Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unlocking the effect of Zn2+ on crystal structure, optical properties, and photocatalytic degradation of perfluoroalkyl substances (PFAS) of Bi2WO6†
Mirabbos
Hojamberdiev
 *a,
Ana Laura
Larralde
*a,
Ana Laura
Larralde
 bc,
Ronald
Vargas
bc,
Ronald
Vargas
 de,
Lorean
Madriz
de,
Lorean
Madriz
 de,
Kunio
Yubuta
f,
Lokesh Koodlur
Sannegowda
de,
Kunio
Yubuta
f,
Lokesh Koodlur
Sannegowda
 g,
Ilona
Sadok
g,
Ilona
Sadok
 h,
Agnieszka
Krzyszczak-Turczyn
hi,
Patryk
Oleszczuk
i and
Bożena
Czech
*i
h,
Agnieszka
Krzyszczak-Turczyn
hi,
Patryk
Oleszczuk
i and
Bożena
Czech
*i
aInstitut für Chemie, Technische Universität Berlin, Straße des 17. Juni 135, 10623 Berlin, Germany. E-mail: hmirabbos@gmail.com
bConsejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Buenos Aires, Argentina
cInstituto Nacional de Tecnología Industrial, Avenida General Paz 5445, San Martín (B1650WAB), Buenos Aires, Argentina
dInstituto Tecnológico de Chascomús (INTECH), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Avenida Intendente Marino, Km 8,2, Chascomús (B7130IWA), Provincia de Buenos Aires, Argentina
eEscuela de Bio y Nanotecnologías, Universidad Nacional de San Martín (UNSAM), Avenida Intendente Marino, Km 8,2, Chascomús (B7130IWA), Provincia de Buenos Aires, Argentina
fDepartment of Applied Quantum Physics and Nuclear Engineering, Kyushu University, Fukuoka 819-0395, Japan
gDepartment of Studies in Chemistry, Vijayanagara Sri Krishnadevaraya University, Cantonment, Vinayakanagara, Ballari, 583105, India
hDepartment of Chemistry, Institute of Biological Sciences, Faculty of Medicine, The John Paul II Catholic University of Lublin, Konstantynów 1J, 20-708 Lublin, Poland
iDepartment of Radiochemistry and Environmental Chemistry, Institute of Chemical Sciences, Faculty of Chemistry, Maria Curie-Skłodowska University in Lublin, 3 Maria Curie-Skłodowska Sq., 20-031 Lublin, Poland. E-mail: bozena.czech@mail.umcs.pl
First published on 6th September 2023
Abstract
Bismuth tungstate (Bi2WO6) with a layered structure and visible light response exhibits excellent photocatalytic activity. To enhance its photocatalytic activity for the degradation of perfluoroalkyl substances (PFAS), Zn2+ is partially substituted for Bi3+ in the Bi2WO6 lattice in this study. Particularly, the effect of Zn2+ content (0–22.5 at%) on the crystal structure, optical property, and photocatalytic activity for the photodegradation of PFAS of Bi2WO6 is investigated. According to the Le Bail fits, the unit-cell volume is slightly reduced from 487.7 Å3 to 480.8 Å3 by the partial substitution of smaller Zn2+ (0.74 Å for CN = 6) for larger Bi3+ (1.03 Å for CN = 6) in the Bi2WO6 crystal lattice, and the solubility of Zn2+ in the Bi2WO6 lattice is found to be below 17.5 at%. The partial substitution of Zn2+ influences the self-aggregation of nanoparticles, Ostwald ripening, and self-organization of nanoplates, resulting in different morphologies. Although the optical bandgap energy of Bi2WO6 is not significantly altered upon the partial substitution of Zn2+, the conduction and valence bands simultaneously shift upward. Among the Bi2−xZnxWO6+δ photocatalysts, 2.5 at% Zn2+-substituted Bi2WO6 exhibits larger water oxidation photocurrent density (0.316 mA cm−2 at 1.23 VRHE) and the highest photocatalytic activity for the photodegradation of PFHxA (k1 = 0.012 min−1). The trapping experiments confirm that the photo-excited holes (h+) and superoxide radicals (O2˙−) are the major reactive species involved in the photodegradation of PFHxA. Liquid chromatography quadrupole time-of-flight mass spectrometry (LC-QTOF/MS) reveals that decarboxylation and defluorination are the main possible routes for the photodegradation of PFHxA over Bi2−xZnxWO6+δ photocatalysts. Our findings suggest that the partial Zn2+-to-Bi2+ substitution can enhance the photocatalytic activity of Bi2WO6 for the degradation of PFAS.
Water impactThe contamination of water with per- and poly-fluoroalkyl substances (PFAS) leads to adverse health effects because of their toxicity, extreme persistency, high mobility, and accumulative nature. The conventional water treatment process is ineffective in removing PFAS from contaminated water. The partial substitution of Zn2+ for Bi3+ in the Bi2WO6 crystal lattice can enhance the photocatalytic removal of PFAS from the model and real wastewater. |
1. Introduction
Per- and poly-fluoroalkyl substances (PFAS) are a class of persistent, water-soluble synthetic organic compounds, including perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA).1–3 Particularly, perfluorooctane sulfonate (PFOS) has been designated as one of the target chemicals by the Stockholm Convention on Persistent Organic Pollutants (POPs). PFAS have also adverse health effects due to their toxicity, persistency, mobility, and accumulative nature.4 The United States Environmental Protection Agency (EPA) proposed maximum contaminant level goals (MCLG) of 4 ng L−1 and set a health recommendation of 70 ng L−1 for perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA).5 PFAS are specifically characterized by having a fully or partially fluorinated carbon chain. Their high electronegativity and small ionic radius give them an extremely strong C–F bond (up to 544 kJ mol−1) and high chemical and thermal stability, respectively.6 Therefore, the degradation of PFAS remains challenging because of this strong C–F bond, risks associated with the persistency and toxicity of intermediates and final products, long treatment time, high energy requirement, and high capital cost.7 For the elimination of PFAS, the conventional water treatment process has been found to be ineffective.8Perfluorohexanoic acid (PFHxA) is a short-chain, six-carbon perfluoroalkyl acid, and it is a principal contaminant, degradant, and metabolite linked to short-chain fluorotelomer-based compounds.9,10 PFHxA was detected in various water samples at concentrations reaching up to 1400 and 4000 ppb for drinking water and groundwater.10 Therefore, the European Chemical Agency (ECHA) suggested listing PFHxA as a “substance of very high concern” because of its extreme persistence, multiple sources, and high mobility in the aquatic environment.11 Various techniques, including adsorption,12 membrane filtration,13 anion exchange resin,14 foam fractionation,15 advanced oxidation processes,16etc., were applied for the removal of PFAS.
One of the methods applied for the removal of PFAS is physical adsorption.17 In addition to its slow sorption rate because of the sluggish diffusion of PFAS molecules, physical adsorption simply converts PFAS from the liquid phase to the solid phase without completely mineralizing them. Therefore, to avoid secondary pollution, various redox treatment processes, including electrochemical,18 photocatalytic,19 photolytic,20 photochemical,21 sonochemical,22 radiochemical,23 thermochemical,24 subcritical,25 and plasma,26 have been applied to completely mineralize PFAS compounds. Among the redox treatment processes, photocatalytic degradation is promising because of its operation under mind conditions and higher efficiency.27 The homogeneous photocatalytic degradation of PFAS is based on the photo-Fenton process. Tang et al.28 efficiently degraded PFAS by applying the photo-Fenton process and achieved more than 90% degradation and 53.2% defluorination after 5 h. Unlike P25-TiO2 with low efficiency for PFAS degradation due to the recombination of photoexcited charge carriers,29 In2O3 nanoporous nanospheres exhibited high and fast degradation efficiency towards PFAS (100% within 30 min) because of the presence of abundant oxygen vacancy defects (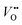 ).30 Having an open layer structure, BiOCl with oxygen vacancies also exhibited a high defluorination efficiency (59.3%) after 3 h.31 The sheaf-like β-Ga2O3 with nanoplates elongating in the [1 0 0] direction and a large number of nanopores exhibited a complete degradation of PFAS after 3 h of reaction.32 The photodegradation of PFAS tightly bound on the surfaces of BiOCl and β-Ga2O3 followed the hole-oxidation path. The limiting factor of the oxidation process was found to be the electronic structures and reactive site distribution on specific surfaces, and facet engineering was suggested to improve the removal efficiency of PFAS.33
).30 Having an open layer structure, BiOCl with oxygen vacancies also exhibited a high defluorination efficiency (59.3%) after 3 h.31 The sheaf-like β-Ga2O3 with nanoplates elongating in the [1 0 0] direction and a large number of nanopores exhibited a complete degradation of PFAS after 3 h of reaction.32 The photodegradation of PFAS tightly bound on the surfaces of BiOCl and β-Ga2O3 followed the hole-oxidation path. The limiting factor of the oxidation process was found to be the electronic structures and reactive site distribution on specific surfaces, and facet engineering was suggested to improve the removal efficiency of PFAS.33
As one of the simplest members of the Aurivillius family (Bi2An−1BnO3n+3), Bi2WO6 has an orthorhombic layer structure constructed from alternating [Bi2O2]n2n+ and perovskite-like [WO4]n2n− layers.34 In general, this layer structure not only favors the separation and transfer of photoexcited charge carriers due to the built-in electric field vertical to the layer direction but also reduces the surface trapping of photoexcited charge carriers.35 The valence band edge is more positively positioned,36 providing the sufficient potential to thermodynamically drive a hole-mediated oxidation reaction. By changing the synthesis parameters and doping, the morphology and exposed facets of Bi2WO6 can be easily tuned, resulting in a large number of oxygen vacancies that act as electron scavengers and binding sites for adsorbates.37
Due to its capability to couple the highest occupied dopant states into the valence band, reduce the band gap, hamper the formation of midgap states in the forbidden band, and distort the crystal structure, Koteski et al.38 and Ren et al.39 computationally investigated the effect of Zn substituted partially for Bi and W lattice sites on the optical and electronic properties of Bi2WO6 using density functional theory (DFT), respectively. The partial substitution of Zn for W could narrow the optical bandgap energy without the direct presence of the localized defect states and affect the mobility of photoexcited charge carriers.38 The partial substitution of Zn for Bi also led to band gap reduction due to the increase in the density of electrons and an upward shift of the conduction and valence band positions, revealing the possibility of the reaction of electrons with molecular oxygen to form active radicals.39 Inspired by these two theoretical studies,38,39 we aim to experimentally validate the effect of partial substitution of Zn for Bi on the crystal structure, optical properties, and photocatalytic activity for the degradation of perfluoroalkyl substances (PFAS) of Bi2WO6 in this study. The kinetics and mechanism of photodegradation of PFAS on Zn-substituted Bi2WO6 are also discussed.
2. Experimental
2.1. Synthesis
Pristine and Zn2+-substituted Bi2WO6 photocatalysts were synthesized by a hydrothermal method.40 For the synthesis of pristine Bi2WO6, Bi(NO3)3·5H2O (98%, Merck) was dissolved in 5 mL of ethylene glycol (>99%, Merck), while Na2WO4·2H2O (>99%, chemPUR) was dissolved in 5 mL of deionized water. For the synthesis of Zn2+-substituted Bi2WO6, both Bi(NO3)3·5H2O (98%, Merck) and Zn(CH3COO)2·2H2O (>98%, Merck) were simultaneously dissolved in 5 mL of ethylene glycol (>99%, Merck), while Na2WO4·2H2O (>99%, chemPUR) was dissolved in 5 mL of deionized water. Both solutions were then mixed under vigorous magnetic stirring, and the pH of the well-homogenized solution was adjusted to 7–9 by adding dropwise an aqueous solution of NaOH (98%, Alfa Aesar) and the mixture was transferred into a Teflon-lined stainless-steel autoclave (Parr Instrument GmbH). The hydrothermal reaction was carried out at 200 °C for 24 h. After the completion of the hydrothermal reaction, the resulting precipitate was washed and collected using a Universal 320 centrifuge (Andreas Hettich GmbH & Co. KG, 9000 rpm for 5 min), and dried at 80 °C for 12 h in a drying oven. The amount of Zn2+ substituted partially for Bi3+ in Bi2WO6 was controlled at 0, 1, 2.5, 7.5, 12.5, 17.5, and 22.5 at%. The synthesized powder samples were denoted as Zn0, Zn1, Zn2.5, Zn7.5, Zn12.5, Zn17.5, and Zn22.5 according to the content of substituted Zn2+.2.2. Characterization
The X-ray diffraction (XRD) patterns were acquired with a Panalytical X'Pert PRO diffractometer with Cu Kα radiation. The diffraction data were collected in a Bragg–Brentano geometry with a θ/θ-arrangement over an angular range of 2θ = 10–120° with a 0.026° step. Initial qualitative analysis of the XRD data was performed using the HighScore plus program (version 4.7) and compared to entries from the ICDD-PDF-2 powder pattern database. Le Bail fits were performed using the method implemented in the FULLPROF Suite41 in order to determine the lattice parameters and proper fit of the crystalline phases. The microstructures of the synthesized samples were examined using a Carl Zeiss GeminiSEM 500 NanoVP scanning electronic microscope (SEM). The bright-field and lattice images and selected-area electron diffraction (SAED) patterns were obtained using an EM-002B high-resolution transmission electron microscope (TOPCON) at an accelerating voltage of 200 kV. The UV-vis diffuse reflectance spectra were recorded on an Evolution 220 UV/vis spectrometer (Thermo Fisher Scientific).Photoelectrochemical tests were performed with a DropSens μSTAT200 potentiostat in 0.1 M Na2SO4 deoxygenated water solution (50 μL). Irradiation was provided with a solar light LED. The Bi2−xZnxWO6+δ photocatalysts were dip-coated on the carbon surface (0.13 cm2) of the working electrode of the commercial screen-printed electrode (DS110), following the procedure previously reported elsewhere.42,43 Linear scanning voltammetry (LSV) at 5 mV s−1 from 0.2 to 1.4 V (V vs. Ag–AgCl) and chronoamperometry (CA) at 1.4 V (vs. Ag–AgCl) for 1 h were conducted.
2.3. Photocatalytic activity tests
The photocatalytic activity of pristine and Zn2+-substituted Bi2WO6 samples was evaluated for the degradation of polyfluoroalkyl and perfluoroalkyl substances (PFAS). First, 0.4 mg L−1 of the synthesized sample was dispersed in an aqueous solution of PFHxA (5 mg L−1) in the photochemical reactor (0.7 L, Heraeus) in the dark to achieve adsorption–desorption equilibrium for 30 min. Then, a 150 W mercury lamp, which was vertically placed in the center of the photoreactor, with emitting radiation centered at 500–550 nm, an intensity of 7.31–7.53 mW cm−2, and a photon flux of 20.83 × 1019 m2 s−1 was turned on. During the photocatalytic reaction, an aliquot was collected at different times (−30, 0, 5, 15, 30, 45, 60, and 120 min), filtered, and analyzed by High-Performance Liquid Chromatography (1200 Series Gradient HPLC System, Agilent Technologies). The extracted ion chromatograms of PFHxA and 13C6-PFHxA are shown in Fig. S1.† The details of the instrumentation and analysis conditions of HPLC are given in the ESI.† The blank test conducted without any photocatalyst sample revealed that PFHxA was not decomposed under visible light irradiation as a stable long-chain PFAS decomposition product.44The ratio of the PFHxA concentrations before and after the photocatalytic reactions C/C0 was used to indicate the amount of PFHxA photocatalytically removed from the aqueous solution. The effects of various parameters, such as the initial pH of PFHxA-containing aqueous solution (controlled using 0.1 mol L−1 NaOH or 0.1 mol L−1 HCl), competing ions (Cl−, NO3−, and H2PO4− from respective sodium salts with a concentration of 5 × 10−3 mol L−1), and water matrix (distilled water – DW, tap water – TW, and treated wastewater – TWW) on the efficiency of the photocatalytic degradation of PFHxA over pristine and Zn2+-substituted Bi2WO6 photocatalysts were studied. TWW was collected from the municipal wastewater treatment plant “Hajdów” in Lublin, which is a mechanical-biological wastewater treatment facility with increased removal of biogenic compounds (nitrogen and phosphorus) from wastewater. TWW was characterized by significantly reduced biological (5.7 mg L−1) and chemical (35.1 mg L−1) oxygen demands, total suspended solids (up to 6.4 mg L−1), and total nitrogen (10.72 mg L−1) and phosphorous (0.27 mg L−1) contents. TW was characterized by total organic carbon (<3 mg L−1), chloride (35 mg L−1), nitrate (<2 μg L−1), and sulfate (41 mg L−1) contents, and pH of 7.2.
Toxicity towards marine bacteria (Aliivibrio fischeri) was also evaluated based on the inhibition of bioluminescence using the Microtox® test.45 The luminescence inhibition was determined after 5 min and 15 min of exposure of Aliivibrio fischeri to the water samples before and after the photocatalytic reaction according to the standard protocol (Microtox®, 1995) in a Microtox M500 analyzer with the Omni software. All obtained data were expressed with the standard deviation.
3. Results and discussion
3.1. Material characterization
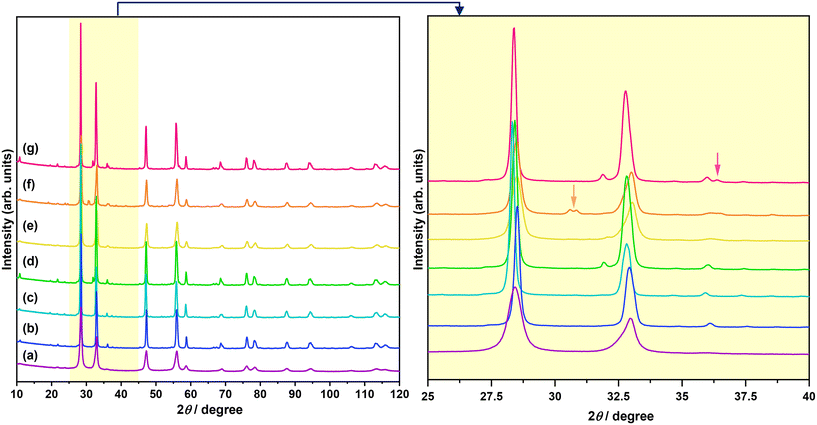
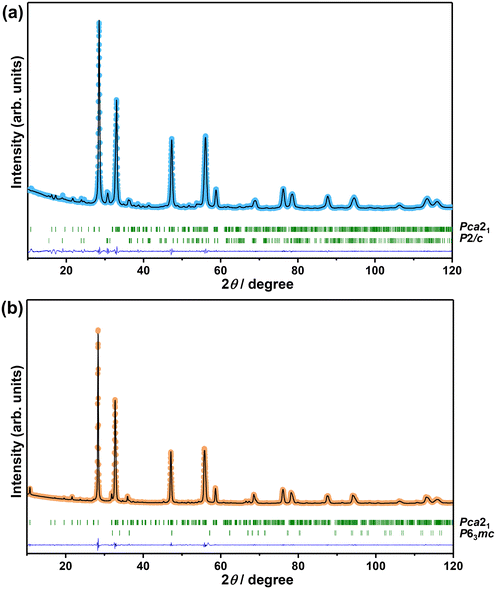
The XRD patterns of Bi2−xZnxWO6+δ photocatalysts with varying contents of Zn2+ substituent are shown in Fig. 1. The main reflections in the XRD patterns of Bi2−xZnxWO6+δ photocatalysts can be indexed to the orthorhombic Bi2WO6 phase with a space group of Pca21 and lattice parameters of a = 5.4370 Å, b = 16.4300 Å, and c = 5.4580 Å (ICSD# 98-006-7647). The Bi2−xZnxWO6+δ photocatalysts maintained the phase purity up to 12.5 at% Zn2+ (sample Zn12.5), and a further increase in the Zn2+ content affected the phase purity of the Bi2−xZnxWO6+δ photocatalysts. That is, additional reflections corresponding to the monoclinic ZnWO4 phase with a space group of P2/c and lattice parameters of a = 4.5160 Å, b = 5.5210 Å, and c = 4.7990 Å (ICSD# 98-016-2236) and the hexagonal ZnO phase with a space group of P63mc and lattice parameters of a = 3.2420 Å, b = 3.2420 Å, and c = 5.1880 Å (ICSD# 98-006-5119) appeared at 17.5 and 22.5 at% Zn2+ (samples Zn17.5 and Zn22.5), respectively. It can be stated that the solubility of Zn in the Bi2WO6 matrix is below 17.5 at%. Table 1 summarizes the results from the Le Bail fits and the agreement factors of the calculations. Profile matching of the Bi2−xZnxWO6+δ photocatalysts with 17.5 and 22.5 at% Zn2+ (samples Zn17.5 and Zn22.5) was performed using two different phases (Fig. 2a and b). The goodness of the agreement factors confirms a satisfactory fit of the ZnWO4 and ZnO phases. The calculated unit-cell volume decreased with increasing the Zn2+ content up to 12.5 at% (Fig. S2†). This is due to the fact that the ionic radius of Zn2+ (0.74 Å) is much smaller than that of Bi3+ (1.03 Å) in a six-fold coordination.46 Thus, the partial substitution of Zn2+ for Bi3+ in the crystal lattice of Bi2WO6 contracts the unit-cell volume. Interestingly, the unit-cell volume was slightly increased in the Bi2−xZnxWO6+δ photocatalysts containing 22.5 at% Zn2+. In the case of the Bi2−xZnxWO6+δ photocatalysts containing 17.5 and 22.5 at% Zn2+ (samples Zn17.5 and Zn22.5), the unit-cell volumes could not be properly estimated due to the simultaneous presence of Zn2+ in Bi2WO6 and minor impurity phases.| Sample | Zn0 | Zn1 | Zn2.5 | Zn7.5 | Zn12.5 | Zn17.5 | Zn22.5 | ||
|---|---|---|---|---|---|---|---|---|---|
| Crystalline phase | Bi2WO6 | Bi2WO6 | Bi2WO6 | Bi2WO6 | Bi2WO6 | Bi2WO6 | ZnWO4 | Bi2WO6 | ZnO |
| Space group | Pca21 | Pca21 | Pca21 | Pca21 | Pca21 | Pca21 | P2/c | Pca21 | P63mc |
| a (Å) | 5.4482 (7) | 5.4318 (2) | 5.4384 (2) | 5.4355 (2) | 5.4280 (4) | 5.4239 (3) | 4.684 (1) | 5.4354 (2) | 3.2255 (7) |
| b (Å) | 16.423 (2) | 16.4185 (5) | 16.4278 (6) | 16.4299 (5) | 16.426 (1) | 16.4090 (9) | 5.724 (1) | 16.4351 (6) | |
| c (Å) | 5.4507 (6) | 5.4473 (2) | 5.4551 (2) | 5.4511 (2) | 5.3924 (3) | 5.3829 (3) | 4.9318 (8) | 5.4549 (2) | 5.288 (3) |
| β (°) | — | — | — | — | — | — | 90.58 (2) | — | — |
| Volume (Å3) | 487.7 (1) | 485.81 (3) | 487.36 (3) | 486.81 (3) | 480.80 (5) | 479.08 (4) | 132.22 (5) | 485.56 (3) | 47.65 (3) |
| R p | 2.19 | 2.34 | 2.55 | 2.55 | 2.62 | 2.51 | 7.50 | 2.47 | 5.83 |
| R wp | 3.15 | 3.23 | 3.90 | 3.66 | 3.53 | 3.45 | 7.30 | 3.60 | 6.41 |
| R exp | 1.53 | 1.68 | 1.66 | 1.57 | 1.67 | 1.68 | 3.55 | 1.59 | 2.83 |
| χ 2 | 4.26 | 3.71 | 5.54 | 5.39 | 4.47 | 4.23 | 4.23 | 5.14 | 5.14 |
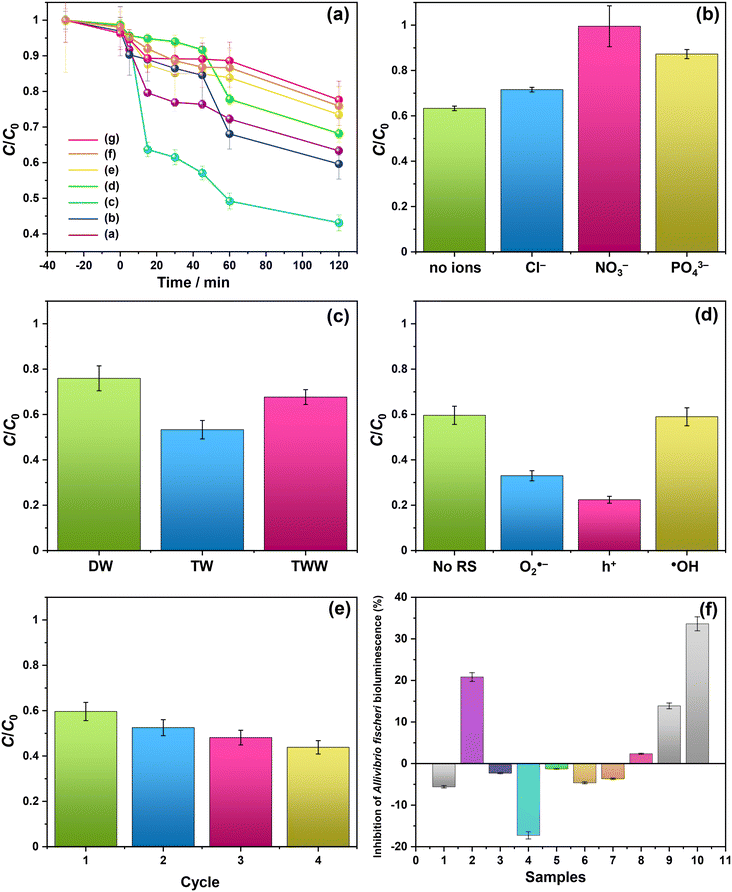
Fig. 3 shows the SEM images of Bi2−xZnxWO6+δ photocatalysts with varying contents of Zn2+ substituent. Unlike the Bi2WO6 particles with three-dimensional morphologies,47–49 pristine Bi2WO6 (sample Zn0) synthesized by a hydrothermal method in this study possesses a platelet morphology with an average diameter of <600 nm (Fig. 3a), which was possibly formed by the self-assembly of ultrathin nanosheets. At 1 at% Zn2+ substituent, the platelet morphology was altered, and large anisotropic nanoplates with irregular shapes were formed due to the dominance of the dissolution–recrystallization (Oswald ripening) process over self-assembly (Fig. 3b). According to a recent study by Iversen and co-workers,50 the preferential growth of Bi2WO6 nanoplates under hydrothermal conditions is governed by the initial presence of Bi2O22+ molecular complexes that interact with WO42− tetrahedra, forming disordered Bi0.933W0.067O1.6, and when there are sufficient WO42−units intertwined the Bi2WO6 nanoplates are eventually formed by the sideways addition of units in the ac plane, which has a three times faster growth rate than the b direction. Strikingly, flower-like microstructures were formed when the content of Zn2+ substituent was set to 2.5 at% (Fig. 3c). Clearly, 2.5 at% Zn2+ substituent balanced the self-aggregation of nanoparticles, Ostwald ripening, and self-organization of nanoplates with high anisotropic surface energy,51 resulting in flower-like microstructures. By adjusting the amount of Zn2+ substituent to 7.5, 12.5, and 17.5 at%, the governing roles of Oswald ripening and self-organization were subdued, forming some nanoplates along with nanoparticles (Fig. 3d–f). A further increase in the concentration of Zn2+ to 22.5 at% gave rise to the Ostwald ripening process but not to the self-organization process (Fig. 3g). It is evident that the partial substitution of Zn2+ for Bi3+ in the Bi2WO6 crystal lattice changed the content of Bi2O22+ molecular complexes interacting with WO42− tetrahedra, surface atomic structure, and surface free energy, which ultimately affected the particle morphology of Bi2−xZnxWO6+δ photocatalysts.
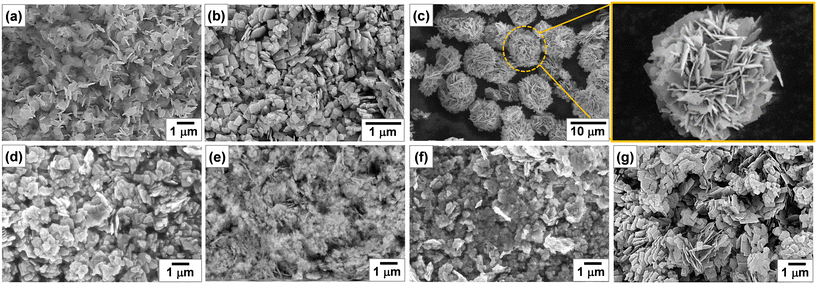 | ||
| Fig. 3 SEM images of Bi2−xZnxWO6+δ powders with varying contents of Zn2+ substituent: (a) Zn0, (b) Zn1, (c) Zn2.5, (d) Zn7.5, (e) Zn12.5, (f) Zn17.5, and (g) Zn22.5. | ||
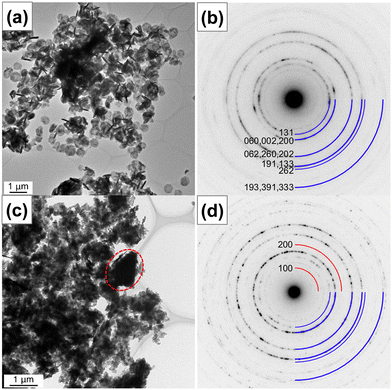
Further, the nanostructures were characterized by transmission electron microscopy. Fig. 4 shows the bright-field TEM and HRTEM images and SAED patterns of pristine (sample Zn0) and 17.5 at% Zn2+-substituted (sample Zn17.5) Bi2WO6 photocatalysts. In Fig. 4a, the low-magnification TEM image of sample Zn0 shows that pristine Bi2WO6 is in the form of platelets with a diameter of 580 nm and a thickness of about 75 nm. The high-magnification TEM image of sample Zn0 in Fig. S3a† confirms that the platelets were formed by the self-assembly of ultrathin nanosheets. The observed lattice fringes with a d-spacing value of 0.390 nm in Fig. S3b† manifest that the ultrathin nanosheets have exposed (1 3 0) facets along the [![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) 1 6] direction. No obvious defects are noticed in the examined area, suggesting the high crystallinity of ultrathin nanosheets. In the corresponding selected area electron diffraction pattern (Fig. 4b), a ring diffraction pattern can be seen due to the random orientation of crystallites. The observed Debye–Scherrer ring patterns are indexed to the different Miller indices of orthorhombic Bi2WO6. In Fig. 4c, the bright-field TEM image of sample Zn17.5 indicates the change in the nanostructure upon 17.5 at% Zn2+ substitution and the formation of large irregular particles, which are enclosed by a dotted oval, along with ultrathin nanosheets. In the corresponding selected area electron diffraction pattern in Fig. 4d, the Debye–Scherrer ring patterns are indexed to the different Miller indices of orthorhombic Bi2WO6 and monoclinic ZnWO4, which is consistent with the XRD result.
1 6] direction. No obvious defects are noticed in the examined area, suggesting the high crystallinity of ultrathin nanosheets. In the corresponding selected area electron diffraction pattern (Fig. 4b), a ring diffraction pattern can be seen due to the random orientation of crystallites. The observed Debye–Scherrer ring patterns are indexed to the different Miller indices of orthorhombic Bi2WO6. In Fig. 4c, the bright-field TEM image of sample Zn17.5 indicates the change in the nanostructure upon 17.5 at% Zn2+ substitution and the formation of large irregular particles, which are enclosed by a dotted oval, along with ultrathin nanosheets. In the corresponding selected area electron diffraction pattern in Fig. 4d, the Debye–Scherrer ring patterns are indexed to the different Miller indices of orthorhombic Bi2WO6 and monoclinic ZnWO4, which is consistent with the XRD result.
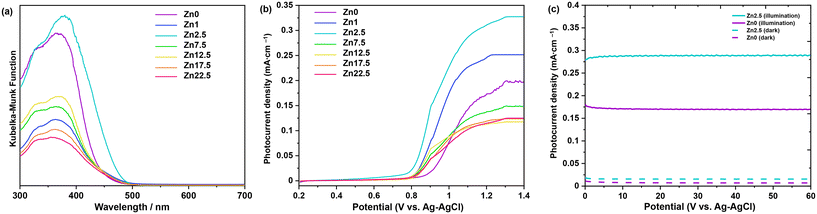
The optical properties of the synthesized samples were analyzed by UV-vis spectroscopy. Fig. 5a shows the UV-vis diffuse reflectance spectra of Bi2−xZnxWO6+δ photocatalysts with varying contents of Zn2+ substituent. A typical absorption edge at approximately 445 nm was noted for pristine Bi2WO6 (sample Zn0), which corresponds to an optical bandgap energy of 2.78 eV according to the Kubelka–Munk function vs. the energy of absorbed light. This is due to the transition from filled anti-bonding states, formed by the hybridization of Bi 6s and O 2p orbitals at the top of the valence band to the empty W 5d orbitals in the conduction band.52 With increasing the Zn2+ content to 1, 2.5, 7.5, and 12.5 at%, the absorption edges of Bi2−xZnxWO6+δ photocatalysts were slightly redshifted toward 453, 475, 451, and 448 nm, respectively. A slight reduction in the optical bandgap energy upon partial substitution of Zn2+ for Bi3+ stemmed from the merging and coupling of the highest occupied substituent states (the Zn 3d–levels) into the valence band39 and the split-off of states near the top of the valence band as a result of a substituent–host interaction (Zn–3d–states and O–2p–state).38 The partial substitution led to the upward shift of the conduction band minimum and valence band maximum.39 A further increase in the Zn2+ content to 17.5 and 22.5 at% resulted in the blueshift in the light absorption due possibly to the existence of ZnWO4 and ZnO with absorption edges at about 355 nm (ref. 53) and 385 nm,54 respectively. Among the Bi2−xZnxWO6+δ samples, 2.5 at% Zn2+-substituted Bi2WO6 is more effective in absorbing visible light, which is important for enhancing the visible-light-driven photocatalytic activity. Unlike in previous studies,38,55,56 no background absorption beyond the absorption edges was observed upon partial substitution of Zn2+, suggesting the absence of deep impurity states within the band gap.
The photoelectrochemical studies allow the elucidation of the characteristics of a photocatalyst for the conversion of light energy in chemical reactions. Fig. 5b presents the LSV results. The LSV response of Bi2−xZnxWO6+δ photocatalysts is due to the photoinduced oxidation of water and is qualitatively consistent with previous reports.57,58 Under light illumination, all the photocatalysts define a photocurrent with the following order: Zn2.5 > Zn1.0 > Zn0 > Zn7.5 > Zn12.5 = Zn17.5 = Zn22.5. Therefore, Bi2WO6 partially substituted with 2.5 at% Zn2+ exhibits the highest water oxidation photocurrent density (0.316 mA cm−2 at 1.23 VRHE), projecting itself as a photocatalyst capable of effectively promoting different photo-redox reactions. In general, the potential at which the photocurrent begins to be representative is relatively positive for all the samples, suggesting that charge carrier recombination dominates at low overpotentials. However, as the potential increases, the photocurrent is observed to increase to a pseudo-steady value at very high overpotentials. The latter is evidence that electron transfer phenomena become important at higher overpotentials, since polarization allows photo-excited electrons to be collected, effectively slowing down the recombination process. It is worth mentioning that Zn0 and Zn2.5 define the CA response with a constant photocurrent for 1 h (Fig. 5c), indicating the stability of the photocatalysts under light irradiation and high polarization conditions. Therefore, the Bi2−xZnxWO6+δ photocatalysts studied have a consistent photoelectrochemical response that results from the interplay of light absorption and the balance between recombination and charge transfer processes.59
3.2. Photocatalytic degradation of PFHxA
The photocatalytic activity of Bi2−xZnxWO6+δ photocatalysts was evaluated for the photodegradation of PFHxA in an aqueous solution for 120 min. Fig. 6 shows the reaction time course of the photodegradation of PFHxA over Bi2−xZnxWO6+δ photocatalysts. As shown in Fig. 6a, the content of the Zn2+ substituent influenced the photodegradation kinetics of PFHxA over Bi2−xZnxWO6+δ photocatalysts. Although the photodegradation of PFHxA was slow, the pronounced change in the concentration of PFHxA was noted in the first 45 min under light irradiation. The highest photodegradation rate of PFHxA was observed for sample Zn2.5 (up to 57%), followed by Zn1.0 (40%), Zn0 (37%), and Zn7.5 (32%). Samples Zn12.5, Zn17.5, and Zn22.5 are characterized to have a lower photodegradation rate of PFHxA (<30%). Clearly, 2.5 at% Zn2+ substitution was favorable to enhance the adsorption and photodegradation of PFHxA over Bi2−xZnxWO6+δ photocatalysts due to the optimum concentration of oxygen vacancies.Recently, Lovisa et al.55 experimentally studied the impact of partial substitution of Zn for Bi on the photocatalytic and photoluminescence properties of Bi2WO6 and found that Zn could not effectively favor the photocatalytic property but the photoluminescence property due to the increase in the recombination rate of photoexcited charge carriers in the oxygen vacancy-related defects. On the contrary, In2O3 nanostructures with pores showed high photocatalytic activity toward PFAS degradation because of the presence of abundant oxygen vacancy defects on their surface,30 which received an O atom from the –COOH group of PFOA molecules, forming a close contact with In2O3 and improving the charge transfer and photocatalytic activity under UV irradiation. The oxygen vacancy in the BiOI microspheres was tuned by Zn2+ substitution and an increase in the concentration of oxygen vacancies enhanced the photocatalytic activity for NO removal.60 Also, the flower-like microstructures of sample Zn2.5 permit multiple light scattering and offer a longer optical path, which improves the efficiency of light harvesting and increases the number of photo-excited charge carriers available for the photodegradation of PFHxA. Similarly, flower-like microspheres of Bi2WO6 exhibited superior photocatalytic activity for the photodegradation of RhB in comparison to the plate-like and clew-like structures due to their efficient separation and transfer of photo-excited charge carriers and enhanced light-harvesting efficiency.61 Previously, P25-TiO2 exhibited 31.1% degradation efficiency and 3.3% de-fluorination efficiency for PFOA,62 while In2O3 with graphene showed 100% degradation efficiency and 60.9% de-fluorination efficiency for PFOA although it requires a thermal treatment at 400 °C.63 As shown in Table S1 in the ESI,† although some photocatalysts and processes showed high efficiency in the degradation of PFAS, they were conducted under UV light irradiation. In contrast, 2.5 at% Zn2+-substituted Bi2WO6 exhibited a 57% removal efficiency of PFHxA in 45 min under visible light irradiation. Apparently, the photocatalytic removal of PFHxA by Bi2−xZnxWO6+δ photocatalysts followed a pseudo-first-order kinetics, implying that the adsorption process was a rate-limiting step of the reaction, and the highest pseudo-first-order kinetic constant (k1) was observed for sample Zn2.5 (0.012 min−1 in Table 2). With increasing the concentration of Zn2+ substituent, the k1 value gradually decreased. Table 2 shows the kinetic parameters, such as k1, half-life time (t1/2), and pseudo-second-order kinetic (k2), for the photocatalytic degradation of PFHxA over Bi2−xZnxWO6+δ photocatalysts.
| Photocatalyst | k 1 (×103) | t 1/2 | R 2 | k 2 (×103) | R 2 |
|---|---|---|---|---|---|
| min−1 | (min) | (mg g−1 min−1) | |||
| Zn0 | 5.32 | 130 | 0.9690 | 9.10 | 0.9686 |
| Zn1.0 | 5.23 | 133 | 0.9456 | 12.13 | 0.9289 |
| Zn2.5 | 12.24 | 57 | 0.9923 | 17.64 | 0.9980 |
| Zn7.5 | 3.49 | 198 | 0.9032 | 8.67 | 0.8855 |
| Zn12.5 | 3.19 | 217 | 0.9844 | 6.86 | 0.9870 |
| Zn17.5 | 3.05 | 227 | 0.9734 | 6.86 | 0.9684 |
| Zn22.5 | 2.39 | 290 | 0.9809 | 5.66 | 0.9756 |
The natural components of the water matrix, including inorganic ions, are recognized as potential competitors of active sites on the surface of a photocatalyst. Thus, additional photodegradation tests were conducted using sample Zn2.5 in the presence of Cl−, NO3−, or PO43−. As shown in Fig. 6b, the photocatalytic removal efficiency of PFHxA was lowered in the presence of these inorganic anions. Particularly, the PO43− ions were more active in competition with PFHxA molecules on the surface of Bi2−xZnxWO6+δ photocatalysts, significantly reducing the photocatalytic removal efficiency of PFHxA. Depending on the type of water matrix, the photocatalytic removal efficiency of PFHxA can be varied due to the simultaneous existence of inorganic ions and organic molecules (dissolved organic matter). Fig. 6c presents the effect of the water matrix (distilled water – DW, tap water – TW, and treated wastewater – TWW) on the photocatalytic removal of PFHxA over sample Zn2.5. It can be seen that the photocatalytic removal efficiency of PFHxA was much higher in tap water in comparison to distilled water and treated wastewater possibly due to the simultaneous presence of Fe (49 mg L−1) or Mg (22.9 mg L−1) ions. Interestingly, the presence of Cl− (35 mg L−1) in treated wastewater did not reduce the photocatalytic removal efficiency of sample Zn2.5.
3.3. Photodegradation mechanism
To gain insights into the possible mechanism for the degradation of PFHxA over Bi2−xZnxWO6+δ photocatalysts, trapping experiments were conducted using sample Zn2.5 in the presence of isopropyl alcohol – IPA (1000 mg L−1), p-benzoquinone – PBQ (1 mg L−1), and ethylenediaminetetraacetic acid disodium salt dihydrate – Na2EDTA (100 mg L−1) as scavengers of ˙OH, O2˙−, and h+, respectively. Fig. 6d shows that the photodegradation of PFHxA over Bi2−xZnxWO6+δ photocatalysts was mainly driven by the involvement of photo-excited holes and superoxide radicals. This is because the conduction band minimum and valence band maximum of Bi2WO6 shifted upward, preferentially generating O2˙− radicals since the reduction potential of O2/O2˙− is −0.33 eV. Similarly, photo-excited holes and O2˙− were found to be the major contributors to the photodegradation of fluoroquinolones over Mg-substituted Bi2WO6.64 Interestingly, hydroxyl radicals were not involved in the photodegradation of PFHxA according to the results of trapping experiments. It is then inferred that the direct electron transfer (DET) from PFHxA is an important step. In fact, this evidence is in good agreement with the proposed mechanistic study.65 It has been reported that the formation of the radical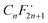 is a determining step in the reaction.65,66 Then, to complete the oxidation process, different chemical and redox steps took place, such as the reaction with holes, electrons, and O2˙− as possible steps to react with
is a determining step in the reaction.65,66 Then, to complete the oxidation process, different chemical and redox steps took place, such as the reaction with holes, electrons, and O2˙− as possible steps to react with 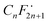 formed and continue the photocatalytic reaction (Fig. 8).
formed and continue the photocatalytic reaction (Fig. 8).
To understand the reaction pathway of the photodegradation of PFHxA over Bi2−xZnxWO6+δ photocatalysts, LC-QTOF/MS operating in a scan mode was used. By applying collision energy equal to 0 eV, we attempt to minimize a possible fragmentation of PFHxA in an MS source and to identify specific products of its photodegradation. As shown in Fig. 7a, the deprotonated molecular ion of m/z = 312.97 and an ion of m/z = 268.98, which are associated with the loss of CO2 from the carboxyl group, and an ion of m/z = 68.99 delivered from fragment CF3 were observed in each MS spectra. This suggests the partial decomposition of PFHxA in an MS source instead of its degradation by photocatalysis. Generally, dealkylation is the main process that takes place during the photocatalytic removal of PFAS,44 and longer PFAS are degraded into shorter ones.67 Interestingly, fragments indicating the photodegradation of PFHxA into short-chain PFAS, such as perfluoropentanoic acid (Mw = 264.05 g mol−1) or perfluorobutanoic acid (Mw = 214.4 g mol−1) were not found. However, a thorough inspection of MS spectra acquired for the photocatalytically treated samples confirms the presence of smaller fragments of m/z = 248.96, m/z = 226.98, and m/z = 180.97, which are associated with the loss of HF, CO, CO2, and CnFm (Fig. 7b) and the formation of [M–HF–CO2]-, [M + H–CF4]- and [M–CO + H2–CF5]-fragments.68 Decarboxylation and defluorination are the main possible routes for the transformation of PFAS during the photocatalytic processes.69 The possible mechanism for the photodegradation of PFHxA over Zn2+substituted Bi2WO6 is shown in Fig. 8. The reusability of the synthesized photocatalyst (sample Zn2.5) for the degradation of PFHxA in an aqueous solution was tested in four cycles. The obtained results in Fig. 6e revealed that sample Zn2.5 has good reusability since only a 27% reduction in photocatalytic activity after four runs was observed due to the loss of photocatalyst powders during collection and drying after each cycle.
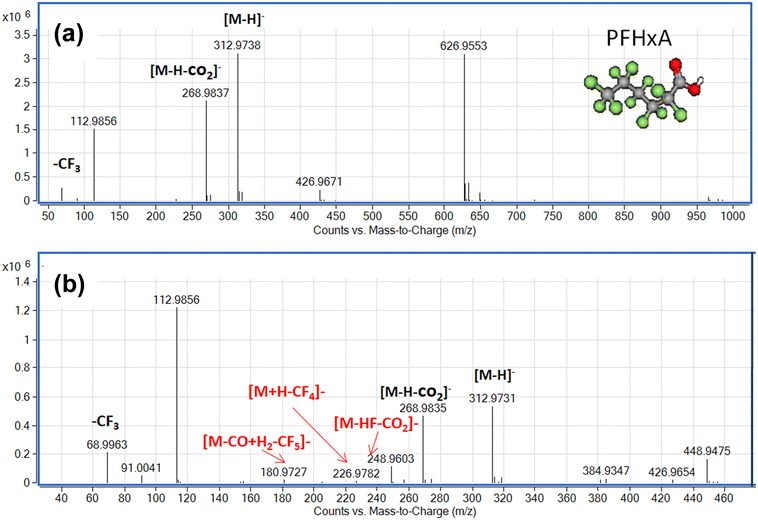 | ||
| Fig. 7 Mass spectra of PFHxA-containing water samples before (a) and after (b) photocatalytic treatment in the presence of sample Zn2.5. | ||
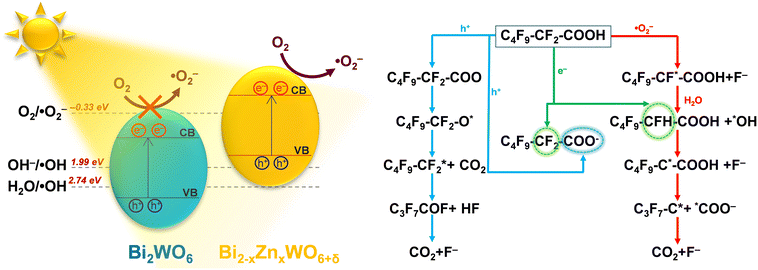 | ||
| Fig. 8 Possible mechanism for the photodegradation of PFHxA in an aqueous solution over the Zn2+-substituted Bi2WO6 photocatalyst. | ||
3.4. Toxicity
The toxicity of photocatalytically treated water samples containing PFHxA to Aliivibrio fischeri was estimated to be low (EC50 = 1.27 g).70 The presence of PFHxA in distilled water before and after photocatalytic treatment (Fig. 6f) did not affect the bioluminescence of Aliivibrio fischeri. However, slight toxicity (<20%) was observed in the water samples containing PFHxA after photocatalytic treatment using sample Zn0. With the Zn2+ substituent, the toxicity was reduced, which needs further investigation to understand this phenomenon. The toxicity of the photocatalytically treated PFHxA-containing TWW sample was significant (nearly 34%), inhibiting the bioluminescence of Aliivibrio fischeri.4. Conclusions
Zn2+-substituted Bi2WO6 has the potential to transform solar energy into redox reactions effectively because the partial substitution of Zn2+ for Bi3+ in the Bi2WO6 crystal lattice contracts the unit-cell volume, improves the absorption of visible light, and decreases the recombination of photo-excited charge carriers, favoring the photo-redox processes. The Bi2WO6 substituted with 2.5 at% Zn2+ differed from the unmodified Bi2WO6 by the possibility of generating O2˙− due to the displacement of the conduction band to more negative potentials. The Zn2+-substituted Bi2WO6 photocatalysts exhibited the highest pseudo-first-order kinetic constant for PFHxA photodegradation (k1 = 0.012 min−1). Trapping experiments confirmed that photo-excited holes and superoxide radicals were the main reactive species involved in the photodegradation of PFHxA. The direct electron transfer of PFHxA was the important step and the reaction of holes, electrons, and O2˙− with the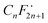 formed supported the photocatalytic oxidation reaction. Considering the complexity of wastewater, which simultaneously contains organic (dissolved organic matter) and inorganic substances (anions, such as nitrates, carbonates), the low photocatalytic efficiency in TTW still indicates the potential of the synthesized photocatalysts for practical application in the removal of PFAS under visible light irradiation. Furthermore, there is also a possibility to regenerate the used photocatalysts by heat treatment at temperatures above 157 °C, which is the boiling point of PFHxA. Although the number of studies on the photocatalytic removal of PFAS has recently increased, the efficiency, stability, and scalability of such systems need to be further explored along with understanding their detailed mechanisms.
formed supported the photocatalytic oxidation reaction. Considering the complexity of wastewater, which simultaneously contains organic (dissolved organic matter) and inorganic substances (anions, such as nitrates, carbonates), the low photocatalytic efficiency in TTW still indicates the potential of the synthesized photocatalysts for practical application in the removal of PFAS under visible light irradiation. Furthermore, there is also a possibility to regenerate the used photocatalysts by heat treatment at temperatures above 157 °C, which is the boiling point of PFHxA. Although the number of studies on the photocatalytic removal of PFAS has recently increased, the efficiency, stability, and scalability of such systems need to be further explored along with understanding their detailed mechanisms.
Author contributions
Mirabbos Hojamberdiev: conceptualization, investigation, methodology, validation, visualization, writing – original draft, writing – review & editing; Ana Laura Larralde: investigation, software, validation, writing – original draft; Ronald Vargas: formal analysis, investigation, software, validation, writing – original draft, writing – review & editing; Lorean Madriz: formal analysis, investigation, software, validation, writing – original draft, writing – review & editing; Kunio Yubuta: formal analysis, investigation, software, validation, writing – original draft; Lokesh Koodlur Sannegowda: investigation, writing – review & editing; Ilona Sadok: formal analysis, investigation, software, validation, writing – original draft; Agnieszka Krzyszczak-Turczyn: formal analysis, investigation, software, validation, writing – original draft; Patryk Oleszczuk: formal analysis, investigation, software, validation, writing – original draft; Bożena Czech: conceptualization, investigation, methodology, supervision, validation, visualization, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank MPWIK Lublin and The John Paul II Catholic University of Lublin, Poland, for providing wastewater samples and giving access to services and research facilities, which were co-funded by the European Regional Development Fund (POPW.01.03.00-06-003/09-00), respectively. India-Uzbekistan Collaborative Grants (No. INT/Uzbek/P-21 and UZB-Ind-2021-91) are also acknowledged. The authors would like to thank Dipl. Phys. Christoph Fahrenson from ZELMI, TU Berlin, for his kind technical support in SEM analysis.References
- R. Mahinroosta and L. Senevirathna, A review of the emerging treatment technologies for PFAS contaminated soils, J. Environ. Manage., 2020, 255, 109896 CrossRef CAS PubMed.
- B. C. Crone, T. F. Speth, D. G. Wahman, S. J. Smith, G. Abulikemu, E. J. Kleiner and J. G. Pressman, Occurrence of Pre- and Polyfluoroalkyl Substances (PFAS) in Source Water and Their Treatment in Drinking Water, Crit. Rev. Environ. Sci. Technol., 2019, 49, 2359–2396 CrossRef PubMed.
- J. L. Domingo and M. Nadal, Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature, Environ. Res., 2019, 177, 108648 CrossRef CAS PubMed.
- F. Suja, B. K. Pramanik and S. M. Zain, Contamination, Bioaccumulation and Toxic Effects of Perfluorinated Chemicals (PFCs) in the Water Environment: A Review Paper, Water Sci. Technol., 2009, 60, 1533–1544 CrossRef CAS PubMed.
- United States Environmental Protection Agency, Technical Fact Sheet – Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA), EPA 505-F-17-001, United States Environmental Protection Agency, 2017 https://19january2021snapshot.epa.gov/sites/static/files/2017-12/documents/ffrrofactsheet_contaminants_pfos_pfoa_11-20-17_508_0.pdf, Per- and Polyfluoroalkyl Substances (PFAS) Proposed PFAS National Primary Drinking Water Regulation (Docket ID: EPA-HQ-OW-2022-0114), https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas.
- M. Wang, Y. Cai, B. Zhou, R. Yuan, Z. Chen and H. Chen, Removal of PFASs from water by carbon-based composite photocatalysis with adsorption and catalytic properties: A review, Sci. Total Environ., 2022, 836, 155652 CrossRef CAS PubMed.
- S. Kancharla, P. Alexandridis and M. Tsianou, Sequestration of per- and polyfluoroalkyl substances (PFAS) by adsorption: Surfactant and surface aspects, Curr. Opin. Colloid Interface Sci., 2022, 58, 101571 CrossRef CAS.
- D. M. Wanninayake, Comparison of currently available PFAS remediation technologies in water: A review, J. Environ. Manage., 2021, 283, 111977 CrossRef CAS PubMed.
- A. L. Luz, J. K. Anderson, P. Goodrum and J. Durda, Perfluorohexanoic acid toxicity, part I: Development of a chronic human health toxicity value for use in risk assessment, Regul. Toxicol. Pharmacol., 2019, 103, 41–55 CrossRef CAS PubMed.
- J. K. Anderson, A. L. Luz, P. Goodrum and J. Durda, Perfluorohexanoic acid toxicity, part II: Application of human health toxicity value for risk characterization, Regul. Toxicol. Pharmacol., 2019, 103, 10–20 CrossRef CAS PubMed.
- Annex XV Report: Proposal for Identification of a Substance of Very High Concern on the Basis of the Criteria Set Out in REACH Article 57, Identification of Undecafluorohexanoic Acid and its Ammonium Salt as SVHC Search PubMed.
- E. T. Hernandez, B. Koo, L. E. Sofen, R. Amin, R. K. Togashi, A. I. Lall, D. J. Gisch, B. J. Kern, M. A. Rickard and M. B. Francis, Proteins as adsorbents for PFAS removal from water, Environ. Sci.: Water Res. Technol., 2022, 8, 1188–1194 RSC.
- T. Jin, M. Peydayesh, H. Joerss, J. Zhou, S. Bolisetty and R. Mezzenga, Amyloid fibril-based membranes for PFAS removal from water, Environ. Sci.: Water Res. Technol., 2021, 7, 1873–1884 RSC.
- T. H. Boyer, Y. Fang, A. Ellis, R. Dietz, Y. J. Choi, C. E. Schaefer, C. P. Higgins and T. J. Strathmann, Anion exchange resin removal of per- and polyfluoroalkyl substances (PFAS) from impacted water: A critical review, Water Res., 2021, 200, 117244 CrossRef CAS PubMed.
- T. Buckley, K. Karanam, X. Xu, P. Shukla, M. Firouzi and V. Rudolph, Effect of mono- and di-valent cations on PFAS removal from water using foam fractionation – A modelling and experimental study, Sep. Purif. Technol., 2022, 286, 120508 CrossRef CAS.
- M. B. Ahmed, Md. M. Alam, J. L. Zhou, B. Xu, Md. A. H. Johir, A. K. Karmakar, Md. S. Rahman, J. Hossen, A. T. M. K. Hasan and M. A. Moni, Advanced treatment technologies efficacies and mechanism of per- and poly-fluoroalkyl substances removal from water, Process Saf. Environ. Prot., 2020, 136, 1–14 CrossRef CAS.
- D. Zhang, Q. Luo, B. Gao, S.-Y. D. Chiang, D. Woodward and Q. Huang, Sorption of perfluorooctanoic acid, perfluorooctane sulfonate and perfluoroheptanoic acid on granular activated carbon, Chemosphere, 2016, 144, 2336–2342 CrossRef CAS PubMed.
- Q. Zhuo, S. Deng, B. Yang, J. Huang and G. Yu, Efficient Electrochemical Oxidation of Perfluorooctanoate Using a Ti/SnO2-Sb-Bi Anode, Environ. Sci. Technol., 2011, 45, 2973–2979 CrossRef CAS PubMed.
- X. Li, P. Zhang, L. Jin, T. Shao, Z. Li and J. Cao, Efficient Photocatalytic Decomposition of Perfluorooctanoic Acid by Indium Oxide and Its Mechanism, Environ. Sci. Technol., 2012, 46, 5528–5534 CrossRef CAS PubMed.
- R. R. Giri, H. Ozaki, T. Okada, S. Taniguchi and R. Takanami, Factors influencing UV photodecomposition of perfluorooctanoic acid in water, Chem. Eng. J., 2012, 180, 197–203 CrossRef CAS.
- H. V. Lutze, J. Brekenfeld, S. Naumov, C. von Sonntag and T. C. Schmidt, Degradation of perfluorinated compounds by sulfate radicals – New mechanistic aspects and economical considerations, Water Res., 2018, 129, 509–519 CrossRef CAS PubMed.
- C. D. Vecitis, Y. Wang, J. Cheng, H. Park, B. T. Mader and M. R. Hoffmann, Sonochemical Degradation of Perfluorooctanesulfonate in Aqueous Film-Forming Foams, Environ. Sci. Technol., 2010, 44, 432–438 CrossRef CAS PubMed.
- Z. Zhang, J.-J. Chen, X.-J. Lyu, H. Yin and G.-P. Sheng, Complete mineralization of perfluorooctanoic acid (PFOA) by γ-irradiation in aqueous solution, Sci. Rep., 2014, 4, 7418 CrossRef CAS PubMed.
- P. J. Krusic, A. A. Marchione and D. C. Roe, Gas-phase NMR studies of the thermolysis of perfluorooctanoic acid, J. Fluorine Chem., 2005, 126, 1510–1516 CrossRef CAS.
- H. Hori, M. Murayama and S. Kutsuna, Oxygen-induced efficient mineralization of perfluoroalkylether sulfonates in subcritical water, Chemosphere, 2009, 77, 1400–1405 CrossRef CAS PubMed.
- H. Obo, N. Takeuchi and K. Yasuoka, Decomposition of perfluorooctanesulfonate (PFOS) by multiple alternating argon plasmas in bubbles with gas circulation, Int. J. Plasma Environ. Sci. Technol., 2015, 9, 62–68 Search PubMed.
- S. Wang, Q. Yang, F. Chen, J. Sun, K. Luo, F. Yao, X. Wang, D. Wang, X. Li and G. Zeng, Photocatalytic degradation of perfluorooctanoic acid and perfluorooctane sulfonate in water: A critical review, Chem. Eng. J., 2017, 328, 927–942 CrossRef CAS.
- H. Tang, Q. Xiang, M. Lei, J. Yan, L. Zhu and J. Zou, Efficient degradation of perfluorooctanoic acid by UV–Fenton process, Chem. Eng. J., 2012, 184, 156–162 CrossRef CAS.
- Y. Wang and P. Zhang, Photocatalytic decomposition of perfluorooctanoic acid (PFOA) by TiO2 in the presence of oxalic acid, J. Hazard. Mater., 2011, 192, 1869–1875 CrossRef CAS PubMed.
- Z. Li, P. Zhang, T. Shao and X. Li, In2O3 nanoporous nanosphere: A highly efficient photocatalyst for decomposition of perfluorooctanoic acid, Appl. Catal., B, 2012, 125, 350–357 CrossRef CAS.
- Z. Song, X. Dong, N. Wang, L. Zhu, Z. Luo, J. Fang and C. Xiong, Efficient photocatalytic defluorination of perfluorooctanoic acid over BiOCl nanosheets via a hole direct oxidation mechanism, Chem. Eng. J., 2017, 317, 925–934 CrossRef CAS.
- T. Shao, P. Zhang, L. Jin and Z. Li, Photocatalytic decomposition of perfluorooctanoic acid in pure water and sewage water by nanostructured gallium oxide, Appl. Catal., B, 2013, 142–143, 654–661 CrossRef CAS.
- W. Ding, X. Tan, G. Chen, J. Xu, K. Yu and Y. Huang, Molecular-Level Insights on the Facet-Dependent Degradation of Perfluorooctanoic Acid, ACS Appl. Mater. Interfaces, 2021, 13, 41584–41592 CrossRef CAS PubMed.
- N. Zhang, R. Ciriminna, M. Pagliaro and Y.-J. Xu, Nanochemistry-derived Bi2WO6 nanostructures: towards production of sustainable chemicals and fuels induced by visible light, Chem. Soc. Rev., 2014, 43, 5276–5287 RSC.
- L. Zhang, H. Wang, Z. Chen, P. K. Wong and J. Liu, Bi2WO6 micro/nano-structures: Synthesis, modifications and visible-light-driven photocatalytic applications, Appl. Catal., B, 2011, 106, 1–13 CAS.
- O. Núñez, L. Madriz, D. Carvajal, J. Tatá and R. Vargas, Unprecedented large solvent (H2O vs D2O) isotope effect in semiconductors photooxidation, J. Phys. Org. Chem., 2019, 32, e3952 CrossRef.
- M. Arif, M. Zhang, J. Yao, H. Yin, P. Li, I. Hussain and X. Liu, Layer-assembled 3D Bi2WO6 hierarchical architectures by Ti-doping for enhanced visible-light driven photocatalytic and photoelectrochemical performance, J. Alloys Compd., 2019, 792, 878–893 CrossRef CAS.
- V. Koteski, J. Belošević-Čavor, V. Ivanovski, A. Umićević and D. Toprek, Ab initio calculations of the optical and electronic properties of Bi2WO6 doped with Mo, Cr, Fe, and Zn on the W–lattice site, Appl. Surf. Sci., 2020, 515, 146036 CrossRef CAS.
- F. Ren, J. Zhang and Y. Wang, Enhanced photocatalytic activities of Bi2WO6 by introducing Zn to replace Bi lattice sites: a first-principles study, RSC Adv., 2015, 5, 29058–29065 RSC.
- M. Hojamberdiev, Z. C. Kadirova, E. Zahedi, D. Onna, M. C. Marchi, G. Zhu, N. Matsushita, M. Hasegawa, S. A. Bilmes and K. Okada, Tuning the morphological structure, light absorption, and photocatalytic activity of Bi2WO6 and Bi2WO6-BiOCl through cerium doping, Arabian J. Chem., 2020, 13, 2844–2857 CrossRef CAS.
- J. Rodríguez-Carvajal, FULLPROF: A Program for Rietveld Refinement and Pattern Matching Analysis, Abstract of the Satellite Meeting on Powder Diffraction of the XV Congress of the IUCr, Toulouse, France, 1990, p. 127 Search PubMed.
- M. Hojamberdiev, B. Czech, A. Wasilewska, A. Boguszewska-Czubara, K. Yubuta, H. Wagata, S. S. Daminova, Z. C. Kadirova and R. Vargas, Detoxifying SARS-CoV-2 antiviral drugs from model and real wastewaters by industrial waste-derived multiphase photocatalysts, J. Hazard. Mater., 2022, 429, 128300 CrossRef CAS PubMed.
- L. S. Gómez-Velázquez, L. Madriz, M. Rigoletto, E. Laurenti, M. Bizarro, M. L. Dell’Arciprete and M. C. González, Structural and Physicochemical Properties of Carbon Nitride Nanoparticles via Precursor Thermal Treatment: Effect on Methyl Orange Photocatalytic Discoloration, ACS Appl. Nano Mater., 2023, 6, 14049–14062 CrossRef.
- S. Taniyasu, N. Yamashita, E. Yamazaki, G. Petrick and K. Kannan, The environmental photolysis of perfluorooctanesulfonate, perfluorooctanoate, and related fluorochemicals, Chemosphere, 2013, 90, 1686–1692 CrossRef CAS PubMed.
- Microtox® Acute Toxicity Manual, Modern Water Inc., https://www.modernwater.com/wp-content/uploads/2022/09/MW-Microtox-Acute-Toxicity-Manual-Brochure-WEB.pdf Search PubMed.
- R. D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- M. Hojamberdiev, K.-I. Katsumata, N. Matsushita and K. Okada, Preparation of Bi2WO6- and BiOI-allophane composites for efficient photodegradation of gaseous acetaldehyde under visible light, Appl. Clay Sci., 2014, 101, 38–43 CrossRef CAS.
- M. Hojamberdiev, Z. C. Kadirova, R. V. Gonçalves, K. Yubuta, N. Matsushita, K. Teshima, M. Hasegawa and K. Okada, Reduced graphene oxide-modified Bi2WO6/BiOI composite for the effective photocatalytic removal of organic pollutants and molecular modeling of adsorption, J. Mol. Liq., 2018, 268, 715–727 CrossRef CAS.
- M. Hojamberdiev, Z. C. Kadirova, Y. Makinose, G. Zhu, S. Emin, N. Matsushita, M. Hasegawa, M. Hasegawa and K. Okada, Involving CeVO4 in improving the photocatalytic activity of a Bi2WO6/allophane composite for the degradation of gaseous acetaldehyde under visible light, Colloids Surf., A, 2017, 529, 600–612 CrossRef CAS.
- D. Saha, E. D. Bøjesen, A. H. Mamakhel and B. B. Iversen, Why Does Bi2WO6 Visible-Light Photocatalyst Always Form as Nanoplatelets?, Inorg. Chem., 2020, 59, 9364–9373 CrossRef CAS PubMed.
- L. Zhang, W. Wang, Z. Chen, L. Zhou, H. Xu and W. Zhu, Fabrication of flower-like Bi2WO6 superstructures as high performance visible-light driven photocatalysts, J. Mater. Chem., 2007, 17, 2526–2532 RSC.
- H. Fu, C. Pan, W. Yao and Y. Zhu, Visible-Light-Induced Degradation of Rhodamine B by Nanosized Bi2WO6, J. Phys. Chem. B, 2005, 109, 22432–22439 CrossRef CAS PubMed.
- M. Hojamberdiev, K.-I. Katsumata, K. Morita, S. A. Bilmes, N. Matsushita and K. Okada, One-step hydrothermal synthesis and photocatalytic performance of ZnWO4/Bi2WO6 composite photocatalysts for efficient degradation of acetaldehyde under UV light irradiation, Appl. Catal., A, 2013, 457, 12–20 CrossRef CAS.
- M. Hojamberdiev, R. M. Prasad, K. Morita, M. A. Schiavon and R. Riedel, Polymer-derived mesoporous SiOC/ZnO nanocomposite for the purification of water contaminated with organic dyes, Microporous Mesoporous Mater., 2012, 151, 330–338 CrossRef CAS.
- L. X. Lovisa, T. B. O. Nunes, R. R. Y. O. V. Wilson, E. Longo, M. D. Teodoro, M. R. D. Bomio and F. V. Motta, Synthesis and evaluation of photocatalytic and photoluminescent properties of Zn2+-doped Bi2WO6, Dalton Trans., 2022, 51, 17700–17710 RSC.
- A. Etogo, R. Liu, J. Ren, L. Qi, C. Zheng, J. Ning, Y. Zhong and Y. Hu, Facile one-pot solvothermal preparation of Mo-doped Bi2WO6 biscuit-like microstructures for visible-light-driven photocatalytic water oxidation, J. Mater. Chem. A, 2016, 4, 13242–13250 RSC.
- L. Madriz, J. Tatá, D. Carvajal, O. Núñez, B. R. Scharifker, J. Mostany, C. Borrás, F. M. Cabrerizo and R. Vargas, Photocatalysis and photoelectrochemical glucose oxidation on Bi2WO6: conditions for the concomitant H2 production, Renewable Energy, 2020, 152, 974–983 CrossRef CAS.
- C. Bhattacharya, H. C. Lee and A. J. Bard, Rapid Screening by Scanning Electrochemical Microscopy (SECM) of Dopants for Bi2WO6 Improved Photocatalytic Water Oxidation with Zn Doping, J. Phys. Chem. C, 2013, 117, 9633–9640 CrossRef CAS.
- O. Núñez and R. Vargas, The interplay between RedOx, photophysics and surface process in Bi2WO6 photocatalyst, in A Closer Look at Chemical Kinetics, ed. V. Martinez-Luaces, Nova Science Publishers, 2023, pp. 205–222 Search PubMed.
- F. Rao, G. Zhu, M. Hojamberdiev, W. Zhang, S. Li, J. Gao, F. Zhang, Y. Huang and Y. Huang, Uniform Zn2+-Doped BiOI Microspheres Assembled by Ultrathin Nanosheets with Tunable Oxygen Vacancies for Super-Stable Removal of NO, J. Phys. Chem. C, 2019, 123, 16268–16280 CrossRef CAS.
- H. Lv, Y. Liu, J. Guang, Z. Ding and J. Wan, Shape-selective synthesis of Bi2WO6 hierarchical structures and their morphology-dependent photocatalytic activities, RSC Adv., 2016, 6, 80226–80233 RSC.
- Z. Li, P. Zhang, J. Li, T. Shao and L. Jin, Synthesis of In2O3-graphene composites and their photocatalytic performance towards perfluorooctanoic acid decomposition, J. Photochem. Photobiol., A, 2013, 271, 111–116 CrossRef CAS.
- M. Li, Z. Yu, Q. Liu, L. Sun and W. Huang, Photocatalytic decomposition of perfluorooctanoic acid by noble metallic nanoparticles modified TiO2, Chem. Eng. J., 2016, 286, 232–238 CrossRef CAS.
- F. Zhu, Y. Lv, J. Li, J. Ding, X. Xia, L. Wei, J. Jiang, G. Zhang and Q. Zhao, Enhanced visible light photocatalytic performance with metal-doped Bi2WO6 for typical fluoroquinolones degradation: Efficiencies, pathways and mechanisms, Chemosphere, 2020, 252, 126577 CrossRef CAS PubMed.
- J. Radjenovic, N. Duinslaeger, S. S. Avval and B. P. Chaplin, Facing the Challenge of Poly- and Perfluoroalkyl Substances in Water: Is Electrochemical Oxidation the Answer?, Environ. Sci. Technol., 2020, 54, 14815–14829 CrossRef CAS PubMed.
- Y. S. Khalid, S. N. Misal, S. Mehraeen and B. P. Chaplin, Reactive-Transport Modeling of Electrochemical Oxidation of Perfluoroalkyl Substances in Porous Flow-through Electrodes, ACS ES&T Engg, 2022, 2, 713–725 Search PubMed.
- B. Xu, J. L. Zhou, A. Altaee, M. B. Ahmed, M. A. H. Johir, J. Ren and X. Li, Improved photocatalysis of perfluorooctanoic acid in water and wastewater by Ga2O3/UV system assisted by peroxymonosulfate, Chemosphere, 2020, 239, 124722 CrossRef CAS PubMed.
- M. Altarawneh, M. H. Almatarneh and B. Z. Dlugogorski, Thermal decomposition of perfluorinated carboxylic acids: Kinetic model and theoretical requirements for PFAS incineration, Chemosphere, 2022, 286, 131685 CrossRef CAS PubMed.
- C. Fu, X. Xu, C. Zheng, X. Liu, D. Zhao and W. Qiu, Photocatalysis of aqueous PFOA by common catalysts of In2O3, Ga2O3, TiO2, CeO2 and CdS: influence factors and mechanistic insights, Environ. Geochem. Health, 2022, 44, 2943–2953 CrossRef CAS PubMed.
- E. Mulkiewicz, B. Jastorff, A. C. Składanowski, K. Kleszczyński and P. Stepnowski, Evaluation of the acute toxicity of perfluorinated carboxylic acids using eukaryotic cell lines, bacteria and enzymatic assays, Environ. Toxicol. Pharmacol., 2007, 23, 279–285 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ew00430a |
| This journal is © The Royal Society of Chemistry 2023 |