Phosphorus recovery by re-dissolution from activated sludge – effects of carbon source and supplementation level revisited†
Annika
Anders
 *,
Harald
Weigand
*,
Harald
Weigand
 and
Harald
Platen
and
Harald
Platen

Competence Centre for Sustainable Engineering and Environmental Systems (ZEuUS), THM University of Applied Sciences, Wiesenstr. 14, D-35390 Giessen, Germany. E-mail: annika.anders@lse.thm.de; harald.weigand@lse.thm.de; harald.platen@lse.thm.de
First published on 15th November 2022
Abstract
Municipal sewage sludge is a sink for wastewater-borne phosphorus (P) and a source for P recovery. Many wastewater treatment plants (WWTPs) employ the enhanced biological P removal (EBPR) which relies on the ability of polyphosphate accumulating organisms (PAOs) to store P in the biomass. Inversion of EBPR may provide a tool for on-site P recovery from activated sludge (AS). Key features of anaerobic P release and the metabolism of acclimated PAOs are well known from laboratory experiments. However, uncertainty persists regarding the behavior of non-acclimated sludge which hampers the practical implementation of P recovery. In this light, we revisited the effects of volatile fatty acid supplementation (formate, acetate, propionate, and butyrate) on the anaerobic P re-dissolution from non-acclimated AS of a full-scale WWTP. All supplementations induced P re-dissolution but the highest re-dissolution was observed with acetate (1.54–1.68 mmol P L−1) with a Pyield/VFAconsumed ratio of 0.45. For AS with 6.3 gTSS L−1, a supplementation level of 200 mg L−1 acetate was most efficient. Recovery amounted to 21–24% of total P within 300 min. Surprisingly, P re-dissolution continued even after acetate had been fully consumed. From the energetic viewpoint, this seems contradictory. Therefore, we integrated the process stoichiometry with known metabolic pathways accounting for the main electron, energy, carbon and P flows for the acetate-induced P re-dissolution. Results show that induction of anaerobiosis in AS from the EBPR process is, indeed, a viable technical option for P recovery. Yet, efficiency needs to be improved since P re-dissolution was either limited by acetate uptake capacity or by available polyphosphate.
Water impactSustainable nutrient recovery from wastewater is crucial and the German Sewage Sludge Ordinance enforces phosphorus recovery by 2029. Therefore, we investigated the potential of a biological phosphorus re-dissolution from activated sludge by C supplementation for crystallization of a phosphorus fertilizer in a fluidized bed reactor. Results highlight persisting limitations as well as perspectives for full-scale technology implementation. |
1 Introduction
On a global basis, each year around 1.3 Mt of sewage-borne phosphorus (P) are removed in wastewater treatment plants (WWTPs).1 To avoid eutrophication of water bodies and to comply with regulatory P discharge values, many WWTPs have either implemented a chemical P precipitation (CPR, chemical phosphorus removal) or an enhanced biological phosphorus removal (EBPR) process. Thereby, influent P is removed from the aqueous phase and incorporated in the solid sludge.In the EBPR process, P removal is achieved by alternatingly cycling the activated sludge (AS) through aerobic and anaerobic zones. Ortho-phosphate (ortho-P) from the influent wastewater is used by sludge-borne microorganisms for metabolism and growth. According to the classical understanding of the EBPR process, polyphosphate accumulating organisms (PAOs) intracellularly store excess P as polyphosphate (polyP) under aerobic conditions.2,3 Energy for P uptake is provided by oxidation of carbon sources from the wastewater or by degradation of stored polyhydroxyalkanoates (PHAs). Under anaerobic conditions, PAOs can hydrolyze polyP as an energy source and ortho-P is released into the aqueous phase. Simultaneously, volatile fatty acids (VFAs), such as acetate, are taken up for PHA replenishment.2,4 The reducing equivalents required for PHA formation may be obtained from glycolysis or from the tricarboxylic acid (TCA) cycle.4–6 Repeated cycling of the AS through aerobic/anaerobic conditions leads to an enrichment of PAOs in the sludge providing the basis for an efficient EBPR process.
Candidatus Accumulibacter and Tetrasphaera are two important genera of PAOs in full-scale EBPR systems.7,8 Both accumulate polyP, yet based on individual metabolic properties they likely occupy different ecological niches within the EBPR consortium.9,10 In the case of Tetrasphaera spp. several authors have observed an additional fermentation and denitrification capability and a more versatile substrate utilization without PHA storage.9,11,12 This differs from the classical PAO metabolism of Candidatus Accumulibacter spp. (henceforth called Accumulibacter) described above. Moreover, the metabolic versatility of PAOs has been shown to depend on operational and physiological conditions, such as pH, availability of influent P, source of reducing power as well as biomass polyP and glycogen content.13–15 A broad range of carbon sources (VFA, amino acids, sugars), as well as the accumulation of storage polymers such as polyP, glycogen, PHA and yet unidentified storage reserves, can play a role in metabolic behavior.9,16 This illustrates the complexity of metabolic processes taking place in the AS.
As P is eliminated from the sewage by incorporation into the solid phase, the AS could be used as a gateway for P recovery instead of directly applying it to farmland. In Germany, such sustainable P recovery strategies have gained importance since the 2017 amendment of the Sewage Sludge Ordinance. The regulation obliges large WWTPs to recycle P from their process streams as of 2029 and bans the direct application of AS to farmland by WWTPs serving >50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 population equivalents.17 Current approaches focus either on the ash derived from the mono-incineration of sewage sludge or aim at a P re-dissolution and selective precipitation after chemical or thermal hydrolysis of the sludge.18 Compliance with the mandatory P recovery rates of 80% and 50%, respectively, requires a relatively high energy input and consumption of additional chemical compounds.18,19 Thus, sustainability and cost-efficiency of such processes may be questioned. In this light, the fundamentals of EBPR may be used in an alternative engineering approach where the biological P uptake process is simply inverted to provide a P-rich aqueous phase for further processing. Acevedo et al.20 and Xia et al.21 have shown that it is possible to re-dissolve high amounts of intracellularly stored P from laboratory PAO-enriched sludge by supplementation of a carbon source. Based on the consumption of acetate, in a previous study, we delineated treatment costs for such a process in the range of 210–484 € per tonne of AS dry matter.22
000 population equivalents.17 Current approaches focus either on the ash derived from the mono-incineration of sewage sludge or aim at a P re-dissolution and selective precipitation after chemical or thermal hydrolysis of the sludge.18 Compliance with the mandatory P recovery rates of 80% and 50%, respectively, requires a relatively high energy input and consumption of additional chemical compounds.18,19 Thus, sustainability and cost-efficiency of such processes may be questioned. In this light, the fundamentals of EBPR may be used in an alternative engineering approach where the biological P uptake process is simply inverted to provide a P-rich aqueous phase for further processing. Acevedo et al.20 and Xia et al.21 have shown that it is possible to re-dissolve high amounts of intracellularly stored P from laboratory PAO-enriched sludge by supplementation of a carbon source. Based on the consumption of acetate, in a previous study, we delineated treatment costs for such a process in the range of 210–484 € per tonne of AS dry matter.22
Advantages include the easy on-site integration of the re-dissolution process at WWTPs, reduction of transport costs, recycling of P-depleted AS into the aerobic stage to re-new aerobic P uptake, and chemical precipitant savings. Further, reduced sludge volume may help to increase storage capacities and cut disposal costs. Most importantly, in accordance with the German Sewage Ordinance the P-depleted sludge can be disposed of by co-fueling (e.g. in cement kilns) and thus thermal disposal is no longer bound to mono-incineration with posterior P recovery from the ash.
Using the biological P re-dissolution as a strategy for P recovery at full-scale WWTPs entails the identification of a suitable carbon source. P re-dissolution and VFA utilization are interdependent and PAOs may synthesize different PHAs from individual VFAs.16 It is well known that P can be re-dissolved from the intracellular polyP pool of Accumulibacter by supplementation of acetate.7,10 However, uptake capacity and preference for certain carbon sources often depend on the habitat of the AS organisms and prevailing environmental conditions. Therefore, substrates may not be universally suitable for an extensive P re-dissolution in full-scale WWTPs. As opposed to laboratory-acclimated PAO-sludge, in full-scale EBPR processes, microbial competition and interaction within the heterogenic AS community play an important role and will likely affect P re-dissolution efficiency. Specifically, glycogen accumulating organisms (GAOs), often abundant in AS derived from full-scale systems, compete with PAOs for anaerobic VFA uptake without contributing to aerobic P removal as they do not accumulate polyP.23,24
So far, intensive research focused on the mechanisms of EBPR activity and the role of the bacterial communities in P removal. Factors such as pH,25 carbon source type,26,27 temperature28 and influent composition14 have been studied in the light of EBPR optimization. By contrast, the potential benefits of inverting the process in a sustainable and biological P recovery strategy that can be implemented along the wastewater treatment line remain largely unexplored. A previous study has shown high efficiency of P re-dissolution from AS from pure EBPR systems with acetate supplementation.22 However, in full-scale WWTPs operators often secure P removal by additional CPR, thereby potentially affecting activity and behavior of the microbial population.29,30 Especially, the usage of iron-based precipitation agents has been recognized to lower the P recovery potential.31 Due to the strict effluent standards, WWTP operators often have not yet been able to refrain from the addition of chemical precipitants. Thus, investigation of AS biomass from full-scale systems that rely on combined EBPR and CPR is of importance to advance a future implementation of a sludge-based P recovery strategy that makes use of the EBPR anaerobiosis induced by the addition of a carbon source. Because of the high diversity and versatile metabolism of PAOs, different carbon sources may be suitable for a biological P re-dissolution. In a recent publication, Zhang et al.32 outlined that full-scale mainstream P recovery from EBPR sludge could be a promising approach for on-site implementation on the medium term. The authors highlighted that future works should among other aspects focus on real municipal wastewater and pointed out the additional costs when EBPR sludge is supplemented with external carbon sources. In terms of costs, it is important not only to determine the effect of carbon source type but also to quantify the required supplementation level. To this end, we revisited the re-dissolution of P from real municipal AS (non-acclimated AS) of a full-scale WWTP under varied carbon supplementation. Formate, acetate, propionate, and butyrate were evaluated as these are economical carbon sources that can be present in the WWTP influent or can be produced by hydrolysis and pre-fermentation processes. A series of batch tests with freshly sampled EBPR-CPR sludge served to study the effect of the carbon source type on P recovery and to identify its optimal dosage.
2 Materials and methods
Laboratory studies regarding the P re-dissolution upon VFA supplementation were conducted with AS collected at a WWTP located in central Germany. The plant has a capacity of 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 population equivalents and uses the EBPR process. To comply with the strict discharge limits for P set out by the European Water Framework Directive as well as the regional effluent standards33 of 0.4 mg L−1 total P, the plant has additionally implemented a CPR step where ferrous chloride is added to the aeration tanks. All AS samples were taken as a mixed liquor AS suspension at the outlet of the aerated biological treatment stage before the sludge is transferred into the settling tanks. Biological replicates were obtained by sampling AS at different dates (on eight occasions) during the months of August, October and November in 2019 and 2020 thereby accounting for sludge variability. The in situ temperature of the sampled AS was 18–19 °C, throughout. Periods of heavy rainfall or drought and out-of-line events of WWTP operation were excluded from sampling. The re-dissolution experiments were performed within one hour after sampling to prevent changes in bacterial activity and composition during storage.
000 population equivalents and uses the EBPR process. To comply with the strict discharge limits for P set out by the European Water Framework Directive as well as the regional effluent standards33 of 0.4 mg L−1 total P, the plant has additionally implemented a CPR step where ferrous chloride is added to the aeration tanks. All AS samples were taken as a mixed liquor AS suspension at the outlet of the aerated biological treatment stage before the sludge is transferred into the settling tanks. Biological replicates were obtained by sampling AS at different dates (on eight occasions) during the months of August, October and November in 2019 and 2020 thereby accounting for sludge variability. The in situ temperature of the sampled AS was 18–19 °C, throughout. Periods of heavy rainfall or drought and out-of-line events of WWTP operation were excluded from sampling. The re-dissolution experiments were performed within one hour after sampling to prevent changes in bacterial activity and composition during storage.
All samples were characterized in terms of total suspended solids (TSS) as well as total P, iron, calcium, potassium and magnesium content according to analytical methods described below. Volatile suspended solids (VSS) and chemical oxygen demand (COD) values were reported by the plant staff.
2.1 Effect of carbon source on P re-dissolution
Four different VFAs were tested for their impact on P release from AS. A defined volume (5 mL) of VFA stock solution (20 g L−1) was added to 500 mL of the AS to obtain a spike concentration of 200 mg L−1 formate, acetate, propionate, and butyrate, respectively. All VFA were added as sodium salts (purity ≥98–99%) purchased from Merck KGaA, Darmstadt, Germany and Carl Roth GmbH & Co. KG, Karlsruhe, Germany. Experiments were carried out in closed 500 mL Erlenmeyer flasks. Biological triplicates were conducted with independently samples AS and were accompanied by a non-supplemented control. The batches were kept at room temperature for 220 min and were stirred at 100 rpm using a magnetic stirrer to maintain the AS in suspension. The pH was not adjusted. Aliquots were sampled with a syringe for time-resolved determination of P release and VFA uptake.2.2 Effect of acetate supplementation level on P re-dissolution
To investigate a potential effect of the carbon supplementation level, AS was supplemented with 100–600 mg L−1 acetate added as sodium acetate trihydrate (p.a., purity ≥99%, Merck KGaA, Darmstadt, Germany) employing the setup described in section 2.1 but using a volume of 1 L AS. Acetate was added to the AS as a concentrated stock solution (20 g L−1). Additionally, the pH, redox potential (EH) and dissolved oxygen (DO) concentration were monitored using WTW Multi 3630 IDS (WTW, Xylem Analytics, Weilheim, Germany) equipped with SenTix®940, SenTix®ORP-T900 and FDO®925 sensors. The ortho-P and acetate concentrations in the aqueous phase were followed over time. Experiments were performed as five biological replicates with acetate concentrations of 100–400 mg L−1 and in biological triplicates with 600 mg L−1 acetate to check for reproducibility. AS without acetate supplementation served as a control.2.3 Analytical methods
Ortho-P was determined in 0.45 μm filtered samples using the molybdenum blue method.34 The VFA concentration was determined using an ion chromatograph (Metrohm 861 Advanced Compact IC). Sample splits of 20 μL obtained after 0.2 μm membrane filtration were separated with a 1.0 mmol L−1 NaHCO3/3.2 mmol L−1 Na2CO3 eluent on a Metrosep A Supp 5 column (150 mm × 4.0 mm, particle size 5 μm, Metrohm, Herisau, Switzerland). Runs were conducted at a flow rate of 0.7 mL min−1 and had a duration of 20 min. Detection took place after chemical suppression by measuring conductivity. The samples' signals were aligned according to the retention times of external standards. TSS was determined according to the APHA standard method.34 Homogenized sludge biomass was dried at 105 °C for 24 h and ground with a ball mill prior to aqua regia digestion according to the European standard procedure DIN EN 16174:2012-11.35Total phosphate in the digestates was determined as ortho-P as described above. The iron, calcium, potassium and magnesium concentration in the digestates was determined by atomic absorption spectroscopy (acetylene/air-flame, AAnalyst 100, Perkin Elmer, Waltham, MA, USA).
2.4 Data evaluation
A modified version of the Gompertz model36 was fitted to the P release curves to describe the changes in the P release rate (eqn (1)). The model has been previously used to fit sigmoidal kinetics in different research areas from bacterial growth to microbial product formation and re-dissolution processes.36–39 In eqn (1), the term Amax [mmol P L−1] represents the upper asymptote of P release (saturation value), μ [mmol P (L−1 min−1)] the maximum release rate, λ [min] the lag phase, and t [min] the time after supplementation. Model-fits were obtained by minimizing the sum of squared residues between observed and model-predicted values using the MS Excel SOLVER add-in. Quality of the model-fit was assessed using the coefficient of determination (R2).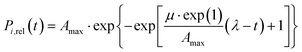 | (1) |
3 Results and discussion
In the investigated WWTP, P is removed from the influent in an EBPR process supported by the additional dosage of ferrous chloride to meet the strict effluent standards. The P content in the final stabilized sewage sludge exceeds 20 mg P gTSS−1. Thus, the WWTP falls under the obligation to recover P as of 2029.17During the sampling period, the mixed liquor AS suspension from the outlet of the aerated biological treatment stage had a stable TSS concentration of (6.3 ± 0.5) g L−1 and the VSS/TSS ratio was 0.65. The COD (determined in the supernatant of WWTP settling tanks after aerobic treatment) was around 17 mg L−1. The dissolved ortho-P concentration of the aqueous phase of AS was (0.10 ± 0.03) mg P L−1. Acetate was not detected in the aqueous phase. Total P, Fe, Ca, K and Mg content in the AS dry matter was (36.21 ± 3.39) mg P gTSS−1, (40.83 ± 10.84) mg Fe gTSS−1, (16.14 ± 5.89) mg K gTSS−1, (15.11 ± 0.95) mg Ca gTSS−1, and (6.60 ± 2.77) mg Mg gTSS−1, respectively.
3.1 Effect of carbon source on P re-dissolution
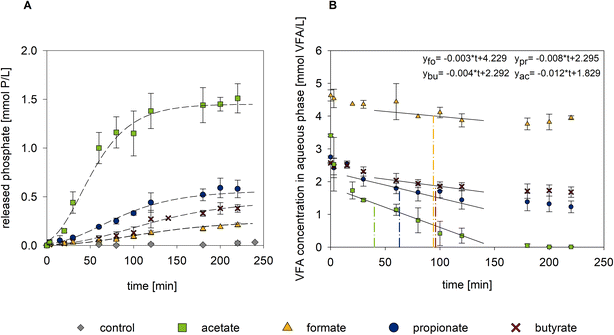
We examined the short-term effects of different VFAs on the P re-dissolution from AS under laboratory conditions. VFAs were supplied to the AS batches as 200 mg L−1 spikes. The pH of the AS suspension was around 7.05 ± 0.08 at all times.The P re-dissolution kinetics observed upon supplementation of the different carbon sources are shown in Fig. 1B and the parameters of the Gompertz model are summarized in ESI† Table S1. The error bars indicate only small variation among the biological replicates. The non-supplemented control showed a minimal P release of 0.03 mmol P L−1 within the experimental period and no VFAs (formate, acetate, propionate or butyrate) were detected. By contrast, all VFA-spiked batches, released P in the form of ortho-P. The P re-dissolution decreased in the order acetate > propionate > butyrate > formate. In the acetate-supplemented batches, P release started immediately after spiking and exhibited a high release rate of 17.20 × 10−3 mmol P (L−1 min−1) (see values of μ in ESI† Table S1). For propionate, butyrate and formate spiking resulted in lower P release rates of 4.29 × 10−3, 2.58 × 10−3 and 1.41 × 10−3 mmol P (L−1 min−1), respectively. The onset of P release was delayed by a 15–30 min lag phase (see values of λ in ESI† Table S1). Substrate consumption varied with the type of carbon source (Fig. 1B). While acetate was rapidly and entirely consumed, the cumulative uptake of propionate, butyrate and formate amounted to 55%, 34%, and 15% of the supplementation level, respectively. Complete acetate consumption suggested that under anaerobic conditions all of the carbon source was utilized by PAOs or the competing GAO population. This is in accordance with literature reporting on acetate utilization during anaerobic cell maintenance of Accumulibacter40,41 and supports the findings of Lemos et al.16 on the suitability of acetate for PHA production. High P re-dissolution upon acetate supplementation agrees with the abundance of this VFA in the influent of WWTPs26,42 whereby sludge organisms are likely adapted to utilize this carbon source.
| Supplemented VFA | Prelease/Cuptakea,b [mol P mol C−1] | Pyield/VFAspikeb [mol P mol VFA−1] | Pyield/VFAconsumedb [mol P mol VFA−1] |
|---|---|---|---|
a Calculated at the timing of maximum P release rate μ (model-fit) obtained with the second derivative 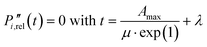 .
b For further information on indicator calculation please refer to the ESI.† .
b For further information on indicator calculation please refer to the ESI.†
|
|||
| Formate | 0.15 | 0.05 | 0.31 |
| Acetate | 0.13 | 0.45 | 0.45 |
| Propionate | 0.07 | 0.21 | 0.39 |
| Butyrate | 0.06 | 0.15 | 0.43 |
The Prelease/Cuptake ratio was higher with formate (0.15 mol P mol C−1) and acetate (0.13 mol P mol C−1) than with propionate (0.07 mol P mol C−1) and butyrate (0.06 mol P mol C−1).
In previous studies on full-scale acetate-fed EBPR systems, the Prelease/Cuptake ratio was in the range of 0.29–0.66 mol P mol C−1.24,42,43 Compared to this, our Prelease/Cuptake ratio with acetate was lower. This could be due to a higher abundance of GAOs competing with PAOs for available substrate, as members of both lineages have similar acetate uptake rates.26,42 Alternatively, carbon consumption by other groups of microorganisms is also possible. Predominance of GAO over PAO is unlikely given that the abundance of Candidatus Competibacter spp. amounts to 2% of the total bacterial population which is clearly below the PAO Accumulibacter abundance (9% of the total bacterial population).22 More importantly, acetate uptake in combination with glycogen catabolism for PHA formation is not exclusive to GAOs, but also PAOs can utilize the glycogen pool depending on their metabolic state and energy storage pool.14,20,44,45 Therefore, in our case, the Prelease/Cuptake may have been limited by low polyP hydrolysis and a mixed polyP-/glycogen-based metabolism of PAOs rather than by the competing GAOs themselves. Generally, the Prelease/Cuptake indicator is suited to characterize within-study effects of substrate utilization rather than for comparisons among different studies. In the latter case, comparability may be particularly hampered if microbial community data are lacking.
With propionate, the Prelease/Cuptake ratio was only half that of acetate and the rate of propionate uptake was lower (acetate: 42.73 μmol C (L−1 min−1), propionate: 26.26 μmol C (L−1 min−1) during the first 2 h). The reduced rate of propionate utilization is in line with the inability of Candidatus Competibacter spp. to take up propionate at standard conditions (20 °C, pH 7),26,27 while Accumulibacter PAOs possess a similar preference towards uptake of both acetate and propionate.46
The P release rate was further reduced in the case of supplementation with formate and butyrate, with similar profiles throughout the experiment. Only few studies have examined the effect of formate on P re-dissolution10,47,48 and anaerobic formate utilization by PAOs has so far been ruled out.10 In our study, uptake of formate went along with moderate P re-dissolution, indicating PAO activity. Direct formate utilization for polyhydroxybutyrate (PHB) synthesis by PAOs is unlikely since formate would need to undergo carboxylation to form acetyl-CoA as a precursor.47 We hypothesize that formate was metabolized to PHA via the glycine–serine interconversion through glycine and acetyl-CoA. The reductive glycine pathway is a possible route for carbon fixation in formate assimilating organisms.49 Recently, it was shown that glycine can induce P release from PAOs.15,50,51Accumulibacter15 and Tetrasphaera spp.50 were able to consume glycine with and without PHA synthesis, respectively. Oyserman et al.15 found that acetate induced the expression of genes involved in the anaerobic glycine metabolism in Accumulibacter. In our case, these genes may have been readily active at the time of AS sampling and may have allowed formate utilization. Otherwise, formate itself may have induced gene expression. Low uptake of formate might be explained by feedback inhibition to avoid high intracellular concentrations of substrate or conversion products.
For butyrate, an initial adaptation period (lag-phase) preceded P re-dissolution. While the butyrate concentration decreased at a rate of 25.5 μmol C (L−1 min−1) during the first 2 h, P release started 30 min after supplementation. Butyrate may directly be used for PHB formation or indirectly via intermediates produced by Tetrasphaera spp. or other sludge bacteria.9 In any case, concomitant P re-dissolution can be expected. Dependence of the involved lineages may also explain contradictory results regarding the utilization of butyrate in previous literature.10,16 In our study, the batches of the butyrate supplementation were devoid of other VFA, which may indicate that potential by-products like acetate were immediately taken up. Since the acetate uptake rate was most likely higher than its production from butyrate, the lack of observable VFA formation within the experimental time frame of 220 min is reasonable.
Literature suggests that substrate uptake rates and utilization efficiencies differ not only due to PAO–GAO competition but also within specific groups and lineages within either type of organism.41 This precludes the elucidation of metabolic pathways when real and non-acclimated AS is studied, as was the case in our study. Under these conditions the Prelease/Cuptake ratio may be ambiguous. Regarding the cost–benefit state of a full-scale process implementation, the Pyield/VFAspike as well as the total Pyield/VFAconsumed may be useful indicators. Spike efficiencies of formate, acetate, propionate and butyrate were 0.05, 0.45, 0.21 and 0.15 mol P mol VFAspike−1, respectively (Table 1). Compared to acetate, spike efficiency was reduced by 53–89% for the other tested substrates. The ratio of Pyield/VFAconsumed decreased in the order acetate > butyrate > propionate > formate. For acetate and propionate, the values of Pyield/VFAspike and Pyield/VFAconsumed were close. We propose that this could be used as an indication for P re-dissolution combined with efficient substrate usage. Identical values for acetate confirm the superiority of this VFA in terms of P release and substrate utilization. From an engineering perspective and regarding process economics we suggest the use of Pyield/VFAspike and Pyield/VFAconsumed in future studies on the optimization of P recovery from AS.
3.2 Effect of acetate supplementation level on P re-dissolution
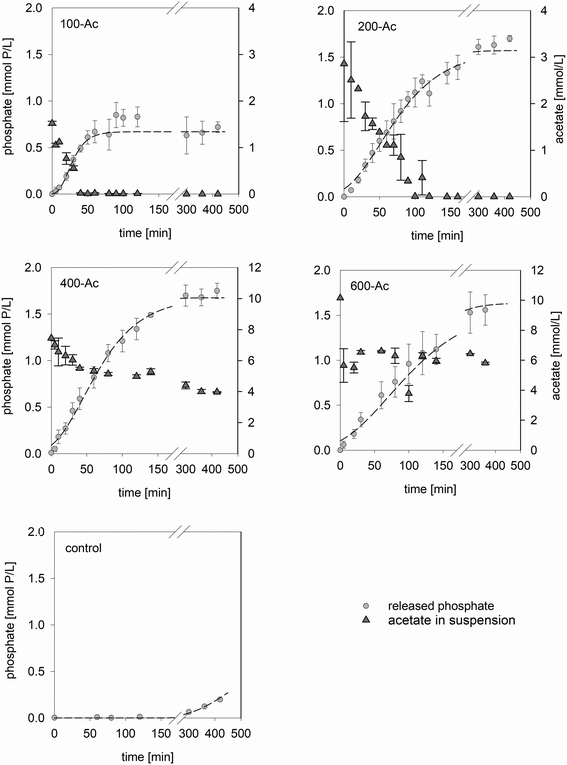
Having seen that supplementation with acetate yielded the highest P re-dissolution from AS, we studied the effects of acetate dosage by varying the spike levels between 100 and 600 mg L−1, henceforth referred to as 100-Ac, 200-Ac, 400-Ac and 600-Ac. Irrespective of the initial acetate spike level, the pH and DO concentration were comparable among the batches and were broadly constant over time. The pH was between 6.8 and 7.1 and the DO was virtually zero. The EH dropped from 320–200 mV (values reported against standard hydrogen electrode) to below 50 mV within 60 min of incubation indicating that, ultimately, anaerobic conditions prevailed in all batches.All spike levels induced a re-dissolution of intracellular P into the aqueous phase (Fig. 2). Variation among the biological replicates of the same spike level was small, demonstrating that independent samples collected during the normal WWTP operation responded reproducibly. This is important in terms of a potential full-scale implementation as a P recovery scheme. The control batch without acetate supplementation exhibited no P release over a period of 300 min but showed low concentrations of dissolved P towards the end of the experiment (Control, Fig. 2). The latter may have resulted from cell decay. In agreement with previous studies,20,26 we observed sigmoidal P re-dissolution curves (Fig. 2). Again, P release kinetics could be satisfactorily described using the modified Gompertz model (eqn (1)). The best-fit parameters are shown in Table 2. The modeled maximum P release rate μ was 0.02 mmol P (L−1 min−1) for 100-Ac and 0.01 mmol P (L−1 min−1) for 200-Ac through 600-Ac. As opposed to the sigmoidal P re-dissolution, the acetate uptake in 100-Ac and 200-Ac was broadly linear over time and led to a full consumption of the spike at a rate of 26 μmol acetate (L−1 min−1). Full acetate consumption occurred within 60 min and 100 min, respectively. In 100-Ac the fast depletion resulted in a short-term P release from AS. Increasing the acetate spike from 100 mg L−1 to 200 mg L−1 led to a prolonged and two times higher P re-dissolution. The maximum P yield of (1.54 ± 0.09) mmol P L−1 was reached after 300 min of incubation. Increasing the acetate spike beyond 200 mg L−1 (400-Ac and 600-Ac, Fig. 2) did not increase the P yield further nor did it result in faster release kinetics. In the respective batches, more than 50% of the supplemented acetate remained in the aqueous phase at the end of the experiment (no substrate limitation). Inhibitory effects due to undissociated acetic acid are unlikely since the circumneutral pH of the batches was above its pKa of 4.76.
| 100-Ac | 200-Ac | 400-Ac | 600-Ac | Control | |
|---|---|---|---|---|---|
| A max [mmol P L−1] | 0.73 | 1.54 | 1.68 | 1.63 | 0.92 |
| μ [mmol P (L−1 min−1)] | 0.02 | 0.01 | 0.01 | 0.01 | 0.002 |
| λ [min] | 7.72 | 5.76 | 0.98 | 0.88 | 309.80 |
| R 2 | 0.997 | 0.989 | 0.993 | 0.985 | 0.982 |
Overall, results show, that P re-dissolution was influenced by the acetate spike level but was also limited by the ability of AS to completely take up acetate at higher concentrations. Substrate limiting conditions may have prevailed in 100-Ac. The final P release was very similar for acetate levels equal to or greater than 200-Ac. In 400-Ac and 600-Ac, P re-dissolution may have been limited by acetate uptake capacity or by available polyP content of the cells. In 400-Ac and 600-Ac, acetate uptake levelled off with increasing P re-dissolution. This is in line with previous observations on saturation of uptake and storage capacity for acetate in PAOs after 2–3 h (ref. 10 and 12) and reduction of PHA synthesis at high PHA concentrations.52 In total the uptake of around 3 mol acetate yielded 1.5–1.7 mol P. It is likely that the ratio of P release and acetate uptake was influenced by the ratio of PAO and GAO levels in the sewage sludge, as both groups of organisms use acetate but only PAOs release P.23,24,26 Please note in Fig. 2 that P re-dissolution and substrate uptake were not synchronous. Specifically, P release continued after acetate uptake ceased or acetate was fully consumed. A similar phenomenon was also reported by Pijuan et al.53 and Puig et al.,54 who observed a secondary P release rate. The authors hypothesized that this was caused by additional polyP hydrolysis to cover energy demands of cellular maintenance. However, in our case this explanation is not valid since the control batch exhibited increasing levels of P release after 300 min, only. Therefore, we propose the following model.
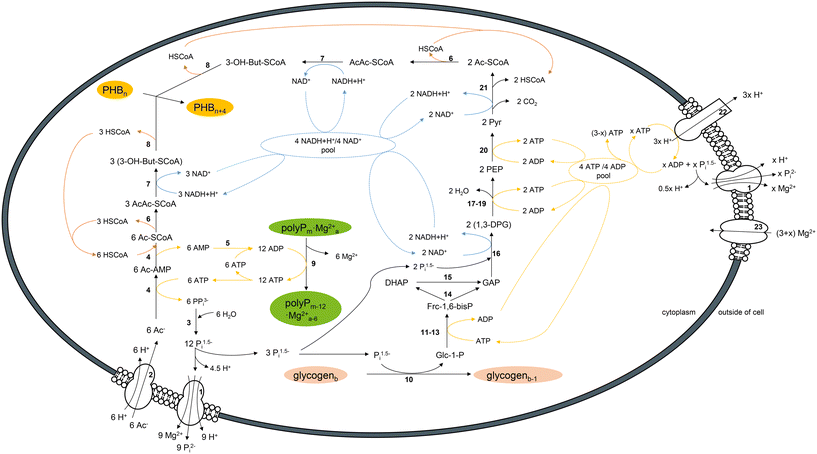 | ||
| Fig. 3 Conceptual model and integrated balance for the main flows of carbon, P, reducing equivalents and energy during polyP hydrolysis and glycogen degradation for PHB synthesis during acetate-induced P re-dissolution in PAOs. Carbon and P routes are presented in black, flows of NAD+/NADH + H+ and AMP/ADP/ATP are presented in blue and yellow, respectively. Numbers indicate involved enzymes and transporter proteins (see ESI† Table S2). Abbreviations: 3-hydroxybutyryl-CoA (3-OH-But-SCoA), 1,3-bisphosphoglycerate (1,3-DPG), acetate (Ac), acetoacetyl-CoA (AcAc-SCoA), acetyl-AMP (Ac-AMP), acetyl-CoA (Ac-SCoA), coenzyme A (HSCoA), dihydroxyacetone phosphate (DHAP), fructose-1,6-bis-phosphate (Frc-1,6-bisP), glucose-1-phosphate (Glc-1-P), glyceraldehyde 3-phosphate (GAP), phosphoenolpyruvate (PEP), pyruvate (Pyr). | ||
The observed P re-dissolution is primarily a result of ATP/AMP conversion during acetate assimilation and acetyl-CoA formation. Therein, each mol acetate consumes 1 mol ATP, obtained by cleavage of polyP units. The by-product pyrophosphate is hydrolyzed to Pi, which is released from the cell or used for glycogen degradation. Acetate uptake occurs through an acetate permease (ActP) and is driven by the proton motive force (pmf).56,57 It has been suggested that pmf can be generated by proton efflux with transport of Pi and counter cations (e.g. Mg2+) via the low affinity inorganic phosphate transporter (pit).6,15,55 We suggest that, per mol P, 0.5 mol Mg2+ can be obtained from polyP (see ESI†). Fig. 3 shows that Mg requirements for efflux of Pi cannot be compensated through polyP hydrolysis alone but may require extracellular Mg uptake.
Reducing equivalents are required for 3-hydroxybutyryl-CoA formation from 2 mol acetyl-CoA and elongation of one unit of the PHB polymer. These could potentially originate from the TCA cycle and/or from glycolysis.4–6 Maintenance of the TCA cycle under anaerobic conditions requires a re-oxidation of quinone, e.g. by a proposed novel cytochrome complex.6 Alternatively, here we focused on glycogen degradation as a source for reducing equivalents. Therein, degradation of 1 mol of glucose produces 3 mol of NADH + H+. These are available for PHB formation from assimilated acetate. During glycogen degradation to pyruvate by the Embden–Meyerhof–Parnas pathway 3 mol ATP are provided if glucose and glyerinealdehyde-3-phosphate are phosphorylated with Pi originating from polyP degradation. A maximum of 3 ATP is available for cellular maintenance such as generating pmf by reverse H+-ATPase activity and additional P release. Intracellular accumulation of ATP may eventually lead to inhibition of glycogen degradation.
We propose the following chemical eqn (2) for the conversion of educts
| 6Ac−(out) + 6H+(out) + polyPm·Mg2+a(in) + glycogenb(in) + PHBn(in) → (9 + x)P2−i(out) + 6Mg2+(out) + (9 + 4x)H+(out) + polyPm−12·Mg2+a−6(in) + glycogenb−1(in) + PHBn+4(in) | (2) |
With Ac−/H+ uptake, 6 mol positive charge equivalents are transported into the cell and with P2−i release 18 to 24 mol negative and 21 to 33 mol positive (H+ and Mg2+) charge equivalents are transported to the outside, depending on ATPase activity. Compared to the cytoplasm, the outer cell will become positively charged which disrupts cell potential and halts the metabolism. As glycogen degradation is repressed, internal Pi previously used for the induction of glycogen cleavage58 can be released thus explaining the P re-dissolution after cessation of acetate uptake. As an alternative to disruption of pmf, the metabolism may also be halted by (i) polyP limitation and subsiding NADH requirement, (ii) glycogen limitation or (iii) ATP accumulation.
We propose the above metabolic model as a hypothesis that may explain, why P release continued after full substrate consumption or after cessation of substrate uptake. To validate the model, future experiments should include pertinent analyses (e.g. Mg2+, Ca2+, intracellular glycogen concentration, enzyme activity). Possibly, validation also would need to be performed in less buffered systems to verify the proposed net proton release.
4 Implications and conclusion
EBPR-CPR sludge from a full-scale WWTP was investigated in terms of P re-dissolution under supplementation with various carbon sources. Results show that acetate had the highest efficiency for a fast P re-dissolution. An acetate spike level of 200 mg L−1 proved best in terms of substrate utilization and P yield, while higher acetate levels seemed to exhaust the P re-dissolution capacity. For all spike levels, P re-dissolution extended beyond the uptake of acetate. Known metabolic pathways of PAO were integrated into a conceptual model that accounts for the stoichiometric balance of carbon, P, reducing equivalents and ATP flows. The model suggests that the extended P re-dissolution may be explained by the halting of glycogen degradation.Although additional CPR is believed to hamper P recovery from AS it still allowed for a partial microbial P re-dissolution. Acetate treatment recovered 7.6–8.6 mg P gTSS−1 or 11.7–13.2 mg P gVSS−1 which equaled 21–24% of the total sludge P content. With the applied strategy of acetate-induced P re-dissolution, yields are limited to the recycling of P from the intracellular polyP pool at maximum. Our previous results showed, that also with pure EBPR sludge from full-scale plants (excluding the influence of CPR) a complete re-dissolution of sludge P was not possible.22 By supplementation of 0.07 g gTSS−1 (ref. 22) up to 0.23 g gTSS−1 (unpublished data) acetate the re-dissolved P yield was 56–60% of total sludge P. This limitation may be a protective mechanism to avoid extensive PAO activity loss. Thus, EBPR performance may be sustained by recycling leached AS into the aerobic stage to re-new aerobic P uptake. A maximum P recovery value of 60% of influent P has been proposed by Zhang et al.32 allowing for stable PAO activity and EBPR operation. We hypothesize that repeated re-dissolution cycles may also improve adaption of PAOs to ultimately meet the regulatory P recovery limits, provided the use of chemical precipitants is reduced. This may suggest that in the future WWTP operators reduce the use of chemical precipitants or implement a post-EBPR precipitation step to allow higher P re-dissolution efficiencies and practical application of the process in WWTPs. In terms of substrate availability, a biological re-dissolution approach with acetate seems promising, since acetate is often produced during pre-fermentation or anaerobic sludge digestion. This may be boosted by addition of low-cost carbon sources such as fermentable industrial or food waste.
For on-site integration and final P recovery, the re-dissolution process needs to be followed by a phase separation step (e.g. sedimentation). The P-depleted AS may be returned to the aerobic stage of the WWTP and the P-enriched aqueous phase may be used to precipitate a P-fertilizer. Ongoing work is related to the implementation of a pilot-scale fluidized bed reactor where milk of lime is used to increase the pH and recover a calcium phosphate as proposed in Anders et al.22
Author contributions
Annika Anders: conceptualization, investigation, formal analysis, visualization, writing – original draft, review & editing. Harald Weigand: conceptualization, writing – review & editing, supervision, funding acquisition. Harald Platen: conceptualization, writing – review & editing, supervision, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded in the framework of the Industrial Collective Research program (IGF) [Re-Bio-P-Cycle, grant number 19746N], supported by the Federal Ministry for Economic Affairs and Energy (BMWi) through the AiF (German Federation of Industrial Research Associations eV) based on a decision taken by the German Bundestag. Additional funding was received by the Strategic Research Fund of the THM University of Applied Sciences. The authors thank the WWTP Klärwerk Giessen-Mittelhessische Wasserbetriebe for allowing sludge sampling and data collection. We thank Lisa Voigt, Nicolai Bannwitz and Lukas Künkel for their practical support and Frank Ohnemüller and Harun Cakir for valuable discussions in the frame of our research project.References
- R. Li and X. Li, Recovery of phosphorus and volatile fatty acids from wastewater and food waste with an iron-flocculation sequencing batch reactor and acidogenic co-fermentation, Bioresour. Technol., 2017, 245, 615–624 CrossRef CAS.
- T. Mino, M. C. M. van Loosdrecht and J. J. Heijnen, Microbiology and biochemistry of the enhanced biological phosphate removal process, Water Res., 1998, 32, 3193–3207 CrossRef CAS.
- G. W. Fuhs and M. Chen, Microbiological basis of phosphate removal in the activated sludge process for the treatment of wastewater, Microb. Ecol., 1975, 2, 119–138 CrossRef CAS PubMed.
- Y. Comeau, K. J. Hall, R. E. W. Hancock and W. K. Oldham, Biochemical model for enhanced biological phosphorus removal, Water Res., 1986, 20, 1511–1521 CrossRef CAS.
- T. Mino, V. Arun, Y. Tsuzuki and T. Matsuo, Effect of phosphorus accumulation on acetate metabolism in the biological phosphorus removal process, in Biological phosphate removal from wastewaters, ed. R. Ramadori, Pergamon Press, Oxford, 1987, pp. 27–38 Search PubMed.
- H. G. Martín, N. Ivanova, V. Kunin, F. Warnecke, K. W. Barry, A. C. McHardy, C. Yeates, S. He, A. A. Salamov, E. Szeto, E. Dalin, N. H. Putnam, H. J. Shapiro, J. L. Pangilinan, I. Rigoutsos, N. C. Kyrpides, L. L. Blackall, K. D. McMahon and P. Hugenholtz, Metagenomic analysis of two enhanced biological phosphorus removal (EBPR) sludge communities, Nat. Biotechnol., 2006, 24, 1263–1269 CrossRef PubMed.
- E. Y. Fernando, S. J. McIlroy, M. Nierychlo, F.-A. Herbst, F. Petriglieri, M. C. Schmid, M. Wagner, J. L. Nielsen and P. H. Nielsen, Resolving the individual contribution of key microbial populations to enhanced biological phosphorus removal with Raman–FISH, ISME J., 2019, 13, 1933–1946 CrossRef CAS PubMed.
- M. Stokholm-Bjerregaard, S. J. McIlroy, M. Nierychlo, S. M. Karst, M. Albertsen and P. H. Nielsen, A critical assessment of the microorganisms proposed to be important to enhanced biological phosphorus removal in full-scale wastewater treatment systems, Front. Microbiol., 2017, 8, 1–18 Search PubMed.
- R. Kristiansen, H. T. T. Nguyen, A. M. Saunders, J. L. Nielsen, R. Wimmer, V. Q. Le, S. J. McIlroy, S. Petrovski, R. J. Seviour, A. Calteau, K. L. Nielsen and P. H. Nielsen, A metabolic model for members of the genus Tetrasphaera involved in enhanced biological phosphorus removal, ISME J., 2013, 7, 543–554 CrossRef CAS PubMed.
- Y. Kong, J. L. Nielsen and P. H. Nielsen, Microautoradiographic study of Rhodocyclus-related polyphosphate-accumulating bacteria in full-scale enhanced biological phosphorus removal plants, Appl. Environ. Microbiol., 2004, 70, 5383–5390 CrossRef CAS PubMed.
- Y. Kong, J. L. Nielsen and P. H. Nielsen, Identity and ecophysiology of uncultured actinobacterial polyphosphate-accumulating organisms in full-scale enhanced biological phosphorus removal plants, Appl. Environ. Microbiol., 2005, 71, 4076–4085 CrossRef CAS PubMed.
- H. T. T. Nguyen, V. Q. Le, A. A. Hansen, J. L. Nielsen and P. H. Nielsen, High diversity and abundance of putative polyphosphate-accumulating Tetrasphaera-related bacteria in activated sludge systems, FEMS Microbiol. Ecol., 2011, 76, 256–267 CrossRef CAS.
- B. Acevedo, M. Murgui, L. Borrás and R. Barat, New insights in the metabolic behaviour of PAO under negligible poly-P reserves, Chem. Eng. J., 2017, 311, 82–90 CrossRef CAS.
- L. Welles, B. Abbas, D. Y. Sorokin, C. M. Lopez-Vazquez, C. M. Hooijmans, M. C. M. van Loosdrecht and D. Brdjanovic, Metabolic response of “Candidatus Accumulibacter Phosphatis” clade II C to changes in influent P/C ratio, Front. Microbiol., 2017, 7, 2121 Search PubMed.
- B. O. Oyserman, D. R. Noguera, T. G. Del Rio, S. G. Tringe and K. D. McMahon, Metatranscriptomic insights on gene expression and regulatory controls in Candidatus Accumulibacter phosphatis, ISME J., 2016, 10, 810–822 CrossRef CAS.
- P. C. Lemos, C. Viana, E. N. Salgueiro, A. M. Ramos, J. P. S. G. Crespo and M. A. M. Reis, Effect of carbon source on the formation of polyhydroxyalkanoates (PHA) by a phosphate-accumulating mixed culture, Enzyme Microb. Technol., 1998, 22, 662–671 CrossRef CAS.
- AbfKlärV, Verordnung über die Verwertung von Klärschlamm, Klärschlammgemisch und Klärschlammkompost: Klärschlammverordnung (Ordinance on the utilization of sewage sludge, sewage sludge mixtures and sewage sludge compost: Sewage Sludge Ordinance), BGBl, 2017, pp. 3465–3511 Search PubMed.
- C. Kabbe and S. Rinck-Pfeiffer, Global compendium on phosphorus recovery from sewage/sludge/ash, London Global Water Research Coalition, 2019 Search PubMed.
- A. Amann, O. Zoboli, J. Krampe, H. Rechberger, M. Zessner and L. Egle, Environmental impacts of phosphorus recovery from municipal wastewater, Resour., Conserv. Recycl., 2018, 130, 127–139 CrossRef.
- B. Acevedo, C. Camiña, J. E. Corona, L. Borrás and R. Barat, The metabolic versatility of PAOs as an opportunity to obtain a highly P-enriched stream for further P-recovery, Chem. Eng. J., 2015, 270, 459–467 CrossRef CAS.
- C.-W. Xia, Y.-J. Ma, F. Zhang, Y.-Z. Lu and R. J. Zeng, A novel approach for phosphorus recovery and no wasted sludge in enhanced biological phosphorus removal process with external COD addition, Appl. Biochem. Biotechnol., 2014, 172, 820–828 CrossRef CAS PubMed.
- A. Anders, H. Weigand, H. Cakir, U. Kornhaas and H. Platen, Phosphorus recycling from activated sludge of full-scale wastewater treatment plants by fast inversion of the biological phosphorus elimination mechanism, J. Environ. Chem. Eng., 2021, 106403 CrossRef CAS.
- J. S. Cech and P. Hartman, Competition between polyphosphate and polysaccharide accumulating bacteria in enhanced biological phosphate removal systems, Water Res., 1993, 27, 1219–1225 CrossRef CAS.
- A. M. Saunders, A. Oehmen, L. L. Blackall, Z. Yuan and J. Keller, The effect of GAOs (glycogen accumulating organisms) on anaerobic carbon requirements in full-scale Australian EBPR (enhanced biological phosphorus removal) plants, Water Sci. Technol., 2003, 47, 37–43 CrossRef CAS.
- A. Oehmen, M. Teresa Vives, H. Lu, Z. Yuan and J. Keller, The effect of pH on the competition between polyphosphate-accumulating organisms and glycogen-accumulating organisms, Water Res., 2005, 39, 3727–3737 CrossRef CAS PubMed.
- A. Oehmen, Z. Yuan, L. L. Blackall and J. Keller, Comparison of acetate and propionate uptake by polyphosphate accumulating organisms and glycogen accumulating organisms, Biotechnol. Bioeng., 2005, 91, 162–168 CrossRef CAS PubMed.
- M. Carvalheira, A. Oehmen, G. Carvalho and M. A. M. Reis, The effect of substrate competition on the metabolism of polyphosphate accumulating organisms (PAOs), Water Res., 2014, 64, 149–159 CrossRef CAS.
- C. M. Lopez-Vazquez, C. M. Hooijmans, D. Brdjanovic, H. J. Gijzen and M. C. M. van Loosdrecht, Temperature effects on glycogen accumulating organisms, Water Res., 2009, 43, 2852–2864 CrossRef CAS PubMed.
- Y. Liu, H. Shi, W. Li, Y. Hou and M. He, Inhibition of chemical dose in biological phosphorus and nitrogen removal in simultaneous chemical precipitation for phosphorus removal, Bioresour. Technol., 2011, 102, 4008–4012 CrossRef CAS PubMed.
- I. Röske and C. Schönborn, Interactions between chemical and advanced biological phosphorus elimination, Water Res., 1994, 28, 1103–1109 CrossRef.
- P. Wilfert, P. S. Kumar, L. Korving, G.-J. Witkamp and M. C. M. van Loosdrecht, The relevance of phosphorus and iron chemistry to the recovery of phosphorus from wastewater: A review, Environ. Sci. Technol., 2015, 49, 9400–9414 CrossRef CAS.
- C. Zhang, A. Guisasola and J. A. Baeza, A review on the integration of mainstream P-recovery strategies with enhanced biological phosphorus removal, Water Res., 2022, 212, 118102 CrossRef CAS PubMed.
- HMUKLV-Hessisches Ministerium für Umwelt Klimaschutz Landwirtschaft und Verbraucherschutz, Maßnahmenprogramm 2015–2021 zu Umsetzung der EG-Wasserrahmenrichtlinie in Hessen, ISBN 978-3-89274-380-4, Wiesbaden, Germany, 2015 Search PubMed.
- APHA, Standard methods for the examination of water and wastewater, American Public Health Association, American Water Works Association, Water Environment Federation, Washington DC, 20th edn, 1999, vol. 2 Search PubMed.
- CEN, DIN EN 16174:2012:11 Sludge, treated biowaste and soil-Digestion of aqua regia soluble fractions of elements, European committee for standardization, Brussels, 2012 Search PubMed.
- M. H. Zwietering, I. Jongenburger, F. M. Rombouts and K. van't Riet, Modeling of the bacterial growth curve, Appl. Environ. Microbiol., 1990, 56, 1875–1881 CrossRef CAS PubMed.
- J. Li, J. D. Gu and L. Pan, Transformation of dimethyl phthalate, dimethyl isophthalate and dimethyl terephthalate by Rhodococcus rubber Sa and modeling the processes using the modified Gompertz model, Int. Biodeterior. Biodegrad., 2005, 55, 223–232 CrossRef CAS.
- J. Wang and W. Wan, Kinetic models for fermentative hydrogen production: A review, Int. J. Hydrogen Energy, 2009, 34, 3313–3323 CrossRef CAS.
- D. M. Easton, Gompertz pharmacokinetic model for drug disposition, Pharm. Res., 2002, 19, 463–469 CrossRef CAS.
- P. H. Nielsen, A. T. Mielczarek, C. Kragelund, J. L. Nielsen, A. M. Saunders, Y. Kong, A. A. Hansen and J. Vollertsen, A conceptual ecosystem model of microbial communities in enhanced biological phosphorus removal plants, Water Res., 2010, 44, 5070–5088 CrossRef CAS PubMed.
- A. Oehmen, G. Carvalho, C. M. Lopez-Vazquez, M. C. M. van Loosdrecht and M. A. M. Reis, Incorporating microbial ecology into the metabolic modelling of polyphosphate accumulating organisms and glycogen accumulating organisms, Water Res., 2010, 44, 4992–5004 CrossRef CAS PubMed.
- C. M. López-Vázquez, C. M. Hooijmans, D. Brdjanovic, H. J. Gijzen and M. C. M. van Loosdrecht, A Practical Method for Quantification of Phosphorus- and Glycogen-Accumulating Organism Populations in Activated Sludge Systems, Water Environ. Res., 2007, 79, 2487–2498 CrossRef PubMed.
- G. Qiu, R. Zuniga-Montanez, Y. Law, S. S. Thi, T. Q. N. Nguyen, K. Eganathan, X. Liu, P. H. Nielsen, R. B. H. Williams and S. Wuertz, Polyphosphate-accumulating organisms in full-scale tropical wastewater treatment plants use diverse carbon sources, Water Res., 2019, 149, 496–510 CrossRef CAS PubMed.
- S. Kolakovic, E. B. Freitas, M. A. M. Reis, G. Carvalho and A. Oehmen, Accumulibacter diversity at the sub-clade level impacts enhanced biological phosphorus removal performance, Water Res., 2021, 199, 117210 CrossRef CAS PubMed.
- N. Majed, T. Chernenko, M. Diem and A. Z. Gu, Identification of functionally relevant populations in enhanced biological phosphorus removal processes based on intracellular polymers profiles and insights into the metabolic diversity and heterogeneity, Environ. Sci. Technol., 2012, 46, 5010–5017 CrossRef CAS PubMed.
- A. Oehmen, R. J. Zeng, Z. Yuan and J. Keller, Anaerobic metabolism of propionate by polyphosphate-accumulating organisms in enhanced biological phosphorus removal systems, Biotechnol. Bioeng., 2005, 91, 43–53 CrossRef CAS PubMed.
- Z. H. Abu-Ghararah and C. W. Randall, The effect of organic compounds on biological phosphorus removal, Water Sci. Technol., 1991, 23, 585–594 CrossRef CAS.
- L. C. Burow, Y. Kong, J. L. Nielsen, L. L. Blackall and P. H. Nielsen, Abundance and ecophysiology of Defluviicoccus spp., glycogen-accumulating organisms in full-scale wastewater treatment processes, Microbiology, 2007, 153, 178–185 CrossRef CAS PubMed.
- A. Bar-Even, Formate assimilation: The metabolic architecture of natural and synthetic pathways, Biochemistry, 2016, 55, 3851–3863 CrossRef CAS PubMed.
- H. T. T. Nguyen, R. Kristiansen, M. Vestergaard, R. Wimmer and P. H. Nielsen, Intracellular accumulation of glycine in polyphosphate-accumulating organisms in activated sludge, a novel storage mechanism under dynamic anaerobic-aerobic conditions, Appl. Environ. Microbiol., 2015, 81, 4809–4818 CrossRef CAS.
- Y. Tian, H. Chen, L. Chen, X. Deng, Z. Hu, C. Wang, C. Wei, G. Qiu and S. Wuertz, Glycine adversely affects enhanced biological phosphorus removal, Water Res., 2022, 209, 117894 CrossRef CAS.
- R. Wang, Y. Li, W. Chen, J. Zou and Y. Chen, Phosphate release involving PAOs activity during anaerobic fermentation of EBPR sludge and the extension of ADM1, Chem. Eng. J., 2016, 287, 436–447 CrossRef CAS.
- M. Pijuan, A. M. Saunders, A. Guisasola, J. A. Baeza, C. Casas and L. L. Blackall, Enhanced biological phosphorus removal in a sequencing batch reactor using propionate as the sole carbon source, Biotechnol. Bioeng., 2004, 85, 56–67 CrossRef CAS PubMed.
- S. Puig, M. Coma, H. Monclús, M. C. M. van Loosdrecht, J. Colprim and M. D. Balaguer, Selection between alcohols and volatile fatty acids as external carbon sources for EBPR, Water Res., 2008, 42, 557–566 CrossRef CAS PubMed.
- H. W. van Veen, T. Abee, G. J. J. Kortstee, W. N. Konings and A. J. B. Zehnder, Translocation of metal phosphate via the phosphate inorganic transport system of Escherichia coli, Biochemistry, 1994, 33, 1766–1770 CrossRef CAS PubMed.
- L. C. Burow, A. N. Mabbett, A. G. McEwan, P. L. Bond and L. L. Blackall, Bioenergetic models for acetate and phosphate transport in bacteria important in enhanced biological phosphorus removal, Environ. Microbiol., 2008, 10, 87–98 CAS.
- A. M. Saunders, A. N. Mabbett, A. G. McEwan and L. L. Blackall, Proton motive force generation from stored polymers for the uptake of acetate under anaerobic conditions, FEMS Microbiol. Lett., 2007, 274, 245–251 CrossRef CAS PubMed.
- J. O. Cifuente, N. Comino, B. Trastoy, C. D'Angelo and M. E. Guerin, Structural basis of glycogen metabolism in bacteria, Biochem. J., 2019, 476, 2059–2092 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ew00356b |
| This journal is © The Royal Society of Chemistry 2023 |