Enhanced sulfur resistance by constructing MnOx–Co3O4 interface on Ni foam in the removal of benzene†
Dawei
Han
a,
Menglan
Xiao
b,
Yuechang
Wei
 a,
Xueqin
Yang
d,
Yucong
Guo
b,
Lingjuan
Ma
c,
Xiaolin
Yu
a,
Xueqin
Yang
d,
Yucong
Guo
b,
Lingjuan
Ma
c,
Xiaolin
Yu
 *b and
Maofa
Ge
*b and
Maofa
Ge
 b
b
aState Key Laboratory of Heavy Oil Processing, China University of Petroleum, Beijing 102249, P. R. China
bState Key Laboratory for Structural Chemistry of Unstable and Stable Species, Beijing National Laboratory for Molecular Sciences (BNLMS), CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: icecoolyu@iccas.ac.cn
cSchool of Chemistry and Chemical Engineering, Qufu Normal University, Qufu 273165, P. R. China
dCollege of Forestry, Henan Agricultural University, Zhengzhou 450002, P. R. China
First published on 18th November 2022
Abstract
The catalytic degradation of volatile organic compounds (VOCs) in the presence of SO2 remains an urgent issue for industrial applications. Herein, we constructed an MnOx–Co3O4 interface on Ni foam (MnxCoy–NF catalysts) to improve SO2 resistance for benzene degradation. The surface decoration of MnOx on MnxCoy–NF catalysts could generate a Co–Mn interface to tune the redox ability and active oxygen species. The Mn1Co1–NF catalyst showed high Co3+/Co2+ and Mn3+/Mn4+ ratios as well as a high Olatt/Oads ratio, which are conducive to excellent low-temperature reducibility. Benefiting from abundant interfacial active sites, the Mn1Co1–NF catalyst exhibited superior catalytic activity with T50 and T90 values of 259 and 290 °C and SO2-tolerance for benzene degradation. Results of in situ diffuse reflectance infrared Fourier transform spectroscopy and density functional theory calculation revealed that surface metal sulfate species were preferentially formed on surface Mn sites rather than Co sites, thereby retarding the poisoning of Co–Mn interfacial active sites. Correspondingly, the ring-opening of benzoquinone into maleate species on the Mn1Co1–NF catalyst was only slightly inhibited by the introduction of SO2. This work provides a novel route to design SO2-resistant catalysts for VOC degradation in practical applications.
Environmental significanceVolatile organic compounds (VOCs) pose a serious threat to the environment and human health. Catalytic oxidation is an effective technology to eliminate VOCs. However, the presence of SO2 can lead to the severe deactivation of catalysts due to the preferential reaction of SO2 with active metal oxides to form inactive metal sulfates. Hence, to improve SO2 tolerance, constructing an MnOx–Co3O4 interface can not only improve the efficiency of benzene oxidation, but also effectively prevent SO2 from poisoning the active sites, because of the preferential contact of SO2 with MnOx in catalysts, thereby protecting the active sites from poisoning. This work provides a novel route to design SO2-resistant catalysts for VOC degradation in practical application. |
1. Introduction
Volatile organic compounds (VOCs) are the major factors in environmental pollution, and many VOCs are known to be toxic, causing harm and even death to humans.1–3 Therefore, legitimate control over VOC emissions is one of the top priorities to maintain the sustainable development of human society.4,5 Among the available ways to eliminate VOCs, catalytic oxidation is an effective technology due to its high efficiency, low operating temperature and high product selectivity.6,7 However, the key issue is to explore catalysts with high catalytic activity and superior anti-poisoning for VOC elimination.Transition-metal oxides (TMOs) and their composites have been considered promising candidates for VOC elimination. Among TMOs, Co3O4 and MnOx have received considerable attention owing to their excellent catalytic performance and low cost. Co3O4 has a spinel structure with Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m symmetry, containing Co3+ on octahedral coordination sites (CoOh3+) and Co2+ on tetrahedral coordination sites (CoTd2+), and is widely used in photocatalysis,8,9 electric catalysis9,10 and thermal catalysis.11,12 Because of the relatively low ΔH of vaporization of O2, Co3O4 exhibits high activity for the removal of most VOCs.13 Manganese oxides, such as Mn3O4, Mn2O3, and MnO2, are known for exhibiting relatively high activity in catalytic oxidation.14,15 Compared with single-component catalysts, multi-component composite catalysts generally show excellent catalytic activity due to the synergistic and interfacial effects of the junction interface. Putla et al. reported that an MnOx/CeO2 catalyst exhibited outstanding catalytic activities toward both diesel soot oxidation and benzylamine oxidation compared to a pure CeO2 catalyst, which was due to the catalytically favorable properties at the MnOx/CeO2 interface.16 Liu et al. prepared a 12CoCu–R catalyst with better catalytic activity for acetone oxidation, which was attributed to the fracturing of the Co–O bond by interfacial interaction between Co and Cu.17 Han et al. prepared a series of cobalt/manganese oxides with different Co/Mn ratios through the pyrolysis of CoMn–MOF-71, which exhibited improved catalytic activity for toluene oxidation owing to a synergistic effect.18 In this regard, the fabrication of Co–Mn double component composites is an effective method to improve the low-temperature activity of VOC elimination.
m symmetry, containing Co3+ on octahedral coordination sites (CoOh3+) and Co2+ on tetrahedral coordination sites (CoTd2+), and is widely used in photocatalysis,8,9 electric catalysis9,10 and thermal catalysis.11,12 Because of the relatively low ΔH of vaporization of O2, Co3O4 exhibits high activity for the removal of most VOCs.13 Manganese oxides, such as Mn3O4, Mn2O3, and MnO2, are known for exhibiting relatively high activity in catalytic oxidation.14,15 Compared with single-component catalysts, multi-component composite catalysts generally show excellent catalytic activity due to the synergistic and interfacial effects of the junction interface. Putla et al. reported that an MnOx/CeO2 catalyst exhibited outstanding catalytic activities toward both diesel soot oxidation and benzylamine oxidation compared to a pure CeO2 catalyst, which was due to the catalytically favorable properties at the MnOx/CeO2 interface.16 Liu et al. prepared a 12CoCu–R catalyst with better catalytic activity for acetone oxidation, which was attributed to the fracturing of the Co–O bond by interfacial interaction between Co and Cu.17 Han et al. prepared a series of cobalt/manganese oxides with different Co/Mn ratios through the pyrolysis of CoMn–MOF-71, which exhibited improved catalytic activity for toluene oxidation owing to a synergistic effect.18 In this regard, the fabrication of Co–Mn double component composites is an effective method to improve the low-temperature activity of VOC elimination.
As we all know, an inevitable problem is the SO2-induced deactivation of catalysts for VOC elimination due to the complex composition of fuel gas. SO2 can react with active metal oxides via competitive adsorption to form inactive metal sulfates, resulting in blocking of the active sites.19,20 To improve SO2-tolerance, one strategy is to regulate the surface electronic properties of catalysts; the other approach is to introduce an active phase as a scavenger to preferentially capture SO2.21,22 Xu et al. revealed that V doping into a 13Cu/γ-Al2O3 catalyst exhibited acceptable SO2 resistance of the 13Cu/γ-Al2O3 catalyst for toluene oxidation owing to the synergy between Cu and V oxide.23 Deng et al. confirmed that a W-doped Pt/TiO2 catalyst could inhibit SO2 adsorption and activity, thus improving SO2 resistance for acetone oxidation.24 As previously reported, Mn-based catalysts suffered from severe SO2-induced deactivation because the active phase MnOx was preferentially sulfated by SO2 to form MnSO4.25,26 Thus, it is reasonable to expect the preferential contact of SO2 with MnOx in catalysts, thereby protecting the active sites from poisoning.
In this study, MnxCoy–NF catalysts were successfully fabricated by the pyrolysis of ZIF-67 and impregnation. The Mn1Co1–NF catalyst had abundant interfacial active sites and surface-exposed MnOx, thus exhibiting excellent catalytic activity for benzene as well as SO2 resistance. The mechanism of SO2 resistance was unraveled through in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) spectra and (density functional theory) DFT calculation. This work can benefit the design of catalysts with abundant interfaces and excellent SO2 resistance for the abatement of VOCs.
2. Experimental section
2.1 Materials
All reagents were of analytical grade and used without further purification. Ethyl alcohol and methanol (>99.7% purity) were purchased from Concord Technology. Co(NO3)2·6H2O (99.99% purity) was obtained from Sinopharm Chemical Regent Co., Ltd. 2-Methylimidazole (purity > 98%) was obtained from Saen Chemical Technology (Shanghai) Co., Ltd. Mn(NO3)2·6H2O solution (50 wt%) was obtained from J&K Scientific Ltd.2.2 Preparation of catalysts
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) on the Co3O4–NF catalyst. Then these samples were collected and dried in a vacuum oven. Finally, the samples were calcined at 500 °C for 2 h to obtain the MnxCoy–NF catalysts, which were denoted Mn1Co2–NF, Mn1Co1–NF, and Mn2Co1–NF, respectively, according to the molar ratio of Mn/Co.
2) on the Co3O4–NF catalyst. Then these samples were collected and dried in a vacuum oven. Finally, the samples were calcined at 500 °C for 2 h to obtain the MnxCoy–NF catalysts, which were denoted Mn1Co2–NF, Mn1Co1–NF, and Mn2Co1–NF, respectively, according to the molar ratio of Mn/Co.
2.3 Characterization of catalysts
The X-ray power diffraction (XRD) patterns of the samples were obtained on a Rigaku D/MAX 2500 diffractometer using monochromatized Cu Kα radiation with a scanning range from 5 to 80° and rate of 2 °C min−1. The nitrogen sorption isotherms of the catalysts were conducted with an Autosorb-IQ at 77 K. The morphology and microstructure of these samples were characterized by a field-emission scanning electron microscope (SEM, Hitachi S-4800) and a transmission electron microscope (TEM, HT-7700). Raman spectra of the catalysts were performed with a Lab-RAM spectrometer (HORIBA Jobin Yvon S.A.S.) using a 532 nm laser. X-ray photoelectron spectroscopy (XPS) was performed with an ESCALAB250XI instrument equipped with Al Kα radiation. H2 temperature-programmed reduction (H2-TPR) measures were carried out on Micromeritics AutoChem II 2920. For H2-TPR, the samples were pretreated at 300 °C for 30 min in He flow to remove adsorbed species and then cooled to room temperature. The TPR profiles were obtained with temperatures rising from room temperature to 600 °C at a rate of 10 °C min−1 in 10% H2/He flow. The method for calculating the initial H2 consumption rate is as follows: firstly, the data (time, temperature and corresponding cumulative H2 consumption), where there was less than 25% of the first reduction peak, was calculated and selected. Secondly, the H2 consumption rate, which is designated as the ratio of cumulative H2 consumption to time, can be calculated. Finally, the curve of initial H2 consumption rate can be obtained which takes 1000/(T + 273.15) as the abscissa and the H2 consumption rate as the ordinate. The GC-MS spectra were obtained with GC-MS (7890B-5977B, Agilent Technology, USA). The column used in the study was 30 m × 250 μm × 0.25 μm (19091S-433UI, Agilent, USA). In situ diffuse reflectance infrared Fourier transform spectroscopy (in situ DRIFT) was conducted on a Nicolet iS50 spectrometer in the range of 1000–4000 cm−1 with 32 scans at a resolution of 4 cm−1. For the toluene adsorption experiment, the samples were pretreated at 310 °C in N2 flow (100 mL min−1) for 2 h, and then the background spectrum was recorded. The mixture flow (500 ppm benzene + 20% O2 + N2 balance) was let into the reaction cell at 310 °C, and the in situ DRIFTS spectra were collected. After that, the mixture flow (500 ppm benzene + 20% O2 + 1 ppm SO2 + N2 balanced) was let into the cell and the in situ DRIFTS spectra were collected.2.4 Catalytic activity test
The activity tests for the catalytic oxidation of benzene over the samples were determined in a continuous-flow fixed-bed quartz reactor (i.d. = 4 mm) with 50 mg of catalyst. The reactant feed was 100 ppm benzene, 20% (vol) of O2 and N2 equilibrium gas. The total gas flow rate was 100 mL min−1, and the corresponding weight hourly space velocity (WHSV) was 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1. The conversion of benzene (Xbenzene) was calculated from the inlet benzene concentration (cinlet) and outlet benzene concentration (coutlet) according to eqn (1):
000 mL g−1 h−1. The conversion of benzene (Xbenzene) was calculated from the inlet benzene concentration (cinlet) and outlet benzene concentration (coutlet) according to eqn (1): | (1) |
2.5 DFT calculations
DFT calculations were employed to investigate the contribution of introduced Mn to the adsorption of molecular SO2. The density function theory (DFT) calculations were performed by using the GGA-PBE function and CASTEP module. A 1 × 1 × 1 Monkhorst–Pack k-point sampling density was employed with a plane-wave cutoff energy of 400 eV. The adsorption and desorption energy were calculated with the equation:| Eads = ESO2/surf − Esurf − ESO2 | (2) |
3. Results and discussion
3.1 Catalytic activities
As shown in Fig. 1a, the benzene-catalytic activities of all the catalysts increased with an increase in temperature. For comparison, benzene oxidation was also performed over the pure support Ni foam, revealing that the temperatures of 50% (T50) and 90% (T90) conversion were above 400 °C. However, the Co3O4–NF catalyst obtained by direct calcination of ZIF-67–NF could achieve a T50 of 314 °C and T90 of 348 °C for benzene oxidation. Notably, the introduction of Mn species into the Co3O4–NF catalyst remarkably improved the catalytic activity, and the T50 and T90 values were further lowered to 293 °C and 318 °C (Mn1Co2–NF), 259 °C and 290 °C (Mn1Co1–NF), 274 °C and 300 °C (Mn2Co1–NF), respectively. It is worth noting that both Co and Mn were beneficial to lowering the temperature of benzene oxidation, which could be due to the synergistic effects between Co and Mn species. The Mn1Co1–NF catalyst exhibited the best catalytic performance, presumably due to the largest amount of active interface between Co and Mn. As shown in Fig. 1b, no apparent attenuation of benzene conversion occurred during the 80 h test over the Mn1Co1–NF catalyst, indicating its excellent stability. The apparent activation energy (Ea) of the prepared catalysts in Fig. 1c showed that Co3O4–NF presented an Ea of 105.4 kJ mol−1, notably higher than those of Mn1Co2–NF (92.2 kJ mol−1), Mn1Co1–NF (61.8 kJ mol−1) or Mn2Co1–NF (70.5 kJ mol−1), consistent with the catalytic activity. Conversion of the normalized benzene by surface area (Fig. S2†) was performed to exclude the influence of surface area, indicating that surface area is not the main factor in the catalytic performance.Sulfur compounds and water vapor are general in the process of VOC oxidation. Therefore, the catalytic performance of as-prepared catalysts under 1 ppm SO2 or water vapor was also examined. As shown in Fig. 1d, the on-stream reaction on all catalysts was performed at ca. 90% conversion through controlling the reaction temperature. When the gaseous SO2 was let into the reaction system, the benzene conversion over as-prepared catalysts exhibited an obvious decrease with reaction time. In particular, the NF catalyst was totally deactivated for benzene oxidation within 1 h, and the benzene conversion of Co3O4–NF decreased to ca. 10% after 2 h. Such a large loss in catalytic activity should be due to the strong adsorption of SO2 on the active sites of the catalysts. In contrast, for the MnxCoy–NF catalysts, the decrease in the benzene conversion was retarded. The slope of the curves in Fig. 1d could represent the inactivation rate of the catalysts. Obviously, the inactivation rates of the MnxCoy–NF catalysts were smaller than those of the NF catalyst or Co3O4–NF catalyst, and the Mn1Co1–NF catalyst showed outstanding sulfur resistance. Once the gaseous SO2 had been cut off from the feed gas, the as-prepared catalysts were no longer deactivated, and retained stable benzene conversion except for the NF catalyst. For the NF catalyst, the benzene conversion could be slowly recovered, indicating that the active sites were exposed again due to the removal of adsorbed SO2 from the active sites at a high temperature, which was possibly attributed to the weak interaction of SO2 with Ni foam. It is known that water vapor always exists in exhaust emissions. Many studies have shown that water generally causes a deterioration in catalytic performance, and it is considered a typical poison in VOC oxidation.13,28–30 As shown in Fig. S3,† benzene conversion by the Co3O4–NF and Mn1Co1–NF catalysts decreased with the introduction of water, especially for the Co3O4–NF catalyst, which exhibited inferior water tolerance. Compared with the Co3O4–NF catalyst, the Mn1Co1–NF catalyst improved water resistance for benzene oxidation under the different water vapor concentrations.
3.2 Structural characterization
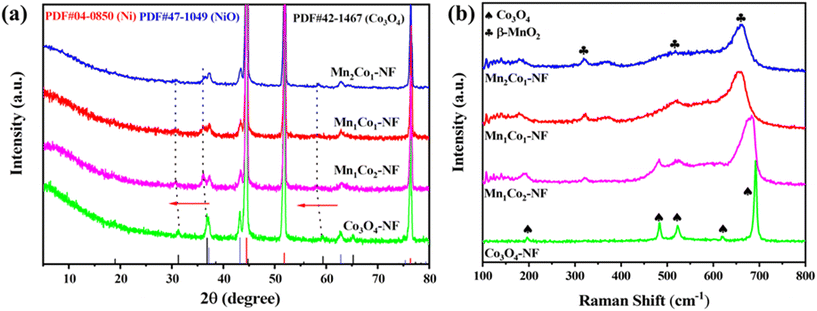
XRD analysis was carried out to investigate the crystalline phase of the catalysts. As shown in Fig. 2a, the strong peaks at 44.6°, 51.9° and 76.5° could be well indexed to the (111), (200) and (220) planes of nickel metal (JCPDS no. 04-0850), and the diffraction peaks at 37.4° and 43.4° could be attributed to the (111) and (200) planes of nickel oxide (JCPDS no. 47-1049). For the Co3O4–NF catalyst, obvious peaks were observed at 31.1°, 36.8°, 59.2° and 65.1°, corresponding to the spinel Co3O4 phase (JPCDS no. 42-1467). After introduction of MnOx, the MnxCoy–NF catalysts still mainly displayed characteristic diffraction peaks of Co3O4, indicating that the loading a suitable amount of MnOx on the surface of Co3O4 could not result in an obvious change in the crystal structure of Co3O4, but these peaks showed a slight shift to a low angle with the introduction of Mn species, indicating the doping of Mn into the Co3O4 lattice. No peaks of the MnOx species were detected in the XRD pattern, which could be attributed to the high dispersion of MnOx in the MnxCoy–NF catalysts. Compared with the Co3O4–NF catalyst, the MnxCoy–NF catalysts exhibited the weak and broad diffraction peaks of the Co3O4 phase, implying that the small crystal size was prone to the formation of lattice defects.31 Distinct Raman spectra for all catalysts were clearly observed, as displayed in Fig. 2b. For the Co3O4–NF catalyst, five distinct bands at 197, 484, 523, 620 and 691 cm−1 corresponded to the F2g, Eg and A1g Raman-active modes of the spinel Co3O4 phase.12,28,32,33 With the decoration of Mn species on the surface of the Co3O4–NF catalyst, the MnxCoy–NF catalysts presented similar Raman peaks to the Co3O4–NF catalyst, but these Raman peaks were weakened and downshifted to lower wavenumbers (a red-shift), suggesting that the MnxCoy–NF catalysts caused a lattice distortion or residual stress in the spinal matrix due to the incorporation of Mn ions.34,35 For the Mn1Co1–NF and Mn2Co1–NF catalysts, peaks at 180, 321, 518 and 661 cm−1 emerged gradually, which were assigned to the characteristic peaks for MnO2.31,36,37 Note that the Raman peaks of related Co3O4 disappeared in the Mn1Co1–NF and Mn2Co1–NF catalysts, indicating that the surface of Co3O4 should be covered by MnOx, which was beneficial for the increase in the interface between MnOx and Co3O4.The morphologies and structural features of Co3O4–NF and MnxCoy–NF catalysts are characterized by SEM and TEM. Ni foam presented a three-dimensional and interconnected porous structure (Fig. S4a†), which facilitated the growth of ZIF-67 on Ni foam, and a series of nanoplates with a smooth surface were uniformly distributed in ZIF-67–NF (Fig. S4b†). After annealing treatment for ZIF-67–NF, the obtained Co3O4–NF sample comprised many dense leaflike nanosheets (Fig. 3a). The TEM image in Fig. 3b confirmed that the Co3O4 nanosheets had a size of ca. 1 μm, and lattice spacings of ca. 0.24 and 0.28 nm were observed in the HRTEM image of the Co3O4–NF catalyst (Fig. 3c), which are ascribed to the (311) and (220) planes of Co3O4, respectively. Compared with the Co3O4–NF catalyst, the MnxCoy–NF catalyst showed loose honeycomb shapes with numerous thin nanosheets (Fig. 3d and S5†). As shown in Fig. 3e and S5c and d,† the triangular nanosheets with numerous pores consisted of small nanoparticles, and many nanoparticles had accumulated on the edge of the nanosheets, consistent with the BET result (Fig. S6 and Table S1†). Moreover, the line scanning images (Fig. S7†) confirmed the absence of a core–shell structure. The TEM results indicated that the leaf shape of Co3O4 became thinner and smaller with the introduction of Mn species. No lattice spacings of Mn species were observed in the HRTEM images of the Mn1Co1–NF catalyst (Fig. 3f) except the lattice spacings of Co3O4. The elemental mapping results of Mn1Co1–NF in Fig. 3g showed that Co and Mn species had a homogeneous distribution.
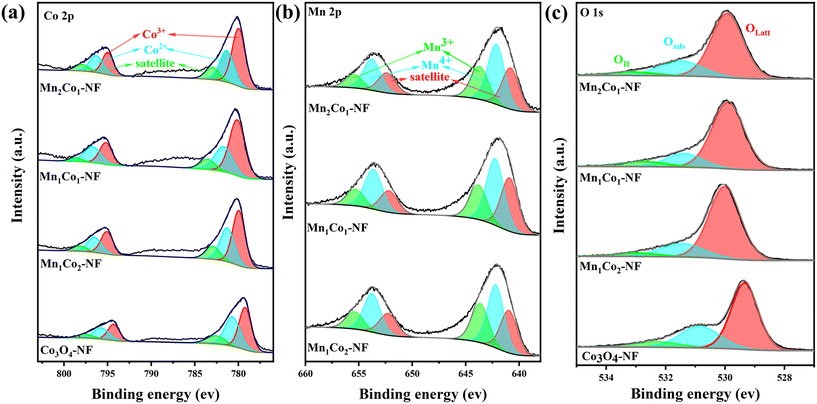
To explore the valence state of elements and active oxygen species on the surface of the prepared samples, X-ray photoelectron spectroscopy (XPS) was performed to characterize the Co3O4–NF and MnxCoy–NF catalysts. As shown in Fig. 4a, the Co 2p spectra of all catalysts could be fitted into two main components (Co3+ and Co2+) together with two shake-up satellites.38 The signals at binding energies of 780.0 and 795.1 eV were indicative of surface Co3+, whereas the peaks at 781.6 and 796.5 eV demonstrated the presence of surface Co2+.39 As listed in Table 1, the Co3+/Co2+ molar ratio increased in the sequence Co3O4–NF < Mn1Co2–NF < Mn2Co1–NF < Mn1Co1–NF, consistent with the activity of the catalysts. As previously reported, the greater amount of surface Co3+ in the Co-based catalysts was beneficial to the catalytic activity.40 The Mn 2p features in Fig. 4b exhibited two main peaks, where the peaks at 640.8 and 652.4 eV were assigned to Mn3+, and the peaks at 642.1 and 653.8 eV were attributed to Mn4+.41 The surface Mn3+/Mn4+ ratio was calculated and is shown in Table 1, and the Mn1Co1–NF catalyst displayed the highest Mn3+/Mn4+ ratio, followed by Mn2Co1–NF and Mn1Co2–NF catalysts. Combining the Co 2p and Mn 2p results, it was concluded that there should be interfacial interaction between Co and Mn species, resulting in electron transfer between Co and Mn species. In the case of the O 1s spectra (Fig. 4c), the broad asymmetrical O 1s XPS peaks were fitted into three peaks, corresponding to three kinds of surface oxygen species. The peak at 529.9 eV could be ascribed to the lattice oxygen species (Olatt), the peak at 531.5 eV belonged to chemisorbed oxygen species and the peak at 532.9 eV was related to hydroxyl species/adsorbed water molecules (OH).42 In general, VOC oxidation on transition metal oxides follows the Mars–van Krevelen (MvK) mechanism, where the Olatt species should play a vital role in the deep oxidation of VOCs. It was noted that the incorporation of Mn species increased the Olatt/Oads ratio in MnxCoy–NF catalysts (Table 1), and the Olatt/Oads ratio followed the order: Co3O4–NF < Mn1Co2–NF < Mn2Co1–NF < Mn1Co1–NF, revealing the activation of lattice oxygen by interfacial interaction between Co and Mn species.
| Samples | T 50 (°C) | T 90 (°C) | Ea (kJ mol−1) | Surface element ratio | Mn/Coa | Mn/Cob | ||
|---|---|---|---|---|---|---|---|---|
| Co3+/Co2+ | Mn3+/Mnα+ | Olatt/Oads | ||||||
| a The data was calculated from XPS. b The data was calculated from ICP-MS. | ||||||||
| Co3O4–NF | 314 | 348 | 105.4 | 1.12 | — | 0.61 | — | — |
| Mn1Co2–NF | 293 | 318 | 92.2 | 1.35 | 1.69 | 0.73 | 0.61 | 0.54 |
| Mn1Co1–NF | 259 | 290 | 61.8 | 1.59 | 1.92 | 0.77 | 1.34 | 0.96 |
| Mn2Co1–NF | 274 | 300 | 70.5 | 1.49 | 1.81 | 0.71 | 0.98 | 0.95 |
The redox properties of the Co3O4–NF and MnxCoy–NF catalysts were further investigated by H2-TPR. As illustrated in Fig. 5a, the pure Co3O4–NF catalyst exhibited three main broad peaks at 268, 310 and 351 °C, belonging to the stepwise reduction of Co3+ → Co2+ → Co0. For MnxCoy–NF catalysts, the first reduction peak (Co3+ → Co2+) showed a slight shift to the low-temperature region, while the last two peaks (Co2+ → Co0) made an opposite movement. The improvement in low-temperature reducibility was attributed to the occupation by Mn3+/Mn4+ ions of octahedral Co3+ sites, which was conducive to catalytic activity. There is no reduction peak of MnOx, presumably due to the reduction of MnOx with small size at low temperature. The initial H2 consumption rate in Fig. 5b exhibited an increasing order of low-temperature reducibility as follows: Co3O4 < Mn1Co2–NF < Mn1Co1–NF < Mn2Co1–NF. The results confirmed that the low-temperature reducibility was remarkably improved by the incorporation of Mn species.
 | ||
| Fig. 5 (a) H2-TPR profiles and (b) initial H2 consumption rate at low temperature over all catalysts. | ||
3.3 Degradation and SO2-tolerance mechanisms
In situ DRIFTS experiments were carried out to detect the possible intermediate species and further infer the reaction path of benzene oxidation. As depicted in Fig. 6a, the bands of intermediate species for the MnxCoy–NF catalyst appeared during the initial 60 min, and the intensity became stronger with time. The bands at 3099, 3042 and 1054 cm−1 were ascribed to the C–H stretching vibration in the benzene rings, indicating the adsorption of benzene on the surface of Mn1Co1–NF.1,43 In addition, the band at 1194 cm−1 was ascribed to phenolate species,44 and the band at 1418 cm−1 was attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of quinone species.45 It is worth noting that the abundant maleate species were detected at 1556, 1314 and 1233 cm−1, implying sufficient active oxygen species to accelerate the ring-opening of benzoquinone. Similar intermediate species were also observed in the Co3O4–NF catalyst (Fig. S8a†). However, the phenolate species was the most abundant in the Co3O4–NF catalyst, indicating the lack of active oxygen species. After introducing SO2 into the reaction, it was observed that the corresponding bands of the intermediate species still existed, but the intensities became weaker. Meanwhile, the band at 1110 cm−1 in the MnxCoy–NF catalyst was attributed to sulfate species, possibly resulting from the reaction between SO3/SO32− and surface Mn species,46 which could inhibit SO2 poisoning to some extent.
O stretching vibrations of quinone species.45 It is worth noting that the abundant maleate species were detected at 1556, 1314 and 1233 cm−1, implying sufficient active oxygen species to accelerate the ring-opening of benzoquinone. Similar intermediate species were also observed in the Co3O4–NF catalyst (Fig. S8a†). However, the phenolate species was the most abundant in the Co3O4–NF catalyst, indicating the lack of active oxygen species. After introducing SO2 into the reaction, it was observed that the corresponding bands of the intermediate species still existed, but the intensities became weaker. Meanwhile, the band at 1110 cm−1 in the MnxCoy–NF catalyst was attributed to sulfate species, possibly resulting from the reaction between SO3/SO32− and surface Mn species,46 which could inhibit SO2 poisoning to some extent.
To further study the change in Co3O4–NF and Mn1Co1–NF after introducing SO2, we established the model of Co3O4 and MnO2 (Fig. S9†) according to the XRD and Raman results. As two aspects of the data, the parameters for adsorption energy and bond lengths were analyzed. The adsorption energy (Eads) of SO2 increased from −0.66 eV on the surface Co sites over Co3O4–NF to −0.83 eV on the surface Mn sites over Mn1Co1–NF (Fig. 6b and S10†). In general, the adsorption energy is related to strong interaction. The high Eads for SO2 indicated that the formation of surface metal sulfate species on Mn sites was easier than on Co sites, in good agreement with the results of the DRIFTS experiments. In addition, SO2 was adsorbed on the Mn sites with an O–Mn bond length of 0.17 nm and on the Co sites with an O–Mn bond length of 0.21 nm, indicating that SO2 showed relatively stable chemical adsorption on the Mn1Co1–NF surface. Therefore, the surface MnO2 on the Mn1Co1–NF catalyst could serve as a sacrificial site to slow down the poisoning of Co–Mn interfacial active sites.
To identify the influence of SO2 on the changes in intermediate species more clearly, Fig. 6c shows the normalized variation in benzoquinone and maleate species over Mn1Co1–NF. It is worth noting that with an increase in time, the amounts of benzoquinone and maleate species were gradually reduced, but the decreasing rate of benzoquinone species was slower than that of maleate species. The results revealed that the introduction of SO2 had no obvious influence on benzene oxidation into benzoquinone, but slightly inhibited the ring-opening of benzoquinone into maleate species on the Mn1Co1–NF catalyst. In contrast, benzoquinone and maleate species on the Co3O4–NF catalyst exhibited an obvious decreasing trend of benzoquinone and maleate species (Fig. S8b†). Thus, the introduction of Mn species could protect the Co–Mn interfacial active sites from SO2 poisoning, thus maintaining continuous oxidation. As shown in Fig. 6d, the GC-MS spectra exhibited similar intermediate species over the Co3O4–NF and MnxCoy–NF catalysts. Benzaldehyde was observed as a new intermediate species over the Mn1Co1–NF catalyst, due to the reaction between benzene and intermediate species.47 Meanwhile, there was a large number of isopropanol and acetic acid species over the Mn1Co1–NF catalyst, indicating further oxidation by the surficial active oxygen species.
Based on characterizations and in situ DRIFTS results, the degradation mechanism for benzene oxidation is proposed in Fig. 7. The benzene and gas oxygen molecules were first adsorbed on the surface of the catalyst, and then the active oxygen reacted with the benzene rings to produce phenolate species via the interaction of the C–H bond with the interface between MnOx and Co3O4. After further oxidation, the phenolate species were oxidized into benzoquinone species, and then the consumed active oxygen was supplemented via Co–Mn interfacial active sites by gaseous molecular oxygen. The benzoquinone species were deeply oxidized into maleate species, which could finally be decomposed into CO2 and H2O. The Mn1Co1–NF catalyst had abundant Co–Mn interfacial active sites, which were conducive to the replenishment of consumed active oxygen by gaseous molecular oxygen, thus exhibiting excellent performance for benzene degradation. As shown in Fig. 7, the surface MnO2 served as a sacrificial site to capture SO2 preferentially via the formation of manganese sulfate to retard the poisoning of Co–Mn interfacial active sites, thereby enhancing SO2 tolerance.
 | ||
| Fig. 7 Schematic illustration of the degradation mechanism for benzene oxidation on the Mn1Co1–NF catalyst. | ||
4. Conclusions
In conclusion, we elaborately constructed a series of MnxCoy–NF catalysts with an MnOx–Co3O4 interface, and the influence of SO2 on benzene oxidation was deeply explored. The surface decoration of MnOx on MnxCoy–NF catalysts could enlarge the Co–Mn interface to enhance the redox ability and interfacial interaction. The Mn1Co1–NF catalyst had abundant Co–Mn interfacial active sites, resulting in a high amount of Co3+ and Mn3+. The large amount of Olatt species provided sufficient active oxygen species to trigger benzene degradation. As confirmed by in situ DRIFTS and DFT calculation, the surface Mn sites rather than Co sites on the Mn1Co1–NF catalyst could capture SO2 to form metal sulfate, thereby retarding the poisoning of Co–Mn interfacial active sites. Thus, the Mn1Co1–NF catalyst exhibits high catalytic activity and SO2 tolerance during benzene oxidation. This work provides a route to construct high-performance catalysts for enhancing catalytic activity and SO2 tolerance in the degradation of VOCs in practical applications.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This project was supported by the National Natural Science Foundation of China (22076192, 21777166, 42175133 and 21806169), and Beijing National Laboratory for Molecular Sciences (BNLMS-CXXM-202011). The authors wish to thank facility support of the 4B9A beamline of Beijing Synchrotron Radiation Facility (BSRF).References
- Z. Hou, L. Dai, Y. Liu, J. Deng, L. Jing, W. Pei, R. Gao, Y. Feng and H. Dai, Highly efficient and enhanced sulfur resistance supported bimetallic single-atom palladium-cobalt catalysts for benzene oxidation, Appl. Catal., B, 2021, 285, 119844 CrossRef CAS.
- D. Han, X. Ma, X. Yang, M. Xiao, H. Sun, L. Ma, X. Yu and M. Ge, Metal organic framework-templated fabrication of exposed surface defect-enriched Co3O4 catalysts for efficient toluene oxidation, J. Colloid Interface Sci., 2021, 603, 695–705 CrossRef CAS.
- M. C. Wen, S. N. Song, W. N. Zhao, Q. X. Liu, J. Y. Chen, G. Y. Li and T. C. An, Atomically dispersed Pd sites on Ti-SBA-15 for efficient catalytic combustion of typical gaseous VOCs, Environ. Sci.: Nano, 2021, 8, 3735–3745 RSC.
- Y. Shen, J. Deng, S. Impeng, S. Li, T. Yan, J. Zhang, L. Shi and D. Zhang, Boosting Toluene Combustion by Engineering Co-O Strength in Cobalt Oxide Catalysts, Environ. Sci. Technol., 2020, 54, 10342–10350 CrossRef CAS.
- C. Dong, Z. Qu, Y. Qin, Q. Fu, H. Sun and X. Duan, Revealing the Highly Catalytic Performance of Spinel CoMn2O4 for Toluene Oxidation: Involvement and Replenishment of Oxygen Species Using In Situ Designed-TP Techniques, ACS Catal., 2019, 9, 6698–6710 CrossRef CAS.
- K. X. Cao, X. X. Dai, Z. B. Wu and X. L. Weng, Unveiling the importance of reactant mass transfer in environmental catalysis: Taking catalytic chlorobenzene oxidation as an example, Chin. Chem. Lett., 2021, 32, 1206–1209 CrossRef CAS.
- M. L. Xiao, X. L. Yu, Y. C. Guo and M. F. Ge, Boosting Toluene Combustion by Tuning Electronic Metal-Support Interactions in In Situ Grown Pt@Co3O4 Catalysts, Environ. Sci. Technol., 2022, 56, 1376–1385 CrossRef CAS.
- W. M. Singh, T. Baine, S. Kudo, S. Tian, X. A. Ma, H. Zhou, N. J. DeYonker, T. C. Pham, J. C. Bollinger, D. L. Baker, B. Yan, C. E. Webster and X. Zhao, Electrocatalytic and photocatalytic hydrogen production in aqueous solution by a molecular cobalt complex, Angew. Chem., Int. Ed., 2012, 51, 5941–5944 CrossRef CAS PubMed.
- L. Ge, C. Han, X. Xiao and L. Guo, In situ synthesis of cobalt–phosphate (Co–Pi) modified g-C3N4 photocatalysts with enhanced photocatalytic activities, Appl. Catal., B, 2013, 142–143, 414–422 CrossRef CAS.
- Y. Min, X. Zhou, J. J. Chen, W. Chen, F. Zhou, Z. Wang, J. Yang, C. Xiong, Y. Wang, F. Li, H. Q. Yu and Y. Wu, Integrating single-cobalt-site and electric field of boron nitride in dechlorination electrocatalysts by bioinspired design, Nat. Commun., 2021, 12, 303 CrossRef CAS PubMed.
- F. Wyrwalski, J. F. Lamonier, S. Siffert and A. Aboukais, Additional effects of cobalt precursor and zirconia support modifications for the design of efficient VOC oxidation catalysts, Appl. Catal., B, 2007, 70, 393–399 CrossRef CAS.
- B. Bai and J. Li, Positive Effects of K+ Ions on Three-Dimensional Mesoporous Ag/Co3O4 Catalyst for HCHO Oxidation, ACS Catal., 2014, 4, 2753–2762 CrossRef CAS.
- X. Ma, M. Xiao, X. Yang, X. Yu and M. Ge, Boosting benzene combustion by engineering oxygen vacancy-mediated Ag/CeO2-Co3O4 catalyst via interfacial electron transfer, J. Colloid Interface Sci., 2021, 594, 882–890 CrossRef CAS PubMed.
- S. C. Kim and W. G. Shim, Catalytic combustion of VOCs over a series of manganese oxide catalysts, Appl. Catal., B, 2010, 98, 180–185 CrossRef CAS.
- J. Xiong, Z. Li, P. Zhang, Q. Yu, K. Li, Y. Zhang, Z. Zhao, J. Liu, J. Li and Y. Wei, Optimized Pt-MnOx interface in Pt-MnOx/3DOM-Al2O3 catalysts for enhancing catalytic soot combustion, Chin. Chem. Lett., 2021, 32, 1447–1450 CrossRef CAS.
- S. Putla, M. H. Amin, B. M. Reddy, A. Nafady, K. A. Al Farhan and S. K. Bhargava, MnO(x) Nanoparticle-Dispersed CeO2 Nanocubes: A Remarkable Heteronanostructured System with Unusual Structural Characteristics and Superior Catalytic Performance, ACS Appl. Mater. Interfaces, 2015, 7, 16525–16535 CrossRef CAS.
- Y. F. Zheng, Y. Su, C. H. Pang, L. Z. Yang, C. F. Song, N. Ji, D. G. Ma, X. B. Lu, R. Han and Q. L. Liu, Interface-Enhanced Oxygen Vacancies of CoCuOx Catalysts In Situ Grown on Monolithic Cu Foam for VOC Catalytic Oxidation, Environ. Sci. Technol., 2022, 56, 1905–1916 CrossRef PubMed.
- W. Han, F. Dong, W. Han and Z. Tang, Fabrication of homogeneous and highly dispersed CoMn catalysts for outstanding low temperature catalytic oxidation performance, New J. Chem., 2019, 43, 12846–12857 RSC.
- H. Z. Chang, X. Y. Chen, J. H. Li, L. Ma, C. Z. Wang, C. X. Liu, J. W. Schwank and J. M. Hao, Improvement of Activity and SO2 Tolerance of Sn-Modified MnOx-CeO2 Catalysts for NH3-SCR at Low Temperatures, Environ. Sci. Technol., 2013, 47, 5294–5301 CrossRef CAS PubMed.
- Z. Q. Xu, S. Impeng, X. Y. Jia, F. L. Wang, Y. J. Shen, P. L. Wang and D. S. Zhang, SO2-Tolerant catalytic reduction of NOx by confining active species in TiO2 nanotubes, Environ. Sci.: Nano, 2022, 9, 2121–2133 RSC.
- Z. X. Ma, L. P. Sheng, X. W. Wang, W. T. Yuan, S. Y. Chen, W. Xue, G. R. Han, Z. Zhang, H. S. Yang, Y. H. Lu and Y. Wang, Oxide Catalysts with Ultrastrong Resistance to SO2 Deactivation for Removing Nitric Oxide at Low Temperature, Adv. Mater., 2019, 31, 1903719 CrossRef CAS.
- J. Mao, D. D. He, Y. T. Zhao, L. Zhang and Y. M. Luo, Sulfur-resistance iron catalyst in sulfur-containing VOCs abatement modulated through H(2 )reduction, Appl. Surf. Sci., 2022, 584, 152631 CrossRef CAS.
- Y. Lyu, J. Xu, Q. Cao, Z. Zhou, W. Hu and X. Liu, Highly efficient removal of toluene over Cu-V oxides modified gamma-Al2O3 in the presence of SO2, J. Hazard. Mater., 2022, 436, 129041 CrossRef.
- Z. W. Wang, S. Li, S. H. Xie, Y. X. Liu, H. X. Dai, G. S. Guo and J. G. Deng, Supported ultralow loading Pt catalysts with high H2O-, CO2-, and SO2-resistance for acetone removal, Appl. Catal., A, 2019, 579, 106–115 CrossRef CAS.
- W. S. Kijlstra, M. Biervliet, E. K. Poels and A. Bliek, Deactivation by SO2 of MnOx/Al2O3 catalysts used for the selective catalytic reduction of NO with NH3 at low temperatures, Appl. Catal., B, 1998, 16, 327–337 CrossRef.
- R. B. Jin, Y. Liu, Y. Wang, W. L. Cen, Z. B. Wu, H. Q. Wang and X. L. Weng, The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature, Appl. Catal., B, 2014, 148, 582–588 CrossRef.
- A. Muthurasu, G. P. Ojha, M. Lee and H. Y. Kim, Integration of Cobalt Metal–Organic Frameworks into an Interpenetrated Prussian Blue Analogue to Derive Dual Metal–Organic Framework-Assisted Cobalt Iron Derivatives for Enhancing Electrochemical Total Water Splitting, J. Phys. Chem. C, 2020, 124, 14465–14476 CrossRef CAS.
- X. Ma, X. Yu, X. Yang, M. Lin and M. Ge, Hydrothermal Synthesis of a Novel Double-Sided Nanobrush Co3O4 Catalyst and Its Catalytic Performance for Benzene Oxidation, ChemCatChem, 2019, 11, 1214–1221 CrossRef CAS.
- X. Yang, X. Yu, M. Jing, W. Song, J. Liu and M. Ge, Defective Mn xZr1- xO2 Solid Solution for the Catalytic Oxidation of Toluene: Insights into the Oxygen Vacancy Contribution, ACS Appl. Mater. Interfaces, 2019, 11, 730–739 CrossRef CAS.
- M. Xiao, X. Yang, Y. Peng, Y. Guo, Y. Wei, M. Ge and X. Yu, Confining shell-sandwiched Ag clusters in MnO2-CeO2 hollow spheres to boost activity and stability of toluene combustion, Nano Res., 2022, 1–10 Search PubMed.
- T. Chang, Z. Shen, Y. Huang, J. Lu, D. Ren, J. Sun, J. Cao and H. Liu, Post-plasma-catalytic removal of toluene using MnO2–Co3O4 catalysts and their synergistic mechanism, Chem. Eng. J., 2018, 348, 15–25 CrossRef CAS.
- D. Kong, J. Luo, Y. Wang, W. Ren, T. Yu, Y. Luo, Y. Yang and C. Cheng, Three-Dimensional Co3O4@MnO2Hierarchical Nanoneedle Arrays: Morphology Control and Electrochemical Energy Storage, Adv. Funct. Mater., 2014, 24, 3815–3826 CrossRef CAS.
- L. Miao, Y. Xie, Y. Xia, N. Zou and J. Wang, Facile photo-driven strategy for the regeneration of a hierarchical C@MnO2 sponge for the removal of indoor toluene, Appl. Surf. Sci., 2019, 481, 404–413 CrossRef CAS.
- S. Mo, Q. Zhang, Y. Sun, M. Zhang, J. Li, Q. Ren, M. Fu, J. Wu, L. Chen and D. Ye, Gaseous CO and toluene co-oxidation over monolithic core–shell Co3O4-based hetero-structured catalysts, J. Mater. Chem. A, 2019, 7, 16197–16210 RSC.
- P. Liu, H. P. He, G. L. Wei, X. L. Liang, F. H. Qi, F. D. Tan, W. Tan, J. X. Zhu and R. L. Zhu, Effect of Mn substitution on the promoted formaldehyde oxidation over spinel ferrite: Catalyst characterization, performance and reaction mechanism, Appl. Catal., B, 2016, 182, 476–484 CrossRef CAS.
- Y. Luo, Y. Zheng, J. Zuo, X. Feng, X. Wang, T. Zhang, K. Zhang and L. Jiang, Insights into the high performance of Mn-Co oxides derived from metal-organic frameworks for total toluene oxidation, J. Hazard. Mater., 2018, 349, 119–127 CrossRef CAS PubMed.
- C. M. Julien, M. Massot and C. Poinsignon, Lattice vibrations of manganese oxides, Spectrochim. Acta, Part A, 2004, 60, 689–700 CrossRef CAS.
- Q. Zhao, Y. Zheng, C. Song, Q. Liu, N. Ji, D. Ma and X. Lu, Novel monolithic catalysts derived from in-situ decoration of Co3O4 and hierarchical Co3O4@MnOx on Ni foam for VOC oxidation, Appl. Catal., B, 2020, 265, 118552 CrossRef CAS.
- C. Huang, Q. Ji, H. Zhang, Y. Wang, S. Wang, X. Liu, Y. Guo and C. Zhang, Ru-incorporated Co3O4 nanoparticles from self-sacrificial ZIF-67 template as efficient bifunctional electrocatalysts for rechargeable metal-air battery, J. Colloid Interface Sci., 2022, 606, 654–665 CrossRef CAS PubMed.
- G. Li, M. Chen, Y. Ouyang, D. Yao, L. Lu, L. Wang, X. Xia, W. Lei, S.-M. Chen, D. Mandler and Q. Hao, Manganese doped Co3O4 mesoporous nanoneedle array for long cycle-stable supercapacitors, Appl. Surf. Sci., 2019, 469, 941–950 CrossRef CAS.
- Z. Bi, C. H. Feng, D. S. Wang, X. P. Ge and H. X. Tang, Transformation of planar Mogel Al-13 to epsilon Keggin Al-13 in dissolution process, Colloids Surf., A, 2012, 407, 91–98 CrossRef CAS.
- Y. Yang, S. Zhao, F. Bi, J. Chen, Y. Li, L. Cui, J. Xu and X. Zhang, Oxygen-vacancy-induced O2 activation and electron-hole migration enhance photothermal catalytic toluene oxidation, Cell Rep. Phys. Sci., 2022, 3, 101011 CrossRef CAS.
- K. Yang, Y. Liu, J. Deng, X. Zhao, J. Yang, Z. Han, Z. Hou and H. Dai, Three-dimensionally ordered mesoporous iron oxide-supported single-atom platinum: Highly active catalysts for benzene combustion, Appl. Catal., B, 2019, 244, 650–659 CrossRef CAS.
- Y. Liang, Y. Liu, J. Deng, K. Zhang, Z. Hou, X. Zhao, X. Zhang, K. Zhang, R. Wei and H. Dai, Coupled Palladium-Tungsten Bimetallic Nanosheets/TiO2 Hybrids with Enhanced Catalytic Activity and Stability for the Oxidative Removal of Benzene, Environ. Sci. Technol., 2019, 53, 5926–5935 CrossRef CAS PubMed.
- H. Guo, Z. Zhang, Z. Jiang, M. Chen, H. Einaga and W. Shangguan, Catalytic activity of porous manganese oxides for benzene oxidation improved via citric acid solution combustion synthesis, J. Environ. Sci., 2020, 98, 196–204 CrossRef CAS PubMed.
- Y. Lyu, J. Xu, Q. Cao, Z. Zhou, W. Hu and X. Liu, Highly efficient removal of toluene over Cu-V oxides modified gamma-Al2O3 in the presence of SO2, J. Hazard. Mater., 2022, 436, 129041 CrossRef PubMed.
- H. Yi, L. Miao, J. Xu, S. Zhao, X. Xie, C. Du, T. Tang and X. Tang, Palladium particles supported on porous CeMnO3 perovskite for catalytic oxidation of benzene, Colloids Surf., A, 2021, 623, 126687 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: More detailed information of long-term stability experiments over the Mn1Co1–NF catalyst, Arrhenius plots of all catalysts, the conversion of benzene per unit area over all the catalysts, water effect on benzene conversion over Co3O4–NF and Mn1Co1–NF catalyst, SEM images, TEM images, N2 adsorption–desorption isotherms and pore size distributions, XPS spectra, in situ DRIFTS spectra of reactant adsorption, side and top views of Co3O4–NF and Mn1Co1–NF, the top view of the SO2-adsorbed on Co3O4–NF and Mn1Co1–NF, Table S1 (physicochemical properties of the samples). See DOI: https://doi.org/10.1039/d2en00893a |
| This journal is © The Royal Society of Chemistry 2023 |