothermal at 100 °C for 2 h | 0.05 mm | 0.06 mm | 10.39 MPa | 22.01 MPa | — | — | 18.5% | 24.04% | Antibacterial, antioxidant, and UV blocking | 32 |
| Silk sericin protein/chitosan to silk sericin protein/chitosan/carbon dots | Silkworm cocoon | Hydrothermal at 100 °C for 2 h | 0.06 mm | 0.07 mm | 8.21 MPa | 19.83 MPa | — | — | 24.0% | 54.7% | Anti-counterfeiting, antioxidant, and antimicrobial | 32 |
| Chitosan/gelatin to chitosan/gelatin/carbon dots | — | Hydrothermal | 51.8 μm | 52.9 μm | 79.3 MPa | 82.1 MPa | 2.8 GPa | 3.1 GPa | 6.4% | 7.3% | Antioxidant, and antimicrobial | 54 |
| Cellulose nanofiber-based graphene carbon dots to N-functionalized graphene carbon dots | Glucose | Hydrothermal at 200 °C for 6 h | 32.8 μm | 36.1 μm | 75.2 MPa | 76.0 MPa | 6.2 GPa | 5.9 GPa | 3.9% | 4.1% | UV-blocking and antimicrobial | 25 |
| Polyvinyl alcohol to polyvinyl alcohol/waste tea residue carbon dots | Waste tea residue | Muffle furnace at 200 °C | 0.040 mm | 0.07 mm | 249.8% | 209.99% | — | — | 4929.12 gf | 4926.847 gf | UV-blocking | 13 |
| Gelatin/carrageenan to gelatin/carrageenan/enoki mushroom-based carbon dots | Enoki mushroom | Hydrothermal at 200 °C for 6 h | 54.5 μm | 61.4 μm | 52.8 MPa | 81.2 MPa | 1.3 GPa | 0.8 GPa | 3.9% | 6.4% | Antimicrobial | 3 |
| Pectin/gelatin to pectin/gelatin/carbon dots | Potato | Hydrothermal at 200 °C for 6 h | _ | _ | 55.3 MPa | 60.6 MPa | 4.3 GPa | 2.0 GPa | 8.9% | 6.4% | Antioxidant, and antimicrobial | 31 |
| Pectin/gelatin/carbon dots to pectin/gelatin/sulphur-doped carbon dots | Potato | Hydrothermal at 200 °C for 6 h | _ | _ | 52.8 MPa | 20.1 MPa | 2.3 GPa | 1.1 GPa | 6.4% | 12.5% | Antioxidant, and antimicrobial | 31 |
| Cellulose nanofiber to cellulose nanofiber-based graphene carbon dots | Glucose | Hydrothermal at 200 °C | 32.8 μm | 33.1 μm | 73.3 MPa | 75.2 MPa | 6.3 GPa | 6.2 GPa | 3.8% | 3.9% | Antioxidant and antimicrobial | 25 |
5.1. Mechanical properties
Mechanical properties affect the film's behaviour during processing, preservation, transportation, and distribution until the consumer uses the food product. Packaging protects food products from various external mechanical loads during food supply chain processes.52 Mechanical properties such as tensile strength (TS), elastic modulus (EM), and tensile strain of the film are increased by adding carbon dots in biodegradable polymers and forming composite films for food packaging.53The use of thinner film packaging is made by improved mechanical performance, which lowers the cost and amount of the material used.12 The addition of carbon dots in the gelatin/carrageenan polymer matrix creates a suitable composite film with excellent improvement in mechanical properties.3 The tensile strength depends on the intermolecular interaction between the two biopolymers. The addition of enoki mushroom-based carbon dots in the gelatin/carrageenan film increases the tensile strength of the composite film because the interaction between the –OH group of carrageenan and the –COOH group of gelatin forms an ester bond, and the enhanced tensile strength was 61 MPa. Elastic modulus (EM) and elongation at break (EB) are also affected by the addition of enoki mushroom-based carbon dots (mCDs). The elongation at break and the thickness value without adding mCDs were 4.0% and 55 μm, respectively, and after the addition of mCDs, the values increased by nearly 4.4% and 57 μm, respectively. Similarly, the value of elastic modulus linearly decreases with an increased concentration of enoki mushroom-based carbon dots (mCDs).3
The addition of carbon dots derived from waste tea residue (WTR-CD) in the PVA matrix increases the mechanical properties of the polymer film. The stress–strain study of PVA and PVA/WTR-CDs composite films was conducted at a high concentration of waste tea residue-carbon dots. The tensile strain, tensile stress, and extension properties of PVA films are 249.78%, 194.06 kg cm−2, and 63.44 nm, respectively. After adding WTR-CDs to the PVA film, the tensile strain, tensile stress, and extension were 209.97%, 104.53 kg cm−2, and 53.44 nm, respectively. From the above results, Patil et al.13 (2020) observed that the value of extension and stress–strain parameters in the PVA film were high compared to the PVA/WTR-CDs films. The gelatin film's thickness, tensile strength, and elongation at break are 60.7 MPa, 62.3 μm, and 12.2%, and after adding carbon dots, they increase to 65.3 MPa, 69.0 μm, and 12.0%, respectively. The flexibility of the gelatin/CD film did not change the cruciality, but the stiffness of the composite film decreased significantly. Min et al. (2022) reported that a low concentration of carbon dots increased the mechanical strength of the gelatin/CD composite film. This result indicates that hydrogen bonding and interfacial interaction between the gelatin/CD matrix increased at low concentrations.29
The addition of carbon dots increased the tensile strength of the biopolymer matrix. The tensile strength of pectin/gelatin-based films depends on the solid intermolecular interaction between the –OH group of pectin and the –COOH group of gelatin. The interfacial interaction between the pectin/gelatin-based biopolymer and carbon dots is strong compared to the pectin/gelatin-based composite film. Adding carbon dots in pectin/gelatin-based films increased the tensile strength and modulus of elasticity and decreased elongation at break.52 The addition of sulfur-doped carbon dots (S-CDs) reduced tensile strength and increased elongation at break. The different results of mechanical properties of CDs and S-CDs composite films depend on the surface functional groups on CDs.31 The addition of carbon dots into cellulose nanofiber (CNF) does not significantly alter the thickness of the film. The tensile parameters of the CNF film are relatively high; for example, tensile strength was nearly 74 MPa, young modulus of elasticity was 6.1 GPa, and elongation at break was 4.1% compared to pectin, gelatin/carrageenan-based polymeric films. The addition of graphene quantum dots and the nitrogen-doped graphene quantum dots in the CNF did not affect the mechanical properties of the composite film.25
5.2. Barrier properties
Barrier properties are essential for retaining food refreshment, nutritional values, and quality. Ultra-violet (UV) barriers, water vapor permeability (WVP), water contact angle (WCA), and gas barriers help to protect food from the atmosphere. This section provides a brief idea about the significance of barriers in food packaging.29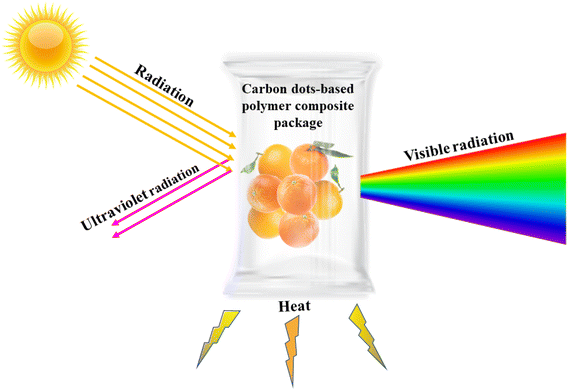 | ||
| Fig. 5 Ultraviolet light barrier mechanism of carbon dots based on biodegradable packaging illustrating food product's protection from ultraviolet radiation. | ||
Patil et al. (2020) reported that increasing the concentration of tea waste residue-based carbon dot nanoparticles into neat polyvinyl alcohol film (WTR-CDs/PVA) increased the UV-blocking properties of the composite film. The UV-barrier properties of the composite films depend on the various carbon dot concentrations in the polymeric films. The UV-barrier capability changes dramatically when the concentration of waste tea residue-derived carbon dots in PVA increase. Increasing the 0.5 mg concentration of the waste tea residue-derived carbon dots in PVA can prevent the transmission of light of UV-A (310 nm to 400 nm) and UV-B (280 nm to 310 nm) and also reduce UV-C (235 nm to 280 nm). This result indicated that enhancement of the UV adsorption capability changes dramatically when the concentration of waste tea residue-derived carbon dots in PVA increases in the PVA film. There was no significant change in thickness, or mechanical characteristics, such as tensile strength, along with the transparency of the film after adding WTR/CDs UV blocking agent in the polyvinyl alcohol film.13
Further, another study showed that adding carbon dots in chitosan/gelatin composite film increased UV-blocking of the radiation without changing other properties, such as transparency and tensile characteristics. Ultraviolet radiation generally encourages the oxidation and decomposition of nutrients in foods due to various chemical compounds, which form hazardous compounds, and leads to the decolorization of food products. The pectin/gelatin film was excellent and had visible light transparency. Incorporating carbon dots and S-CDs decreased the transparency of the films while increasing the light transmission to visible light. The addition of carbon dots and sulfur-doped carbon dots in the pectin/gelatin film decreased the transparency of the composite film but increased the UV barrier properties. At 280 nm, light transmittance blocks UV-A (320 nm to 400 nm) and UV-B radiation.31 Glucose carbon dots (GCD) and N-functionalized carbon dots (NGCD) were used to prepare cellulose nanofiber (CNF)-based composite films. GCD and NGCD provided high UV blocking properties to the CNF film without changing the transparency of the composite film. At 280 nm light transmittance, the transparency of the CNF film was considerably reduced from 53.8% to 5.2% and 1.3%, while the addition of the glucose carbon dots and N-functionalized carbon dots in the CNF film at 660 nm light transmittance decreased from 86.7% to 80.2% and 78.4%, respectively.25
Carboxymethyl cellulose (CMC) films had good ultraviolet (280 nm) and visible light (660 nm) transmittances of 61.0% and 84.2%, respectively. The ultraviolet-barrier characteristics increased with the addition of carbon quantum dots to CMC. At the carbon quantum dot (CQDs) concentrations of 1.0%, 3.0%, and 5.0%, the ultraviolet-barrier characteristics of the composite film increased by 81%, 98%, and 100%, respectively, because CQDs have good light-adsorbing properties. But at the other hand, the inclusion of carbon dots did not affect the transparency of the composite film. The size of CQDs is much smaller than the 660 nm wavelength of light, which does not interact with the transmitted light. As such, it showed that the addition of carbon quantum dots in the CMC blocks the UV radiation without any impact on the transparency of the composite film.33 The above results indicated that adding carbon dots to the composite film enhanced the composite film's ultraviolet barrier characteristics, which can be utilized in the food packaging sector.56
Research suggested that the WVP of the pure CNF films did not altered with the addition of graphene carbon dots in the polymer matrix. But the addition of nitrogen-doped graphene carbon dots to the CNF composite film enhanced the WVP activity of the film. This might be due to the high hydrophilic tendency of CNF films containing nitrogen-based graphene carbon dots compared to the CNG films with graphene carbon dots. This claim can also be justified by the water contact angle values of both films. The water contact angle of pure cellulose nanofiber film was nearly 30°, indicating that the CNF film was highly hydrophilic. The addition of graphene carbon dots in the cellulose nanofiber composite film decreased the water contact angle because carbon dots have hydrophilicity properties. The surface wettability and hydrophilicity of the CNF composite film depend on the mode of the interaction between the polymer and additives.25
The addition of carbon dots (2 wt%) in a neat gelatin film increased the water vapor permeability of the composite film, which decreased the water vapor because of the surface hydrophilic functional groups of the carbon dots. At the same time, the water contact angle of the composite film was significantly reduced by adding carbon dots. The water contact angle and water vapor permeability of gelatin/carrageenan film were 58.3° and 0.95 × 10−9 g × m m−2×Pa × s, respectively.29 The addition of enoki mushroom-based carbon dots (mCDs) in the gelatin/carrageenan had no significant change in the water barrier properties. Enoki mushroom-derived carbon dots (mCDs) had surface hydrophilicity properties, which increased water vapor permeability but decreased the water contact angle in the gelatin/carrageenan/mCDs composite film. As such, it is concluded that the hydrophilic nature of the composite film depends on the concentration of carbon dots and nature polymers.58
6. Carbon dots as an active material
Active packaging interacts with the packaged product effectively, either chemically or biologically. It affects the product in the packaging, for instance, preventing microbial growth, maintaining the quality and freshness of food, extending shelf-life or inhibiting fungal growth, and providing information about the product's condition. Active packaging mainly includes antimicrobial and antioxidant properties.596.1. As an antioxidant agent
Antioxidant-releasing packaging techniques effectively reduce the food oxidation rate, increasing the food shelf life.60 Currently, a series of CDs, such as GQD, selenium-doped CDs, nitrogen-doped CDs, and chlorine-doping CDs are widely used as antioxidants for reducing reactive oxygen species. The sources utilized to synthesize carbon dots with antioxidant properties are primarily plant-origin compounds, and the formation technique typically involves an autoclave. Das et al. (2021) found that the DPPH free-radical blocking properties of nitrogen, phosphorous, and sulfur-doped (NPSC) carbon dots synthesized using a one-step autoclave method were reduced. By coating with nitrogen, phosphorous, and sulfur-doping of carbon dots polypropylene composite film, the antioxidant capacity of the NPSC dots was efficiently employed on plastic packages. Many types of radicals, such as DPPH radicals, hydroxyl radicals, KMnO4 radicals, and superoxides anion radicals, elevated the antioxidant properties in the NPSC-dots. DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) is a kind of electron transfer and stable radical used to evaluate the antioxidant properties of the compounds. This method is both economical and practical for the measurement of antioxidant properties. When DPPH reacts with an antioxidant, it absorbs the hydrogen-radical to form the DPPH-hydrogen molecule, which has a pale-yellow appearance. Because it contains nitrogen atoms with lone-pair electrons and is enclosed by 3 benzene rings, DPPH is a stabilized free radical. The antioxidant properties of the NPSC dots were enhanced as the concentration increased nearly to 417 μg mL−1. The nitrogen, phosphorous, and sulfur-doped CDs (NPSC-dots) have the highest scavenging activity of 74% for DPPH radicals.37The gelatin/CDs film shows high antioxidant activity and is intended to inhibit the degradability of packaged foods sensitive to oxidative degradation. Depending on the concentration of carbon dots, considerable antioxidant activity in the ABTS (2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) and DPPH techniques were determined. Carbon dots have good shielding ability, which could be attributed to surface structural features that contribute to removing free radical scavenging to produce more developed systems in the ABTS and DPPH methods, and in most of the films, antioxidant properties from the DPPH method are less compared to ABTS methods. Gelatin-based films have some scavenging activity assessed using the DPPH and ABTS methods. Antioxidant activity in gelatin film was 27.0% because of some antioxidant peptides present in gelatin. The addition of carbon dots at 2.0% and 4.0% levels enhances the scavenging capacity of the film to 99.0% and 99.6% in the ABTS method.29
Similarly, incorporating carbon dots in the gelatin film increases the free radical scavenging activities by 72.1% and 93.2%, respectively, for 2.0% and 4.0%, when assessed using the DPPH method. From the above results, it can be concluded that the antioxidant activity of the carbon dot incorporated films was more significant when measured using the ABTS method, as compared to the DPPH method, which could be due to the higher expanding rate of the gelatin film in an aqueous ABTS mixture than in a methanolic DPPH solution. Carbon dots' solid antioxidant activity can be linked to their surface functional groups capable of scavenging free radicals. Carbon dots added in the biopolymer-based composite films have more antioxidant activity, such as pectin/gelatin and gel/carr composite films.29 Some parameters influencing the antioxidant activity are electron flow, unpaired electrons, hydrogen donating behavior, hetero-atom doping, sp2 hybrid carbon domain, and surface functional group type. Research indicates that the presence of carbon dots at 2 or 3 mg mL−1 concentration helps improve the films' antioxidant activity by about 18-fold and prevents oxidative rancidity.31 The antioxidant properties of the cellulose-based film were determined by DPPH and ABTS methods. The value of antioxidants for the bacterial nanocellulose (BNC) film without adding carbon dots was 14.0% and 5.0%, from the ABTS and DPPH methods, respectively. After adding carbon dots, the antioxidant activity of the BNC composite film was enhanced.
The addition of GCD and NGCD in the CNF film increased the capacity to scavenge free radicals to nearly 98.1% and 98.0%, respectively, in the ABTS method, but for the DPPH method, the values were 79.0% and 84.0%, respectively. The antioxidant activities of graphene carbon dots/BNC composite film were more significant in the ABTS method as compared to the DPPH method. The surface functional groups of the graphene carbon dots that take part in free radical scavenging are responsible for good antioxidant activity in the CNF film. CNF/NGCD and CNF/GCD composite films presented the same antioxidant properties in the ABTS method. But, the NGCD/CNF composite films presented a more excellent antioxidant activity in the DPPH method as compared to NGCD/BNC, and due to the high antioxidant properties, these films can be used in the active packaging domains to reduce the rate of food oxidation.25 The enoki mushroom-based carbon dot/gelatin/carrageenan composite film significantly increased the free-radical release effect. Similarly, they increased the antioxidant properties compared with pure gelatin/carrageenan films.3 The increased activity in ABTS is because mCDs are more soluble in an aqueous ABTS solution than in the DPPH methanol solution. The occurrence of surface hydroxyl groups on mCDs may explain their excellent antioxidant property. Enoki mushroom-based carbon dot/gelatin/carrageenan composite film significantly increased the free radical release effect, resulting in a film with higher antioxidant properties as compared to pure gelatin/carrageenan films. Carbon dots work as active agents with heteroatom doping in the films in the above process.3
6.2. As an antimicrobial agent
Carbon dots from various sources exhibit antimicrobial properties against a broad spectrum of food-borne pathogens and spoilage microorganisms. The antimicrobial properties of carbon dots depend on their shape, size, surface charge, and various functional groups.52 It was accomplished by incorporating an active ingredient into the product package, depositing a coating on the product, or incorporating it within the packaging matter. Carbon dot's antibacterial properties are affected by numerous mechanisms such as cytoplasmic leakage, cell structure breakdown, and reactive oxygen species (ROS) production of genomic deoxyribonucleic acid (DNA) fragmentation/condensation.38 The addition of carbon dots to the films demonstrated different antibacterial actions in the gelatine-based film based on the number of carbon dots and bacterium test.38 The composite films inhibited the development of E. coli but disturbed the growth of L. monocytogenes, and the action was enhanced when the concentration of the carbon dots was increased. Paper is commonly used as packaging material for food packaging and other fields since it is recyclable, ecological, and affordable. Li et al. (2021) developed a paper with antibacterial or waterproofing properties that can be utilized for food packing to increase the shelf-life of food. The antibacterial activity of paper, nano-ZnO, and graphene quantum dots was produced on the surface using a hydrothermal synthesis approach. The BNC paper displayed antimicrobial properties against Gram-negative E. coli and L. monocytogenes. Some CDs were implanted with nanocellulose using an ex-situ process to produce antimicrobial/ultraviolet-protected nanopaper. The antibacterial potential of carbon dots at different concentrations, such as 100, 250, and 500 mg mL−1, was investigated using the agar well diffusion methods.25,61In another research, the viable cell colony count method was used to assess the antibacterial activity of the pectin/gelatin composite films towards food-borne pathogenic bacteria, L. monocytogenes, and E. coli. The pure pectin/gelatin layer did not show any antibacterial properties, but the addition of carbon dots in pectin/gelatin showed antibacterial properties that prevented the growth of E.coli and L.monocytogenes by nearly 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU mL−1. Nevertheless, the addition of sulfur-doped carbon dots (S-CDs) in the pectin/gelatin composite film showed antimicrobial properties that increased rapidly because S-CDs have some functional groups in sulfur, such as sulfonic acids and sulfonates. These functional groups help bind the enzymes, produce reactive oxygen species, and degrade lipids or proteins.31 The carbon dots derived from the turmeric and sulfur-functionalized carbon dot composite film showed good antimicrobial properties through ABTS and DPPH methods. The zone inhibition method was used to determine the antimicrobial activity of carbon dots and sulfur-based carbon dots.62 Carbon dots are also used in biopolymer solutions to create an antibacterial coating around food. In this way, the food is dipped into the antimicrobial solution. For example, the coating on freshly-cut cucumber dipped in the antimicrobial additive polymer solution. The kelp-derived carbon dots were dipped into the chitosan solution and coated on the cucumber because the coating on the fresh cucumber under modified atmosphere conditions prohibited the growth of microorganisms, mold, and yeast.44
log CFU mL−1. Nevertheless, the addition of sulfur-doped carbon dots (S-CDs) in the pectin/gelatin composite film showed antimicrobial properties that increased rapidly because S-CDs have some functional groups in sulfur, such as sulfonic acids and sulfonates. These functional groups help bind the enzymes, produce reactive oxygen species, and degrade lipids or proteins.31 The carbon dots derived from the turmeric and sulfur-functionalized carbon dot composite film showed good antimicrobial properties through ABTS and DPPH methods. The zone inhibition method was used to determine the antimicrobial activity of carbon dots and sulfur-based carbon dots.62 Carbon dots are also used in biopolymer solutions to create an antibacterial coating around food. In this way, the food is dipped into the antimicrobial solution. For example, the coating on freshly-cut cucumber dipped in the antimicrobial additive polymer solution. The kelp-derived carbon dots were dipped into the chitosan solution and coated on the cucumber because the coating on the fresh cucumber under modified atmosphere conditions prohibited the growth of microorganisms, mold, and yeast.44
The addition of nitrogen-doped carbon dots in a cellulose nanofiber film demonstrated more excellent antimicrobial properties than graphene carbon dots. Therefore, NGCD/CNF-based nanofiber showed excellent antimicrobial properties for food packaging to prevent food spoilage and enhance the shelf-life of food. The graphene carbon dots inhibit the growth of the microorganism; meanwhile, microbial killing was demonstrated by the NGCD-added film. Various studies have demonstrated that generating carbon dot-generated reactive oxygen species leads to cell death. Therefore, the antimicrobial properties of carbon dots were performed using the oxygen radical absorbance capability method, and the formation of reactive oxygen species solutions by adding carbon dots and sulfur-doped carbon dots increased over time. The aforementioned research studies on carbon dots conclude that a broad spectrum of antibacterial action and antimicrobial properties are greatly influenced by the type of carbon used, particle size, doping atom and shape, and the production process.38
7. Challenges in carbon dots for food packaging
The advanced improvement of nanotechnology is to the creating a novel sensor for food packaging that includes a platform that enhances food quality, reduces unwanted waste, assesses protection, and enhances shelf-life.63 Numerous innovative nanoparticles are now being studied for application in food packaging. The center of attention on nanomaterials for their safety in food packaging is their toxicity and higher surface region to volume proportion, leading to hazardous reactions. Introducing any novel products into the market must thoroughly examine the impact of the products on the health of the consumer and the atmosphere across the food chain, after which they can be used in the food packaging industry, sales, and marketing.64The nanomaterial utilized in the current technology has the potential to transfer and penetrate the body via breathing, ingestion, and skin absorption; it is going to affect consumers' health. Toxicity is a significant factor in determining the capacity-environmental impact of carbon dots.65 Current research shows that biological hazards demonstrated that carbon dots are biocompatibility and do not induce noticeable pathological changes in the body after insertion. The release of carbon dots from packaging material is a factor of the possible toxicity of carbon dots and requires specific consideration. Furthermore, a few research articles have investigated the effect of incorporating carbon dots into food-inert substances. Many factors influence the liberation of active components from films or active packages to food simulants, including the type of food additives, the suitability of the active material with the film, the solubility of the film, and the quantity or concentration of the substance. For instance, the concentration of mushroom-derived CDs and the food additives affected how quickly they were released from a gelatin/carrageenan-based polymer matrix. When the concentration of carbon dots was increased from 1.0% to 6.0%, the release rate of enoki mushroom-derived carbon dots in all simulants improved with the increase in concentration. The release rates of carbon dots derived from enoki mushrooms in 95.1% and 50.2% ethanol were lower than 10.0% ethanol and water. Carbon dots are tiny nanoparticles, which may be a concern for some consumers.3
8. Future recommendation
The application of carbon dots as nanoscale food additives for extending food's shelf life is still lacking. As such, realistic food samples must be used to prove the practical use of carbon dots as additives in packaging materials.The heteroatom-doped carbon dots also form a composite film; for example, sulfur-functionalized turmeric-based carbon dots like sulfur-based carbon dots (S-CDs) and nitrogen-doped carbon dots. Thus, it would also be crucial to investigate the possible hazards or toxicity of various types of carbon dots to verify that they may be used securely in food applications.
There are two approaches to synthesizing carbon dots: The top-down and the bottom-up. In the bottom-up approach, the hydrothermal method is an easy, environmentally friendly, and one-step synthesis quickly dispersed in solution, and the reaction operates below 300 °C. Furthermore, hydrothermal synthesis is less expensive than other solution synthesis processes in terms of instruments, power, and time. It will be essential to develop cost-effective techniques for generating carbon dots on a broad scale to be economically feasible for culinary applications.
More research is needed to determine the effect of specific carbon dots' features, such as shape, composition, charge, and functional groups, on the properties of packaging materials synthesized from various polymers.
Consumers' opinions on using carbon dots in meals and manufacturing processes are essential. By thermal or pyrolysis methods, brown-colored carbon dots may be restricted to using carbon dots on a wide scale in various meals. For this issue, large-scale production can be handled using new non-thermal methods for carbon dots.
Carbon dots are tiny nanoparticles, which may be a worrying point for some people. Due to their nanosize and dimensions, they can enter the human body through the skin-inhaling process and affect our health. The release of carbon dots from the packages is the main issue. It is necessary to research and determine the carbon dots' migration into the gastrointestinal food system and their potential toxicity. This paper showed that solid waste and residues from food manufacturing could be converted into significant CDs, potentially boosting the sustainability of the world's food supply and economics.
9. Conclusion
Carbon dots are a wide source of raw materials at low cost, good photoluminescence, UV blocking, high stability, easy interaction with substrates, and photoelectrochemical capabilities. The carbon dots offer numerous advantages for advanced or innovative food packaging, including enhanced mechanical properties, water barrier, thermal activity, barrier properties (radiation blocking, water, and gas), and antimicrobial and antioxidant properties. Carbon dots can be combined with biological–chemical indicators to create rapid and sensitive packages for detecting food quality and originality. C-dots can be synthesized with different compositions and characteristics depending on the primary sources and processing conditions. They have a high potential for usage as active material, nanolevel food additives, coating agents, and intelligent agents to improve the shelf-life of food products. Carbon dots provide superior safety to other nanoparticles. At suitable concentrations of carbon dots, nontoxicity is observed for human and animal cells. Carbon dots help protect cells from oxidative stress harm in exceptional cases. Research on carbon dots used to create intelligent packaging that detects food freshness and active packaging with antibacterial and antioxidant capabilities is a prospective research area. Carbon dots have been studied as multipurpose nanoparticles for food packaging films, with significant improvements. It expands the range of possible applications for radically-new quantum materials. The migration of carbon dots in the food is the main reason for toxicity. Additional research should be conducted to investigate the potential migration patterns of carbon dots from packaging films into various food systems and create ways to regulate the release of carbon dots.Author contributions
DG: investigation, writing – original draft, writing–review, and editing, LK: writing and editing. KKG: conceptualization, supervision, project administration, funding acquisition, writing–review, and editing.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
K. K. Gaikwad would like to sincerely thank the Department of Science and Technology (DST), Government of India, for the financial support provided under the DST INSPIRE Faculty (DST/INSPIRE/04/2018/002544).References
- A. K. Singh, D. Ramakanth, A. Kumar, Y. S. Lee and K. K. Gaikwad, Active packaging technologies for clean label food products: a review, J. Food Meas. Char., 2021, 15(5), 4314–4324 CrossRef.
- L. Kumar, D. Ramakanth, K. Akhila and K. K. Gaikwad, Edible films and coatings for food packaging applications: a review, Environ. Chem. Lett., 2022, 20(1), 875–900, DOI:10.1007/s10311-021-01339-z.
- S. Roy, P. Ezati and J. W. Rhim, Gelatin/Carrageenan-Based Functional Films with Carbon Dots from Enoki Mushroom for Active Food Packaging Applications, ACS Appl. Polym. Mater., 2021, 3(12), 6437–6445 CrossRef CAS.
- S. Singh, P. K. Maji, Y. S. Lee and K. K. Gaikwad, Applications of gaseous chlorine dioxide for antimicrobial food packaging: a review, Environ. Chem. Lett., 2021, 19(1), 253–270 CrossRef CAS [Internet]. [cited 2022 Apr 15]. Available from: https://link.springer.com/article/10.1007/s10311-020-01085-8.
- C. Andreeßen and A. Steinbüchel, Recent developments in non-biodegradable biopolymers: Precursors, production processes, and future perspectives, Appl. Microbiol. Biotechnol., 2019, 103(1), 143–157 CrossRef PubMed.
- R. Tanwar, V. Gupta, P. Kumar, A. Kumar, S. Singh and K. K. Gaikwad, Development and characterization of PVA-starch incorporated with coconut shell extract and sepiolite clay as an antioxidant film for active food packaging applications, Int. J. Biol. Macromol., 2021, 185, 451–461 CrossRef CAS PubMed.
- P. Kumar, R. Tanwar, V. Gupta, A. Upadhyay, A. Kumar and K. K. Gaikwad, Pineapple peel extract incorporated poly(vinyl alcohol)-corn starch film for active food packaging: Preparation, characterization and antioxidant activity, Int. J. Biol. Macromol., 2021, 187, 223–231 CrossRef CAS PubMed.
- R. K. Deshmukh, K. Akhila, D. Ramakanth and K. K. Gaikwad, Guar gum/carboxymethyl cellulose based antioxidant film incorporated with halloysite nanotubes and litchi shell waste extract for active packaging, Int. J. Biol. Macromol., 2022, 201, 1–13 CrossRef CAS PubMed.
- L. Kumar, R. K. Deshmukh and K. K. Gaikwad, Antimicrobial packaging film from cactus (Cylindropuntia fulgida) mucilage and gelatine, Int. J. Biol. Macromol., 2022, 596–605 CrossRef CAS PubMed , https://linkinghub.elsevier.com/retrieve/pii/S0141813022013757.
- F. Zu, F. Yan, Z. Bai, J. Xu, Y. Wang and Y. Huang, et al. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications, Microchim. Acta, 2017, 184(7), 1899–1914 CrossRef CAS.
- I. Siró, Intelligent Packaging and Food Safety, 2014, pp. 375–394 Search PubMed.
- J. H. Qu, Q. Wei and D. W. Sun, Carbon dots: Principles and their applications in food quality and safety detection, Crit. Rev. Food Sci. Nutr., 2018, 58(14), 2466–2475 CrossRef CAS PubMed.
- A. S. Patil, R. D. Waghmare, S. P. Pawar, S. T. Salunkhe, G. B. Kolekar and D. Sohn, et al., Photophysical insights of highly transparent, flexible and re-emissive PVA @ WTR-CDs composite thin films: A next generation food packaging material for UV blocking applications, J. Photochem. Photobiol., A, 2020, 400 Search PubMed.
- H. K. M. Ng, G. K. Lim and C. P. Leo, Comparison between hydrothermal and microwave-assisted synthesis of carbon dots from biowaste and chemical for heavy metal detection: A review, Microchem. J., 2021, 165, 106116 CrossRef CAS.
- J. Wang and J. Qiu, A review of carbon dots in biological applications, J. Mater. Sci., 2016, 51(10), 4728–4738 CrossRef CAS.
- M. Tuerhong, Y. Xu and X. B. Yin, Review on Carbon Dots and Their Applications, Chin. J. Anal. Chem., 2017, 45(1), 139–150 Search PubMed.
- T. S. Atabaev, Doped Carbon Dots for Sensing and Bioimaging Applications: A Minireview, Nanomater, 2018, 8(5), 342 CrossRef PubMed , [Internet]. Available from: https://www.mdpi.com/2079-4991/8/5/342/htm.
- L. Zhao, M. Zhang, A. S. Mujumdar and H. Wang, Application of carbon dots in food preservation: a critical review for packaging enhancers and food preservatives, Crit. Rev. Food Sci. Nutr., 2022, 1–19 Search PubMed.
- H. Wang, M. Zhang, Y. Ma, B. Wang, M. Shao and H. Huang, et al., Selective inactivation of Gram-negative bacteria by carbon dots derived from natural biomass: Artemisia argyi leaves, J. Mater. Chem. B, 2020, 8(13), 2666–2672 RSC [Internet]. [cited 2022 Oct 18];Available from: https://pubs.rsc.org/en/content/articlehtml/2020/tb/c9tb02735a.
- M. Moradi, R. Molaei, S. A. Kousheh, T. J. Guimarães and D. J. McClements, Carbon dots synthesized from microorganisms and food by-products: active and smart food packaging applications, Crit. Rev. Food Sci. Nutr., 2021, 1–17 CrossRef CAS PubMed.
- X. Luo, Y. Han, X. Chen, W. Tang, T. Yue and Z. Li, Carbon dots derived fluorescent nanosensors as versatile tools for food quality and safety assessment: A review, Trends Food Sci. Technol., 2020, 95, 149–161 CrossRef CAS.
- M. L. Liu, B. B. Chen, C. M. Li and C. Z. Huang, Carbon dots: synthesis, formation mechanism, fluorescence origin and sensing applications, Green Chem., 2019, 21(3), 449–471 RSC.
- T. V. De Medeiros, J. Manioudakis, F. Noun, J. R. Macairan, F. Victoria and R. Naccache, Microwave-assisted synthesis of carbon dots and their applications, J. Mater. Chem. C, 2019, 7(24), 7175–7195 RSC.
- Y. Zhao, S. Jing, X. Peng, Z. Chen, Y. Hu and H. Zhuo, et al. Synthesizing green carbon dots with exceptionally high yield from biomass hydrothermal carbon, Cellulose, 2020, 27(1), 415–428 CrossRef CAS.
- P. Ezati, J. W. Rhim, R. Molaei, R. Priyadarshi and S. Han, Cellulose nanofiber-based coating film integrated with nitrogen-functionalized carbon dots for active packaging applications of fresh fruit, Postharvest Biol. Technol., 2022, 186 Search PubMed.
- K. Ghosal and A. Ghosh, Carbon dots: The next generation platform for biomedical applications, Mater. Sci. Eng. C, 2019, 96, 887–903 CrossRef CAS PubMed.
- L. Zhao, M. Zhang, A. S. Mujumdar and H. Wang, Application of carbon dots in food preservation: a critical review for packaging enhancers and food preservatives, Crit. Rev. Food Sci. Nutr., 2022, 1–19 Search PubMed.
- F. Radnia, N. Mohajeri and N. Zarghami, New insight into the engineering of green carbon dots: Possible applications in emerging cancer theranostics, Talanta, 2020, 209, 120547 CrossRef CAS PubMed.
- S. Min, P. Ezati and J. W. Rhim, Gelatin-based packaging material incorporated with potato skins carbon dots as functional filler, Ind. Crops Prod., 2022, 181 Search PubMed.
- B. Fu, Q. Liu, M. Liu, X. Chen, H. Lin and Z. Zheng, et al., Carbon dots enhanced gelatin/chitosan bio-nanocomposite packaging film for perishable foods, Chin. Chem. Lett., 2022, 4577–4582 CrossRef.
- P. Ezati, S. Roy and J. W. Rhim, Pectin/gelatin-based bioactive composite films reinforced with sulfur functionalized carbon dots, Colloids Surf. A Physicochem. Eng. Asp., 2022, 636, 1–7 CrossRef.
- S. Mei, B. Fu, X. Su, H. Chen, H. Lin and Z. Zheng, et al. Developing silk sericin-based and carbon dots reinforced bio-nanocomposite films and potential application to litchi fruit, LWT, 2022, 164, 113630 CrossRef CAS.
- Z. Riahi, J. W. Rhim, R. Bagheri, G. Pircheraghi and E. Lotfali, Carboxymethyl cellulose-based functional film integrated with chitosan-based carbon quantum dots for active food packaging applications, Prog. Org. Coating, 2022, 166, 1–14 CrossRef.
- J. Wu, X. Chen, Z. Zhang and J. Zhang, Off-on” fluorescence probe based on green emissive carbon dots for the determination of Cu2+ ions and glyphosate and development of a smart sensing film for vegetable packaging, Microchim. Acta, 2022, 189(3), 1–9 Search PubMed.
- L. Zhao, M. Zhang, A. S. Mujumdar and B. Adhikari, Preparation of a Novel Carbon Dot/Polyvinyl Alcohol Composite Film and Its Application in Food Preservation, ACS Appl. Mater. Interfaces, 2022, 37528–37539 CrossRef CAS PubMed.
- N. Xu, S. Gao, C. Xu, Y. Fang, L. Xu and W. Zhang, Carbon quantum dots derived from waste acorn cups and its application as an ultraviolet absorbent for polyvinyl alcohol film, Appl. Surf. Sci., 2021 Aug, 556, 149774 Search PubMed.
- P. Das, S. Ganguly, S. Margel and A. Gedanken, Immobilization of Heteroatom-Doped Carbon Dots onto Nonpolar Plastics for Antifogging, Antioxidant, and Food Monitoring Applications, 2021, 37, 58 Search PubMed.
- S. A. Kousheh, M. Moradi, H. Tajik and R. Molaei, Preparation of antimicrobial/ultraviolet protective bacterial nanocellulose film with carbon dots synthesized from lactic acid bacteria, Int. J. Biol. Macromol., 2020, 155, 216–225 CrossRef CAS PubMed.
- L. Zhao, M. Zhang, H. Wang and S. Devahastin, Effect of carbon dots in combination with aqueous chitosan solution on shelf life and stability of soy milk, Int. J. Food Microbiol., 2020, 326, 108650 CrossRef CAS PubMed.
- S. Rani, K. D. Kumar, S. Mandal and R. Kumar, Functionalized carbon dot nanoparticles reinforced soy protein isolate biopolymeric film, J. Polym. Res., 2020, 27(10), 1–10 Search PubMed.
- F. Schmitz, M. B. Silva de Albuquerque, M. D. Alberton, I. C. Riegel-Vidotti and L. M. Zimmermann, Zein films with ZnO and ZnO:Mg quantum dots as functional nanofillers: New nanocomposites for food package with UV-blocker and antimicrobial properties, Polym. Test., 2020, 91, 106709 CrossRef CAS.
- L. Xu, Y. Li, S. Gao, Y. Niu, H. Liu and C. Mei, et al. Preparation and Properties of Cyanobacteria-Based Carbon Quantum Dots/Polyvinyl Alcohol/Nanocellulose Composite, Polym., 2020, 12(5), 1143 CAS.
- M. Maruthapandi, K. Sharma, J. H. T. Luong and A. Gedanken, Antibacterial activities of microwave-assisted synthesized polypyrrole/chitosan and poly (pyrrole-N-(1-naphthyl) ethylenediamine) stimulated by C-dots, Carbohydr. Polym., 2020, 243, 116474 CrossRef CAS PubMed.
- K. Fan, M. Zhang, D. Fan and F. Jiang, Effect of carbon dots with chitosan coating on microorganisms and storage quality of modified-atmosphere-packaged fresh-cut cucumber, J. Sci. Food Agric., 2019, 99(13), 6032–6041 CrossRef CAS PubMed.
- A. Cuevas, B. B. Campos, R. Romero, M. Algarra, M. I. Vázquez and J. Benavente, Eco-friendly modification of a regenerated cellulose based film by silicon, carbon and N-doped carbon quantum dots, Carbohydr. Polym., 2019, 206, 238–244 CrossRef CAS PubMed.
- T. da S. Pinto, P. N. S. Rodrigues, L. E. S. Marinho, R. M. Verly, J. P. Bretas Roa and L. C. A. de Oliveira, et al. Self-assembled hybrid nanocomposite films of carbon dots and hydrolyzed collagen, Mater. Chem. Phys., 2019, 230, 44–53 CrossRef CAS.
- L. Xu, Y. Zhang, H. Pan, N. Xu, C. Mei and H. Mao, et al., Preparation and Performance of Radiata-Pine-Derived Polyvinyl Alcohol/Carbon Quantum Dots Fluorescent Films, Mater., 2019, 13(1), 67 CrossRef PubMed.
- P. Uthirakumar, M. Devendiran, T. H. Kim and I. H. Lee, A convenient method for isolating carbon quantum dots in high yield as an alternative to the dialysis process and the fabrication of a full-band UV blocking polymer film, New J. Chem., 2018, 42(22), 18312–18317 RSC.
- X. Feng, Y. Zhao, Y. Jiang, M. Miao, S. Cao and J. Fang, Use of carbon dots to enhance UV-blocking of transparent nanocellulose films, Carbohydr. Polym., 2017, 161, 253–260 CrossRef CAS PubMed.
- S. Han, H. Zhang, Y. Xie, L. Liu, C. Shan and X. Li, et al., Application of cow milk-derived carbon dots/Ag NPs composite as the antibacterial agent, Appl. Surf. Sci., 2015, 328, 368–373 CrossRef CAS.
- L. Bastarrachea, S. Dhawan and S. S. Sablani, Engineering Properties of Polymeric-Based Antimicrobial Films for Food Packaging, Food Eng. Rev., 2011, 3(2), 79–93 CrossRef.
- M. Das Purkayastha, A. K. Manhar, V. K. Das, A. Borah, M. Mandal and A. J. Thakur, et al. Antioxidative, hemocompatible, fluorescent carbon nanodots from an “end-of-pipe” agricultural waste: Exploring its new horizon in the food-packaging domain, J. Agric. Food Chem., 2014, 62(20), 4509–4520 CrossRef CAS PubMed.
- W. Tang, B. Wang, J. Li, Y. Li, Y. Zhang and H. Quan, et al. Facile pyrolysis synthesis of ionic liquid capped carbon dots and subsequent application as the water-based lubricant additives, J. Mater. Sci., 2019, 54(2), 1171–1183 CrossRef CAS.
- P. Ezati, J. W. Rhim, R. Molaei and Z. Rezaei, Carbon quantum dots-based antifungal coating film for active packaging application of avocado, Food Packag. Shelf Life, 2022, 33 Search PubMed.
- L. Zhao, M. Zhang, A. S. Mujumdar, B. Adhikari and H. Wang, Preparation of a Novel Carbon Dot/Polyvinyl Alcohol Composite Film and Its Application in Food Preservation, ACS Appl. Mater. Interfaces, 2022, 14(33), 37528–37539 CrossRef CAS PubMed.
- M. G. Passaretti, M. D. Ninago, C. Di Anibal, C. Pacheco, D. A. Vega and M. A. Villar, et al. Composite films with UV barrier capacity to minimize flavored waters degradation, Food Packag. Shelf Life, 2019, 21, 100334 CrossRef.
- P. Phuhongsung, M. Zhang and B. Bhandari, 4D printing of products based on soy protein isolate via microwave heating for flavor development, Food Res. Int., 2020, 137, 109605 CrossRef CAS PubMed.
- S. Roy, P. Ezati and J. W. Rhim, Gelatin/carrageenan-based functional films with carbon dots from enoki mushroom for active food packaging applications, ACS Appl. Polym. Mater., 2021, 3(12), 6437–6445 CrossRef CAS.
- K. Kraśniewska, S. Galus and M. Gniewosz, Biopolymers-Based Materials Containing Silver Nanoparticles as Active Packaging for Food Applications–A Review, Int. J. Mol. Sci., 2020, 21(3), 698 CrossRef PubMed.
- M. H. Son, S. W. Park and Y. K. Jung, Antioxidant and anti-aging carbon quantum dots using tannic acid, Nanotechnology, 2021, 32(41), 415102 CrossRef CAS PubMed.
- M. Li, Q. Feng, H. Liu, Y. Wu and Z. Wang, In situ growth of nano-ZnO/GQDs on cellulose paper for dual repelling function against water and bacteria, Mater. Lett., 2021, 283, 128838 CrossRef CAS.
- S. Roy, P. Ezati, J. W. Rhim and R. Molaei, Preparation of turmeric-derived sulfur-functionalized carbon dots: antibacterial and antioxidant activity, J. Mater. Sci., 2022, 57(4), 2941–2952 CrossRef CAS.
- D. Enescu, M. A. Cerqueira, P. Fucinos and L. M. Pastrana, Recent advances and challenges on applications of nanotechnology in food packaging. A literature review, Food Chem. Toxicol., 2019, 134, 110814 CrossRef CAS PubMed.
- M. A. Cerqueira, A. A. Vicente and L. M. Pastrana, Nanotechnology in Food Packaging: Opportunities and Challenges, in Nanomaterials in Food Packaging: Materials, Processing Technologies and Safety Issues. 2018, pp. 1–11 Search PubMed.
- M. Havrdova, K. Hola, J. Skopalik, K. Tomankova, M. Petr and K. Cepe, et al. Toxicity of carbon dots – Effect of surface functionalization on the cell viability, reactive oxygen species generation and cell cycle, Carbon NY, 2016, 99, 238–248 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |