Open Access Article
Open Access ArticlePlasma processing: a sustainable technology in agri-food processing
Anbarasan
Rajan
a,
Bhavadharini
Boopathy
b,
Mahendran
Radhakrishnan
 *a,
Lakshminarayana
Rao
b,
Oliver K.
Schlüter
c and
Brijesh K.
Tiwari
d
*a,
Lakshminarayana
Rao
b,
Oliver K.
Schlüter
c and
Brijesh K.
Tiwari
d
aCentre of Excellence in Non-Thermal Processing, National Institute of Food Technology, Entrepreneurship and Management, Thanjavur-613005, Tamil Nadu, India. E-mail: mahendran@iifpt.edu.in
bCenter for Sustainable Technologies, India Indian Institute of Science, Bangalore-560012, Karnataka, India
cQuality and Safety of Food and Feed, Leibniz Institute for Agricultural Engineering and Bioeconomy (ATB), Potsdam, Germany
dTeagasc Food Research Centre, Ashtown, Ireland
First published on 3rd January 2023
Abstract
Globalization and rapid urbanization have led to tremendous improvement in the agriculture and food processing sectors to fulfil the food demands. In this context, managing food product safety and quality throughout the agri-food chain (pre-harvest to post-harvest) becomes vital to avoid food spoilage and increase production. Numerous innovative interventions have been investigated to achieve these goals; however, no single technology can be applied at all processing stages and may require different technologies. Nevertheless, cold plasma is a multifaceted solution for most pre and post-harvest issues, including soil/water contamination, microbial spoilage, insect infestation, and prolonged seed dormancy. In addition, the recent applications of plasma to food shape transformation is an evidence of the versatility of this technique in agri-food processing. Advantages, such as on-site production, residue, and toxic-free treatment, make the plasma process more sustainable. Reactive species, UV photons, and electrons are this plasma treatment's major compounds, giving them the peculiar and unique property to tackle most pre and post-harvest challenges. This review provides comprehensive possibilities for utilizing plasma technology throughout the agri-food chain. Various plasma systems have been developed, but their potential is limited to the lab scale. Research on large-scale applications can utilize cold plasma in future.
1. Introduction
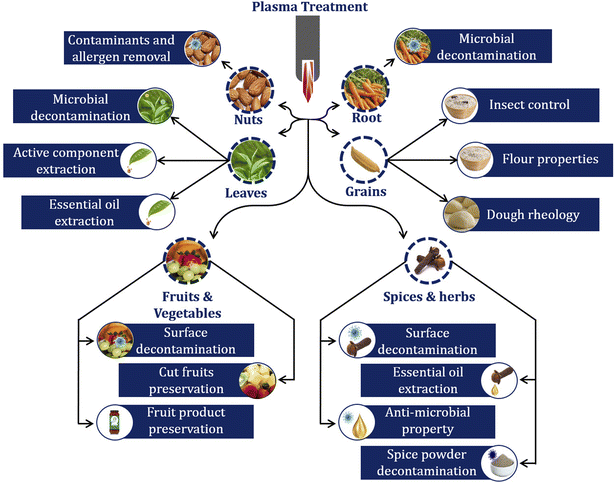
Agriculture and food processing sectors need progressive growth in production and value addition to feed the increasing world population. Nevertheless, the required food production has to be achieved with the available resources without compromising the product quality. Thus, we need a technology that could bring progressive growth in food supply and address food security and quality-related issues. In agri-food processing, irrigation and soil preparation are the two significant steps; any issues associated with these steps affect the entire supply chain and reduce product quality and yield. However, due to industrialization and urbanization, effluents are continuously introduced into environment and contaminate soil and water with heavy metals and cause phytotoxicity.1,2 Further, medical and related waste containing antimicrobial contaminants also affect plant growth based on their type and nature.3 Therefore, removing these contaminants from soil and water before the farming stage could increase agricultural yield and food safety.4–7 Nevertheless, the seed germination rate and adaptability to different environments also affect crop yield. In this regard, selecting a suitable seed treatment would also increase seed tolerance against salinity and drought stress.8,9 However, the real challenge arises at the post-harvesting stage, where the harvested fresh edible agricultural produce needs to be treated to avoid quality deterioration concerning microbes,10–12 enzymes,13 insects, and their secondary toxic contaminants.14 Furthermore, in later stages, most agricultural products reach industries where they will undergo various unit operations before they are stored and distributed to consumers.15 However, these product rheological properties, microbial/pest safety, functional properties, extract quality, and quantity would be low until these products are treated with suitable processing methods.16–18 Hence, while selecting the processing method/treatment, we should focus on its ability to address all these issues, irrespective of the product physical nature and intended final use.Regarding processing, non-thermal technologies are preferred over thermal technologies to avoid undesirable product quality changes. In food processing, non-thermal treatments such as pulsed light, ultrasound, UV, ozone, pulsed electric field, and high-pressure processing technologies are considered alternatives for thermal treatments. As the name suggests, these non-thermal food processing techniques are performed nearly at room temperature to produce safe, nutritive products and retain freshness without inducing thermal damage in the product,19–21 and cold plasma technology is also one among them. Plasma is created by supplying high energy to gas molecules. The supplied energy dissociates molecules into atoms, and a further increase in the energy breaks those gas atoms into wholly or partially charged ions. Irving Langmuir coined the name plasma for this ionized gas in the early 20th century.22 It is the fourth state of matter and can be generated under atmospheric or vacuum conditions.23 Photons, electrons, ions, atoms, free radicals, and excited or unexcited molecules are present in this state. Based on energy given to the plasma system and energy transferred to the molecules in plasma, the plasma is classified as equilibrium (thermal) and non-equilibrium (low temperature) plasma.24 One of the critical advantages of this novel technology is that it can be used for both constructive and destructive applications. Constructive approaches include functional modification,25 seed germination, plant growth,26 and quality improvement of extracts,27 while destructive applications include decontamination,28 disinfestation,29 and pesticide degradation.30 This review explains up-to-date research findings of plasma-assisted agri-food processing studies and the mechanisms involved in each process. Further, the article focuses on pre and post-harvest plasma applications specific to the commodities obtained from different plant parts (leaves, roots, fruits, vegetables, nuts, and spices).
2. Mechanism of plasma in various applications of agri-food processing
2.1. Microbial destruction and inactivation mechanism
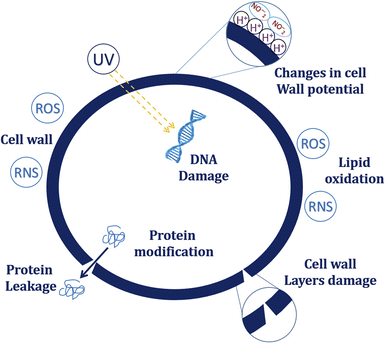
Though various plasma-chemical reactions are initiated during plasma generation, O3, O+, OH−, and H2O2 are the primary species responsible for microbial inactivation.31 These RS breaks peptidoglycan (PG) bonds of the cell wall and causes destruction wherein the PG structural bonds like C–O, C–N, and C–C are destroyed by reactive oxygen species.32 It is also suggested that the intense bombardment of the radicals causes surface lesions leading to etching, severely affecting Gram-negative bacteria.33Firstly, the RS oxidizes lipids, enzymes, and cytoskeletal proteins in the cell membrane and damages the cell wall. In comparison, DNA damage is caused by high-energy UV photons released from excited atoms or molecules at 220 to 280 nm. They form thymine dimers and break plasmid DNA's single and double strands that affect cell replication and other functions.34,35 In addition, RS in plasma increases the solution's acidity and further inactivates microbes.36
2.2. Chemical and toxin removal
Interaction of pesticides with plasma RS results in the oxidation of these chemical compounds and degrades them. Apart from oxidation, other intermediate processes also occur during the degradation and finally end in mineralization.30,37 Pesticide and other organic or inorganic pollutant removal follows different pathways, where pollutants are converted into simple non-toxic components such as CO2, H2O, inorganic carbons, and other organic or inorganic components based on treatment time and reactive species nature. Oxidation, isomerization, and H2O/CO2 removal are some essential reactions that take place during this conversion.382.3. Insect mortality
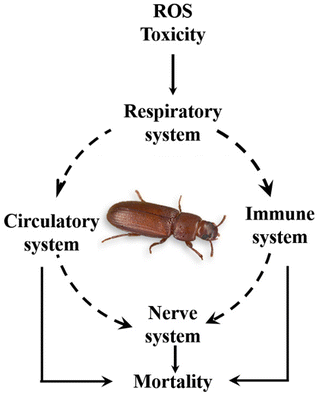
The nerve toxin effect caused by plasma ROS on the neuromuscular systems of insects induces mortality. These species enter through the respiratory pathway of insects and affect the insect behaviour and immune and circulatory systems.39–41 Mechanisms for insect mortality vary concerning the insect life stages. Ramanan et al.29 reported the different mechanisms involved in the insect's mortality of egg, larva, and adult stages.2.4. Seed germination
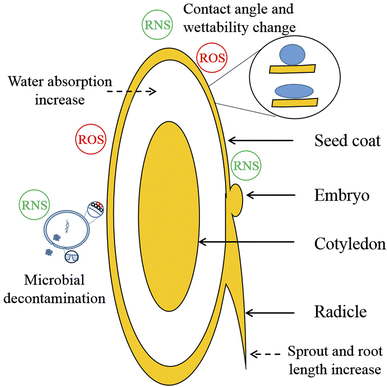
Many factors influence the germination rate of plasma-treated seeds. However, the exact reasons are not clear. However, a few acceptable reasons are etching, the opening of seed coats, increased seed wettability, deposition of small bioactive molecules on the seed coat, and decontamination of seed surface microbes.42,432.5. Functionality modification
Plasma treatment alters the nature of food constituents and their property. ROS reacts with sulfur and aromatic amino acids in the protein, resulting in oxidation. Sometimes oxidation (carbonylation) also assists enzyme inactivation. In addition, the treatment also changes the secondary structure of proteins and alters their functionality. Further, ROS attacks methyl groups near the double bond regions and oxidizes them in lipids. Meanwhile, carbohydrates, aldose, and ketose form formic acids during oxidation.38This review discusses the applications of plasma treatment on agri-food processing in various stages to enhance the agricultural production rate, reduce spoilage & waste, and improve the quantity and quality of the final product.
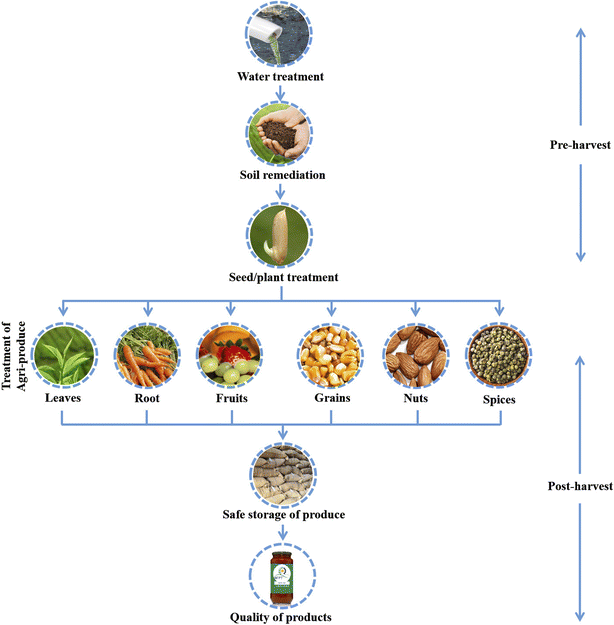
3. Plasma applications in pre-harvest stages
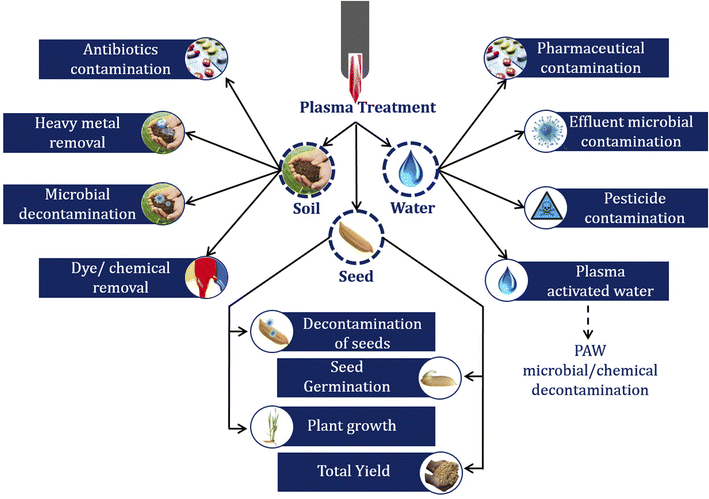
Plasma is used in different stages of pre and post-harvest processing of the agri-food chain (Fig. 1), where the pre-harvest operation includes soil irrigation, soil preparation, sowing, planting, etc., and continues till harvesting (Fig. 2). Each stage has a noticeable impact on final product quality and yield. Hence, plasma technology has been used to obtain the desirable change during pre and post-harvest operations.3.1. Plasma treatment of water
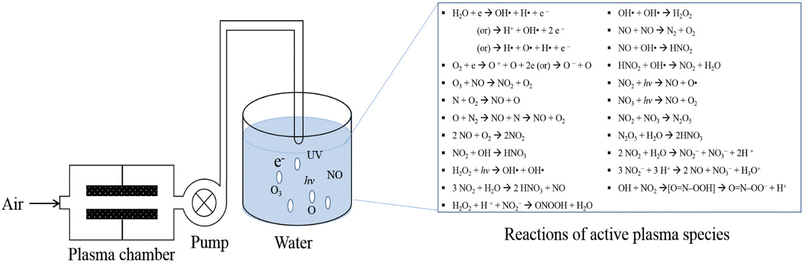
Water is one of the vital sources for agricultural practices and is usually contaminated by microbes, heavy metals, and pharmaceutical compounds.44–46 Removing these contaminants can improve agricultural product quality and overall production. The reactive species present in plasma can oxidize and remove the contaminants from water or effluent.47 Furthermore, plasma-activated water (PAW) can also be used to suppress microbial activity48 and to remove chemical contaminants from water.49 The interaction of reactive species with water during plasma treatment and PAW production varies based on the nature of RS (Fig. 3). The degradation of organic and inorganic compounds is shown in eqn (1).| Chemical compounds + RS → simple carbon/nitrogen compound + CO2 + H2O | (1) |
Though degradation follows different pathways, the end product will likely fall under any of those components mentioned in eqn (1). For instance, pesticide degradation (diuron) by OH− species oxidation releases CO2 and H2O after producing organic acids.50 Similarly, after producing different intermediate components, dimethoate degraded as non-toxic PO43− during plasma treatment.51 In addition to oxidation, mineralization, removal of alkyl groups, CO2, H2O, halogens, and isomerization could take place during the degradation process. This degradation mechanism is often similar to colourant and off-odour-producing compounds.38
Plasma treatment has the potential to degrade individual dyes and their mixtures (i.e., alizarin yellow + orange II + methylene blue) from water.52 Iervolino et al.53 investigated the effect of plasma on different water pollutants by varying treatment time, power, and gas and identified the lower resistance of methylene blue (MB) and ceftriaxone against plasma (degraded within 5 min). While phenol (15 min), paracetamol (15 min), and caffeine (25 min) took more time for degradation. During this process, MB was almost entirely converted into CO2. Instead of focusing on degradation alone, Tampieri et al.54 studied the degradation and mineralization efficiency of plasma-treated rhodamine B, phenol, and metolachlor. For low pollutant concentrations, the highest mineralization efficiency of 59% and 20% was obtained for phenol and metolachlor in 30 min, respectively. To understand the combined effect of plasma with any other non-thermal method, Bradu et al.47 combined ozone treatment with plasma to remove organic pollutants from water. Ozone combined with plasma reduced the treatment time by half to remove 50% chlorophenoxyacetic herbicide (2,4-D) with doubled removal reaction rate. This study explains the role of OH− species on 2,4-D oxidation and the importance of catalytic ozonation for faster pollutant removal and mineralization. Similar to other organic pollutants, Sarangapani et al.55 reported pesticide removal concerning power and treatment time. Higher power and exposure time increased pesticide degradation into simple chemical groups due to the oxidization of active O3 and OH− species. Other than organic pollutants and pesticides, microbes can also contaminate water. So, Pavlovich et al.56 reported the effect of plasma on Escherichia coli present in water and found that ozone concentration alone had a higher correlation with the antimicrobial activity of the treatment than pH and other species-generated acids. This study concluded that 5 s plasma treatment was enough to reduce E. coli to a not detectable level and suggested the importance of mixing on the antimicrobial effect of plasma species. In order to evaluate the effect of plasma on the removal of different pollutant mixes, Hijosa-Valsero et al.57 conducted a study where atrazine, chlorfenvinfos, 2,4-dibromophenol, and lindane pollutants were mixed with water and treated in DBD batch reactor (R1) and co-axial thin-film DBD reactor (R2). Results showed a decrease in the degradation efficiency of chemicals when their concentration increased, and even after pollutant degradation, byproduct removal took more treatment time. Similarly, pentoxifylline and its intermediates removal were analyzed by Magureanu et al.,45 and the author reported that removal was high at a lower concentration of pollutant (100 mg L−1), higher power, high pulse rate, and higher frequency. Intermediate components produced during this degradation were entirely removed after 120 min. Industrial wastewater with higher microbial contamination might also affect the water quality of agri-farms. So, industrial wastewater needs decontamination studies, and Rowan et al.46 conducted a study on poultry wash water to investigate microbial decontamination. In this study, other than RS and UV photons, nitric and carbonic acids formed during plasma treatment resulted in pH reduction (acidified) that helped in microbial destruction. These components resulted in the microbial reduction of 8 logs (less than or equal) to complete removal after 30 s of treatment, and the sensitivity of treatments varied for different microbes (Bacillus cereus endospores < Listeria monocytogenes < Salmonella enterica Typhimurium < Salmonella enterica Enteritidis < E. coli < Campylobacter jejuni < Campylobacter coli).
Apart from using plasma units for treating different water samples, it is also possible to carry the plasma species in water by which the treatments can be done. Ten Bosch et al.40 reported the effect of plasma-treated water on insect mortality (Mealybugs). Though Plasma Treated Water (PTW) and Classically Acidified Water (CAW) had no significant difference, the CAW mortality rate was higher than PTW at the same pH due to the complexity of RS present in PTW. This PTW can also be used as an alternative for sodium nitrite in sausage curing with similar sensory, microbial, and peroxide values as that of control.58 Even though plasma-activated water (PAW) possesses antimicrobial properties, its effectiveness reduces during the storage period. Traylor et al.59 reported that PAW prepared with 15 min and 3 h dosage intervals were found to have a similar antimicrobial effect of more than 5 logs. However, after 30 min, the effectiveness of PAW prepared with 15 min exposure time reduced from 5.6 logs to 2.4 logs, while the other was stable for 2 days. Similarly, the lethal effect of stable RS on Hafnia alvei was reported, and among NO−, NO2−, and H2O2 species, acidification of PAW by NO2− species acted as a primary factor in microbial reduction.60 Kamgang‐Youbi et al.48 reported the influence of pH reduction and substrate absence on saccharomyces destruction and found that due to its cell size, initial population, and adhesiveness of substrate surface, their resistance was higher against plasma treatment.
Table 1 shows the different plasma generation methods and significant results achieved in water treatments. However, further research must be explored to characterize the degradation process's end product. Since the future industrialization of PAW usage may produce a massive quantity of used PAW, there is a need for research on its recyclability and toxicity.
| Sample | Plasma characteristics | Significant results | Reference |
|---|---|---|---|
| (1) Plasma treatment on water | |||
| (a) Effluent decontamination | |||
| Dimethyl phthalate | Self-pulsing discharge (SPD) and multipin corona discharge (MCD) | MCD was more effective in the degradation of dimethyl phthalate than SPD | 184 |
| Input – 30 kV, 12 mA | |||
| Treatment time – 5 to 30 min | |||
| Methylene blue, ceftriaxone, phenol, paracetamol and caffeine | DBD reactor | Degradation and mineralization were higher when O2 was used at 20 kV with a 0.18 nL min−1 flow rate | 53 |
| Gas – air or oxygen (0.09 to 0.36 nL min−1) | |||
| • Input power – 12 to 38 kV | |||
| Treatment time – 5 to 25 min | |||
| Rhodamine B, phenol and metolachlor | Atmospheric plasma reactor | In 5 × 10−4 M phenol solution, 10% residue remained after 30 min. Low concentrations were removed completely | 54 |
| Gas – air (flow rate – 100 mL min−1) | Rhodamine B and metolachlor were removed in 15 and 20 min, respectively | ||
| Input – 5.9 ± 0.7 W | |||
| Treatment time – up to 30 min | |||
| Chlorophenoxyacetic herbicide (2,4-D) | Pulsed corona discharge with zonation reactor | Plasma with ozone provides >99.8% removal of 2,4-D in 30 min of treatment time | 47 |
| • Gas – oxygen (0.3 L min−1) | |||
| • Input power – 11 to 31 W | |||
| • Treatment time – up to 60 min | |||
| Pesticide (dichlorvos, malathion, endosulfan) | DBD plasma reactor | Degradation was maximum for dichlorvos (78.98%), malathion (69.62%), and endosulfan (57.71) at 80 kVRMS after 8 min | 185 |
| • Gas – atmospheric pressure plasma | |||
| • Input power – 60, 70, and 80 kVRMS | |||
| • Treatment time – 0, 2, 4, 6, and 8 min | |||
| Bacterial culture mixed water | DBD plasma | E. coli – vortex (5 s) increases the microbial reduction up to 5 log in 120 s at 0.2 W cm−2 | 56 |
| • Gas – ambient air | Increased power has less effect on microbial reduction | ||
| • Input power – 0.02 to 0.4 W cm−2 | |||
| • Treatment time – 30 to 300 s | |||
| Polluted water (atrazine, chlorfenvinfos, 2,4-dibromophenol, and lindane) | DBD batch reactor (R1) and co-axial thin-film DBD reactor (R2) | R 2 was found to be efficient more efficient. For example, 15 min of treatment degraded the pollutant while the byproducts degradation was low | 57 |
| • Gas – helium at atmospheric pressure (5 L min−1) | |||
| • Input power – 30 W | |||
| • Treatment time – 0 to 15 min | |||
| Pentoxifylline (in water) | DBD plasma with coaxial configuration | 92.5% pentoxifylline reduction after 90 min treatment | 45 |
| • Gas – oxygen (flow rate – 600 SCCM) | Chemical reduction increases with increased O3 consumption | ||
| • Input power – 1.2 W | |||
| • Treatment time – up to 120 min | |||
| Poultry wash water | Pulsed-plasma gas-discharge | Campylobacter and Salmonella contamination was entirely removed to not detectable level | 46 |
| • Gas – sulfur hexafluoride (SF6)/air (flow rate – 10 L min−1) | |||
| • Input power – 23.5 kV | |||
| • Treatment time – 30 s | |||
| Methylene blue (MB) water | DBD plasma with coaxial configuration | Plasma treatment of 30 min with O2 gas removed 95% MB dye | 186 |
| • Gas – oxygen and air (300, 600, and 900 SSCM) | Ozone concentration influences the removal rate | ||
| • Input power – up to 1 W | |||
| • Treatment time – up to 90 min | |||
| (b) PAW treatment | |||
| Plasma treated water (PTW) | DBD plasma | Mortality rate of PTW, tap water, and reference sample after 24 h was around 85, 8 and 2% for mealybug | 40 |
| Gas – air | |||
| Input – 11 W | |||
| Electrode gap – 3 mm | |||
| Treatment time – 1 to 10 min | |||
| PTW | Surface dielectric barrier discharge | PTW used sausage with nitrite (782 ppm) that can provide a curing effect | 58 |
| • Gas – atmospheric air | |||
| • Input power – 3.14 W | |||
| • Treatment time – 120 min | |||
| Plasma-activated water (PAW) | Indirect DBD plasma | Anti-microbial declined during storage | 59 |
| • Gas – atmospheric air | After seven days, 2.4 logs and no reduction were observed in 3 h and 15 min treated PAW, respectively | ||
| • Input power – 5 W | |||
| • Treatment time – 20 min | |||
| PAW | Atmospheric non-thermal quenched plasma | Acidification by nitrites in PAW produces a lethal effect on Hafnia alvei | 60 |
| • Gas – atmospheric air (flow rate – 550 L h−1) | |||
| • Treatment time – 5 min | |||
| PAW | Atmospheric pressure gliding arc plasma | S. cerevisiae – 3 log reduction after 30 min | 48 |
| • Gas – atmospheric air | Without solid substrate H. alvei, Leuc. mesenteroides, Staph. Epidermidis were eradicated after 30 min | ||
| • Input power – 1.2 W | |||
| • Treatment time – 5 min | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| (2) Plasma treatment on soil remediation | |||
| p-Nitrophenol contaminated soil | Novel spray-type coaxial cylindrical dielectric barrier discharge | 54.2% of PNP was degraded after 50 s discharge treatment | 187 |
| Input – 0–30 kV, 200 Hz, carrier gas – 20% O2 + 80% N2 | |||
| Fluorene | Needle-plate pulsed corona discharge plasma | 78.7% fluorene degradation was achieved after 60 min | 64 |
| Input – 30 kV | When washing is done to remove oxidation products, 99% degradation achieved in 45 min | ||
| Treatment time – 60 min | |||
| Electrode gap – 20 mm | |||
| Non-aqueous phase liquid (NAPL)-mixed soils | Ex situ DBD plasma cylinder-to-plane reactor | High energy density eradicates NAPL irrespective of its initial concentration of it | 65 |
| • Gas – atmospheric air (flow rate – 1 L min−1) | |||
• Input power ∼25 W (energy density 675 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 125 J g−1) 125 J g−1) |
|||
| • Treatment time – 2.5 to 33 min | |||
| Dye-polluted soil (acid scarlet GR) | Plane-to-plane DBD plasma | Degradation efficiency was 93% at 19.6 kV and also at 300 Hz (17.6 kV) after 25 min treatment | 66 |
| • Gas – atmospheric air | |||
| • Input power – 15.6 to 19.6 kV (3.51 to 5.72 W) | |||
| • Treatment time – 0, 5, 10, 15, 20, and 25 min | |||
| p-Nitrophenol polluted soil (PNP) | Pulsed discharge plasma-TiO2 catalytic (PDPTC) | Soil depth increase reduces PNP degradation | 70 |
| • Gas – atmospheric air (flow rate – 0.5 L min−1) | Quartz sand containing PNP degraded more than sand and sandy soil. Clay soil has the lowest degradation rate | ||
| • Input power – 23 kV | |||
| • Treatment time – 45 min | |||
| Contaminated soil | Ozonizer combined DBD plasma with TiO2-based surface discharge | The microbial load of ozone treated soil reduced from 5.7 × 106 to 1.7 × 102 | 72 |
| • Gas – oxygen (flow rate – 0.5 dm3 min−1) | |||
| • Input power – 23 kV | |||
| • Treatment time – 45 min | |||
| Chloramphenicol polluted soil | Atmospheric pressure DBD plasma | The presence of oxygen improves the degradation compared to air | 67 |
| • Gas – oxygen, ozone, air, argon, nitrogen (flow rate – 0.15 to 1.5 L min−1) | Higher input voltage and flow rate increase the degradation efficiency | ||
| • Input power – 16.4, 18.4, and 20.4 kV | |||
| • Treatment time – 0, 5, 10, 15, 20, and 25 min | |||
| p-Nitrophenol polluted soil (PNP) | Pulsed discharge plasma – TiO2 catalytic (PDPTC) | Plasma alone caused 78.1% of PNP degradation, while PDPTC caused 88.8% degradation at 20 kV (10 min) | 68 |
| • Gas – atmospheric air (flow rate – 0.5 L min−1) | |||
| • Input power – 20 kV | |||
| • Treatment time – 30 min | |||
| Pentachlorophenol (PCP) in soil | Pulsed corona discharge plasma | An increase in voltage from 12 to 18 kV increased PCP degradation from 64 to 90% (60 min) | 69 |
| • Gas – oxygen, air, argon, nitrogen (flow rate – up to 8 L min−1) | |||
| • Input power – 12 to 18 kV | |||
| • Treatment time – up to 60 min | |||
| Pentachlorophenol (PCP) | Pulsed corona discharge plasma (PCDP) | 11.6, 13.6, 15.6 kV, and 17.6 kV degraded PCP up to 62, 77, 83, and 87%, respectively, after 60 min | 71 |
| • Gas – air (flow rate – 3 L min−1) | |||
| • Input power – 11.6 to 17.6 kV | |||
| • Treatment time – up to 60 min | |||
| Kerosene polluted soil | Cylinder-to-plane DBD plasma | An energy level of 960 J g−1 degrades the kerosene by 90% | 188 |
| • Gas – air (flow rate – 1 L min−1) | |||
| • Input power – 15 to 20 kV | |||
| • Treatment time – up to 60 min | |||
| Contaminated soil | DBD plasma | Ozone produced during the process reduced the microbial count and pH | 62 |
| • Gas – oxygen and air (flow rate – 1, 2 L min−1) | Increased the mineral content of the soil | ||
| • Input power – up to 30 W | |||
| • Treatment time – 60 min | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| (3) Plasma treatment of seeds/plants | |||
| Rice seedling | Non-equilibrium atmospheric pressure plasma | 15% increase in grain yield when the plant was treated in direct plasma during the early period and no change in the later stage | 189 |
| Plasma activated ringer's lactate solution | |||
| Treatment period of solution – twice a week | |||
| Input – 60 Hz, 9 kV | |||
| Maize grains | Diffuse coplanar surface barrier | Wettability increased – the amount of water imbibition was higher | 190 |
| Input – 400 W with air, oxygen, and nitrogen | 90 and more had a negative effect on growth and production indices | ||
| Treatment time – 0, 30, 60, 90, 120, 180, and 300 s | |||
| Buckwheat seeds | Low-pressure radiofrequency system | A shorter exposure time of up to 45 s was safer for the application | 191 |
| Input – 30 W | The longest exposure of 120 s affected the fungal colonisation | ||
| Treatment time – 15, 30, 45. 60, 90, and 120 s | |||
| Carrot seed | DBD | 3 min had a great quantity of flavonoids, phenolics, and plant extracts had high radical scavenging activity | 192 |
| Input – 12.5 kV and 50 Hz | |||
| Treatment time – 1 to 4 min | |||
| Pea seeds (Pisum sativum L.) | DCSBD plasma unit with a silver electrode and Al2O3 as the dielectric material | DNA damage – 240 s treatment reduces the damage by 28 to 30.5%, 3.5%, and 21% for zeocin, distilled water, and hydrogen peroxide treated sample, respectively | 90 |
| • Gas – atmospheric air (flow rate – 3 L min−1) | |||
| • Input power – 400 W | |||
| • Treatment time – 60 to 300 s | |||
| Wheat | DBD plasma with 3 mm electrode distance | Plasma treatment of 15 and 90 s increased HSFA4A by six and four folds after 3 h in the root | 91 |
| Gas – Ar | However, for the shoot after 6 h, a ten-fold increase was observed in the 30 s treatment | ||
| Input – 80 W | |||
| Treatment time – 15, 30, 60, and 120 s | |||
| Wheat | Atmospheric DBD plasma | After treatment, GP and GR in drought stress increased from 39.3 to 50.0% and 62.7 to 80.0%, respectively | 92 |
| Gas – dry air | |||
| Input – 13 kV | |||
| Treatment time – 4 min | |||
| Radish (Raphanus sativus) | Double DBD reactor with plate-to-plate configuration for seed treatment | Seed GR was 40, 60, and 100% for control, PAW-15 and PAW-30, respectively | 42 |
| Tomato (Solanum Lycopersicum) | Gas – air (flow rate – 1 L min−1) | In tap water (TW), growth was higher for control, P10, and P20 tomato plant than PAW watered plants | |
| Sweet pepper (Capsicum annum) | Input – 57 mJ per pulse (max) | More growth in PAW-30 watered plants than TW | |
| Treatment time – 10 (P10) and 20 (P20) min | |||
| Double DBD reactor with cylindrical configuration – water treatment | |||
| Gas – synthetic air (flow rate – 1 L min−1) | |||
| Input – 7 mJ per pulse (max) | |||
| Treatment time – 15 (PAW-15) and 30 (PAW-30) min | |||
| Rapeseed (Brassica napus L.) | CDPJ generation system | Germination was 7.7% higher after 1 min of treatment | 86 |
| • Gas – dry atmospheric air (2.5 m s−1) | The microbial load of the treated sample was maintained lesser than the control sample (1–2 log reduction) | ||
| • Input power – direct current of 20 kV (1.5 A) | |||
| • Treatment time – 0 to 3 min | |||
| Wheat seed | DBD plasma | GP, GR, and GI increased in 4 min treatment from 62.5 to 77.5%, 88.0 to 95.3% and 36.7 to 41.0% compared to control | 74 |
| Gas – air (flow rate 1.5 L min−1) | |||
| Input – 1.5 W | |||
| Treatment time – 0, 1, 4, 7, 10 and 13 min | |||
| Broccoli seed (Brassica oleracea var. kialica plen.) | CDPJ generation system | Plasma reduced microbes in seed and sprout | 75 |
| • Gas – atmospheric air | Less exposure increased germination rate, sprout length, and weight | ||
| • Input power – 20 kV DC | |||
| • Treatment time – 0 to 3 min | |||
| Peanut (Arachis hypogaea L.) seed | Cold helium plasma | After 15 s treatment at 80, 100, and 120 W, germination potential increased by 128, 128, and 150%. Finally, at 120 W germination rate was found to be maximum | 78 |
| • Gas – helium (150 Pa pressure) | |||
| • Input power – 60, 80, 100, 120, and 140 W | |||
| • Treatment time – 15 s | |||
| Wheat seeds (Triticum aestivum L. cv. Eva) | DCSBD – cold atmospheric pressure plasma (CAPP) | Water uptake increased with soaking time and dosage | 76 |
| • Gas – atmospheric air | Bacteria and fungi load on seeds reduced with treatment time | ||
| • Input power – up to 100 W cm−3 | |||
| • Treatment time – 10 to 600 s | |||
| Wheat seeds (Triticum aestivum) | Atmospheric pressure surface discharge plasma reactor with glass plate | Germination rate was similar for plasma treated (98%) and control (95%) wheat seeds | 77 |
| • Atmospheric air (flow rate – 1 L min−1) | |||
| • Input power – 2.7 W | |||
| • Treatment time – 5, 15 and 30 min | |||
| Peas (Pisum sativum ‘Salamanca’) | CAPP – surface dielectric-barrier air-discharge | 5 and 10 min CAPP treatment increased germination to 42 and 50%, respectively, after 24 h | 82 |
| • Atmospheric ambient air | Germination rate reached a maximum (5 min treatment) of 65% after 48 h | ||
| • Input power – 9 kVpp | |||
| • Treatment time – 1 to 10 min | |||
| Bean (Phaseolus vulgaris) | Cold radio frequency air plasma | Water absorption increased in plasma treatment. hence, germination time reduce in plasma-treated samples | 85 |
| • Atmospheric air (pressure – 6.7 × 10−2 Pa) | |||
| • Input power – 20 W | |||
| • Treatment time – plasma treatment (2 min), vacuum treatment (3 min) | |||
| Pea seeds | Coplanar surface discharge – low-temperature plasma | Seed germination increased from 77.5 to 95% after 120 s treatment | 84 |
| • Input – 370 W | |||
| • Time – 60 to 600 s | |||
| Pre germinated rice (Oryza sativa L.) | Plasma jet with quartz tube covered inner electrode | Control – 97% germination | 87 |
| • Argon and oxygen | Treated – 93 and 91% after 5 s at 10 W power with 8, 5 mm distances, respectively | ||
| • Input power – 10–14 W | High γ-aminobutyric acid (GABA) content achieved in 96 h pre-germination | ||
| • Treatment time – 5 to 10 s | |||
| • Distance – 5, 8 mm | |||
| Soybean (Glycine max (L.) Merr) | CDPJ generation system | Maximum germination (G) potential, G rate, G index, and vigour index were observed after 80 W treatment | 81 |
| • Dry atmospheric air (2.5 m s−1) | Water uptake increased by 14.03% | ||
| • Input power – 0 to 120 W | |||
| • Treatment time – 15 s | |||
| Tomato (Solanum Lycopersicum L.) seed | Capacitively coupled plasma (CCP) | Germination potential and rate increased by 8 and 11% | 80 |
| • Helium (150 pa) | Bacterial wilt severity was reduced by 25% in the treated sample | ||
| • Input power – 80 W | |||
| • Treatment time – 15 s | |||
| Wheat (Triticum spp.) | Cold helium plasma | Germination potential and the rate increased by 6, and 6.7%, respectively, after 80 W treatment | 26 |
| • Helium (150 Pa pressure) | |||
| • Input power – 60, 80, 100 W | |||
| • Treatment time – 15 s | |||
| Poppy seed (P. somniferum L.) | Panasonic – microwave generating RF plasma | Germination was 115% in 3 min treatment | 89 |
| Input – 500 W | Plasma treated seedlings were longer than the control seedlings | ||
| • Time – 180 to 5400 s | |||
| • Gas – O2 (50 mL min−1) and Ar (50 ml min−1) | |||
| Maize seed (Zea mays L.) | DCSBD plasma treatment | Plasma treatment for 60 s increased the root length, root fresh, and dry weight by 21, 10, and 14%, respectively | 83 |
| • Atmospheric air | Soluble protein content in the root increased after 60 s treatment | ||
| • Input power – 370 W | |||
| • Treatment time – 60 and 120 s | |||
| Paulownia tomentosa seeds | Glow discharge plasma treatment | Air plasma treatment at 50 W, 100 W and 200 W produced maximum germination at 15, 5, and 1 min, respectively | 88 |
| • Air and argon (200 mTorr) | When glass plates covered the seeds, germination was reduced | ||
| • Input power – 50, 100 and 200 W | |||
| • Treatment time – 1 to 40 min | |||
3.2. Plasma treatment for soil remediation
Industrial and agricultural-related wastes such as heavy metals, pesticides, petroleum, and its products cause soil contamination, which could affect humans and the ecosystem.61 These contaminants' concentration in soil has to be reduced to avoid losses in production and cross-contamination in agricultural produces. Plasma technology has already gained popularity at the industrial level as an emerging technology for different treatments. Hence, this technology can be used in soil decontamination.62In plasma assisted soil remediation, factors such as soil thickness and its type affect the treatment voltage, frequency, soil thickness, and air flow rate. The effectiveness of the method was identified against different types of soil (sandy soil and loam soil).63
Soil pollutant removal varies depending upon the nature of the soil as well as the pollutant. Zhan et al.64 studied the influence of treatment parameters such as time and washing, soil properties such as pH, moisture, and initial concentration of pollutants on fluorene removal from soil. The study showed a significant increase in fluorene degradation (from 33.8 to 57.9%) while reducing the soil moisture content from 20 to 0.6%. In addition, the efficiency increased from 60.6 to 71% when the pH of the soil rose from 3.0 to 9.0. Washing, increasing treatment time, and reducing initial pollutant concentration are reported to be critical factors for pollutant removal. Aggelopoulos et al.65 studied the non-aqueous phase liquid (NAPL)-mixed soil decontamination with high and low energy density plasma treatment to avoid this influence of initial pollutant concentration. When the ex situ DBD plasma treatment energy density was around 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 J g per soil, NAPL was obliterated, irrespective of the initial concentration. However, low energy density was affected by its increased concentration. Lu et al.66 included the effect of frequency, gas flow, and input power in removing acid scarlet GR dye from the soil. It was found that an increase in DBD plasma input power (3.51 to 5.72 W), frequency (200–300 Hz), and gas flow rate (0.5–1.0 L min−1) improved dye degradation. At the same time, a higher air flow rate (1.5 L min−1) reduced efficiency and O3 and OH− reactive species were reported as critical factors in this degradation. However, to find the effectiveness of different gas sources on degradation other than flow rate, Lou et al.67 used O2 and air for chloramphenicol degradation, where the efficiency of O2 (41%) was higher than air (26%). For better efficiency, optimum moisture content (≤10%) and Fe0 addition were needed, and that can increase reactive species and discharge channels, respectively. Similarly, Wang et al.68 used a pulsed discharge plasma-TiO2 catalytic (PDPTC) reactor to enhance the reactor performance for organic component removal. In this study, TDPTC (55.1%) removed higher organic components than the plasma reactor performed without TiO2 (42.9%).
000 J g per soil, NAPL was obliterated, irrespective of the initial concentration. However, low energy density was affected by its increased concentration. Lu et al.66 included the effect of frequency, gas flow, and input power in removing acid scarlet GR dye from the soil. It was found that an increase in DBD plasma input power (3.51 to 5.72 W), frequency (200–300 Hz), and gas flow rate (0.5–1.0 L min−1) improved dye degradation. At the same time, a higher air flow rate (1.5 L min−1) reduced efficiency and O3 and OH− reactive species were reported as critical factors in this degradation. However, to find the effectiveness of different gas sources on degradation other than flow rate, Lou et al.67 used O2 and air for chloramphenicol degradation, where the efficiency of O2 (41%) was higher than air (26%). For better efficiency, optimum moisture content (≤10%) and Fe0 addition were needed, and that can increase reactive species and discharge channels, respectively. Similarly, Wang et al.68 used a pulsed discharge plasma-TiO2 catalytic (PDPTC) reactor to enhance the reactor performance for organic component removal. In this study, TDPTC (55.1%) removed higher organic components than the plasma reactor performed without TiO2 (42.9%).
For pentachlorophenol (PCP) degradation, Wang et al.69 used an optimum flow rate (3 L min−1) and a high O2 environment to increase the degradation rate. However, it was found that prolonged exposure of pollutants (4 to 96 h) to soil reduced the degradation by 13.4% as the pollutant gets into the soil granules deeply. So, apart from process parameters and other soil properties, the depth of the soil also decides the degradation efficiency. To understand this, Wang et al.70 studied p-nitrophenol (PNP) degradation at a different depth from the surface of the soil using plasma. From this study, it was found that an increase in soil depth reduces the degradation rate from 77.9 (0–2 mm depth) to 52.8% (10–12 mm depth), and the removal of PNP in moist clay soil (44.1%) was higher than dry soil (11.3%) with increased discharge voltage. Similarly, Wang et al.71 studied the effect of the size of the soil granule on the degradation of pentachlorophenol (PCP) and found higher degradation in 20 mesh size granular soil (87%) than in 10 mesh size granular soil (72%). In addition to granule size, the high pH of the soil also assists the PCP degradation. Unlike other studies, Stryczewska et al.72 focused on microbial decontamination and plasma-induced soil property change. Here, the soil pH and temperature changes occurred with changes in O3 concentration and treatment time, resulting in decontamination. A similar study was carried out with different electrode configurations, and this study reported the importance of screw or pyramid-shaped electrodes on higher O3 production. O3 and NO− produced in this process altered the soil conductivity (34 to 79 mS m−1) and microbial population.62 Later, Redolfi et al. (2010) studied the RS oxidation effect on kerosene-mixed soil to analyze the pollutant residues in the plasma exhaust gas and its bioavailability. Results indicated that only a negligible amount of organic components came along with the gas outlet, while most of them were retained in the soil and did not convert from a solid to a gas state. Hence the bioavailability of kerosene byproducts increased in the soil.
Extended research is needed to understand the effect of plasma treatment on soil mineral content, residue toxicity, and their effects on plant growth and crop yield after treatment. Apart from the decontamination of soil, the effect of plasma on desirable organisms present in it also needs to be addressed.
3.3. Plasma treatment of seeds/plants
Improving germination rate and agricultural yield is the primary concern in increasing food production,73 and plasma treatment has the potential to improve these two aspects of agriculture processing by altering the germination rate (GR), germination potential (GP), vigor index (VI), water absorbance, contact angle, wettability, and growing tolerance of seed. At optimum process conditions, these changes can influence the overall production of food produce by favouring seed and plant growth.Apart from germination enhancement, plasma also has the ability to change the minerals, pigments, enzymes, and other nutritional compositions of the seeds. For example, in capacitively coupled plasma (CCP) treated tomato seeds, calcium (7.73%) and boron (11.53%) contents were increased;80 whereas in soybean, nutrient fractions such as soluble protein and soluble sugar and enzymes such as peroxidase (POD) and phenylalanine ammonia-lyase (PAL) activities increased after CDPJ treatment.81 In helium plasma-treated wheat (Triticum spp.), chlorophyll content increased by 9.8% more than that in the control.26 However, photosynthesis efficiency and flavonoid content in peas are reduced due to UV-C production.82 Similarly, time-dependent reduction of dehydrogenase (27%) and catalase activities (75%) were observed in the roots of germinated maize seeds.83 In comparison, structural changes were observed with increased indolyl acetic acid (13.7%) in peas.84 For beans, germination time (44 to 40 h) and exotesta contact angle were reduced after plasma treatment.85
In contrast to other research works, Puligundla et al.86 analyzed the negative impact of plasma over dosage on rapeseed germination and suggested the optimum treatment time for better germination since 1 min treatment showed better results in this study. However, the study on pre-germinated brown rice by Sookwong et al.87 showed a reduction in germination rate after the treatment and explained the dependency of treatment time and distance. Along with other dependent variables, gas used in treatment also has a considerable impact on seed germination, and studies by Živković et al.88 on paulownia tomentosa seed proved that by showing maximum germination at 15 min for air plasma, unlike argon plasma. It also noted that the impact of plasma on seeds could not be carried out for a long time since poppy seeds treated at 500 W plasma showed higher GR on the first day of germination but reduced on the sixth day. i.e., 13% reduction was observed in 3 min treated sample.89
Seed treatment results and their effects vary based on the plasma characteristics used in the respective treatments (Table 1). Many studies have shown a significant increase in the germination of seeds, but detailed studies are required to identify the effect on the quality and yield of the final product. In addition, the negative impact of plasma treatment on seeds needs to be addressed with the evident mode of action, which could provide the limitations of plasma treatment for seeds (Table 2).
| Sample | Plasma chamber | Purpose of study | Research goal | Reference |
|---|---|---|---|---|
| (1) Plasma treatment of different agricultural produces | ||||
| (a) Plasma treatment on leaves | ||||
| Baby spinach | High voltage atmospheric cold plasma | Inactivation of Salmonella enterica and E. coli O157:H7 | Significant inactivation after 24 h through 14 days post-treatment | 193 |
| Input – 30–130 kV | After 7 days of refrigerated storage – 2.6 log CFU per g – 2 min | |||
| Treatment time – 2 min and 5 min | 3.5 log CFU per g – 5.0 min | |||
| Date palm leave | Radiofrequency plasma | Surface modification | Wettability of leaf surface increased | 194 |
| Input – 80, 100, 120 W, gas pressure – 0.95 torr | Surface roughness was increased due to the removal of the waxy layer and impurities | |||
| Treatment time – 1, 5, and 8 min | ||||
| Green tea leaves | DBD | Antioxidant activity | 15 W and 15 min, the TPC and antioxidant activity increased by 41.14% and 41.06% | 195 |
| Input – 5, 10 and 15 W | Catechin increased by 103.12% | |||
| Treatment time – 5, 10, 15 min | ||||
| Radicchio (Cichorium intybus L.) | Dielectric barrier discharge (DBD) | Microbial inactivation | L. monocytogenes – 2.20 log CFU per cm2 reduction after 30 min | 94 |
| • Atmospheric air | E. coli O157:H7 – 1.35 log MPN per cm2 reduction after 15 min | |||
| • Input power – 15 kV | ||||
| Treatment time – 15 to 30 min | ||||
| Black and green tea | Plasma jet with the copper electrode and Pyrex tube | Microbial inactivation | E. coli – complete removal in black and green tea after 3 and 4 min, respectively | 95 |
| • 99.99% argon (flow rate – 1 L min−1) | Coliform – inactivated after 5 min in both samples | |||
| • Input power – 10 kV | Yeast and mould – inactivated after 7 min in both the samples | |||
| • Distance – 1.5 cm | ||||
| • Treatment time – 0 to 7 min | ||||
| Romaine lettuce (Lactuca sativa L. var. longifolia) | Atmospheric pressure plasma with nickel coated steel needle array | Microbial inactivation | E. coli – 10 min treatment at 12.83 kV reduces the microbial count to 1.5 logs | 93 |
| • Argon (flow rate – 455.33 standard cm3 min−1) | ||||
| • Treatment time – 30 s to 10 min | ||||
| • Input power −3.95 to 12.83 kV | ||||
| Corn salad leaves | Atmospheric pressure plasma jet | Microbial inactivation | The effect of plasma on microbes was higher (3.6 log reduction) at lower level surface contamination (104 CFU per mL) after 15 s treatment at 20 W | 97 |
| • Argon (flow rate – 20 L min−1) | ||||
| • Input power – 10, 20, 30, and 40 W | ||||
| • Treatment time – up to 5 min | ||||
| Lamb's lettuce (Valerianella locusta) | Atmospheric pressure plasma jet | Bioactive compounds | Chlorogenic acid, caffeic acid luteolin diosmetin content were reduced as time increased at 30 W plasma treatment | 98 |
| • Argon (20 cm3) | ||||
| • Input power – up to 35 W | ||||
| • Treatment time – 0 to 120 s (overall) | ||||
| Lamb's lettuce (Valerianella locusta) | Low-pressure oxygen glow discharge plasma | Flavonoid content | Flavonoid content increased in freeze-dried leaves after 120 s exposure | 99 |
| • Oxygen (0.5 mbar) | ||||
| • Input power – 75 and 150 W | ||||
| • Treatment time – 20 to 300 s | ||||
| (b) Plasma treatment on root | ||||
| Baby carrots (Daucus carota L.) | Atmospheric pressure plasma with nickel coated steel needle array | Microbial inactivation | E. coli – inactivation was less than 0.5 log | 93 |
| • Argon (flow rate – 455.33 standard cm3 min−1) | ||||
| • Treatment time – 30 s to 10 min | ||||
| • Input power – 3.95 to 12.83 kV | ||||
| (c) Plasma treatment on fruits | ||||
| Strawberry | DBD | Decontamination | 14% duty cycle and 20 min effective | 196 |
| Input – 7% and 14% duty cycle | 1.46 log CFU per g – total aerobic mesophilic bacteria | |||
| Treatment time – 5, 10, and 20 min | 2.75 log CFU per g – yeast and mould | |||
| Litchi | DBD | Enzymatic browning | Residual activity of litchi peroxidase decreased to 47.16% on 10 min treatment | 197 |
| Input – 50 kV, 1.5 A | ||||
| Treatment time – 0, 2, 4, 6, 8, and 10 min | ||||
| Blueberry | Atmospheric cold plasma | Improving antioxidant activity and microbial inactivation | Reduced the decay rate of blueberries | 198 |
| Input – 12 KV, 5 kHz | Antioxidant activity increased but maintained at a low ROS level | |||
| Treatment time – 0, 30, 60, 90 s | 60 s is the best exposure time | |||
| Cavendish banana | DBD | Post-harvest crown rot | 0.5 min optimum duration | 199 |
| Input – 15 kV, compressed air | Colletotrichum musae inhibition – 51.89% for 0.5 min | |||
| Treatment time – 0.5, 1, 2, and 3 min | ||||
| Tomatoes | DDP with aluminium-coated electrodes | Pesticide degradation | Chlorpyrifos reduced up to 89.18% at 5 W in 6 min | 37 |
| • Gas – atmospheric air (50–75 mmHg) | Lycopene (84.86 to 15.38 mg kg−1), beta carotene (22.82 to 6.61 mg kg−1) and firmness were reduced | |||
| • Input power – 2 to 5 W | ||||
| Treatment time – 4 to 6 min | ||||
| Blueberries | Circular aluminium electrodes with 2 and 10 mm thick perspex dielectric material at ambient temperature (25 ± 2 °C) | Pesticide degradation | Plasma treatment of 5 min at 80 kV degraded pesticides (75% of boscalid and 80% of imidacloprid) | 104 |
| • Atmospheric air | ||||
| • Input power – 60 and 80 kV | ||||
| • Treatment time – 0, 2, and 5 min | ||||
| Strawberries | DBD atmospheric CP with Al electrode and polypropylene dielectric material (in pack treatment) | Microbial inactivation | G 1 and G2 produced 3.1, 3.4 and 3.7, 3.3 log reduction of mesophiles, yeast/mould, respectively | 100 |
• G1 – O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 of 65 CO2 of 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 ratio 19 ratio |
||||
• G2 – N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 of 90 O2 of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio 10 ratio |
||||
| • Treatment time – 5 min | ||||
| • Input power supply 60 kVrms voltage (50 Hz) | ||||
| Cocktail tomatoes (Lycopersicon lycopersicum) | Atmospheric pressure plasma with nickel coated steel needle array | Microbial inactivation | E. coli – 12.83 kV input reduced microbial count to 1.7 log after 10 min | 93 |
| • Argon (flow rate – 455.33 standard cm3 min−1) | ||||
| • Treatment time – 30 s to 10 min | ||||
| • Input power – 3.95 to 12.83 kV | ||||
| (d) Plasma treatment on nuts | ||||
| Peanut | Novel CAP with rotary jet system | Inactivation of fungi | Aspergillus flavus – not detected at 180 W for 7.5 min and 200 W for 5 min | 200 |
| Input – 180 W, 200 W | Aspergillus niger – not detected at 180 W for 10 min and 200 W for 5 min | |||
| Treatment time – 5, 7.5, 10 min | Aflatoxin concentration after 29 days of storage – 16.5 ppb | |||
| Pistachio nut | DBD | Decontamination of aflatoxin | 4 log reduction after 180 s of treatment | 201 |
| • Air plasma system | Maximum reduction of 52.42% of AFB 1 after 180 s | |||
| • Input – 130 W, 20 kHz, and 15 KV | ||||
| • Treatment time – 15, 30, 60, 90, 120, 150, 180 s | ||||
| Pistachio nut | Cold plasma jet | Pest management | Plasma exposure time – 14.04 min, voltage – 19.99 kV and Ar/air ratio – 51.65% caused increased mortality of Plodia interpunctella | 202 |
| Input – 10, 15 and 20 kV and agon/gas ratio – 0, 50 and 100 | ||||
| Dried walnut kernels | Radiofrequency low-pressure cold plasma | Microbial load reduction | The highest log reduction occurred at 50 W | 203 |
| • Air plasma | 1.09 log CFU per g – total viable count, 0.97 log CFU per g – coliform | |||
| • Input – 20, 30, 40 and 50 W | 0.89 log CFU per g – mold | |||
| • Treatment time – 10, 15 and 20 min | ||||
| Cashew nut | Low-pressure plasma-glow discharge | Allergenicity | Did not affect the allergenicity | 204 |
| Air plasma | Improved lipid extractability | |||
| Input – 80 W and 20 kHz | Anacardic acid content was higher | |||
| Almond slices | Atmospheric argon plasma | Surface disinfection | 20 min treatment was effective | 205 |
| Input – 17 V, 2.26 A current | 2.29 log CFU per g – total count | |||
| Treatment time – 5, 10, 15, and 20 min | 1.81 log CFU per g – yeast and mould | |||
| 2.72 log CFU per g – S. aureus | ||||
| Whole peanut (WP) and defatted peanut flour (DPF) | DBD plasma | Allergen reduction | The protein intensity of the Ara h 1 band was unaffected by plasma treatment | 115 |
| Input – 80 kV | With increased time, Ara h 1 band intensity for IgE binding decreased for WP and DPF | |||
| Gas – air | Antigenicity was reduced by 44 and 9.3% for DPF and WP after 60 min | |||
| Treatment time – 0 to 60 min | ||||
| Tiger nut milk | DBD | Microbial inactivation | Undetectable microflora in 12 min pH reduction after 8 and 12 min protein decreased beyond 4 min | 206 |
| Input – 1.22 A and 30 V | ||||
| Treatment time – 2, 4, 6, 8, and 12 min | ||||
| Peanut (Arachis hypogaea L.) | Coplanar – multi hollow surface DBD unit | Quality characteristics | Oleic and linoleic acid content was reduced from 43.47 to 35.74% and 32.56 to 24.49% for fresh and treated peanuts | 114 |
| • Atmospheric air with 20 to 30% humidity (flow rate – 0.5 to 20 L min−1) | Input power, treatment time, and gas mixer used in the study directly affected peanut quality | |||
| • Input power – 10 to 40 W | ||||
| Treatment time – 1 to 15 min | ||||
| Unpeeled almonds | DCSBD plasma | Microbial decontamination | Air, O2, and N2 plasma reduced microbial count by 5, 4.8 and 2 logs after 15 min | 107 |
| Input – 350 W | ||||
| Gas – dry air, O2, N2, CO2 and CO2/Ar mix (90% + 10%) | ||||
| Gas flow rate – 0.8 L min−1 | ||||
| Treatment time – 15 min | ||||
| Hazelnuts | Atmospheric pressure fluidized bed plasma (APFBP) reactor | Aflatoxin removal | 1st reactor: A. flavus and A. parasiticus count reduced by more than 4 log in 5 min at 655 W | 108 |
| Input – 460 to 655 W | 2nd reactor: both microbes count reduced by more than 3 log | |||
| Gas – dry air and N2 (flow rate – 3000 L h−1) | A similar trend was followed in both reactors when N2 gas was used | |||
| Treatment time – 1 to 5 min | ||||
| 1st reactor – 49 mm diameter, 2nd reactor – 65 mm diameter | ||||
| Walnut fruits (J. Regia L.) | Plasma jet with a copper electrode and Pyrex tube | Microbial decontamination | The fresh and dried walnut microbial load was removed after 10 and 11 min treatment, respectively, for all walnut types | 109 |
| • Argon (1 L min−1) | ||||
| • Input power – 15 kV DC supply | ||||
| • Treatment time – 3 to 11 min | ||||
| Hazelnuts | DBD plasma | Aflatoxin removal | Pure N2 or N2 with O2 (0.1%) provided higher toxin removal | 116 |
| Input – 0.4 to 2 kW | After 12 min exposure, residual AFB1 and AFs in nuts were 29.1 and 30.4% at 1150 W | |||
| Gas – pure N2 or N2 and air mix (flow rate – 120 L min−1 with 7 bar) | ||||
| Treatment time – 1 to 12 min | ||||
| Distance – 50 mm | ||||
| Hazelnuts | APFBP reactor | Microbial decontamination | At 655 W (25 kHz) within 1 min exposure, 2 log reduction was achieved, and the natural flora was reduced by 3.45 log in 2 min | 110 |
| Input – 460 to 655 W | ||||
| Gas – dry air (flow rate – 3000 L h−1) | ||||
| Treatment time – 1 to 5 min | ||||
| Almonds | Gliding arc plasma | Microbial decontamination | Plasma reduced 1.34 log CFU per mL of E. coli O157:H7 C9490 after 20 s treatment with 6 cm distance | 111 |
| • Input – 590 W | ||||
| • Gas – air and nitrogen (60 psi) | ||||
| • Time – 0, 10, and 20 s | ||||
| • Distance – 2, 4, 6 cm | ||||
| Hazelnut, peanut, and pistachio nut | Low-pressure CP (LPCP) sterilization unit | Microbial decontamination | In hazelnuts, SF6 plasma reduces D-value (1.1 min) compared to air plasma (4.2 min) | 113 |
| • Air or sulfur hexafluoride (SF6) | 5 times more log reduction was observed in SF6 than in air plasma (5 min) | |||
| • Input power – 300W | ||||
| • Treatment time – 5 to 20 min | ||||
| Almonds | DBD plasma | Microbial decontamination | 4 to 5 log reduction in E. coli was observed in all almond varieties after 30 min | 112 |
| Input – 16 to 30 kV | ||||
| Discharge gap – 10 mm | ||||
| (e) Plasma treatment on spices | ||||
| Saffron | Low-pressure cold plasma technology | Decontamination | 110 W for 30 min – high microbial reduction | 207 |
| Input – 70, 90, and 110 W | 3.52 log CFU per g – TVC | |||
| Treatment time – 5, 10, 15, and 30 min | 4.62 log CFU per g – coliforms | |||
| 2.38 log CFU per g – mold | ||||
| 4.12 log CFU per g – yeast | ||||
| Black pepper corn | Low-pressure cold plasma technology | Decontamination | 250 W for 20 min showed the highest decontamination | 208 |
| Input – 150 W and 250 W | 0.88 log CFU per g – aerobic plate | |||
| Treatment time – 10 min and 20 min | 3.66 log CFU per g – yeast and mould | |||
| Black pepper | DSCBD – DBD | Decontamination | B. subtilis count reduced by 5.06 log after 5 min exposure | 117 |
| Input – 400 W | B. subtilis spores, E. coli and S. Enteritidis D – values were 142, 47 and 58 s | |||
| Gas – ambient air | ||||
| Treatment time – 60 to 300 s | ||||
| Saffron | Plasma jet | Bioactive compound | Safranal content was reduced by 14% and 21% at 8 kV and 12 kV, respectively | 118 |
| Input – 8 and 12 kV | However, isophorone (4 and 8%) and 4-ketoisophorone (2 and 6%) increased by 8 min Ar plasma at 8 kV and 12 kV, respectively | |||
| Gas – Ar with 5, 10, and 20% O2 (flow rate – 1 L min−1) | ||||
| Treatment time – 6 and 8 min | ||||
| Red pepper flake | Microwave integrated CP treatment with high (HMCPT) and low microwave density (LMCPT) | Decontamination | 1.8 and 0.6 log per cm2 reduction was observed in spore count in HMCPT and LMCPT | 119 |
| Input – 900 W | Based on particle size, the microbial reduction was more for flakes (1.4 and 2.7 log per cm2) than particles (0.8 and 1.2 log per cm2) in LMCPT and HMCPT | |||
| Gas – He (flow rate – 1 std L min−1 with 0.7 kPa) | ||||
| Treatment time – 20 min | ||||
| Onion (Allium cepa L.) powder | Microwave-powered CP treatment with HMCPT and LMCPT | Microbial reduction | B. cereus spores reduction was more in HMCPT (2.1 log per cm2) than LMCPT (1.2 log per cm2) | 120 |
| Input – 400 to 900 W | For microbial reduction, vacuum air drying of the sample was superior to hot air drying | |||
| Gas – He (flow rate – 1 L min−1 at 0.7 kPa) | ||||
| Treatment time – 10 to 40 min | ||||
| Onion powder | DBD – CP treatment | Microbial reduction | E. coli and L. monocytogenes count reduced by more than 1 log after 20 min at 9 kV, while more than 2 log reduction was observed in S. Enteritidis | 121 |
| Input – 9 kV | ||||
| Treatment time – 20 min | ||||
| Whole black pepper | Radiofrequency (G1) and microwave (G2) generated plasma jet | Microbial reduction | G 2 plasma was more effective against aerobics and spores than G1 plasma | 28 |
| • G1 used argon (10 L min−1) | ||||
| • G2 used air (18 L min−1) | ||||
| • Input power – 30 W (G1), 1.2 kW (G2) | ||||
| • Treatment time – 15 min (G1) and (G2) 30 min | ||||
| Black pepper | Atmospheric pressure plasma jet (APPJ) | Microbial reduction | Argon and CO2 gas mixer plasma treatment inactivated B. subtilis after 5 min of treatment | 122 |
| • Argon plasma (flow rate – 20 L min−1) | ||||
| • Argon (0.5 L min−1) + CO2 (20 L min−1) plasma 0.5 (argon) + 20 (CO2) 0 | ||||
| • Air plasma (20 L min−1) | ||||
| • Argon + H2O plasma (20 L min−1) | ||||
| • Input power – 280 V (8 A) | ||||
| • Treatment time – 0 to 10 min | ||||
| Black pepper | Fluid bed APPJ treatment | Microbial reduction | Microbial reduction of 1.5 and 5 log reduction of Salmonella was observed after 20 and 80 s of treatment | 123 |
| • Air and argon (flow rate – 20 and 14 L min−1, respectively) | ||||
| • Treatment time – 0 to 80 s | ||||
| Black pepper | RF – low-pressure cold plasma | Microbial reduction | H2O2 usage (60 min) reduced microbial count by 1 to 3 logs | 124 |
| Input – 300 and 400 W | After 60 min, Ar plasma caused higher microbial reduction than air, O2 and N2 plasma (for all microbes) | |||
| Gas – O2, N2, air, and Ar (0.3 and 9 mbar) | ||||
| Treatment time – 15 to 60 min | ||||
| Red pepper (Capsicum annuum L.) powder | Microwave powered CP | Microbial reduction | 20 min plasma treatment at 900 W reduced Aspergillus flavus (N2 gas at 667 pa) by 2.5 logs | 125 |
• Air and argon (267 to 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680 pa) 680 pa) |
Bacillus cereus required heating for inactivation | |||
| • Input – 300 to 900 W | ||||
| • Treatment time – 0 to 80 s | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| (2) Plasma treatment for safe storage, extraction and quality enhancement | ||||
| (a) Grain safety and quality | ||||
| Wheat | DBD | Degradation of deoxynivalenol | 50 kV and 5 min degraded DON by 83.99% | 209 |
| Input – 30, 40, and 50 kV | ||||
| Treatment time – 4, 8, 12, and 16 min | ||||
| Chickpea | Atmospheric cold plasma | Increased storage period to study the occurrence of Callosobruchus chinensis | In vishal – 40 W, 15 and 20 min – grain damage – 1 to 3% | 210 |
| Input – 40, 50, 60 W | In other samples, the damage was 0% | |||
| Treatment time – 10, 15, and 20 min | ||||
| Canola grain, canola meal and barley grains | ACP DBD and ACP-jet | Degradation of Zearalenone | ACP-DBD – 3 min degradation was 91.6%, 83.2%, and 64.8% for canola grain, canola meal, and barley grain | 211 |
| • ACP – DBD – input – 0–34 kV, 1 A and 300 W, 3500 Hz, duty cycle – 70% and output pulse – 10 µs | ACP jet – 85% Ar + 15% O2 – high degradation | |||
| • ACP-jet – 1500 Hz, voltage – 0–22 kV, 70% duty cycle, 10 µs | ||||
| And 0–0.025 A with 75% Ar + 25% N2 | ||||
| • Treatment time – 0.5, 1, 3, 5, and 15 min | ||||
| Barley | Atmospheric cold plasma (DBD) | Removal of deoxynivalenol | 6 min – 48.9% and 10 min – 54.4% | 212 |
| • Input – 0–34 kV, 1 A, and 300 W, 3500 Hz, duty cycle – 70% and output pulse – 10 µs | ||||
| • Treatment time – 0, 2, 4, 6, 8, and 10 min | ||||
| Wheat | DBD plasma jet | T. castaneum mortality for Ar, Ar + N2 (20 sccm) and Ar + N2 (80 sccm) gas mixture were 90, 70 and 46%, and for T. confusum 93%, 63% and 57%, respectively. A similar trend was observed for O2 admixture | 126 | |
| Input – 90–130 W | ||||
| Gas – Ar (3000 sccm), O2 (1 to 8 sccm) with Ar, N2 (0 to 80 sccm) with Ar | ||||
| Treatment time – 10 to 15 min | ||||
| Wheat, barley | CP with DBD | Long-time exposure reduced microbes on the surface | 127 | |
| • Atmospheric air | Wheat germination was affected due to the long exposure time | |||
| • Input power – 80 kV | ||||
| • Treatment time – 5 and 20 min | ||||
| Wheat germ | DPD plasma | Lipase activity was 27.11 and 25.03% at 20 and 24 kV plasma voltage after 25 min | 129 | |
| Input voltage – 20 and 24 kV | Lipoxygenase activity was 55.18 and 49.98% for the same condition | |||
| Treatment time – 5 to 35 min | ||||
| Distance – 1.5 and 2 cm | ||||
| Winter wheat | CP with DBD | Total isolated colonies were reduced to 27 from 250 after 10 s treatment | 131 | |
| • Atmospheric air | ||||
| • Input power – 80 kV | ||||
| • Treatment time – 3, 10, and 30 s | ||||
| Wheat | DBD plasma with polymethylmethacrylate | 10 kV pulse voltage removed 3 times more endospores than 6 kV treatment | 130 | |
| Gas – Ar (flow rate – 2.8 nm) | ||||
| Input – 6 to 10 kV | ||||
| Time – up to 10 min | ||||
| Wheat | Low-pressure plasma circulating fluidized bed reactor | 700 and 900 W with 10% O2 mix reduced 1.91 and 2.59 log microbes after 10 min | 132 | |
| Gas – Ar and O2 (15 norm. L min−1) | ||||
| Input – 700 and 900 W | ||||
| Time – up to 73.5 s | ||||
| Grains and legumes | Low-pressure CP | Microbial contamination reduced below 1% for Aspergillus spp. and Penicillum spp. | 133 | |
| • Air gases or SF6 (300 torr) | ||||
| • Input power – 300 W | ||||
| • Treatment time – 30 s to 30 min | ||||
| (b) Flour safety | ||||
| Wheat flour | Bell jar type of plasma apparatus | Pest control | 60 W and 30 min showed complete inhibition of larvae, pupae, and egg | 213 |
| Input – 40 and 60 W | ||||
| Treatment time – 20, 25, and 30 min | ||||
| Refined wheat flour | DBD CP treatment | Insect inactivation | The maximum mortality rate was found for 15 min exposure times for eggs (93.33%), larvae (93.33%) and adults (100%) | 29 |
| • Atmospheric air (1 mbar) | ||||
| • Input power – 1 to 10 kV | ||||
| • Treatment time – 2 and 20 min | ||||
| Refined wheat flour | DBD atmospheric CP | Insect control | Complete mortality was achieved by increased voltage or increased time, or reduced electrode distance for Tribolium castaneum adults | 134 |
| • Atmospheric air (flow rate – 1 L min−1) | ||||
| • Input power – 500 to 3000 V | ||||
| • Treatment time – 0.5 to 7 min | ||||
| Maida flour | DBD plasma | Insect control | An increase in exposure time and input power or a decrease in electrode distance gave 100 mortality of T. castaneum | 135 |
| Input – 500 to 3000 V | ||||
| Atmospheric air (1 mbar) | ||||
| Treatment time – 0.5 to 7 min | ||||
| Electrode distance – 2 to 6 min | ||||
| (c) Flour quality | ||||
| Quinoa flour | ACP | Functionality study | Results in protein polymerization and starch depolymerization | 214 |
| Input – 50 kV and 60 kV | Treatment time and voltage affects technological properties | |||
| Treatment time – 5 and 10 min | ||||
| Jackfruit seed flour | Pin-to-plate ACP | Functionality modification | Higher input voltage and exposure time cause starch and protein modification and loss in crystallinity (25.75% to 21.31%) | 215 |
| Input – 170, 200, and 230 V | The hydration properties, like water solubility, increased from 9.65 g g−1 to 14.11 g g−1 water absorption – 6.39 g g−1 to 7.66 g g−1 | |||
| Treatment time – 5, 10, and 15 min | Swelling power – 7.28 g g−1 to 8.79 g g−1 | |||
| Water holding – 2.93 g g−1 to 3.48 g g−1 | ||||
| Little millet flour | Multipin electrical discharge atmospheric cold plasma | Functional property modification | 24 W and 30 min resulted in increased functional properties | 216 |
| Input – 13 and 24 W | Water absorption, oil absorption capacity, swelling, and solubility increased | |||
| Treatment times – 10, 20, and 30 min | A decrease in the viscosity of cooked paste, colour, and dispersibility was observed | |||
| Wheatgrass flour | Radiofrequency CP treatment | Functional modification | No change in protein solubility | 137 |
| Input – 120 W | Starch damage increased, and water absorption was affected after treatment | |||
| Gas – Ar and CO2 (flow rate of 10 and 25 cm3 min−1) | Solvent retention of different flour types increased | |||
| Treatment time – 1 h (2 × 30 min) | ||||
| Rice starch | Bell jar CP reactor | Functional property modification | Amylose content, pH, turbidity, and starch hydrolysis percentage were reduced | 138 |
| • Atmospheric air (0.15 mbar) | At maximum power level and time, GT is reduced | |||
| • Input power – 40 to 60 W | ||||
| • Treatment time – 5 and 10 min | ||||
| Wheat flour | Continuous atmospheric pressure cold plasma | Fatty acid profile | FFA and phospholipid were reduced after 60 and 120 s treatment at 20 V | 139 |
| Input – 15 and 20 V | Pasting properties were unaffected | |||
| Treatment time – 60 and 120 s | ||||
| Rice flour (short and long rice) | DBD plasma ozone treatment | Functional property modification | Higher transmittance and swelling power for treated rice pastes and grains, respectively | 140 |
| Input – 60 and 70 kV | Syneresis increased for both flours | |||
| Gas – atmospheric air | ||||
| Treatment time – 5 and 10 min | ||||
| Grain pea (Pisum sativum) | Surface DBD air plasma | Functional property modification | PPI solubility increased with plasma treatment time up to 191% (10 min) in distilled water. PPF solubility reduced from 71 to 33% for the same treatment time | 141 |
| • Input – 8.8 kVPP | ||||
| • Gas – air | ||||
| • Time – up to 10 min | ||||
| Wheat flour (soft, hard) | DBD atmospheric CP | Functional property modification | Peak time and peak integral of soft and hard flour increased significantly when voltage and treatment time exceeded 60 kV and 5 min | 25 |
| • Atmospheric air (flow rate – 1 L min−1) | ||||
| • Input power – 60 and 70 kV | ||||
| • Treatment time – 5 and 10 min | ||||
| Wheat flour | Counter flow – cold plasma generated | Functional property modification | Wet gluten (34.2 to 32.1%), dry gluten (9.7 to 7.9%) and gluten softening (13 to 10 mm) values decreased after 45 min, while sedimentation (56 to 61 cm3) and bread-making strength index (50 to 56) values increased compared to control | 142 |
| Gas – O3 at 1000 ppm (flow rate – 2.5 L min−1) | ||||
| Treatment time – 30 and 45 min | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| (3) Plasma treatment on minimally processed and processed fruits products quality | ||||
| (a) Minimally processed fruit products | ||||
| Fresh-cut cantaloupe | DBD | Quality and flavour | CP treatment inhibited the growth of bacteria and mould during 10 days storage | 217 |
| Input – 40 kV | ||||
| Treatment time – 90 s | ||||
| Banana slices | DBD reactor | Enzyme activity | Polyphenol oxidase and peroxidase activity decreased to 70% and 100% | 218 |
| • Input voltage: 4.8 to 6.9 kV and 12 to 22 kHz | Total phenol and flavonoid content increased to 50% | |||
| • Treatment time: 0.6 to 2.6 min | Vitamin B6 increased | |||
| Carrot discs | DBD reactor | Surface decontamination | 2.1 log CFU per g reduction in total aerobic mesophiles and yeast and mould at 5 min and 100 KV | 219 |
| • Gas: atmospheric pressure plasma | ||||
| • Input voltage: 60 kV, 80 kV, and 100 kV | ||||
| Treatment time: 0, 1, 2, 3, 4, 5 min | ||||
| Fresh-cut pitaya fruit | DBD reactor | Accumulation of phenolics and antioxidant activity | 60 kV for 5 min reduced the total aerobic bacteria and increased safety | 220 |
| • Input voltage: 40, 50, 60, and 70 kV | The content of individual phenolics increased, resulting in increased antioxidant activity | |||
| Treatment time: 1, 3, 5, and 7 min | ||||
| Fresh-cut pear | Atmospheric cold plasma | Microbial inactivation | 65 kV for 1 min was effective in achieving a shelf-life of 7 days | 221 |
| Input voltage: 45 kV, 65 kV | ||||
| Treatment time: 1 and 5 min | ||||
| Fresh-cut apples (Pink Lady®, Fuji, Modì®, Red Delicious (RD)) | Double barrier discharge type gas plasma with three brass electrodes | Enzymatic browning | Browning area – 17% reduction in RD and 50% in other samples (30 min). Whereas 60 min treatment gave 86% and 58% reduction in Pink Lady® and Modi® | 143 |
| • Atmospheric air | ||||
| • Input power – 150 W | ||||
| • Treatment time – 30 (15 + 15) and 60 (30 + 30) min | ||||
| Apples (Malus domestica cv.) | Dielectric barrier discharge (DBD) CP | Polyphenol content | No significant change in total phenolic content | 145 |
| • Atmospheric air | Extracted polyphenol did not affect the cell viability | |||
| • Input power – 150 W | ||||
| • Treatment time – 30 (15 + 15) and 120 (60 + 60) min | ||||
| Fresh-cut apples | Dielectric barrier discharge (DBD) chamber with brass electrodes | Polyphenol content | Polyphenol oxidase activity – reduced by 12, 32, and 58% after 10, 20, and 30 min treatment | 144 |
| • Atmospheric air | ||||
| • Input power – 9 V (15 kV measured potential difference) | ||||
| • Treatment time – 10, 20 and 30 min (5 + 5, 10 + 10 and 15 + 15 min each major slice side) | ||||
| (b) Processed fruit products | ||||
| Apple juice | DBD and glow discharge plasma | Browning enzyme activity, antioxidant capacity, and total phenolic content | Both systems inactivated polyphenol oxidase and peroxidase and increased total phenolic content and antioxidant activity | 222 |
| DBD | DBD – better at increasing total phenolic and antioxidant capacity | |||
| Input – 50–900 Hz | ||||
| Glow discharge | ||||
| Input – air plasma flow rate: 10–30 mL min−1 | ||||
| Treatment time – 10–30 min | ||||
| Cashew apple juice | ACP | Bio accessibility of vitamin C | Malic acid and phenylalanine increased for ACP 700 | 223 |
| Increased the vitamin C bio accessibility at ACP 700 | ||||
| Camu–camu juice | DBD | Bioavailability of vitamin C | Higher excitation frequency increased the availability of ascorbic acid | 224 |
| Input – 24 kV and frequency – 200, 420, 583, 698, and 960 Hz | Degradation of anthocyanins and peroxidase enzyme at a higher frequency | |||
| Kiwi turbid juice | DBD | Microbial inactivation and quality changes | • 12 kV, volume – 18 mL and discharge time – 1 min was the optimum condition | 225 |
| Input – 10, 25, and 40 kV | • Polyphenol content – 0.18 mg g−1, chlorophyll content – 3.47 | |||
| Treatment time – 1, 3 and 5 min | • Sterilization rate – 18.03% | |||
| Volume – 10, 15, and 20 mL | ||||
| Siriguela juice | Low-pressure plasma processing | Bioactive component | Enhanced the bioactive component content | 226 |
| Nitrogen gas flow rate – 10–30 mL min−1 | Highest pigment increase – 10 min – 10 mL min−1 | |||
| Treatment time – 5 to 15 min | Highest antioxidant activity – 15 min – 20 mL min−1 | |||
| Blueberry juice | Cold plasma jet | Bioavailability of vitamin C | 2 min exhibited high anthocyanin | 227 |
| Input – 11 kV, 1000 Hz and oxygen gas concentration – 0, 0.5 and 1 | Long treatment decrement of vitamin C | |||
| Treatment time – 2, 4, and 6 min | 1% concentration of O2 resulted in the reduction of Bacillus by 7.2 logs CFU per g | |||
| Apple juice | Dielectric barrier discharge (DBD) plasma chamber with 2 mm electrode distance from sample surface at 23 ± 2 °C temperature | Microbial inactivation | 4.0 log CFU per mL at 30 W (40 s) | 146 |
| • Atmospheric air | 4.2 log CFU per mL at 40 W (40 s) | |||
| • Input power – 30, 40, 50 W | 4.34 log CFU per mL at 50 W (30 s) | |||
| • Treatment time – 0 to 40 s | ||||
| Banana starch | Corona electrical discharge (CED) plasma | Starch modification | No changes in amylose and resistant starch contents. The crystalline to amorphous ratio increased after CED plasma treatment | 150 |
| Input – 30, 40, and 50 kV | ||||
| Treatment time – 3 min | ||||
| Sour cheery nectar | APPJ setup | E. coli – 3.34 log reduction at 650 W after 120 s | 148 | |
| Tomato juice | • Atmospheric dry air (flow rate – 3000 L h−1) | Microbial inactivation | E. coli – 1.43 log reduction at 650 W after 120 s | |
| Apple juice | • Input power – 650 W | E. coli – 4.02 log reduction at 650 W after 120 s | ||
| Orange juice | • Treatment time – 0 to 120 s | E. coli – 1.59 log reduction at 650 W after 120 s | ||
| White grape juice | High voltage atmospheric pressure CP with plexiglass and polypropylene dielectric barrier | Microbial inactivation | S. cerevisiae population reduced to 7.4 logs (80 kV for 4 min) | 149 |
| • Atmospheric dry air (Ziploc bag) | ||||
| • Input power – 80 kV (peak) | ||||
| • Treatment time – 1 to 4 min | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| (4) Plasma treatment on essential oil extraction and quality | ||||
| Hyssop | DBD | Changes in essential oil content during storage | Trichomes were vulnerable to plasma treatment | 228 |
| Input – 17, 20, and 23 kV | Increased treatment duration reduced the EO content | |||
| Treatment time – 1, 5 and 10 min | ||||
| Fennel and spearmint leaves | DBD | Essential oil yield | 19 kV and 10 min yield 1.89% (v/w) for fennel and 1.81% (v/w) for spearmint leaves | 229 |
| Input – 17–23 kV | ||||
| Treatment time – 5–15 min | ||||
| Lemon peel | Low-pressure DBD cold plasma | Essential oil yield | Plasma treatment for 10 min at 2.5 kV increased the extraction yield by 149.34% | 152 |
| • Gas – atmospheric air (1 mbar) | ||||
| • Input – 1 to 2.5 kV | ||||
| • Treatment time – 10 min | ||||
| Lemon verbena (Lippa citriodora Kunth.) | Low-pressure CP connected with vacuum pump | Essential oil yield | Essential oil (EO) content increased by 36.7% during the first 1 min of treatment and later decreased due to higher exposure time | 153 |
| • Nitrogen, argon, oxygen | ||||
| • Treatment time – 0, 1, 3, and 5 min | ||||
| Sweet basil (Ocimum basilicum) | APPJ CP | Essential oil yield | Plasma treated basil EO produced more antioxidant activity (94.82%) than untreated (90.64%) basil at a 250 µg mL−1 concentration | 27 |
| • Air/helium | ||||
| • Treatment time – 5 s | ||||
| Lemon peel | DBD plasma treatment | Essential oil yield | Maximum EO yield was obtained in the argon plasma (flow rate – 15 mL min−1) after 1 min | 154 |
| • Argon, oxygen, nitrogen, or air (10–20 mL min−1) | Argon plasma of 1 × 1 × 1 cm size lemon peel produces maximum EO extraction efficiency | |||
| • Input power – 30 kV (peak) | ||||
| • Treatment time – 1 to 15 min | ||||
| Clove oil | Plasma jet with pyrex glass tube at atmospheric pressure | Essential oil yield | The minimum concentration required for microbial inhibition (A. niger, Penicillium sp., and Rhizopus sp.) was reduced to 20 times for clove oil and 8 to 9 times for eugenol after 10 min at 40 W | 151 |
| • Argon (flow rate of 10 L min−1) | ||||
4. Plasma applications in post-harvest stages
4.1. Plasma treatment of different agriculture produces
Some of the edible products, such as leaves, roots, fruits, nuts, and spices, are either contaminated by microbes or will have allergens. Plasma treatment can decrease the contaminant level and increase the active component percentage in food products. However, based on the surface morphology of different commodities, the effect may change and usually produces a smooth surface that will have more microbial removal rate than the unevenly surfaced one, and this could be due to the improper penetration of plasma species into the porous structures.93 Decontamination and disinfestation of grains, higher extraction of EO, and functional modification of flours are some advantages of plasma treatments that will be discussed in this section. However, like any other treatment, plasma produces minor undesirable changes that will also be covered here. Different post-harvest applications of plasma are shown in Fig. 4.As pesticides are used extensively in agricultural commodities, their residues remain on the surface of the commodities even after primary and secondary processing. Hence, Ranjitha Gracy et al.37 applied plasma treatment to tomatoes to validate the chlorpyrifos reduction in DBD plasma with different dosage levels. The results showed around 90% pesticide reduction due to the conversion of the phosphorothiol group (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) into the phosphoryl group (P
S) into the phosphoryl group (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). After the treatment, considerable changes in firmness, total phenolic content, and colour index were observed. Earlier, Sarangapani et al.104 studied the same with blueberries contaminated with boscalid and imidacloprid pesticides and found at least a 75% reduction in both pesticides. Along with this, ascorbic acid content increased from 8.91 to 14.01 mg/100, while time and power level increases reduced the total flavonoids, anthocyanin, and TPC. Different interactions of plasma with water are shown in Fig. 6.
O). After the treatment, considerable changes in firmness, total phenolic content, and colour index were observed. Earlier, Sarangapani et al.104 studied the same with blueberries contaminated with boscalid and imidacloprid pesticides and found at least a 75% reduction in both pesticides. Along with this, ascorbic acid content increased from 8.91 to 14.01 mg/100, while time and power level increases reduced the total flavonoids, anthocyanin, and TPC. Different interactions of plasma with water are shown in Fig. 6.
Apart from plasma treatment, PAW also helps in fruit processing. For example, PAW water can clean the fungicide contaminated tomatoes and can cause up to 85.3% and 79.47% reduction in the chlorothalonil and thiram contents.105 Further, they are also capable of reducing microbial contamination of fruits and vegetables.106
Some quality changes are also associated with nuts during treatment. For instance, peanuts were treated with coplanar DBD plasma; though antioxidant capacity was not changed due to short exposure to ROS, the TPC increased from 200.23 to 341.15 mg (GAE/100 g) after 25 W treatment for 8 min (0.5 L min−1) since phenols protect cells against the damaging effects UV and ROS. However, lipid oxidation and moisture reduction were observed due to the oxidation of strong ROS species.114 Another problem with peanuts is the Ara h 1 allergens present in them; however, it was reduced in the whole peanut (WP) and defatted peanut flour (DPF) through binding epitope modifications caused by protein and lipid functionality changes after the treatment.115 Aflatoxins are commonly found in nuts, which is a hazardous toxin, though it was removed about 70% from hazelnut (Aflatoxin B1-AFB1 and total Aflatoxin-AFs) by DBD plasma treatment at 1150 W. Changes in the lactone ring were considered to be the reason for degradation and this effect was increased with power and time.116
Kim et al.119 reported decontamination of red pepper flakes from B. cereus in high microwave density (HMCPT) and low microwave density (LMCPT) CP treatment. It was observed that IR drying and flaked samples were suited for microbial reduction. Higher sample water activity (aW) produced a more lethal effect on microbial reduction. Kim et al.120 treated onion powder in microwave-powered plasma. Microbial inactivation was high in HMCPT than in LMCPT. In addition, the treatment drying method influenced microbial reduction. Onion Aw reduced from 0.26 to 0.16 and 0.12 in LMCPT and HMCPT. Plasma treatment with low temperature (4 °C) storage prevented microbial growth. Quercetin concentration and DPPH activity followed a reducing trend in HMCPT samples, and for the control sample, an increasing trend was observed during storage at both temperatures (4 °C and 25 °C). Won et al.121 reported the effect of He-plasma on onion preservation. In plasma treatment, increased E. coli, L. monocytogenes and S. Enteritidis inactivation by about 2 log per cm2 when aW increased from 0.4 to 0.8 was noted, and also, the increase in particle size positively influenced inactivation.
Hertwig et al.28 reported the effect of radio frequency (G1) and microwave (G2) generated plasma jet on black pepper. G1 plasma treatment for 15 min caused 0.7 logs and 0.6 log inactivation of aerobic and spore count, whereas G2 plasma treatment caused 1.7 logs and 1.4 inactivation of an aerobic and spore count for the same time interval. The piperine content of both plasma-treated samples was reduced slightly, and G2 plasma was effective against S. enterica. Takemura et al.122 reported the microbial reduction in black pepper in plasma treatment. Argon and CO2 mixed plasma treatment reduced microbial load better than other combinations. The growth retardation of untreated samples was less than air and argon + CO2 mixer plasma. Similarly, Sun et al.123 reported APPJ plasma treatment of black pepper on surface microbial reduction. A sample stored at a higher temperature (37 °C) and low RH (33%) reduced the initial microbial count more than that stored at a low temperature and RH. During the treatment, peppercorn surface temperature reached above 120 °C after 80 s exposure. Combined storage condition and 80 s plasma treatment reduced an average of about seven microbial log counts. Grabowski et al.124 treated black pepper with plasma for decontamination. Spore & non-spore forming aerobic bacteria and anaerobic spore-forming bacteria were removed during the treatment. Increased treatment time increased microbial reduction but resulted in water loss. Lumps on pepper were observed after treatment except in O2 and H2O2 plasma. Kim et al.125 reported the effect of microwave-powered CP on the decontamination of A. flavus from red pepper powder. The treatments performed at higher power levels reduced the water activity of pepper due to evaporation, which further increased the ROS concentration. Colour change was insignificant, and He and O2 gas mix with heating up to 90 °C inactivated B. cereus spores.
Research has shown a considerable microbial reduction in most agricultural products and enhancement in active components. However, specific issues, such as colour change, weight loss, and bioactive quality losses, must be addressed by selecting proper plasma characteristics and treatment time. Apart from that, the mode of action of RS or UV produced from plasma treatment on the food components needs to be explained to understand the reason for positive and negative impacts on quality.
4.2. Plasma treatment for safe storage, extraction, and quality enhancement of agricultural products
Removal of microbes and pests during storage is essential to increase the quality of goods. Fumigation and other thermal treatments result in either residue formation or quality deterioration. Plasma treatment facilitates microbial removal and disinfestation without affecting product quality. Meanwhile, it also inactivates the enzymes that reduce the product quality or shelf life. In some cases, plasma treatment improves the functional properties of food constituents.Tolouie et al.129 reported the plasma effect on wheat germ enzyme activity to enhance its shelf life. Lipase and lipoxygenase activities were reduced more in 24 kV plasma treatment, while antioxidant activity and total phenols were unchanged. Lipase and lipoxygenase activities were recovered by 1.18 and 6.52 U g−1, respectively, after 30 days. Butscher et al.130 reported bacterial inactivation of plasma treatment in wheat grain where water content was reduced by 10.48 to 9.35% gluten, and the falling number was unaffected. Kordas et al.131 reported around ten times reduction in the fungal colonies of wheat seeds when exposed to up to 10 s of plasma treatment, and it also resulted in more than 98% germination energy. However, the root and leaf length decreased during the same treatment time. Butscher et al.132 reported the effect of power level and O2 concentration on microbial destruction in wheat. Higher O2 levels and power levels positively affect microbes, while the extensograph and amylograph parameters such as elasticity, strain resistance, energy, P/L ratio, and gelatinization increased after plasma treatment. Selcuk et al.133 reported the surface structure of grains and legumes on microbial disinfection of air and SF6 plasma. Cooking quality and water absorbance had no significant effect due to treatments. However, the seed germination rate was maintained above 85% after treatment. Wheat disinfection was 99% in SF6 plasma treatment.
Studies have found the effect of plasma treatment on rheological properties and changes in flours. Held et al.137 reported the flour and dough properties of hard and soft wheat. Secondary structure contribution to protein (β-turns, α-helices, random and β-sheet) was significantly affected after plasma treatment. Peak maximum hard, soft and intermediate flour time increased after plasma treatment while extensibility values decreased. Thirumdas et al.138 reported the changes in rice starch after CP treatment. At 60 W power level, gelatinization temperature (GT) reduced after 10 min of treatment. At the same time, the pasting temperature was reduced for all treatments. An increase in peak viscosity, storage modulus (except 60 W to 5 min) and loss modulus (except 60 W to 5 min) was found. Bahrami et al.139 studied the effect of plasma on the functional properties of wheat flour. It was found that protein fractions in flour changed with a higher dosage, while non-polar lipids and glycolipids fractionation had no difference after treatment, and the dough became strong due to protein and lipid modifications. When short and long rice flours were treated with plasma, it showed an increase in transmittance, swelling power, and gel syneresis. Though rice protein was not affected by plasma treatment, the pasting properties of long rice flour increased due to starch molecules' cross-linkage by ozone oxidation. In contrast, after treatment, amino acids were reduced.140
Pea protein-rich flour (PPF), pea starch-rich four (PSF), pea testa flour (PTF), and pea protein isolate (PPI) fractions of pea flour were treated in surface DBD plasma, which led to a mass loss of 2.1, 1.2, 1.3, and 1.1% for PPF, PSF, PTF, and PPI, respectively. Colour changes were increased for PPF and PPI and reduced for PSF when exposure time increased. PPF and PTF water and fat binding increased as treatment time increased to 10 min.141 Misra et al.25 reported plasma's effect on wheat flour's rheological properties. Peak time and peak integral of soft and hard flour increased significantly when voltage and treatment time exceeded 60 kV and 5 min. Ozone concentration increased with respect to time and input voltage increased. α-helix, β-turn + β-sheet percentage were reduced at 60 kV for 5 min exposure in both samples. However, they increased at 70 kV after 10 min time in soft wheat flour.
Plasma-induced flour functionality changes also resulted in end product quality changes. Menkovska et al.142 reported the effect of plasma on wheat flour and dough quality changes. An increase in farinograph values (except dough softening) was observed with respect to treatment time. Similarly, alveograph values increased (except elasticity) with respect to time. Shape formation ratio, specific and total loaf volumes increased from 0.47 to 0.58 h per day, 3.79 to 4.55 cm3, and 560 to 657 cm3, respectively, after 45 min of treatment due to gas retention and crumb cell increase.
Few research studies have focused on the mode of action of ROS on insects, while the effect of UV and RNS on insects is unclear. In addition, PTW and CAW have not produced the same mortality, even though they produced similar pH. Thus, the actual reason behind the mortality of insects in PTW is unclear. Industrial use of PTW has many practical issues since it hydrates/increases the moisture content of produce.
4.3. Processed and minimally processed fruits products quality
The primary reason for minimal processing is to preserve the food with minimal quality changes. However, as non-thermal processing, plasma can help retard browning and microbial activity without adding preservatives and retains the quality of the product. Browning in cut fruit surface is the major problem in preservation. In the case of apples (Pink Lady®, Fuji, Modì®, Red Delicious (RD)), the browning area reduced significantly after plasma treatment due to less residual PPO activity of 10% and 50% in Fuji and Modi, respectively, after 60 min. This could be due to the changes in the secondary structure of proteins and modification of the amino acid side chain of enzymes.143 Along with browning control, other parameters such as soluble solids, dry matter, acidity, firmness, and rupture strength were increased;144 however, antioxidant capacity and total phenol index (TPI) were reduced in apples.145 When the juice was extracted from apples, more than 4 logs of microbial reduction were achieved at 30 W (40 s), 40 W (40 s), and 50 W (30 s) without TSS change. However, the treatment affected total phenolic content (41.7 to 32.4 GAE mg/100 g), antioxidant capacity, pH, and colour values.146 These data confirmed the study of Surowsky et al.147 in which a plasma jet was applied to reduce Citrobacter freundii loads in apple juice by about 5 log cycles after a plasma exposure of 480 s using argon and 0.1% oxygen plus a subsequent storage time of 24 h. Similar effects were found in sour cheery nectar and other juices (tomato, apple, and orange) with a minimum of 1 log reduction in E. coli at 650 W after 120 s treatment time. However, in this case, TPC increased by more than 14% except in orange juice (9.52%).148 However, for white grape juice along with S. cerevisiae inactivation, phenols (720.62 to 445.02 GAE µg per mL), flavonoid (265.21 to 231.04 CE µg per mL), DPPH (88.16 to 82.24), and antioxidant capacity (679.35 to 637.61) were reduced after plasma treatment. The degradation of aromatic rings in phenols due to RS causes a reduction in total phenolic content. Thus it is also reflected in DPPH and antioxidant capacity reduction.149Wu et al.150 reported the effect of corona electrical discharge (CED) plasma on banana starch property changes. DSC's onset temperature increased to 60.0, 60.5, and 61.2 °C from 57.2 °C at 30, 40, and 50 kV cm−1 intensities due to the cross-linkages created by RS in the polymeric chain of starch granules. This effect was seen in peak and conclusion temperatures increase and gelatinization enthalpy reduction. In terms of pasting behaviour, peak viscosity reduced from 100.4 (control) to 44.4 RVU (50 kV cm−1) while pasting temperature reached 92.1 °C from 74.4 °C (control) due to higher crystallinity and swelling resistance of starch.
Though plasma can reduce the microbial load of food products, it also causes certain quality deteriorations in it. Microbial reduction can be correlated with the changes in the acidity of the plasma-treated sample, but matrix effects have to be considered for clear identification of the inactivation mechanisms, and a focus on evaluating the correlation between the plasma treatment parameter and the subsequent product quality changes is needed.
4.4. Essential oil extraction and quality
Essential oils naturally have anti-bacterial properties; when treated with plasma, the properties can be enhanced and reduce the quantity of EO required to perform the same effect. While the treatment also affects the EO components due to higher oxidation in a few studies.EO obtained from plasma-treated sweet basil produced more antioxidant activity with higher eugenol content, while the treated seed growth after a month was 40 mm higher than the control.27 Similarly, an increase in the antimicrobial property of clove oil was achieved after plasma jet processing. Due to this, the minimum concentration required for microbial inhibition (A. niger, Penicillium sp., and Rhizopus sp.) was reduced for clove oil and eugenol.151 Other than improving EO quality, the extraction yields also increased in microwave pretreated lemon peel at different plasma power levels (1.0 kV, 1.5 kV, and 2.0 kV), and it was reported that the increase in lemon peel EO yield is due to the rupture that happened on the oil glands by etching, and it was confirmed by SEM images.152 Similarly, Ebadi et al.153 reported the same in lemon verbena (Lippa citriodora Kunth.) without any pretreatment after short-time plasma exposure and found an increase in spathulenol (8.1%) and globulol (7.3%) content after 5 min treatment. Earlier, Kodama et al.154 studied the lemon peel with different gases and peel sizes to understand the effect of process and product variables on extraction. The results found that DBD used with Ar gas induced damage in lemon peel oil glands and increased the EO extraction in the initial stages of treatment. However, the surface area increase (size reduction) reduced the limonene, γ-terpinene, and β-pinene concentrations in EO due to overexposure. Similarly, plasma treatment induced both negative and positive effects in the bioactive compounds of essential oil extracted from turmeric powder. However, the treatment reduced the microbial count from turmeric powder by 1.5 logs.155
5. Effect on the food products of animal origin
5.1. Dairy products
Milk and its products are highly perishable and often contaminated by bacteria such as Salmonella spp., Streptococcus, Coliforms, Enterococcus, Bacillus, etc. Subjecting the milk to plasma treatment generates free radicals inside the sample and offers antibacterial properties. However, the antibacterial nature of the milk relies on the plasma treatment intensity. Therefore, DBD plasma is the most studied plasma treatment for milk and milk products (fat-free- dry powder and cheese). The treatment was also effective against most milk-contaminating microorganisms (Ex: E. coli, L. monocytogenes, and Salmonella typhimurium). Further, the plasma treatment produced superior quality products compared to pasteurized ones. However, its effectiveness is not superior to UHT treatment.156,157H2O2 reactive species are the major cause for the increase of milk's antibacterial properties following plasma treatment. However, few studies suggest direct damage in the microbial cell walls and protein leakage due to plasma species.158–160 Nevertheless, based on the plasma discharge type, the mechanism of microbial inactivation will vary. Specifically, the microbes present in the liquid of the thin layer will observe more direct cell wall damage than the ones present in the larger volumes. The indirect plasma species effect in the high-volume liquid samples is the plasma species's high reactivity and dissolving property. Therefore, the secondary reactive species generated in the liquid samples will have a greater influence on the microbes when using a larger volume of samples.
Plasma treatment tends to reduce the pH of liquid samples due to the generation of H+ ions. However, in milk samples, the phosphate and milk casein buffering effect limits the pH change and causes only a mild reduction. Short-time plasma treatments did not affect the milk and milk products' colour. However, long exposure resulted in the reduction of yellowness and lightness. Further, the treatment also increases the saturated fatty acid content in the milk by oxidizing the unsaturated fatty acids. Apart from that, lipid oxidation also increases in milk products due to the oxidation of plasma species. Similarly, the reactive species oxidize sulfonate and nitrate the side chains of amino acids.156
5.2. Seafood
Cold plasma is effective against most spoilage-causing microorganisms such as L. monocytogenes, Staphylococcus aureus, and Enterobacteriaceae. However, depending on the packing conditions, the treatment effectiveness varies for different seafood products. For example, using CO2 and O2 gas inside the package of the seafood product enhances CP-assisted microbial reduction. In addition, the natural humidity inside the packaging material allows the plasma reactive species interaction and subsequently provides a bactericidal effect to the microbes post the plasma treatment.161During plasma treatment, the hydrogen in the sample dissociates into H+ ions and reduces the pH of the sample. The increase in acidity subsequently triggers protein breakdown. In addition, reactive species also denature proteins through oxidation and fragmentation. The oxidation process causes the proteins to form aggregates with cross-linkages. Like other lipid sources, the seafood lipids oxidize due to plasma treatment and induce off-flavor upon prolonged exposure. However, CP treatment can retort the activity of several enzymes (α-amylase, lipase, alkaline phosphatase, peroxidase, and lipoxygenase) that spoil seafood. Furthermore, CP improved the colour value of the seafood samples by increasing the L* and b values through lipid oxidation and pigment production (Fig. 8).161
5.3. Meat and poultry
Salmonella spp., E. coli O157:H7, S. aureus, L. monocytogenes, and Campylobacter jejuni contaminate most meat and poultry products. These contaminants can be removed effectively using CP treatment. In addition, studies have shown the decontamination efficiency of cold plasma in many types of meat and poultry products (i.e., pork, beef, poultry meat, and egg). The mechanism of action is similar to that discussed in the earlier sections. However, the quality characteristics vary based on the product's nature.162At minimal exposure level, CP treatment does not induce any colour changes. However, at longer exposure times, the L* (reduce), a (increase), and b (increase) values will change significantly. However, the treated sample partially recovered from the colour degradation during the storage period. Similarly, most of the plasma-treated meat and poultry samples observed a minimal effect on the off-odor and water content. However, it can be avoided by minimizing the treatment duration. When it comes to pH, inert gas CP treatment did not cause any considerable changes in meat or poultry products. However, excess nitrogen reactive species reportedly reduced the pH of the CP-treated samples. Nevertheless, the lipids in meat and poultry products also are oxidized due to the reactive plasma species. Further, the treatment also results in the degradation (oxidation) of proteins due to the acidic compounds (H2O2) produced by CP treatment.162
6. Sensory and consumer perception of plasma treated-food products
Though non-thermal technologies preserve the freshness of food products, some techniques impair the sensory qualities. For example, the oxidation of lipid compounds during plasma treatment results in the production of off-flavor-producing compounds. On the other hand, CP treatment also improved the sensory qualities of certain food products. For instance, cut cantaloupe exhibited better sensory characteristics after 40 kV plasma treatment (90 s). Whereas 15 kV CP treated dairy beverage did not cause any sensory attribute changes to the product. However, products like tender coconut had a negative impact (chemical odour) on the sensory quality due to CP (49.4 kJ kg−1) treatment.163 Therefore, understanding the CP effect on the sensory properties of different food products will be a breakthrough in plasma research. Nevertheless, consumer perception of the plasma-treated products also needs to be counted for the success of plasma-treated food products. In this regard, consumers' perceptions of food products can be analyzed through the brain-computer interface method to obtain unbiased results from the consumers.164 Therefore, Coutinho et al.165 surveyed the perception of chocolate milk with 1085 participants. The study revealed that consumers are more concerned about the CP-treated product price and feel the plasma-treated product will be costlier than the conventionally processed products.7. Other novel applications
7.1. Waterless (hydroponic) plant cultivation
Plasma activated water not only assists plant growth in stress (saline, drought, or heavy metal) conditions but also in soilless conditions. Generally, the nitrogen required for plant growth is supplied directly from the soil or from soil microbes. Hence, when the plants are grown in hydroponic conditions, substrates like coir peats, rice husk, etc., are used to support the plant root and provide nutrients. However, PAW itself contains RNS that act as a nitrogen source for plants and eliminate the requirement of substrates. In addition, ROS species also produce H2O2 in PAW, which helps in controlling infections on the plant roots.166 Nevertheless, the PAW effect on plants may not always be positive, as few studies mentioned a negative effect on plant growth (Lettuce); however, after PAW supply, the secondary metabolites (Epicatechin, rutin, quercetin, total phenolic) were found to be high in both roots and leaves.167,168 However, a recent study utilized the plasma jet generated PAW solely for fulfilling the nitrogen requirement of corn and lettuce plants and assisted in seed germination and plant growth.169,1707.2. Cold plasma as green fertilizer
The rapid climate change and increased population will have an impact on food shortage in the future. Therefore, increased crop production is needed, which is done with the utilization of chemical fertilizers for agricultural crops. Hence a sustainable technology with less energy consumption and reduced chemical fertilizer use are of interest to researchers and producers to meet the demands.171 Cold plasma has the potential to be used as a green fertilizer in the future due to its increased efficiency and eco-friendly production. As the plasma treatment will lead to the production of reactive oxygen species and reactive nitrogen species in water, it acts as a fertilization liquid facilitating plant development. The H2O2 formed during the plasma treatment initiated the abscisic acid hormone and gibberellin, which is responsible for seed germination and dormancy. Similarly, the NO3− generated in PAW has a direct impact on the growth rate of the plant, as nitrate is a crucial element for plant growth. One of the major advantages of PAW as a green fertilizer is the presence of nutrients as ions rather than salts, which helps in easy absorption.170 Matra et al.172 have found that the PAW spray generated from the gliding arc plasma generator enhanced the amount of nitrogenous fertilizer in treated water. Similarly, Subramanian et al.173 have reported that PAW generated from DBD has a higher specific energy of nitrogen, which is desirable for agricultural purposes. Stoleru et al.168 have also concluded that the nitrites and nitrates from PAW worked as a fertilizer and enhanced the growth of plants. Though there are many studies on PAW as green fertilizer, the appropriate quantity of water used and concentration of nitrates could be a major breakthrough in this application.7.3. Shape transformation
3D printing and sessile drop drying are the two commonly used additive manufacturing practices to produce shape-changing 2D food structures (xerogels). Both these techniques produce flat 2D food structures that can transform their existing structure into a defined 3D structure under external stimuli (water, oil, drying, or pH) contact.174–177 However, to achieve the desired shape-shift, flat 2D structures need to be coated with food-grade constraint materials (i.e., ethyl cellulose). These constraints act as a barrier between stimuli and food surfaces and limit the interaction in the coated areas. However, the uncoated areas interact with stimuli and induce stress gradients between coated and uncoated areas. As a result, the relative expansion increase causes the food structure to fold or bend in a specific pattern to produce a desirable 3D structure.178–180 Nevertheless, surface wettability (i.e., water absorption and oil absorption) and the binding nature of food surface with constraint material decide the success of these shape-transforming foods. Both these factors can be improved by treating these 2D foods in plasma. Plasma etching increases the surface area of food, thereby its binding behaviour and water/oil absorption.30,180 Hence, to utilize the advantages of plasma treatment in shape transformation, Gupta et al.181 treated wheat xerogels using glow discharge plasma (power: 7.32 W, duration: 5 min) and then coated them with ethyl cellulose in a defined pattern. Due to plasma treatment, the swelling gradient created between coated and uncoated areas of xerogels increased and resulted in better shape transformation when immersed in 90 °C hot water. Similar results were obtained in Stephen et al.182 study on oil-triggered shape transformation, where the author treated corn xerogels in cold plasma (voltage: 1 kV, duration: 5 min) prior to cellulose acetate linear strip coating. As a result, the 2D structure curled into a spiral shape within 2 s in hot (220 °C) coconut oil due to the high relative extension and constraint material binding. Likewise, Cheeyattil et al.183 obtained flower shape and samosa shape in the barley flour xerogel through oleomorphic shape shifting. However, same xerogel can be used to obtain water and oil-based shape transformation. Therefore, Jaspin et al.179 tried obtaining complicated flower shapes from plasma-treated flat xerogels using hot water and oil stimuli and succeeded in both attempts.8. Conclusion
Plasma treatment significantly affected the agri-food chain in terms of maintaining the quality and safety of products, increasing germination, extraction efficiency, plant growth, and removing hazardous contaminants from water, soil, and agricultural produces. The interaction of plasma with food products, microbes, allergens, toxins, enzymes, insects, and other constituents varied depending on process variables such as gas composition, gas flow rate, power level, frequency, treatment time, and type of plasma chamber; product variables such as cultivar type, moisture, and other compositions; and other variables such as nature of pesticide, microbes, chemical contaminants, and their initial concentrations. Different mechanisms were involved in different plasma applications, such as microbial inactivation by surface etching and DNA modification, chemical removal by oxidation, allergen removal by protein and lipid functionality modification, germination by surface modification (wettability and surface etching), seed tolerance by heat shock protein production, disinfestation by nerve toxin effect, higher EO extraction by oil gland damages, browning retardation by changes in the secondary structure of proteins and modification of amino acid side chain, etc. However, many other reasons, such as species and cultivar-dependent efficiency change of treatment in microbes and agricultural commodities, extract quality improvement, uncertain quantity changes in food properties (i.e., increase and decrease in TPC, colour changes, etc.), the effect of RS on consumption to human health and plant growth needs to be explained in further research studies. Also, plasma treatment's large-scale application in these areas is still not stated. Laboratory experiments were mostly done for a lesser quantity of samples. Cost, efficiency, safety, and productivity will be the concern when plasma treatments are used for large-scale operations. Understanding plasma treatment and RS mode of action can increase the chance of productive utilization of plasma treatment by avoiding deterioration during treatment. Some studies have mentioned the long-term effect of RS on product quality and safety.Conflicts of interest
The authors also declare that there are no conflicts of interest.Acknowledgements
The authors wish to acknowledge the funding received from European Union COST Action: PlAgri (Grant No: CA19110) and Science Foundation Ireland (SFI) under grant number 17/CDA/4653.References
- S.-C. Ma, H.-B. Zhang, S.-T. Ma, R. Wang, G.-X. Wang and Y. Shao, et al., Effects of mine wastewater irrigation on activities of soil enzymes and physiological properties, heavy metal uptake and grain yield in winter wheat, Ecotoxicol. Environ. Saf., 2015, 113, 483–490 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0147651314005867.
- T. M. Salem, S. S. Ahmed, M. A. Hamed and G. H. Abd ElAziz, Risk assessment of hazardous impacts on urbanization and industrialization activities based upon toxic substances, Global J. Environ. Sci. Manage., 2016, 2(2), 163–176 CAS . Available from: https://www.gjesm.net/article_15789_44dbc7e25d290224656bad4969a53895.pdf.
- R. Gothwal and T. Shashidhar, Antibiotic Pollution in the Environment: A Review, Clean: Soil, Air, Water, 2015, 43(4), 479–489 CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1002/clen.201300989.
- L. Y. He, G. G. Ying, Y. S. Liu, H. C. Su, J. Chen and S. S. Liu, et al., Discharge of swine wastes risks water quality and food safety: antibiotics and antibiotic resistance genes from swine sources to the receiving environments, Environ. Int., 2016, 92–93, 210–219 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0160412016301039.
- H. Nazir, H. N. Asghar, Z. A. Zahir, M. J. Akhtar and M. Saleem, Judicious use of kinetin to improve growth and yield of rice in nickel contaminated soil, Int. J. Phytorem., 2016, 18(7), 651–655 CrossRef CAS PubMed . Available from: https://www.tandfonline.com/doi/full/10.1080/15226514.2015.1094444.
- W. Gwenzi, N. Chaukura, C. Noubactep and F. N. D. Mukome, Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision, J. Environ. Manage., 2017, 197, 732–749 CrossRef PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S030147971730302X.
- A. I. Mamedov, B. Bar-Yosef, I. Levkovich, R. Rosenberg, A. Silber and P. Fine, et al., Amending Soil with Sludge, Manure, Humic Acid, Orthophosphate and Phytic Acid: Effects on Infiltration, Runoff and Sediment Loss, Land Degradation and Development, 2016, 27(6), 1629–1639 CrossRef . Available from: https://onlinelibrary.wiley.com/doi/10.1002/ldr.2474.
- M. Ashraf and M. R. Foolad, Pre‐Sowing Seed Treatment—A Shotgun Approach to Improve Germination, Plant Growth, and Crop Yield Under Saline and Non‐Saline Conditions, Adv. Agron., 2005, 223–271 Search PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S006521130588006X.
- L. Ling, L. Jiangang, S. Minchong, Z. Chunlei and D. Yuanhua, Cold plasma treatment enhances oilseed rape seed germination under drought stress, Sci. Rep., 2015, 5(1), 13033 CrossRef PubMed . Available from: https://www.nature.com/articles/srep13033.
- F. Yeni, S. Yavaş, H. Alpas and Y. Soyer, Most Common Foodborne Pathogens and Mycotoxins on Fresh Produce: A Review of Recent Outbreaks, Crit. Rev. Food Sci. Nutr., 2016, 56(9), 1532–1544 CrossRef CAS PubMed . Available from: https://www.tandfonline.com/doi/full/10.1080/10408398.2013.777021.
- M. I. Gil, M. V. Selma, T. Suslow, L. Jacxsens, M. Uyttendaele and A. Allende, Pre- and Postharvest Preventive Measures and Intervention Strategies to Control Microbial Food Safety Hazards of Fresh Leafy Vegetables, Crit. Rev. Food Sci. Nutr., 2015, 55(4), 453–468 CrossRef PubMed . Available from: https://www.tandfonline.com/doi/abs/10.1080/10408398.2012.657808.
- Q. Zhu, R. Gooneratne and M. A. Hussain, Listeria monocytogenes in fresh produce: outbreaks, prevalence and contamination levels, Foods, 2017, 6(3), 1–11 CrossRef PubMed . Available from: https://www.mdpi.com/2304-8158/6/3/21.
- B. Singh, K. Suri, K. Shevkani, A. Kaur, A. Kaur and N. Singh, Enzymatic browning of fruit and vegetables: a review, in Enzymes in Food Technology: Improvements and Innovations, Springer Singapore, Singapore, 2018, p. 73–78, available from: https://link.springer.com/10.1007/978-981-13-1933-4_4 Search PubMed.
- F. Cheli, L. Pinotti, M. Novacco, M. Ottoboni, M. Tretola and V. Dell'Orto, Mycotoxins in Wheat and Mitigation Measures, in Wheat Improvement, Management and Utilization, InTech, 2017, p. 227, available from: https://www.intechopen.com/books/wheat-improvement-management-and-utilization/mycotoxins-in-wheat-and-mitigation-measures Search PubMed.
- T. Bosona and G. Gebresenbet, Food traceability as an integral part of logistics management in food and agricultural supply chain, Food Control, 2013, 33(1), 32–48 CrossRef . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0956713513000790.
- D. Kumar and P. Kalita, Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries, Foods, 2017, 6(1), 1–22 CrossRef PubMed . Available from: https://www.mdpi.com/2304-8158/6/1/8.
- I. Chakraborty and A. Chattopadhyay, Advances in Postharvest Technologies of Vegetable Crops, in Advances in Postharvest Technologies of Vegetable Crops, ed. Singh B., Singh S. and Koley T. K., Apple Academic Press, Waretown, NJ, 2018, Series: Postharvest biology and technology, available from: https://www.taylorfrancis.com/books/9781351664165 Search PubMed.
- U. Tiwari and E. Cummins, Factors influencing levels of phytochemicals in selected fruit and vegetables during pre- and post-harvest food processing operations, Food Res. Int., 2013, 50(2), 497–506 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0963996911005370.
- I. M. Caminiti, F. Noci, A. Muñoz, P. Whyte, D. J. Morgan and D. A. Cronin, et al., Impact of selected combinations of non-thermal processing technologies on the quality of an apple and cranberry juice blend, Food Chem., 2011, 124(4), 1387–1392 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0308814610009647.
- B. Boopathy, A. Rajan and M. Radhakrishnan, Ozone: An Alternative Fumigant in Controlling the Stored Product Insects and Pests: A Status Report, Ozone: Sci. Eng., 2022, 44(1), 79–95 CrossRef CAS . Available from: https://www.tandfonline.com/doi/full/10.1080/01919512.2021.1933899.
- A. Rajan and M. Radhakrishnan, Green Technologies for Sustainable Food Production and Preservation: An Overview of Ohmic Heating, Infrared Heating and UV Light Technology, in Reference Module in Food Science, Elsevier, 2023, available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128239605000664 Search PubMed.
- R. Mahendran, C. V. Kavitha Abirami and K. Alagusundaram, Cold plasma technology: an emerging non-thermal processing of foods—a review, in Engineering Interventions in Agricultural Processing, Apple Academic Press, Waretown, NJ, 2017, Series: Innovations in agricultural & biological engineering: Apple Academic Press, 2018, p. 33–55. available from: https://www.taylorfrancis.com/books/9781771885577/chapters/10.1201/9781315207377-2 Search PubMed.
- S. Potluri, K. Sangeetha, R. Santhosh, G. Nivas and R. Mahendran, Effect of low‐pressure plasma on bamboo rice and its flour, J. Food Process. Preserv., 2018, 42(12), e13846 CrossRef . Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/jfpp.13846.
- R. Mahendran and K. Alagusundaram, Uniform discharge characteristics of non-thermal plasma for superficial decontamination of bread slices, in International Journal of Agricultural Science and Research (IJASR), Transstellar Journal Publications and Research Consultancy Private Limited, 2015, p. 209–212, available from: https://www.academia.edu/download/45165821/25._Agri_Sci_-_IJASR___-Uniform_discharge_characteristics_of_non-thermal___-_Mahendran.pdf Search PubMed.
- N. N. Misra, S. Kaur, B. K. Tiwari, A. Kaur, N. Singh and P. J. Cullen, Atmospheric pressure cold plasma (ACP) treatment of wheat flour, Food Hydrocolloids, 2015, 44, 115–121 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0268005X14002951.
- J. Jiang, X. He, L. Li, J. Li, H. Shao and Q. Xu, et al., Effect of cold plasma treatment on seed germination and growth of wheat, Plasma Sci. Technol., 2014, 16(1), 54–58 CrossRef . Available from: https://iopscience.iop.org/article/10.1088/1009-0630/16/1/12.
- G. J. Buonopane, C. Antonacci and J. L. Lopez, Effect of Cold Plasma Processing on Botanicals and Their Essential Oils, Plasma Medical, 2016, 6(3–4), 315–324 CrossRef . Available from: https://www.dl.begellhouse.com/journals/5a5b4a3d419387fb,389e931927aa49bf,110e9426524acd52.html.
- C. Hertwig, K. Reineke, J. Ehlbeck, D. Knorr and O. Schlüter, Decontamination of whole black pepper using different cold atmospheric pressure plasma applications, Food Control, 2015, 55, 221–229 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0956713515001462.
- K. Ratish Ramanan, R. Sarumathi and R. Mahendran, Influence of cold plasma on mortality rate of different life stages of Tribolium castaneum on refined wheat flour, J. Stored Prod. Res., 2018, 77, 126–134 CrossRef . Available from: https://www.researchgate.net/publication/311370163.
- R. Anbarasan, S. Jaspin, B. Bhavadharini, A. Pare, R. Pandiselvam and R. Mahendran, Chlorpyrifos pesticide reduction in soybean using cold plasma and ozone treatments, LWT--Food Sci. Technol., 2022, 159, 113193 CrossRef CAS . Available from: https://www.sciencedirect.com/science/article/pii/S0023643822001281.
- E. Dolezalova and P. Lukes, Membrane damage and active but nonculturable state in liquid cultures of Escherichia coli treated with an atmospheric pressure plasma jet, Bioelectrochemistry, 2015, 103, 7–14 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1567539414001340.
- M. Yusupov, A. Bogaerts, S. Huygh, R. Snoeckx, A. C. T. van Duin and E. C. Neyts, Plasma-Induced Destruction of Bacterial Cell Wall Components: A Reactive Molecular Dynamics Simulation, J. Phys. Chem. C, 2013, 117(11), 5993–5998 CrossRef CAS . Available from: https://pubs.acs.org/doi/10.1021/jp3128516.
- L. Mao, P. Mhaske, X. Zing, S. Kasapis, M. Majzoobi and A. Farahnaky, Cold plasma: microbial inactivation and effects on quality attributes of fresh and minimally processed fruits and Ready-To-Eat vegetables, Trends Food Sci. Technol., 2021, 116, 146–175 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0924224421004404.
- J.-W. Lackmann, S. Schneider, E. Edengeiser, F. Jarzina, S. Brinckmann and E. Steinborn, et al., Photons and particles emitted from cold atmospheric-pressure plasma inactivate bacteria and biomolecules independently and synergistically, J. R. Soc., Interface, 2013, 10(89), 20130591 CrossRef PubMed . Available from: https://royalsocietypublishing.org/doi/10.1098/rsif.2013.0591.
- E. Stoffels, Y. Sakiyama and D. B. Graves, Cold Atmospheric Plasma: Charged Species and Their Interactions With Cells and Tissues, IEEE Trans. Plasma Sci., 2008, 36(4), 1441–1457 CAS . Available from: https://ieeexplore.ieee.org/document/4598991/.
- S. Ikawa, K. Kitano and S. Hamaguchi, Effects of pH on Bacterial Inactivation in Aqueous Solutions due to Low-Temperature Atmospheric Pressure Plasma Application, Plasma Processes Polym., 2010, 7(1), 33–42 CrossRef CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1002/ppap.200900090.
- T. K. Ranjitha Gracy, V. Gupta and R. Mahendran, Influence of low-pressure nonthermal dielectric barrier discharge plasma on chlorpyrifos reduction in tomatoes, J. Food Process Eng., 2019, 42(6), e13242 Search PubMed . Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/jfpe.13242.
- N. N. Misra, O. Schlüter and P. J. Cullen, Cold Plasma in Food and Agriculture: Fundamentals and Applications, Academic Press, 2016, p. 1–368, available from: https://www.sciencedirect.com/book/9780128013656/cold-plasma-in-food-and-agriculture Search PubMed.
- S. Kumar, J. Park, E. Kim, J. Na, Y. S. Chun and H. Kwon, et al., Oxidative stress induced by chlorine dioxide as an insecticidal factor to the Indian meal moth, Plodia interpunctella, Pestic. Biochem. Physiol., 2015, 124, 48–59 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0048357515000814.
- L. Ten Bosch, R. Köhler, R. Ortmann, S. Wieneke and W. Viöl, Insecticidal effects of plasma treatedwater, Int. J. Environ. Res. Public Health, 2017, 14(12), 1460 CrossRef PubMed . Available from: https://www.mdpi.com/1660-4601/14/12/1460.
- K. V. Donohue, B. L. Bures, M. A. Bourham and R. M. Roe, Mode of Action of a Novel Nonchemical Method of Insect Control: Atmospheric Pressure Plasma Discharge, J. Econ. Entomol., 2006, 99(1), 38–47 CrossRef PubMed . Available from: https://academic.oup.com/jee/article-lookup/doi/10.1093/jee/99.1.38.
- L. Sivachandiran and A. Khacef, Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: combined effect of seed and water treatment, RSC Adv., 2017, 7(4), 1822–1832 RSC . Available from: https://xlink.rsc.org/?DOI=C6RA24762H.
- B. Será, V. Stranák, M. Serý, M. Tichý and P. Spatenka, Germination of Chenopodium Album in Response to Microwave Plasma Treatment, Plasma Sci. Technol., 2008, 10(4), 506–511 CrossRef . Available from: https://iopscience.iop.org/article/10.1088/1009-0630/10/4/22.
- H. M. Kalaji and A. Rastogi, Pharmaceutical compounds: an emerging pollutant (a review on plant-pharmaceuticals interaction), Chiang Mai J. Sci., 2017, 44(2), 287–297 CAS . Available from: https://www.thaiscience.info/Journals/Article/CMJS/10985613.pdf.
- M. Magureanu, N. B. Mandache and V. I. Parvulescu, Degradation of pharmaceutical compounds in water by non-thermal plasma treatment, Water Res., 2015, 81(11), 124–136 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0043135415300208.
- N. J. Rowan, S. Espie, J. Harrower, J. G. Anderson, L. Marsili and S. J. Macgregor, Pulsed-Plasma Gas-Discharge Inactivation of Microbial Pathogens in Chilled Poultry Wash Water, J. Food Prot., 2007, 70(12), 2805–2810 CrossRef CAS PubMed . Available from: https://meridian.allenpress.com/jfp/article/70/12/2805/171258/PulsedPlasma-GasDischarge-Inactivation-of.
- C. Bradu, M. Magureanu and V. I. Parvulescu, Degradation of the chlorophenoxyacetic herbicide 2,4-D by plasma-ozonation system, J. Hazard. Mater., 2017, 336, 52–56 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0304389417302996.
- G. Kamgang-Youbi, J.-M. Herry, T. Meylheuc, J.-L. Brisset, M.-N. Bellon-Fontaine and A. Doubla, et al., Microbial inactivation using plasma-activated water obtained by gliding electric discharges, Lett. Appl. Microbiol., 2009, 48(1), 13–18 CrossRef CAS PubMed . Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1472-765X.2008.02476.x.
- R. Thirumdas, A. Kothakota, U. Annapure, K. Siliveru, R. Blundell and R. Gatt, et al., Plasma activated water (PAW): chemistry, physico-chemical properties, applications in food and agriculture, Trends Food Sci. Technol., 2018, 77, 21–31 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0924224417305873.
- J. Feng, Z. Zheng, J. Luan, K. Li, L. Wang and J. Feng, Gas–liquid hybrid discharge-induced degradation of diuron in aqueous solution, J. Hazard. Mater., 2009, 164(2–3), 838–846 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0304389408012958.
- Y. Hu, Y. Bai, X. Li and J. Chen, Application of dielectric barrier discharge plasma for degradation and pathways of dimethoate in aqueous solution, Sep. Purif. Technol., 2013, 120, 191–197 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1383586613005893.
- R. Zhou, T. Zhang, R. Zhou, A. Mai-Prochnow, S. B. Ponraj and Z. Fang, et al., Underwater microplasma bubbles for efficient and simultaneous degradation of mixed dye pollutants, Sci. Total Environ., 2021, 750, 142295, DOI:10.1016/j.scitotenv.2020.142295.
- G. Iervolino, V. Vaiano and V. Palma, Enhanced removal of water pollutants by dielectric barrier discharge non-thermal plasma reactor, Sep. Purif. Technol., 2019, 215, 155–162 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1383586618337432.
- F. Tampieri, A. Giardina, F. J. Bosi, A. Pavanello, E. Marotta and B. Zaniol, et al., Removal of persistent organic pollutants from water using a newly developed atmospheric plasma reactor, Plasma Processes Polym., 2018, 15(6), 1700207 CrossRef . Available from: https://onlinelibrary.wiley.com/doi/10.1002/ppap.201700207.
- C. Sarangapani, N. N. Misra, V. Milosavljevic, P. Bourke, F. O'Regan and P. J. Cullen, Pesticide degradation in water using atmospheric air cold plasma, J. Water Process. Eng., 2016, 9, 225–232 CrossRef . Available from: https://linkinghub.elsevier.com/retrieve/pii/S221471441630006X.
- M. J. Pavlovich, H.-W. Chang, Y. Sakiyama, D. S. Clark and D. B. Graves, Ozone correlates with antibacterial effects from indirect air dielectric barrier discharge treatment of water, J. Phys. D: Appl. Phys., 2013, 46(14), 145202 CrossRef . Available from: https://iopscience.iop.org/article/10.1088/0022-3727/46/14/145202.
- M. Hijosa-Valsero, R. Molina, H. Schikora, M. Müller and J. M. Bayona, Removal of priority pollutants from water by means of dielectric barrier discharge atmospheric plasma, J. Hazard. Mater., 2013, 262, 664–673 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0304389413006663.
- S. Jung, H. J. Kim, S. Park, H. In Yong, J. H. Choe and H.-J. Jeon, et al., The use of atmospheric pressure plasma-treated water as a source of nitrite for emulsion-type sausage, Meat Sci., 2015, 108, 132–137 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S030917401530036X.
- M. J. Traylor, M. J. Pavlovich, S. Karim, P. Hait, Y. Sakiyama and D. S. Clark, et al., Long-term antibacterial efficacy of air plasma-activated water, J. Phys. D: Appl. Phys., 2011, 44(47), 472001 CrossRef . Available from: https://iopscience.iop.org/article/10.1088/0022-3727/44/47/472001.
- M. Naïtali, G. Kamgang-Youbi, J.-M. Herry, M.-N. Bellon-Fontaine and J.-L. Brisset, Combined Effects of Long-Living Chemical Species during Microbial Inactivation Using Atmospheric Plasma-Treated Water, Appl. Environ. Microbiol., 2010, 76(22), 7662–7664 CrossRef PubMed . Available from: https://journals.asm.org/doi/10.1128/AEM.01615-10.
- M. Chen, P. Xu, G. Zeng, C. Yang, D. Huang and J. Zhang, Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: applications, microbes and future research needs, Biotechnol. Adv., 2015, 33(6), 745–755 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0734975015300021.
- H. D. Stryczewska, K. Ebihara, M. Takayama, Y. Gyoutoku and M. Tachibana, Non-thermal plasma-based technology for soil treatment, Plasma Processes Polym., 2005, 2(3), 238–245 CrossRef CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1002/ppap.200400061.
- M. Hatzisymeon, D. Tataraki, C. Tsakiroglou, G. Rassias and C. A. Aggelopoulos, Highly energy-efficient degradation of antibiotics in soil: extensive cold plasma discharges generation in soil pores driven by high voltage nanopulses, Sci. Total Environ., 2021, 786, 147420 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0048969721024918.
- J. Zhan, Y. Liu, W. Cheng, A. Zhang, R. Li and X. Li, et al., Remediation of soil contaminated by fluorene using needle-plate pulsed corona discharge plasma, Chem. Eng. J., 2018, 334, 2124–2133 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1385894717320156.
- C. A. Aggelopoulos, C. D. Tsakiroglou, S. Ognier and S. Cavadias, Non-aqueous phase liquid-contaminated soil remediation by ex situ dielectric barrier discharge plasma, Int. J. Environ. Sci. Technol., 2015, 12(3), 1011–1020 CrossRef CAS . Available from: https://link.springer.com/10.1007/s13762-013-0489-4.
- N. Lu, J. Lou, C. H. Wang, J. Li and Y. Wu, Evaluating the Effects of Silent Discharge Plasma on Remediation of Acid Scarlet GR-Contaminated Soil, Water, Air, Soil Pollut., 2014, 225(6), 1991 CrossRef . Available from: https://link.springer.com/10.1007/s11270-014-1991-0.
- J. Lou, N. Lu, J. Li, T. Wang and Y. Wu, Remediation of chloramphenicol-contaminated soil by atmospheric pressure dielectric barrier discharge, Chem. Eng. J., 2012, 180, 99–105 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1385894711013921.
- T. C. Wang, N. Lu, J. Li and Y. Wu, Plasma-TiO2 catalytic method for high-efficiency remediation of p-nitrophenol contaminated soil in pulsed discharge, Environ. Sci. Technol., 2011, 45(21), 9301–9307 CrossRef CAS PubMed . Available from: https://pubs.acs.org/doi/10.1021/es2014314.
- T. C. Wang, N. Lu, J. Li and Y. Wu, Degradation of pentachlorophenol in soil by pulsed corona discharge plasma, J. Hazard. Mater., 2010, 180(1–3), 436–441 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0304389410004954.
- T. C. Wang, G. Qu, J. Li, D. Liang and S. Hu, Depth dependence of p-nitrophenol removal in soil by pulsed discharge plasma, Chem. Eng. J., 2014, 239, 178–184 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1385894713014599.
- T. C. Wang, N. Lu, J. Li and Y. Wu, Evaluation of the Potential of Pentachlorophenol Degradation in Soil by Pulsed Corona Discharge Plasma from Soil Characteristics, Environ. Sci. Technol., 2010, 44(8), 3105–3110 CrossRef CAS PubMed . Available from: https://pubs.acs.org/doi/10.1021/es903527w.
- H. D. Stryczewska, J. Pawłat and K. Ebihara, Non-Thermal Plasma Aided Soil Decontamination, J. Adv. Oxid. Technol., 2013, 16(1), 23–30 CAS . Available from: https://www.degruyter.com/document/doi/10.1515/jaots-2013-0103/html.
- E. J. Rifna, K. Ratish Ramanan and R. Mahendran, Emerging technology applications for improving seed germination, Trends Food Sci. Technol., 2019, 86, 95–108 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0924224417307975.
- Y. Li, T. Wang, Y. Meng, G. Qu, Q. Sun and D. Liang, et al., Air Atmospheric Dielectric Barrier Discharge Plasma Induced Germination and Growth Enhancement of Wheat Seed, Plasma Chem. Plasma Process., 2017, 37(6), 1621–1634 CrossRef CAS . Available from: https://link.springer.com/10.1007/s11090-017-9835-5.
- J.-W. Kim, P. Puligundla and C. Mok, Effect of corona discharge plasma jet on surface-borne microorganisms and sprouting of broccoli seeds, J. Sci. Food Agric., 2017, 97(1), 128–134 CrossRef CAS PubMed . Available from: https://onlinelibrary.wiley.com/doi/10.1002/jsfa.7698.
- A. Zahoranová, M. Henselová, D. Hudecová, B. Kaliňáková, D. Kováčik and V. Medvecká, et al., Effect of Cold Atmospheric Pressure Plasma on the Wheat Seedlings Vigor and on the Inactivation of Microorganisms on the Seeds Surface, Plasma Chem. Plasma Process., 2016, 36(2), 397–414 CrossRef . Available from: https://link.springer.com/10.1007/s11090-015-9684-z.
- D. Dobrin, M. Magureanu, N. B. Mandache and M.-D. Ionita, The effect of non-thermal plasma treatment on wheat germination and early growth, Innovative Food Sci. Emerging Technol., 2015, 29, 255–260 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1466856415000429.
- L. Li, J. Li, M. Shen, J. Hou, H. Shao and Y. Dong, et al., Improving Seed Germination and Peanut Yields by Cold Plasma Treatment, Plasma Sci. Technol., 2016, 18(10), 1027–1033 CrossRef CAS . Available from: https://iopscience.iop.org/article/10.1088/1009-0630/18/10/10.
- M. Darmanin, A. Fröhling, S. Bußler, J. Durek, S. Neugart and M. Schreiner, et al., Aqueous and gaseous plasma applications for the treatment of mung bean seeds, Sci. Rep., 2021, 11(1), 19681 CrossRef CAS PubMed . Available from: https://www.nature.com/articles/s41598-021-97823-1.
- J. Jiang, Y. Lu, J. Li, L. Li, X. He and H. Shao, et al., Effect of Seed Treatment by Cold Plasma on the Resistance of Tomato to Ralstonia solanacearum (Bacterial Wilt). Yousfi M, editor, PLoS One, 2014, 9(5), e97753 CrossRef PubMed . Available from: https://dx.plos.org/10.1371/journal.pone.0097753.
- L. Ling, J. Jiafeng, L. Jiangang, S. Minchong, H. Xin and S. Hanliang, et al., Effects of cold plasma treatment on seed germination and seedling growth of soybean, Sci. Rep., 2015, 4(1), 5859 CrossRef PubMed . Available from: https://www.nature.com/articles/srep05859.
- S. Bußler, W. B. Herppich, S. Neugart, M. Schreiner, J. Ehlbeck and S. Rohn, et al., Impact of cold atmospheric pressure plasma on physiology and flavonol glycoside profile of peas (Pisum sativum ‘Salamanca’), Food Res. Int., 2015, 76(P1), 132–141 CrossRef . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0963996915001507.
- M. Henselová, Ľ. Slováková, M. Martinka and A. Zahoranová, Growth, anatomy and enzyme activity changes in maize roots induced by treatment of seeds with low-temperature plasma, Biologia, 2012, 67(3), 490–497 CrossRef . Available from: https://link.springer.com/10.2478/s11756-012-0046-5.
- T. Stolárik, M. Henselová, M. Martinka, O. Novák, A. Zahoranová and M. Černák, Effect of Low-Temperature Plasma on the Structure of Seeds, Growth and Metabolism of Endogenous Phytohormones in Pea (Pisum sativum L.), Plasma Chem. Plasma Process., 2015, 35(4), 659–676 CrossRef . Available from: https://link.springer.com/10.1007/s11090-015-9627-8.
- E. Bormashenko, Y. Shapira, R. Grynyov, G. Whyman, Y. Bormashenko and E. Drori, Interaction of cold radiofrequency plasma with seeds of beans (Phaseolus vulgaris), J. Exp. Bot., 2015, 66(13), 4013–4021 CrossRef CAS PubMed . Available from: https://academic.oup.com/jxb/article-lookup/doi/10.1093/jxb/erv206.
- P. Puligundla, J.-W. Kim and C. Mok, Effects of Nonthermal Plasma Treatment on Decontamination and Sprouting of Radish (Raphanus sativus L.) Seeds, Food Bioprocess Technol., 2017, 10(6), 1093–1102 CrossRef CAS . Available from: https://link.springer.com/10.1007/s11947-017-1886-3.
- P. Sookwong, S. Yodpitak, J. Doungkaew, J. Jurithayo, D. Boonyawan and S. Mahatheeranont, Application of Oxygen-argon Plasma as a Potential Approach of Improving the Nutrition Value of Pre-germinated Brown Rice, J. Food Nutr. Res., 2014, 2(12), 946–951 CrossRef . Available from: https://pubs.sciepub.com/jfnr/2/12/14/index.html.
- S. Živković, N. Puač, Z. Giba, D. Grubišić and Z. L. Petrović, The stimulatory effect of non-equilibrium (low temperature) air plasma pretreatment on light-induced germination of Paulownia tomentosa seeds, Seed Sci. Technol., 2004, 32(3), 693–701 CrossRef . Available from: https://www.ingentaconnect.com/content/ista/sst/2004/00000032/00000003/art00005.
- B. Šerá, I. Gajdová, M. Šerý and P. Špatenka, New Physicochemical Treatment Method of Poppy Seeds for Agriculture and Food Industries, Plasma Sci. Technol., 2013, 15(9), 935–938 CrossRef . Available from: https://iopscience.iop.org/article/10.1088/1009-0630/15/9/19.
- S. Kyzek, Ľ. Holubová, V. Medvecká, J. Tomeková, E. Gálová and A. Zahoranová, Cold Atmospheric Pressure Plasma Can Induce Adaptive Response in Pea Seeds, Plasma Chem. Plasma Process., 2019, 39(2), 475–486 CrossRef CAS . Available from: https://link.springer.com/10.1007/s11090-018-9951-x.
- A. Iranbakhsh, N. O. Ardebili, Z. O. Ardebili, M. Shafaati and M. Ghoranneviss, Non-thermal Plasma Induced Expression of Heat Shock Factor A4A and Improved Wheat (Triticum aestivum L.) Growth and Resistance Against Salt Stress, Plasma Chem. Plasma Process., 2018, 38(1), 29–44 CrossRef CAS . Available from: https://link.springer.com/10.1007/s11090-017-9861-3.
- Q. Guo, Y. Wang, H. Zhang, G. Qu, T. Wang and Q. Sun, et al., Alleviation of adverse effects of drought stress on wheat seed germination using atmospheric dielectric barrier discharge plasma treatment, Sci. Rep., 2017, 7(1), 16680 CrossRef PubMed . Available from: https://www.nature.com/articles/s41598-017-16944-8.
- D. Bermúdez-Aguirre, E. Wemlinger, P. Pedrow, G. Barbosa-Cánovas and M. Garcia-Perez, Effect of atmospheric pressure cold plasma (APCP) on the inactivation of Escherichia coli in fresh produce, Food Control, 2013, 34(1), 149–157 CrossRef . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0956713513002065.
- F. Pasquali, A. C. Stratakos, A. Koidis, A. Berardinelli, C. Cevoli and L. Ragni, et al., Atmospheric cold plasma process for vegetable leaf decontamination: a feasibility study on radicchio (red chicory, Cichorium intybus L.), Food Control, 2016, 60, 552–559 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S095671351530178X.
- M. Amini and M. Ghoranneviss, Black and green tea decontamination by cold plasma, Res. J. Microbiol., 2016, 11(1), 42–46 CrossRef CAS . Available from: https://scialert.net/abstract/?doi=jm.2016.42.46.
- J. Durek, A. Fröhling, S. Bußler, A. Hase, J. Ehlbeck and O. K. Schlüter, Pilot-scale generation of plasma processed air and its influence on microbial count, microbial diversity, and selected quality parameters of dried herbs, Innovative Food Sci. Emerging Technol., 2022, 75, 102890 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1466856421002915.
- M. Baier, J. Foerster, U. Schnabel, D. Knorr, J. Ehlbeck and W. B. Herppich, et al., Direct non-thermal plasma treatment for the sanitation of fresh corn salad leaves: evaluation of physical and physiological effects and antimicrobial efficacy, Postharvest Biol. Technol., 2013, 84, 81–87 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0925521413001063.
- F. Grzegorzewski, J. Ehlbeck, O. Schlüter, L. W. Kroh and S. Rohn, Treating lamb's lettuce with a cold plasma – influence of atmospheric pressure Ar plasma immanent species on the phenolic profile of valerianella locusta, LWT--Food Sci. Technol., 2011, 44(10), 2285–2289 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S002364381100140X.
- F. Grzegorzewski, S. Rohn, L. W. Kroh, M. Geyer and O. Schlüter, Surface morphology and chemical composition of lamb's lettuce (Valerianella locusta) after exposure to a low-pressure oxygen plasma, Food Chem., 2010, 122(4), 1145–1152 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0308814610003985.
- N. N. Misra, T. Moiseev, S. Patil, S. K. Pankaj, P. Bourke and J. P. Mosnier, et al., Cold Plasma in Modified Atmospheres for Post-harvest Treatment of Strawberries, Food Bioprocess Technol., 2014, 7(10), 3045–3054 CrossRef CAS . Available from: https://link.springer.com/10.1007/s11947-014-1356-0.
- A. Limnaios, N. Pathak, G. Grossi Bovi, A. Fröhling, V. P. Valdramidis and P. S. Taoukis, et al., Effect of cold atmospheric pressure plasma processing on quality and shelf life of red currants, LWT--Food Sci. Technol., 2021, 151, 112213 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0023643821013669.
- G. G. Bovi, A. Fröhling, N. Pathak, V. P. Valdramidis and O. Schlüter, Safety control of whole berries by cold atmospheric pressure plasma processing: a review, J. Food Prot., 2019, 82(7), 1233–1243 CrossRef CAS PubMed . Available from: https://meridian.allenpress.com/jfp/article/82/7/1233/421004/Safety-Control-of-Whole-Berries-by-Cold.
- M. Baier, J. Ehlbeck, D. Knorr, W. B. Herppich and O. Schlüter, Impact of plasma processed air (PPA) on quality parameters of fresh produce, Postharvest Biol. Technol., 2015, 100, 120–126 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0925521414002609.
- C. Sarangapani, G. O'Toole, P. J. Cullen and P. Bourke, Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries, Innovative Food Sci. Emerging Technol., 2017, 44, 235–241 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1466856416305586.
- M. Ali, J.-H. Cheng and D.-W. Sun, Effect of plasma activated water and buffer solution on fungicide degradation from tomato (Solanum lycopersicum) fruit, Food Chem., 2021, 350, 129195 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0308814621001990.
- A. Soni, J. Choi and G. Brightwell, Plasma-Activated Water (PAW) as a Disinfection Technology for Bacterial Inactivation with a Focus on Fruit and Vegetables, Foods, 2021, 10(1), 166 CrossRef CAS PubMed . Available from: https://www.mdpi.com/2304-8158/10/1/166.
- C. Hertwig, A. Leslie, N. Meneses, K. Reineke, C. Rauh and O. Schlüter, Inactivation of Salmonella Enteritidis PT30 on the surface of unpeeled almonds by cold plasma, Innovative Food Sci. Emerging Technol., 2017, 44, 242–248 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1466856416306737.
- B. G. Dasan, I. H. Boyaci and M. Mutlu, Nonthermal plasma treatment of Aspergillus spp. spores on hazelnuts in an atmospheric pressure fluidized bed plasma system: Impact of process parameters and surveillance of the residual viability of spores, J. Food Eng., 2017, 196, 139–149 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S026087741630351X.
- M. Amini and M. Ghoranneviss, Effects of cold plasma treatment on antioxidants activity, phenolic contents and shelf life of fresh and dried walnut (Juglans regia L.) cultivars during storage, LWT--Food Sci. Technol., 2016, 73, 178–184 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0023643816303462.
- B. G. Dasan, M. Mutlu and I. H. Boyaci, Decontamination of Aspergillus flavus and Aspergillus parasiticus spores on hazelnuts via atmospheric pressure fluidized bed plasma reactor, Int. J. Food Microbiol., 2016, 216, 250–259 CrossRef PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168160515301197.
- B. A. Niemira, Cold Plasma Reduction of Salmonella and Escherichia coli O157:H7 on Almonds Using Ambient Pressure Gases, J. Food Sci., 2012, 77(3), M171–M175 CrossRef CAS PubMed . Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1750-3841.2011.02594.x.
- S. Deng, R. Ruan, C. K. Mok, G. Huang, X. Lin and P. Chen, Inactivation of Escherichia coli on Almonds Using Nonthermal Plasma, J. Food Sci., 2007, 72(2), M62–M66 CrossRef CAS PubMed . Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1750-3841.2007.00275.x.
- P. Basaran, N. Basaran-Akgul and L. Oksuz, Elimination of Aspergillus parasiticus from nut surface with low pressure cold plasma (LPCP) treatment, Food Microbiol., 2008, 25(4), 626–632 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0740002008000063.
- G. G. Gebremical, S. A. Emire and T. Berhanu, Effects of Multihollow Surface Dielectric Barrier Discharge Plasma on Chemical and Antioxidant Properties of Peanut, J. Food Qual., 2019, 2019, 1–10 CrossRef . Available from: https://www.hindawi.com/journals/jfq/2019/3702649/.
- H. Venkataratnam, C. Sarangapani, O. Cahill and C. B. Ryan, Effect of cold plasma treatment on the antigenicity of peanut allergen Ara h 1, Innovative Food Sci. Emerging Technol., 2019, 52, 368–375 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1466856418312128.
- I. Siciliano, D. Spadaro, A. Prelle, D. Vallauri, M. Cavallero and A. Garibaldi, et al., Use of Cold Atmospheric Plasma to Detoxify Hazelnuts from Aflatoxins, Toxins, 2016, 8(5), 125 CrossRef PubMed . Available from: https://www.mdpi.com/2072-6651/8/5/125.
- S. Mošovská, V. Medvecká, N. Halászová, P. Ďurina, Ľ. Valík and A. Mikulajová, et al., Cold atmospheric pressure ambient air plasma inhibition of pathogenic bacteria on the surface of black pepper, Food Res. Int., 2018, 106, 862–869 CrossRef PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0963996918300747.
- M. Amini, M. Ghoranneviss and S. Abdijadid, Effect of cold plasma on crocin esters and volatile compounds of saffron, Food Chem., 2017, 235, 290–293 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0308814617308580.
- J. E. Kim, H.-S. Choi, D.-U. Lee and S. C. Min, Effects of processing parameters on the inactivation of Bacillus cereus spores on red pepper (Capsicum annum L.) flakes by microwave-combined cold plasma treatment, Int. J. Food Microbiol., 2017, 263, 61–66 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168160517304026.
- J. E. Kim, Y. J. Oh, M. Y. Won, K.-S. Lee and S. C. Min, Microbial decontamination of onion powder using microwave-powered cold plasma treatments, Food Microbiol., 2017, 62, 112–123 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0740002015301908.
- M. Y. Won, H. Y. Choi, K. S. Lee and S. C. Min, Helium dielectric barrier discharge-cold plasma treatment for microbiological safety and preservation of onion powder, Korean J. Food Sci. Technol., 2016, 48(5), 486–491 CrossRef . Available from: https://koreascience.or.kr/journal/view.jsp?kj=SPGHB5%26py=2016%26vnc=v48n5%26sp=486.
- Y. Takemura, S. Umeji, K. Ito, S. Furuya and M. Furuta, Inactivation Treatment of Bacterial Spores Contaminated Spices by Atmospheric Plasma Jet, Plasma Medical, 2014, 4(1–4), 89–100 CrossRef . Available from: https://www.dl.begellhouse.com/journals/5a5b4a3d419387fb,700f28e67d84b510,6ebdef2642136c8d.html.
- S. Sun, N. M. Anderson and S. Keller, Atmospheric Pressure Plasma Treatment of Black Peppercorns Inoculated with Salmonella and Held Under Controlled Storage, J. Food Sci., 2014, 79(12), E2441–E2446 CrossRef CAS PubMed . Available from: https://onlinelibrary.wiley.com/doi/10.1111/1750-3841.12696.
- M. Grabowski, A. Strzelczak and W. Dąbrowski, Low Pressure Cold Plasma as an Alternative Method for Black Pepper Sterilization, J. Life Sci., 2014, 8, 931–939 CAS . Available from: https://www.academia.edu/download/46561076/Journal_of_Life_Sciences_2014.12.pdf#page=11.
- J. E. Kim, D.-U. Lee and S. C. Min, Microbial decontamination of red pepper powder by cold plasma, Food Microbiol., 2014, 38, 128–136 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0740002013001810.
- L. G. Carpen, C. Chireceanu, M. Teodorescu, A. Chiriloaie, A. Teodoru and G. Dinescu, The effect of argon/oxygen and argon/nitrogen atmospheric plasma jet on stored products pests, Rom. J. Phys., 2019, 64(503), 1–11 Search PubMed . Available from: https://rjp.nipne.ro/2019_64_3-4/RomJPhys.64.503.pdf.
- A. Los, D. Ziuzina, S. Akkermans, D. Boehm, P. J. Cullen and J. Van Impe, et al., Improving microbiological safety and quality characteristics of wheat and barley by high voltage atmospheric cold plasma closed processing, Food Res. Int., 2018, 106, 509–521 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0963996918300097.
- J. Durek, O. Schlüter, A. Roscher, P. Durek and A. Fröhling, Inhibition or Stimulation of Ochratoxin A Synthesis on Inoculated Barley Triggered by Diffuse Coplanar Surface Barrier Discharge Plasma, Front Microbiol, 2018, 9, 1–9 CrossRef PubMed . Available from: https://www.frontiersin.org/article/10.3389/fmicb.2018.02782/full.
- H. Tolouie, M. A. Mohammadifar, H. Ghomi and M. Hashemi, Cold atmospheric plasma manipulation of proteins in food systems, Crit. Rev. Food Sci. Nutr., 2018, 58(15), 2583–2597 CrossRef CAS PubMed . Available from: https://www.tandfonline.com/doi/full/10.1080/10408398.2017.1335689.
- D. Butscher, D. Zimmermann, M. Schuppler and P. Rudolf von Rohr, Plasma inactivation of bacterial endospores on wheat grains and polymeric model substrates in a dielectric barrier discharge, Food Control, 2016, 60, 636–645 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0956713515301821.
- L. Kordas, W. Pusz, T. Czapka and R. Kacprzyk, The effect of low-temperature plasma on fungus colonization of winter wheat grain and seed quality, Pol. J. Environ. Stud., 2015, 24(1), 433–438 CAS . Available from: https://www.pjoes.com/The-Effect-of-Low-Temperature-Plasma-r-non-Fungus-Colonization-of-Winter-Wheat-r,89435,0,2.html.
- D. Butscher, T. Schlup, C. Roth, N. Müller-Fischer, C. Gantenbein-Demarchi and P. Rudolf von Rohr, Inactivation of microorganisms on granular materials: reduction of Bacillus amyloliquefaciens endospores on wheat grains in a low pressure plasma circulating fluidized bed reactor, J. Food Eng., 2015, 159, 48–56 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0260877415001004.
- M. Selcuk, L. Oksuz and P. Basaran, Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment, Bioresour. Technol., 2008, 99(11), 5104–5109 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0960852407007894.
- M. Radhakrishnan, K. R. Ramanan, R. Sargunam and R. Sarumathi, Effect of cold plasma on mortality of Tribolium castaneum on refined wheat flour, Proc 10th Int Conf Control Atmos Fumigation Stored Prod (CAF 2016), 2016, pp. 7–11, available from: https://www.researchgate.net/publication/311370163 Search PubMed.
- R. Mahendran, K. Ratish Ramanan, R. Sargunam and R. Sarumathi, Effect of Cold Plasma on Mortality of Tribolium Castaneum on Maida Flour, Agric. Eng., 2016, 37–44 Search PubMed . Available from: https://arhiva.nara.ac.rs/handle/123456789/2038.
- S. Bußler, B. A. Rumpold, A. Fröhling, E. Jander, H. M. Rawel and O. K. Schlüter, Cold atmospheric pressure plasma processing of insect flour from Tenebrio molitor: impact on microbial load and quality attributes in comparison to dry heat treatment, Innovative Food Sci. Emerging Technol., 2016, 36, 277–286 CrossRef . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1466856416301345.
- S. Held, C. E. Tyl and G. A. Annor, Effect of Radio Frequency Cold Plasma Treatment on Intermediate Wheatgrass (Thinopyrum intermedium) Flour and Dough Properties in Comparison to Hard and Soft Wheat (Triticum aestivum L.), J. Food Qual., 2019, 1–8 CrossRef . Available from: https://www.hindawi.com/journals/jfq/2019/1085172/.
- R. Thirumdas, A. Trimukhe, R. R. Deshmukh and U. S. Annapure, Functional and rheological properties of cold plasma treated rice starch, Carbohydr. Polym., 2017, 157, 1723–1731 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0144861716313248.
- N. Bahrami, D. Bayliss, G. Chope, S. Penson, T. Perehinec and I. D. Fisk, Cold plasma: a new technology to modify wheat flour functionality, Food Chem., 2016, 202, 247–253 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0308814616301224.
- P. Pal, P. Kaur, N. Singh, A. Kaur, N. N. Misra and B. K. Tiwari, et al., Effect of nonthermal plasma on physico-chemical, amino acid composition, pasting and protein characteristics of short and long grain rice flour, Food Res. Int., 2016, 81, 50–57 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S096399691530291X.
- S. Bußler, V. Steins, J. Ehlbeck and O. Schlüter, Impact of thermal treatment versus cold atmospheric plasma processing on the techno-functional protein properties from Pisum sativum ‘Salamanca, J. Food Eng., 2015, 167, 166–174 CrossRef . Available from: https://linkinghub.elsevier.com/retrieve/pii/S026087741500254X.
- M. Menkovska, M. Mangova and K. Dimitrov, Effect of cold plasma on wheat flour and bread making quality, Macedonian Journal of Animal Science, 2014, 4(1), 27–30 CrossRef . Available from: https://www.mjas.ukim.edu.mk/files/MJAS-04-1-_2014_-183-Mangova.pdf.
- S. Tappi, L. Ragni, U. Tylewicz, S. Romani, I. Ramazzina and P. Rocculi, Browning response of fresh-cut apples of different cultivars to cold gas plasma treatment, Innovative Food Sci. Emerging Technol., 2019, 53, 56–62 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1466856417306045.
- S. Tappi, A. Berardinelli, L. Ragni, M. Dalla Rosa, A. Guarnieri and P. Rocculi, Atmospheric gas plasma treatment of fresh-cut apples, Innovative Food Sci. Emerging Technol., 2014, 21, 114–122 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1466856413001501.
- I. Ramazzina, S. Tappi, P. Rocculi, G. Sacchetti, A. Berardinelli and A. Marseglia, et al., Effect of Cold Plasma Treatment on the Functional Properties of Fresh-Cut Apples, J. Agric. Food Chem., 2016, 64(42), 8010–80118 CrossRef CAS PubMed . Available from: https://pubs.acs.org/doi/10.1021/acs.jafc.6b02730.
- X. Liao, J. Li, A. I. Muhammad, Y. Suo, S. Chen and X. Ye, et al., Application of a Dielectric Barrier Discharge Atmospheric Cold Plasma (Dbd-Acp) for Eshcerichia Coli Inactivation in Apple Juice, J. Food Sci., 2018, 83(2), 401–408 CrossRef CAS PubMed . Available from: https://onlinelibrary.wiley.com/doi/10.1111/1750-3841.14045.
- B. Surowsky, A. Fröhling, N. Gottschalk, O. Schlüter and D. Knorr, Impact of cold plasma on Citrobacter freundii in apple juice: Inactivation kinetics and mechanisms, Int. J. Food Microbiol., 2014, 174, 63–71 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168160514000026.
- B. G. Dasan and I. H. Boyaci, Effect of Cold Atmospheric Plasma on Inactivation of Escherichia coli and Physicochemical Properties of Apple, Orange, Tomato Juices, and Sour Cherry Nectar, Food Bioprocess Technol., 2018, 11(2), 334–343 CrossRef CAS . Available from: https://link.springer.com/10.1007/s11947-017-2014-0.
- S. K. Pankaj, Z. Wan, W. Colonna and K. M. Keener, Effect of high voltage atmospheric cold plasma on white grape juice quality, J. Sci. Food Agric., 2017, 97(12), 4016–4021 CrossRef CAS PubMed . Available from: https://onlinelibrary.wiley.com/doi/10.1002/jsfa.8268.
- T.-Y. Wu, N.-N. Sun and C.-F. Chau, Application of corona electrical discharge plasma on modifying the physicochemical properties of banana starch indigenous to Taiwan, J. Food Drug Anal., 2018, 26(1), 244–251 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1021949817300807.
- N. Matan, M. Nisoa, N. Matan and T. Aewsiri, Effect of cold atmospheric plasma on antifungal activities of clove oil and eugenol against molds on areca palm (Areca catechu) leaf sheath, Int. Biodeterior. Biodegrad., 2014, 86, 196–201 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0964830513003417.
- C. H. Pragna, T. K. Ranjitha Gracy, R. Mahendran and C. Anandharamakrishnan, Effects of Microwave and Cold Plasma Assisted Hydrodistillation on Lemon Peel Oil Extraction, Int. J. Food Eng., 2019, 15(10), 1–10 Search PubMed . Available from: https://www.degruyter.com/document/doi/10.1515/ijfe-2019-0093/html.
- M. Ebadi, S. Abbasi, A. Harouni and F. Sefidkon, Effect of cold plasma on essential oil content and composition of lemon verbena, Food Sci. Nutr., 2019, 7(4), 1166–1171 CrossRef CAS PubMed . Available from: https://onlinelibrary.wiley.com/doi/10.1002/fsn3.876.
- S. Kodama, B. Thawatchaipracha and H. Sekiguchi, Enhancement of Essential Oil Extraction for Steam Distillation by DBD Surface Treatment, Plasma Processes Polym., 2014, 11(2), 126–132 CrossRef CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1002/ppap.201300047.
- V. Hemmati, F. Garavand, M. Goudarzi, Z. Sarlak, I. Cacciotti and B. K. Tiwari, Cold atmospheric‐pressure plasma treatment of turmeric powder: microbial load, essential oil profile, bioactivity and microstructure analyses, Int. J. Food Sci. Technol., 2021, 56(5), 2224–2232 CrossRef CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1111/ijfs.14838.
- N. B. Rathod, S. P. Kahar, R. C. Ranveer and U. S. Annapure, Cold plasma an emerging nonthermal technology for milk and milk products: a review, Int. J. Dairy Technol., 2021, 74(4), 615–626 CrossRef CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1111/1471-0307.12771.
- D. Manoharan, J. Stephen and M. Radhakrishnan, Study on low-pressure plasma system for continuous decontamination of milk and its quality evaluation, J. Food Process. Preserv., 2021, 45(2), e15138 CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1111/jfpp.15138.
- S. B. Ponraj, J. Sharp, J. R. Kanwar, A. J. Sinclair, L. Kviz, K. R. Nicholas, et al., Sterilization of cow's milk using liquid plasma, 22nd Int. Symp. Plasma Chem, 2015, pp. 5–7. available from: https://www.ispc-conference.org/ispcproc/ispc22/P-III-10-25.pdf Search PubMed.
- X. Wu, Y. Luo, F. Zhao, M. Safian Murad and G. Mu, Influence of dielectric barrier discharge cold plasma on physicochemical property of milk for sterilization, Plasma Processes Polym., 2021, 18(1), 1900219 CrossRef CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1002/ppap.201900219.
- D. Manoharan, J. Stephen and M. Radhakrishnan, Study on the effect of atmospheric and low‐pressure plasma and its combination on the microbial reduction and quality of milk, J. Food Saf., 2022, e13018 Search PubMed ; Available from: https://onlinelibrary.wiley.com/doi/10.1111/jfs.13018.
- N. B. Rathod, R. C. Ranveer, P. K. Bhagwat, F. Ozogul, S. Benjakul and S. Pillai, et al., Cold plasma for the preservation of aquatic food products: an overview, Compr. Rev. Food Sci. Food Saf., 2021, 20(5), 4407–4425 CrossRef CAS PubMed . Available from: https://onlinelibrary.wiley.com/doi/10.1111/1541-4337.12815.
- S. Wei, R. Chelliah, D. Oh and S. Liu, Applications of Cold Plasma, ed. Ding T., Cullen P. J. and Yan W., in Applications of Cold Plasma in Food Safety, Singapore, Springer Singapore, 2022, available from: https://link.springer.com/10.1007/978-981-16-1827-7 Search PubMed.
- C. dos Santos Rocha, M. Magnani, G. L. de Paiva Anciens Ramos, F. F. Bezerril, M. Q. Freitas and A. G. Cruz, et al., Emerging technologies in food processing: impacts on sensory characteristics and consumer perception, Curr. Opin. Food Sci., 2022, 47, 100892 CrossRef . Available from: https://linkinghub.elsevier.com/retrieve/pii/S2214799322000947.
- R. Anbarasan, D. Gomez Carmona and R. Mahendran, Human Taste-Perception: Brain Computer Interface (BCI) and Its Application as an Engineering Tool for Taste-Driven Sensory Studies, Food Eng. Rev., 2022, 408–434 CrossRef . Available from: https://link.springer.com/10.1007/s12393-022-09308-0.
- N. M. Coutinho, M. R. Silveira, J. T. Guimarães, L. M. Fernandes, T. C. Pimentel and M. C. Silva, et al., Are consumers willing to pay for a product processed by emerging technologies? The case of chocolate milk drink processed by cold plasma, LWT--Food Sci. Technol., 2021, 138, 110772 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0023643820317618.
- J. Ferrell, T.-C. Tsai, S. Kalghatgi, J. S. Louis and R. L. Gray, Plasma Activated Water for an enhanced soil-free horticulture, WO2017/049263Al, 2017, https://patents.google.com/patent/WO2017049263A1/en.
- S. W. Noh, J. S. Park, S. J. Kim, D.-W. Kim and W. S. Kang, Effect of Plasma-activated Water Process on the Growth and Functional Substance Content of Lettuce during the Cultivation Period in a Deep Flow Technique System, Protected horticulture and Plant Factory, 2020, 29(4), 464–472 CrossRef . Available from: https://www.ksbec.org/articles/doi/10.12791/KSBEC.2020.29.4.464.
- V. Stoleru, R. Burlica, G. Mihalache, D. Dirlau, S. Padureanu and G.-C. Teliban, et al., Plant growth promotion effect of plasma activated water on Lactuca sativa L. cultivated in two different volumes of substrate, Sci. Rep., 2020, 10(1), 20920 CrossRef CAS PubMed . Available from: https://www.nature.com/articles/s41598-020-77355-w.
- P. Lamichhane, M. Veerana, J. S. Lim, S. Mumtaz, B. Shrestha and N. K. Kaushik, et al., Low-Temperature Plasma-Assisted Nitrogen Fixation for Corn Plant Growth and Development, Int. J. Mol. Sci., 2021, 22(10), 5360 CrossRef CAS PubMed . Available from: https://www.mdpi.com/1422-0067/22/10/5360.
- T.-C. Wang, S.-Y. Hsu, Y.-T. Lai and J.-G. Duh, Improving the Growth Rate of Lettuce Sativa Young Plants via Plasma-Activated Water Generated by Multitubular Dielectric Barrier Discharge Cold Plasma System, IEEE Trans. Plasma Sci., 2022, 50(7), 2104–2109 CAS . Available from: https://ieeexplore.ieee.org/document/9797287/.
- D. Pańka, M. Jeske, A. Łukanowski, A. Baturo-Cieśniewska, P. Prus and M. Maitah, et al., Can Cold Plasma Be Used for Boosting Plant Growth and Plant Protection in Sustainable Plant Production?, Agronomy, 2022, 12(4), 841 CrossRef . Available from: https://www.mdpi.com/2073-4395/12/4/841.
- K. Matra, Y. Tanakaran, V. Luang-In and S. Theepharaksapan, Enhancement of Lettuce Growth by PAW Spray Gliding Arc Plasma Generator, IEEE Trans. Plasma Sci., 2022, 50(6), 1430–1439 CAS . Available from: https://ieeexplore.ieee.org/document/9524451/.
- P. S. G. Subramanian, J. Ananthanarasimhan, P. Leelesh, H. Rao, A. M. Shivapuji and P.-L. Girard-Lauriault, et al., Plasma-activated water from DBD as a source of nitrogen for agriculture: specific energy and stability studies, J. Appl. Phys., 2021, 129(9), 093303 CrossRef . Available from: https://aip.scitation.org/doi/10.1063/5.0039253.
- K. Ratish Ramanan and R. Mahendran, Morphogenesis and characterization of wheat xerogel structure and insights into its 4D transformation, Food Struct., 2021, 28, 100170 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213329120300344.
- B. Boopathy, A. Rajan, J. Stephen and M. Radhakrishnan, Development and characterisation of structurally reforming engineered flat‐rice xerogel for hot water cooking, Int. J. Food Sci. Technol., 2022 Search PubMed ; Available from:https://onlinelibrary.wiley.com/doi/10.1111/ijfs.16128.
- B. Boopathy, J. Stephen, A. Rajan and M. Radhakrishnan, Evaluation of temperature and concentration on the development of rice hydrogel and 2D xerogel, J. Food Process. Preserv., 2021, 45(10), e15853 CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1111/jfpp.15853.
- J. Stephen, D. Manoharan, B. Boopathy, A. Rajan and M. Radhakrishnan, Investigation of hydrogel temperature and concentration on tapioca xerogel formation, J. Food Process Eng., 2021, 44(11), e13833 CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1111/jfpe.13833.
- F. Momeni and J. Ni, Laws of 4D Printing, Engineering, 2020, 6(9), 1035–1055 CrossRef . Available from: https://linkinghub.elsevier.com/retrieve/pii/S2095809920302101.
- S. Jaspin, R. Anbarasan, M. Dharini and R. Mahendran, Structural analysis of tapioca xerogel and its water and oil triggered shape change, Food Struct., 2021, 30, 100226 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213329121000502.
- S. Jaspin, R. Anbarasan, M. Dharini and R. Mahendran, Morphological analysis of corn xerogel and its shape shifting in water, J. Food Eng., 2022, 330, 111107 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0260877422001613.
- V. Gupta, T. K. Ranjitha Gracy, J. Stephen and M. Radhakrishnan, Cold plasma-assisted shape-shifting of a flat two-dimensional wheat xerogel and its morphological behavior, J. Food Process Eng., 2020, 43(9), e13456 CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1111/jfpe.13456.
- J. Stephen, D. Manoharan and M. Radhakrishnan, Corn morphlour hydrogel to xerogel formation and its oleomorphic shape-shifting, J. Food Eng., 2021, 292, 110360 CrossRef CAS . Available from: https://doi.org/10.1016/j.jfoodeng.2020.110360.
- S. Cheeyattil, A. Rajan, J. Stephen and M. Radhakrishnan, Study on the optimization of barley flour xerogel and its programed oleomorphic 3D shape-shifting, J. Food Process Eng., 2022, e14197 Search PubMed ; https://onlinelibrary.wiley.com/doi/10.1111/jfpe.14197.
- K. Ulucan-Altuntas, M. Saleem, G. Tomei, E. Marotta and C. Paradisi, Atmospheric plasma-based approaches for the degradation of dimethyl phthalate (DMP) in water, J. Environ. Manage., 2022, 301, 113885 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0301479721019472.
- C. Sarangapani, N. N. Misra, V. Milosavljevic, P. Bourke, F. O'Regan and P. J. Cullen, Pesticide degradation in water using atmospheric air cold plasma, J. Water Process. Eng., 2016, 9, 225–232 CrossRef . Available from: https://linkinghub.elsevier.com/retrieve/pii/S221471441630006X.
- M. Magureanu, D. Piroi, F. Gherendi, N. B. Mandache and V. Parvulescu, Decomposition of Methylene Blue in Water by Corona Discharges, Plasma Chem. Plasma Process., 2008, 28(6), 677–688 CrossRef CAS . Available from: https://link.springer.com/10.1007/s11090-008-9155-x.
- N. Jiang, Y. Qu, Z. Yu, B. Peng, J. Li and K. Shang, et al., p-Nitrophenol contaminated soil remediation in a spray-type coaxial cylindrical dielectric barrier discharge plasma system, Environ. Sci. Pollut. Res., 2022, 29(38), 58110–58120 CrossRef CAS PubMed . Available from: https://link.springer.com/10.1007/s11356-022-19912-6.
- M. Redolfi, C. Makhloufi, S. Ognier and S. Cavadias, Short communication: oxidation of kerosene components in a soil matrix by a dielectric barrier discharge reactor, Process Saf. Environ. Prot., 2010, 88(3), 207–212 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0957582010000091.
- H. Hashizume, H. Kitano, H. Mizuno, A. Abe, G. Yuasa and S. Tohno, et al., Improvement of yield and grain quality by periodic cold plasma treatment with rice plants in a paddy field, Plasma Processes Polym., 2021, 18(1), 2000181 CrossRef CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1002/ppap.202000181.
- Ľ. Holubová, R. Švubová, Ľ. Slováková, B. Bokor, V. Chobotová Kročková and J. Renčko, et al., Cold Atmospheric Pressure Plasma Treatment of Maize Grains—Induction of Growth, Enzyme Activities and Heat Shock Proteins, Int. J. Mol. Sci., 2021, 22(16), 8509 CrossRef PubMed . Available from: https://www.mdpi.com/1422-0067/22/16/8509.
- J. Mravlje, M. Regvar, P. Starič, M. Mozetič and K. Vogel-Mikuš, Cold Plasma Affects Germination and Fungal Community Structure of Buckwheat Seeds, Plants, 2021, 10(5), 851 CrossRef CAS PubMed . Available from: https://www.mdpi.com/2223-7747/10/5/851.
- R. P. Guragain, H. B. Baniya, S. P. Pradhan, S. Dhungana, G. K. Chhetri and B. Sedhai, et al., Impact of non-thermal plasma treatment on the seed germination and seedling development of carrot (Daucus carota sativus L.), J. Phys. Commun., 2021, 5(12), 125011 CrossRef CAS . Available from: https://iopscience.iop.org/article/10.1088/2399-6528/ac4081.
- A. Sudarsan and K. Keener, Inactivation of spoilage organisms on baby spinach leaves using high voltage atmospheric cold plasma (HVACP) and assessment of quality, Innovative Food Sci. Emerging Technol., 2022, 79, 103023 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1466856422001084.
- N. Dawood, Surface modification of date palm leaves by cold plasma treatment, J. King Saud Univ., Sci., 2021, 33(5), 101465 CrossRef . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1018364721001269.
- M. Keshavarzi, G. Najafi, H. Ahmadi Gavlighi, P. Seyfi and H. Ghomi, Enhancement of polyphenolic content extraction rate with maximal antioxidant activity from green tea leaves by cold plasma, J. Food Sci., 2020, 85(10), 3415–3422 CrossRef CAS PubMed . Available from: https://onlinelibrary.wiley.com/doi/10.1111/1750-3841.15448.
- M. Ahmadnia, M. Sadeghi, R. Abbaszadeh and H. R. Ghomi Marzdashti, Decontamination of whole strawberry via dielectric barrier discharge cold plasma and effects on quality attributes, J. Food Process. Preserv., 2021, 45(1), e15019 CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1111/jfpp.15019.
- Y. Wang, Z. Ye, J. Li, Y. Zhang, Y. Guo and J.-H. Cheng, Effects of dielectric barrier discharge cold plasma on the activity, structure and conformation of horseradish peroxidase (HRP) and on the activity of litchi peroxidase (POD), LWT--Food Sci. Technol., 2021, 141, 111078 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0023643821002310.
- Y. Ji, W. Hu, J. Liao, A. Jiang, Z. Xiu and S. Gaowa, et al., Effect of atmospheric cold plasma treatment on antioxidant activities and reactive oxygen species production in postharvest blueberries during storage, J. Sci. Food Agric., 2020, 100(15), 5586–5595 CrossRef CAS PubMed . Available from: https://onlinelibrary.wiley.com/doi/10.1002/jsfa.10611.
- T. Sandanuwan, D. Attygalle, S. Amarasinghe, S. C. Weragoda, B. Ranaweera, K. Rathnayake, et al., Shelf Life Extension of Cavendish Banana Fruit Using Cold Plasma Treatment, in 2020 Moratuwa Engineering Research Conference (MERCon), IEEE, 2020, p. 182–186, available from: https://ieeexplore.ieee.org/document/9185237/ Search PubMed.
- C.-M. Lin, A. K. Patel, Y.-C. Chiu, C.-Y. Hou, C.-H. Kuo and C.-D. Dong, et al., The application of novel rotary plasma jets to inhibit the aflatoxin-producing Aspergillus flavus and the spoilage fungus, Aspergillus niger on peanuts, Innovative Food Sci. Emerging Technol., 2022, 78, 102994 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1466856422000790.
- M. Makari, M. Hojjati, S. Shahbazi and H. Askari, Elimination of Aspergillus flavus from Pistachio Nuts with Dielectric Barrier Discharge (DBD) Cold Plasma and Its Impacts on Biochemical Indices, J. Food Qual., 2021, 1–12 CrossRef . Available from: https://www.hindawi.com/journals/jfq/2021/9968711/.
- Z. Esmaeili, B. Hosseinzadeh Samani, A. Nemati, F. Nazari and S. Rostami, Development of novel green pesticide system by using cold plasma to control Plodia interpunctella in pistachio, J. Food Process. Preserv., 2021, 45(7), e15621 CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1111/jfpp.15621.
- M. Ahangari, Y. Ramezan and M. R. Khani, Effect of low pressure cold plasma treatment on microbial decontamination and physicochemical properties of dried walnut kernels (Juglans regia L.), J. Food Process Eng., 2021, 44(1), e13593 CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1111/jfpe.13593.
- E. G. Alves Filho, L. M. A. Silva, F. Oiram Filho, S. Rodrigues, F. A. N. Fernandes and M. I. Gallão, et al., Cold plasma processing effect on cashew nuts composition and allergenicity, Food Res. Int., 2019, 125, 108621 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0963996919304995.
- K. Shirani, F. Shahidi and S. A. Mortazavi, Investigation of decontamination effect of argon cold plasma on physicochemical and sensory properties of almond slices, Int. J. Food Microbiol., 2020, 335, 108892 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S016816052030386X.
- A. I. Muhammad, Y. Li, X. Liao, D. Liu, X. Ye and S. Chen, et al., Effect of dielectric barrier discharge plasma on background microflora and physicochemical properties of tiger nut milk, Food Control, 2019, 96, 119–127 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0956713518304638.
- H. Darvish, Y. Ramezan, M. R. Khani and A. Kamkari, Effect of low‐pressure cold plasma processing on decontamination and quality attributes of Saffron (Crocus sativus L.), Food Sci. Nutr., 2022, 10(6), 2082–2090 CrossRef CAS PubMed . Available from: https://onlinelibrary.wiley.com/doi/10.1002/fsn3.2824.
- G. De Silva, S. Amarasena, N. Amunugoda, S. Gunawardena and A. De Alwis, Effect of Low-Pressure Cold Plasma Treatment on Microbiological and Physicochemical Properties of Black Peppercorns, in 2021 From Innovation To Impact (FITI), IEEE, 2021, p. 1–6, available from: https://ieeexplore.ieee.org/document/9833050/ Search PubMed.
- X. Chen, Y. Qiu, J. Zhang, Y. Guo, Y. Ding and F. Lyu, Degradation efficiency and products of deoxynivalenol treated by cold plasma and its application in wheat, Food Control, 2022, 136, 108874 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0956713522000676.
- F. L. Pathan, R. R. Deshmukh and U. S. Annapure, Potential of cold plasma to control Callosobruchus chinensis (Chrysomelidae:Bruchinae) in chickpea cultivars during four year storage, Sci. Rep., 2021, 11(1), 13425 CrossRef CAS PubMed . Available from: https://www.nature.com/articles/s41598-021-92792-x.
- E. Feizollahi and M. S. Roopesh, Degradation of Zearalenone by Atmospheric Cold Plasma: Effect of Selected Process and Product Factors, Food Bioprocess Technol., 2021, 14(11), 2107–2119 CrossRef CAS . Available from: https://link.springer.com/10.1007/s11947-021-02692-1.
- E. Feizollahi, B. Iqdiam, T. Vasanthan, M. S. Thilakarathna and M. S. Roopesh, Effects of Atmospheric-Pressure Cold Plasma Treatment on Deoxynivalenol Degradation, Quality Parameters, and Germination of Barley Grains, Appl. Sci., 2020, 10(10), 3530 CrossRef CAS . Available from: https://www.mdpi.com/2076-3417/10/10/3530.
- S. A. Sutar, R. Thirumdas, B. B. Chaudhari, R. R. Deshmukh and U. S. Annapure, Effect of cold plasma on insect infestation and keeping quality of stored wheat flour, J. Stored Prod. Res., 2021, 92, 101774 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022474X21000138.
- L. Zare, N. Mollakhalili-Meybodi, H. Fallahzadeh and M. Arab, Effect of atmospheric pressure cold plasma (ACP) treatment on the technological characteristics of quinoa flour, LWT--Food Sci. Technol., 2022, 155, 112898 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S002364382102051X.
- J. K Joy, R. G. T. Kalaivendan, G. Eazhumalai, S. P. Kahar and U. S. Annapure, Effect of pin-to-plate atmospheric cold plasma on jackfruit seed flour functionality modification, Innovative Food Sci. Emerging Technol., 2022, 78, 103009 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1466856422000947.
- S. Jaddu, R. C. Pradhan and M. Dwivedi, Effect of multipin atmospheric cold plasma discharge on functional properties of little millet (Panicum miliare) flour, Innovative Food Sci. Emerging Technol., 2022, 77, 102957 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S146685642200042X.
- D. Zhou, T. Li, K. Cong, A. Suo and C. Wu, Influence of cold plasma on quality attributes and aroma compounds in fresh-cut cantaloupe during low temperature storage, LWT--Food Sci. Technol., 2022, 154, 112893 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0023643821020466.
- A. Khoshkalam Pour, S. Khorram, A. Ehsani, A. Ostadrahimi and Z. Ghasempour, Atmospheric cold plasma effect on quality attributes of banana slices: Its potential use in blanching process, Innovative Food Sci. Emerging Technol., 2022, 76, 102945 CrossRef . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1466856422000303.
- N. Kumar Mahnot, L.-P. Siyu, Z. Wan, K. M. Keener and N. N. Misra, In-package cold plasma decontamination of fresh-cut carrots: microbial and quality aspects, J. Phys. D: Appl. Phys., 2020, 53(15), 154002 CrossRef . Available from: https://iopscience.iop.org/article/10.1088/1361-6463/ab6cd3.
- X. Li, M. Li, N. Ji, P. Jin, J. Zhang and Y. Zheng, et al., Cold plasma treatment induces phenolic accumulation and enhances antioxidant activity in fresh-cut pitaya (Hylocereus undatus) fruit, LWT--Food Sci. Technol., 2019, 115, 108447 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0023643819307893.
- C. Chen, C. Liu, A. Jiang, Q. Guan, X. Sun and S. Liu, et al., The Effects of Cold Plasma-Activated Water Treatment on the Microbial Growth and Antioxidant Properties of Fresh-Cut Pears, Food Bioprocess Technol., 2019, 12(11), 1842–1851 CrossRef CAS . Available from: https://link.springer.com/10.1007/s11947-019-02331-w.
- T. R. B. Farias, S. Rodrigues and F. A. N. Fernandes, Comparative study of two cold plasma technologies on apple juice antioxidant capacity, phenolic contents, and enzymatic activity, J. Food Process. Preserv., 2022, 46(10), e16871 CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1111/jfpp.16871.
- K. F. Leite, T. V. Fonteles, B. A. R. Miguel, G. Silvestre da Silva, E. Sousa de Brito and E. G. Alves Filho, et al., Atmospheric cold plasma frequency imparts changes on cashew apple juice composition and improves vitamin C bioaccessibility, Food Res. Int., 2021, 147, 110479 CrossRef PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0963996921003781.
- D. R. G. de Castro, J. M. Mar, L. S. da Silva, K. A. da Silva, E. A. Sanches and J. de Araújo Bezerra, et al., Dielectric barrier atmospheric cold plasma applied on camu-camu juice processing: effect of the excitation frequency, Food Res. Int., 2020, 131, 109044 CrossRef PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0963996920300697.
- Z. Liu, W. Zhao, Q. Zhang, G. Gao and Y. Meng, Effect of cold plasma treatment on sterilizing rate and quality of kiwi turbid juice, J. Food Process Eng., 2021, 44(6), e13711 CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1111/jfpe.13711.
- L. M. N. Paixão, T. V. Fonteles, V. S. Oliveira, F. A. N. Fernandes and S. Rodrigues, Cold Plasma Effects on Functional Compounds of Siriguela Juice, Food Bioprocess Technol., 2019, 12(1), 110–121 CrossRef . Available from: https://link.springer.com/10.1007/s11947-018-2197-z.
- Y. Hou, R. Wang, Z. Gan, T. Shao, X. Zhang and M. He, et al., Effect of cold plasma on blueberry juice quality, Food Chem., 2019, 290, 79–86 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0308814619306065.
- F. Jangi, M.-T. Ebadi and M. Ayyari, Qualitative changes in hyssop (Hyssopus officinalis L.) as affected by cold plasma, packaging method and storage duration, J. Appl. Res. Med. Aromat. Plants, 2021, 22, 100289 CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S2214786120300504.
- S. Rezaei, M.-T. Ebadi, B. Ghobadian and H. Ghomi, Optimization of DBD-Plasma assisted hydro-distillation for essential oil extraction of fennel (Foeniculum vulgare Mill.) seed and spearmint (Mentha spicata L.) leaf, J. Appl. Res. Med. Aromat. Plants, 2021, 24, 100300 CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S2214786121000097.
| This journal is © The Royal Society of Chemistry 2023 |