Open Access Article
Open Access ArticleManaging PMT/vPvM substances in consumer products through the concepts of essential-use and functional substitution: a case-study for cosmetics†
Joanke
van Dijk
 *ab,
Romain
Figuière
c,
Stefan C.
Dekker
a,
Annemarie P.
van Wezel
*ab,
Romain
Figuière
c,
Stefan C.
Dekker
a,
Annemarie P.
van Wezel
 b and
Ian T.
Cousins
b and
Ian T.
Cousins
 c
c
aCopernicus Institute of Sustainable Development, Utrecht University, 3584 CB Utrecht, The Netherlands. E-mail: j.vandijk3@uu.nl
bInstitute for Biodiversity and Ecosystem Dynamics, University of Amsterdam, PO Box 94240, 1090 GE Amsterdam, The Netherlands
cDepartment of Environmental Science, Stockholm University, SE-10691 Stockholm, Sweden
First published on 3rd May 2023
Abstract
Measures are needed to protect water sources from substances that are mobile, persistent and toxic (PMT) or very persistent and very mobile (vPvM). PMT/vPvM substances are used in a diverse range of applications, including consumer products. The combined application of the essential-use and functional substitution concepts has been proposed to phase out substances of concern and support the transition to safer and more sustainable chemicals, a key goal of the European Commission’s Chemicals Strategy for Sustainability. Here, we first identified the market share of PMT/vPvM containing cosmetic products. We found that 6.4% of cosmetic products available on the European market contain PMT or vPvM substances. PMT/vPvM substances were most often found in hair care products. Based on their high occurrence, the substances Allura red (CAS 25956-17-6), benzophenone-4 (CAS 4065-45-6) and climbazole (CAS 38083-17-9) were selected as case-studies for assessment of their functionality, availability of safer alternatives and essentiality. Following the functional substitution framework, we found that the technical function of Allura red was not necessary for the performance of some cosmetic products, making the use non-essential. For other applications of Allura red, as well as all applications of benzophenone-4 and climbazole, the technical function of the chemical was considered necessary for the performance. Via the alternative’s assessment procedure, which used experimental and in silico data and three different multicriteria decision analysis (MCDA) strategies, safer alternatives were identified for all case-study chemicals. All assessed uses of PMT/vPvM substances were thus deemed non-essential and should consequently be phased out.
Environmental significancePersistent, mobile and toxic (PMT) and very persistent and very mobile (vPvM) substances pose a risk to water resources worldwide, and thus also to environmental and human health. Although PMT/vPvM substances are widely used, their exact use in consumer and industrial applications is often not well known or well characterised. The essential use and functional substitution concepts have recently been developed as tools to characterise and manage the many uses of chemicals of concern. Here, as recently recommended in the literature, the two concepts are applied in combination to facilitate the transition away from using PMT/vPvM substances in cosmetic products. Minimising the use of PMT/vPvM substances in “open uses”, such as in cosmetic products, will lower environmental emissions and thus human and wildlife exposure. |
1 Introduction
Synthetic chemicals are present in approximately 95% of all manufactured goods and are considered as indispensable for modern societies.1 Over 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 chemicals and mixtures are reportedly registered for usage and production globally.2 Chemical production, use and disposal result, however, in pollution of the environment, affecting both human and environmental health.3,4 Furthermore, pollution, including that from chemicals, has been identified as one out of five main drivers for global biodiversity loss.5 Chemical pollution (now known as “novel identities”) is also one of the planetary boundaries which humanity has already crossed.6,7
000 chemicals and mixtures are reportedly registered for usage and production globally.2 Chemical production, use and disposal result, however, in pollution of the environment, affecting both human and environmental health.3,4 Furthermore, pollution, including that from chemicals, has been identified as one out of five main drivers for global biodiversity loss.5 Chemical pollution (now known as “novel identities”) is also one of the planetary boundaries which humanity has already crossed.6,7
Driven by safety concerns, regulations are in place world-wide in order to reduce the risks associated with the use of chemicals. In the EU, the main chemical legislation REACH (Reg. No. 1907/2006 EC) should ‘ensure a high level of protection of human health and the environment as well as the free movement of substances… while enhancing competitiveness and innovation’. REACH should also promote the development of alternative methods for the assessment of hazards of substances. Under REACH, substances that are persistent, bioaccumulative and toxic (PBT) and substances that are very persistent and very bioaccumulative (vPvB) are identified as substances of very high concern (SVHC), resulting in a limit or ban of their production and consumption. Current SVHC classification criteria, however, do not capture all potentially hazardous substances, including substances that negatively impact water resources. The so-called mobile substances are readily transported in water, soil layers, river banks, and aquifers, or pass through natural or artificial barriers (e.g. in wastewater treatment plants). When combined with persistence, these mobile substances accumulate in the aquatic environment and drinking water sources.8–10 Mobility (M) has therefore been proposed as an additional hazard criterion to identify SVHCs, as substances that are mobile, persistent and toxic (PMT) or very persistent and very mobile (vPvM).11,12
PMT/vPvM substances are currently used in a diverse range of applications, including in cosmetic products.10 An estimated 2320 thousand tonnes of cosmetic products are sold per year in the European Economic Area.13 Furthermore, it has been reported that cosmetic products contain a large number of chemicals with potential hazardous properties.14 Environmental risks are currently not taken into account for market approval of cosmetics under the Cosmetic Products Regulation (CPR, Regulation (EC) No. 1223/2009),15 despite the use of cosmetic products contributing to the occurrence of chemicals in the environment.13 Several improvements are expected, however, in light of the European Green Deal's Chemicals Strategy for Sustainability (CSS).12 As part of the CSS, a new legislative proposal to amend the CPR will be presented. This will include an aim to minimise and substitute the use of chemicals that have a chronic effect on human health and the environment, and incorporation of the essential-use concept in order to phase out the most hazardous chemicals.16
The essential-use concept was first introduced in 1987 as part of the Montreal Protocol to phase-out ozone-depleting chlorofluorocarbons. Under the Montreal Protocol, the use of a substance is considered essential if: “(1) it is necessary for health and safety or is critical for the functioning of society; and (2) there are no available technically and economically feasible alternatives or substitutes that are acceptable from the standpoint of environment and health”. More recently, the essential-use concept was proposed as a tool to guide the phase-out of PFAS.17,18 Following this approach, uses of PFAS can be classified into three different categories: (1) essential and non-substitutable, (2) essential but substitutable by safer chemicals and (3) non-essential; and only the uses that would be judged as being essential and non-substitutable should be authorised.17,18
Essentiality assessments have been proposed to focus on the technical function provided by a chemical for a specific use. Hence, it was recently suggested to combine the essential-use concept with the functional substitution approach.17,19 The identification of non-essential uses is key to prevent the continued use of hazardous substances. In order to identify uses that are substitutable, the implementation of the essential-use concept requires a sufficient understanding of the current uses of substances, but also of the availability, suitability, and hazardous properties of alternatives.17,20 Identifying alternative non-hazardous chemicals that are functional and affordable is key to prevent regrettable substitution of chemicals.21,22
First described by Tickner et al. (2015), functional substitution aims to evaluate whether the function of a chemical is necessary for the application, and then examines through alternative assessment whether safer and effective chemicals, product/process design, or product service alternatives exist to provide a similar function.23 By combining the essential-use and functional substitution concepts, and thus focussing on both the essentiality, function and performance of substances of concern, a solution-oriented approach is obtained that can help to effectively support the transition to safer and more sustainable chemicals.19
The aim of this study is to explore the potential of the combined application of the essential-use and functional substitution concepts to facilitate the phase-out of PMT/vPvM substances in cosmetic products. First, the market share of PMT/vPvM-containing cosmetic products is identified through database searches. Then, the most frequently occurring substances are selected as case-study chemicals for which an assessment is performed per use case by (1) assessing the functional use of these chemicals, (2) identifying suitable and safer alternatives through the process of alternative assessment and (3) considering whether the use of the chemical is necessary for health and safety or critical for the functioning of society, in case no suitable and safer chemical alternatives are available.
2 Methods
An overview of the method used to determine the essentiality of specific potential PMT/vPvM substances used in cosmetic products is presented in Fig. 1. An integrated method has been developed based on the concepts of essential-use,18 function substitution23 and the decision tree suggested by Roy et al. (2022).19 The hazard assessment of alternatives was based on ECHA24 and OECD25 guidances. Each step is explained in more detail below.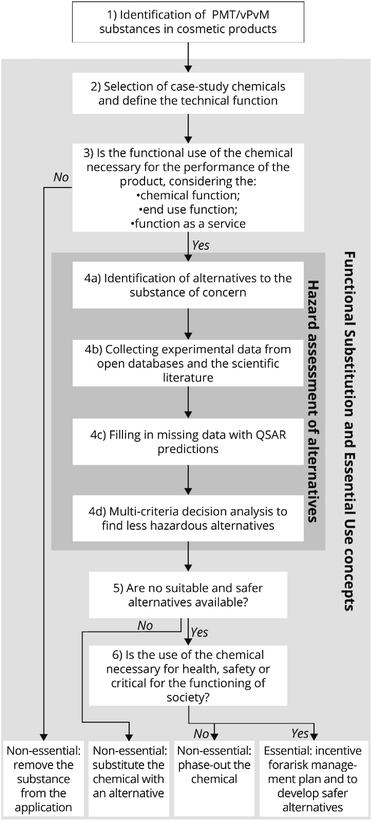 | ||
| Fig. 1 Integrated methodology for the identification and assessment of chemical alternatives to PMT/vPvM substances in cosmetics, incorporating aspects from the concepts of functional substitution, essential-use and alternative assessment.18,19,23,25 | ||
2.1 Identifying PMT/vPvM substances in cosmetic products
The most comprehensive analysis of PMT/vPvM substances registered under REACH has been published by the German Environment Agency in which any substance with a logarithm of the organic carbon–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc) lower than 4 is classified as being mobile.26 In total, 211 substances have been identified as potential PMT/vPvM substances. These substances were compared with entries in the cosmetic ingredient database (CosIng) in order to identify substances which can be used in cosmetic products.27 As of November 2021, CosIng had a total of 53
Koc) lower than 4 is classified as being mobile.26 In total, 211 substances have been identified as potential PMT/vPvM substances. These substances were compared with entries in the cosmetic ingredient database (CosIng) in order to identify substances which can be used in cosmetic products.27 As of November 2021, CosIng had a total of 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 028 entries. These entries contained many substances without CAS numbers. In addition, the list contains substances that are mentioned twice or more, due to their multiple functions. When substances without a CAS number are removed and substances with multiple functions are merged, this results in 10
028 entries. These entries contained many substances without CAS numbers. In addition, the list contains substances that are mentioned twice or more, due to their multiple functions. When substances without a CAS number are removed and substances with multiple functions are merged, this results in 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 unique CAS numbers.
000 unique CAS numbers.
PMT/vPvM substances listed in the CosIng database were screened in The Danish Consumer Council Think Chemicals (Kemiluppen, screened in November 2021) and Cosmethics (screened in December 2021) databases to identify cosmetic products containing PMT/vPvM substances. Kemiluppen is an initiative under the Danish Consumer Council and contains information on cosmetic products available on the Danish market. A full database search was performed for this study, only leaving out outdated products as these are no longer on the market. Cosmethics is a company that has a data repository of cosmetic products. For our study, 1000 products registered in Cosmethics in 2021 were randomly selected and analysed for PMT/vPvM substances. Data beyond the publicly available information of these databases were obtained via personal communication with Stine Müller (Kemiluppen) and Katariina Rantanen (Cosmethics). The three highest occurring substances in the datasets were selected as case studies for the assessment of alternatives and for further analysis of essentiality.
2.2 Technical function and application of substances
The general technical function(s) provided by the case-study chemicals was derived from the CosIng database. Interviews with Gerald Renner (Cosmetic Europe) and Héloïse Le Levier (IDUN Minerals) helped to provide a better understanding of the precise technical function provided by the substances of interest and their necessity for the performance in each application.2.3 Investigating the availability of suitable alternatives
A suitable alternative includes any type of alternative (i.e., chemical, material, process and/or product alternative) which is safer for the environment and human health, and technically and economically feasible.24 To determine whether suitable alternatives to each case-study chemical were available, an alternative assessment based on chemical hazards (PBMT) was performed following the framework described in the ECHA guidance for the preparation for application for authorisation, and the suggestions provided in the OECD guidance for key considerations for the identification and selection of safer chemical alternatives.24,25The potential chemical alternatives were shortlisted in order to filter out substances known (or suspected) to be hazardous. To achieve that goal, the potential alternatives were screened in the SUBSPORT database, which includes 32 lists of substances from industry, authorities and non-governmental organisations (NGOs) that are legally or voluntarily restricted, or are recommended for restriction due to their hazardous properties.29 All the lists screened in the SUBSPORT database are listed in the ESI (SI 1).† Annex III to the CPR (listing restricted substances in cosmetic products) was used as an additional priority list to filter out substances. The ECHA database of registered substances was screened to identify classifications under CLP (classification, focussing labelling and packaging) and C&L (classification and labelling) notifications for potential alternatives. Lastly, the REACH Annex III inventory was screened to determine if the potential alternatives are suspected to present CMR (carcinogenic, mutagenic or reprotoxic), PBT and ED (endocrine disruption) properties based on QSAR outcomes. The precise workflow, which has been followed to shortlist chemical alternatives for further assessment, is presented in the ESI (SI 1).† Other types of potential alternatives (e.g., change in product and change in material) were identified based on the information collected during semi-structured interviews with Gerald Renner (Cosmetic Europe) and Héloïse Le Levier (IDUN Minerals).
2.3.3.1 Heatmap. Hazard profiles of each alternative were compared by using a heatmap. The range of values for each hazard endpoint was divided into four colour-coded categories. The threshold values to define the categories for each hazard endpoint were taken from Zheng et al. (2019), who used CLP and PBT classification criteria to assign hazard categories.34 All threshold values used in this study are available in the ESI (SI 1).† In order to compare the hazard profiles of the potential alternatives, a score was assigned to each alternative following the recommendations of the OECD guidance. In short, for a given alternative, a score of 1 was assigned for every endpoint coloured green (low hazard), of 2 for endpoints coloured yellow (moderate hazard), of 3 for endpoints coloured orange (high hazard), and 4 for endpoints coloured red (hazard criterion exceeded). To test the sensitivity of the approach to the data gaps, three different scenarios were tested (i.e., risk neutral, risk seeking, and risk averse scenarios) as has been performed in previous studies.34,35 For the risk neutral scenario, data gaps were assigned a score of 2.5. The method to assign the score to data gaps under the other scenarios is detailed in SI 1.† The final score of the alternative was obtained by summing up the scores of each endpoint. The alternative with the lowest score was considered to be the potentially safest alternative.
2.3.3.2 MAUT and ELECTRE III. A multi-criteria analysis based on the multi-attribute utility theory (MAUT) principle was performed on the hazard endpoints based on the study by Zheng et al. (2019).34 In short, the data collected for all alternatives were normalised from 0 to 1 for each endpoint considered in the assessment, with 0 corresponding to the worst level, and 1 to the best level for a given endpoint. As for the heatmap, three different scenarios were used to test the sensitivity of the approach to the data gaps. Under the risk neutral scenario, data gaps were assigned a value of 0.5. Further information on the approach is provided in the ESI (SI 1).†
The scores for P, B, M, Thuman, and Tenv were considered as equally important (equal weight approach) and added up to obtain a final MAUT score for a chemical. The chemical with the highest MAUT score was assumed to be the most preferred alternative. The MAUT assessment was complemented with ELECTRE III (an outranking method), for which calculations were performed according to Zheng et al. (2019, 2021).34,35 The determination of the thresholds for the pairwise comparison for each endpoint is detailed in the ESI (SI 1).†
2.4 Necessity of the use of the compound of concern for health, safety, and functioning of society
As no clear criteria to evaluate the necessity of the use of a chemical for health, safety and functioning of society are available at the time of the study, only a qualitative assessment was performed.3 Results
3.1 Occurrence of PMT/vPvM substances in cosmetic products
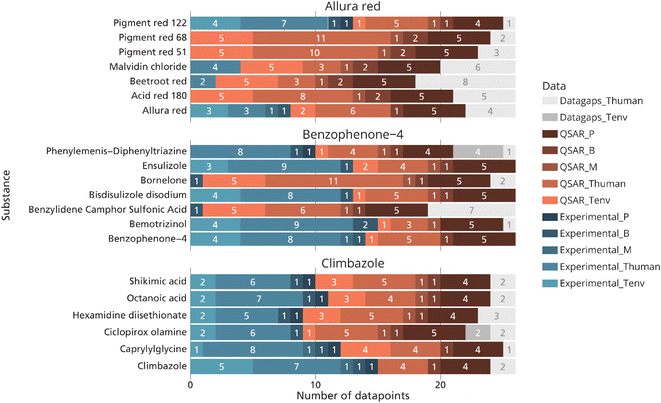
50 PMT/vPvM substances were identified in CosIng (SI 2†). These include REACH registered substances and the pharmaceuticals ibuprofen, naproxen, and progesterone and the biocidal active substance, triclosan. Six substances (dimethoxydiglycol, pigment orange 5, progesterone, chloroform, DMSO and methylthiophenyl morpholino isobutanone) on this list were listed as Annex II substances, meaning their use in cosmetic products is prohibited. These six substances were subsequently not considered for further analysis as they should not be present in cosmetic products. The remaining 44 substances were screened in the cosmetic product databases. Out of these substances, 21 were identified in cosmetic products. In total, 20 of these substances were found in 6.6% (897) of the cosmetic products listed in Kemiluppen, and 8 substances were found in 6.2% (ref. 62) of the cosmetic products screened in the Cosmethics database (Fig. 2). In both databases, the product group containing the most PMT/vPvM substances was hair care products. Table S1† lists all PMT/vPvM substances which have been identified in the Cosing database along with their technical function(s), and the type of cosmetic products they are used in. Based on their high occurrence, Allura red (CAS 25956-17-6), benzophenone-4 (CAS 4065-45-6) and climbazole (CAS 38083-17-9) were selected as case-study chemicals for the assessment of alternatives and essentiality.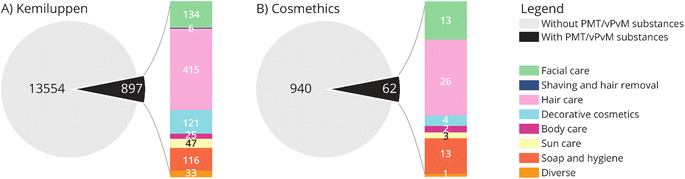 | ||
| Fig. 2 Total number of cosmetic products with and without PMT/vPvM substances in the databases from (A) Kemiluppen and (B) Cosmethics. | ||
3.2 Technical function provided by case-study chemicals
3.3 Identifying suitable chemical alternatives
| Use case | Chemical name | CAS number |
|---|---|---|
| Pigment | Allura red | 25956-17-6 |
| Malvidin chloride | 643-84-5 | |
| Beetroot red | 7659-95-2 | |
| Pigment red 51 | 5850-87-3 | |
| Pigment red 68 | 5850-80-6 | |
| Acid red 180 | 6408-26-0 | |
| Pigment red 122 | 980-26-7 | |
| UV-filter | Benzophenone-4 | 4065-45-6 |
| Ensulizole | 27503-81-7 | |
| Benzylidene camphor sulfonic acid | 56039-58-8 | |
| Bisdisulizole disodium | 180898-37-7 | |
| Bemotrizinol | 187393-00-6 | |
| Bornelone | 2226-11-01 | |
| Phenylemenis-diphenyltriazine | 55514-22-2 | |
| Anti-seborrheic | Climbazole | 38083-17-9 |
| Octanoic acid | 102731-54-4 | |
| Caprylylglycine | 14246-53-8 | |
| Shikimic acid | 138-59-0 | |
| Ciclopirox olamine | 41621-49-2 | |
| Hexamidine diisethionate | 659-40-5 |
For benzophenone-4, 39 potential alternatives were selected from annex VI of the CPR (allowed UV-filters). Six of these substances were selected for the alternative assessment based on known hazard data, classification, and by compromising both low priority substances for environmental assessment40 and UV filters with a similar absorbance compared to that of benzophenone-4.41
As climbazole was only found in shampoos, only its anti-seborrheic functionality was considered for which 10 potential alternatives were identified. Based on known hazard data and classification five of these substances were selected for the alternative assessment.
The exact process to shortlist chemical alternatives for further assessment is presented in SI 1,† and the collected data are shown in SI 3.† All case-study chemicals and the alternatives selected for this study are presented in Table 1, and S3.2.2† provides further information on the chemical structure and physico-chemical properties of the shortlisted alternatives.
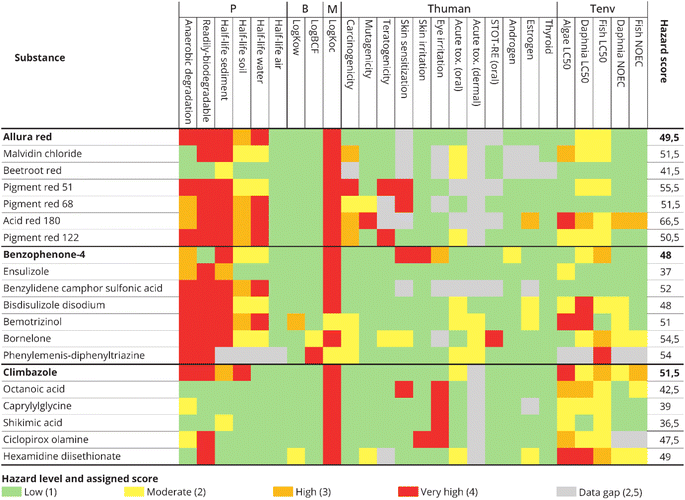 | ||
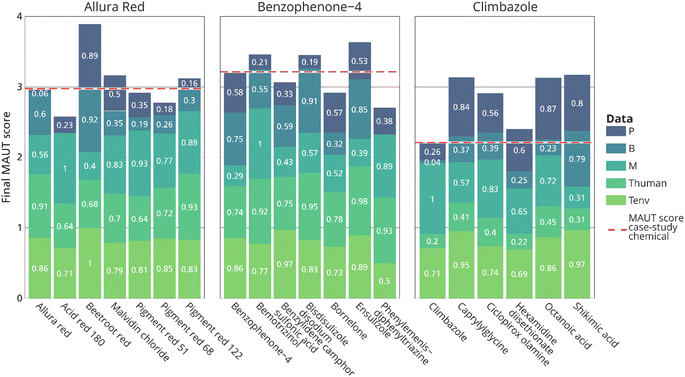
| Fig. 4 Heat map and total hazard score of the three case-study chemicals and their potential alternatives for the P (persistency), B (bioaccumulation), M (mobility), human toxicity (Thuman) and ecotoxicity (Tenv) endpoints. The thresholds for the colour codes were based on ref. 34 and the scores were based on ref. 25 Colour codes indicate whether a hazard criterion is exceeded according to current regulatory standards and a very high hazard (red) is assigned. When hazard criteria fell below regulatory standards a high (orange), moderate (yellow) or low (green) hazard level is assigned. Chemicals with lower hazard scores compared to the case-study chemical are potentially safer alternatives. | ||
For benzophenone-4, all substances were assigned at least one red indicator for persistency and, except for bemotrizinol, the mobility hazard criterion was met for all substances. The lowest total hazard score of 37 was obtained for ensulizole, making this the most preferred alternative.
Climbazole and potential alternatives were all assigned a red indicator for mobility as well. The lowest hazard score was obtained for shikimic acid, which obtained a score of 36.5.
Heat maps for the other scenarios (i.e. risk seeking and risk averse scenarios) to test the sensitivity of the approach to the data gaps are available in the ESI (SI 5).† This, however, did not change the ranking of the most favourable alternative compared to the case-study chemical.
Similar ranking of the alternatives was furthermore observed in the ELECTRE III method, which was performed to complement MAUT results. From Fig. 5, it can be observed that, even though the total MAUT score is higher for some potential alternatives, the individual criteria do not always have a better score. For example, benzophenone-4 has a P score of 0.51, but most assessed alternatives are more persistent based on the available data, and therefore have a lower P score.
| Use case | Chemical name | Heatmap OECD scoring | MAUT | ELECTRE III |
|---|---|---|---|---|
| Pigment | Allura red | 2 | 4 | 3 |
| Malvidin chloride | 4 | 2 | 2 | |
| Beetroot red | 1 | 1 | 1 | |
| Pigment red 51 | 6 | 5 | 5 | |
| Pigment red 68 | 4 | 6 | 3 | |
| Acid red 180 | 7 | 7 | 7 | |
| Pigment red 122 | 3 | 3 | 5 | |
| UV-filter | Benzophenone-4 | 2 | 4 | 3 |
| Ensulizole | 1 | 1 | 1 | |
| Benzylidene camphor sulfonic acid | 5 | 5 | 7 | |
| Bisdisulizole disodium | 2 | 3 | 5 | |
| Bemotrizinol | 4 | 2 | 2 | |
| Bornelone | 7 | 6 | 5 | |
| Phenylemenis-diphenyltriazine | 6 | 7 | 3 | |
| Anti-seborrheic | Climbazole | 6 | 6 | 5 |
| Octanoic acid | 3 | 2 | 1 | |
| Caprylylglycine | 2 | 3 | 2 | |
| Shikimic acid | 1 | 1 | 4 | |
| Ciclopirox olamine | 4 | 4 | 2 | |
| Hexamidine diisethionate | 5 | 5 | 6 |
3.4 Chemical management step based on functionality and essentiality
The technical function of Allura red was not considered necessary for the performance of some cosmetic products, and it is thus considered as non-essential. The chemical should therefore be removed from those cosmetic products where its function is unnecessary (Table 3). For other applications of Allura red, as well as all applications of climbazole and benzophenone-4, the technical function of the chemical was considered necessary for the desired performance of the cosmetic products. Even though no hazard-free alternatives exist, potentially better alternatives were identified for all case-study chemicals in the alternative assessment of hazards. This assessment indicated that Allura red, benzophenone-4 and climbazole can be substituted. Following the concept of essential-use, the use of these chemicals of concern is considered as non-essential for these products, as they can be substituted.| Case-study chemical | Cosmetic products in which the chemical is present, ordered per product group | Technical function of the substance in the cosmetic products | Is the functional use of the chemical necessary for the performance of the product? | Are there no alternatives available? | Is the use of the chemical necessary for health, safety or critical for the functioning of society? | Management of the substance in a product |
|---|---|---|---|---|---|---|
| Benzo phenone-4 | Bath and body products: body wash/shower gel, body scrub, body oil, and bath salt | UV protection | Yes, find drop-in chemical replacement | No | Not relevant | Substitute |
| Facial care: cleansers | Yes, find drop-in chemical replacement | No | Not relevant | Substitute | ||
| Hair care: conditioner, hair spray, shampoo, and styling cream | ||||||
| Hands and nails: hand wash | Yes, find drop-in chemical replacement | No | Not relevant | Substitute | ||
| Make-up: lipstick/lip gloss/lip pencil | Yes, find drop-in chemical replacement | No | Not relevant | Substitute | ||
| Suncare: self-tanning, and suncream/lotion/gel | Yes, find drop-in chemical replacement | No | Not relevant | Substitute | ||
| Climbazole | Hair care: shampoo | Anti-dandruff agent | Yes, find drop-in chemical replacement | No | Not relevant | Substitute |
| Allura red | Bath and body products: shower gel, and bath bomb | Colourant | No, redesign product or packaging | Not relevant | Not relevant | Remove from application |
| Facial care: cleansers, eye makeup remover, face mask, lip balm, and scrub/peeling | No, redesign product or packaging | Not relevant | Not relevant | Remove from application | ||
| Hair care: conditioner, hair spray, shampoo, hair colour, and hair mask | Hair colours: yes, find drop-in chemical replacement | No | Not relevant | Substitute in hair colour | ||
| All other products: no, redesign product or packaging | Remove from all other formulations | |||||
| Foot care: foot wash/bath | No, redesign product or packaging | Not relevant | Not relevant | Remove from application | ||
| Fragrances: parfum/eau de toilette/body mist | No, redesign product or packaging | Not relevant | Not relevant | Remove from application | ||
| Hands and nails: hand disinfection, hand wash, and nail polish remover | No, redesign product or packaging | Not relevant | Not relevant | Remove from application | ||
| Make-up: pressed powders, (foundation, bronzer, primer, blush and eye make-up), eyebrow pencil, eyeliner, lipstick/lip gloss/lip pencil, mascara, and nail polish | Yes, find drop-in chemical replacement | No | Not relevant | Substitute | ||
| Mouth/toothcare: mouthwash and toothpaste | No, redesign product or packaging | Not relevant | Not relevant | Remove from application | ||
| Suncare: self-tanning, suncream/lotion/gel, and sunspray | No, redesign product or packaging | Not relevant | Not relevant | Remove from application |
4 Discussion and conclusion
4.1 PMT/vPvM substances in cosmetics
Our analysis revealed that approximately 6.4% of all cosmetic products contain PMT or vPvM substances, and that these substances have a wide variety of functions in cosmetics. The highest share of PMT/vPvM substances was found in hair care products, followed by facial care products. The list of (potential) PMT/vPvM substances from Arp and Hale (2019) was used as a starting point in this study which classified substances as mobile if the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc is 4.5 or lower.26 In the proposed CLP revision, the mobility criterion, however, is set as log
Koc is 4.5 or lower.26 In the proposed CLP revision, the mobility criterion, however, is set as log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc ≤ 3,42 so a more stringent analysis can be considered. Thus, the list from Arp and Hale (2019)26 might not provide an accurate overview of all relevant PMT/vPvM substances. Previous studies ranked PMT/vPvM substances based on their emission potential to the environment43 or their mobility through waste water treatments.44 Half of the PMT/vPvM substances identified in the CosIng database are listed on these prioritisation lists (Table SI2.1†). This emphasises that, whilst we selected the most frequently occurring PMT/vPvM substances in cosmetics based on data from Kemiluppen and Cosmethics, many other relevant substances, uses and consequent emissions need to be addressed in future studies.
Koc ≤ 3,42 so a more stringent analysis can be considered. Thus, the list from Arp and Hale (2019)26 might not provide an accurate overview of all relevant PMT/vPvM substances. Previous studies ranked PMT/vPvM substances based on their emission potential to the environment43 or their mobility through waste water treatments.44 Half of the PMT/vPvM substances identified in the CosIng database are listed on these prioritisation lists (Table SI2.1†). This emphasises that, whilst we selected the most frequently occurring PMT/vPvM substances in cosmetics based on data from Kemiluppen and Cosmethics, many other relevant substances, uses and consequent emissions need to be addressed in future studies.
4.2 Assessing functionality and identifying alternatives
In this study, only chemical-by-chemical substitution was assessed as no assessment could be performed to compare chemicals to other types of alternatives for functional substitution, such as a change of material and system changes.23 It is key that an alternative chemical provides the same chemical function to preserve the overall performance of products, whilst lowering hazards. An extensive search was conducted to identify candidate alternatives with a known chemical function. However, this search might not be exhaustive as other, perhaps better, alternatives exist that are not listed in open databases. In addition, inorganic substances and substances without a CAS number were purposely left out as no hazard data were available. The other identified substances were short-listed based on already known hazard and classification data.29–31,39 Chemicals that are not classified as hazardous under current regulations are, however, not necessarily safer compared to classified chemicals, as effects of many chemicals are not sufficiently studied, both in the scientific literature and within REACH due to varying information requirements based on the annual tonnage.45–47 In addition, information on the specific end function (i.e. function of the chemical in the product) is often unknown, for many types of chemical uses. Information contained in the CosIng database provided a generic function such as a UV filter, but detailed information on the functionality (such as the UV absorbance spectrum) is not given. Approaches to predict chemical function based on structural and physicochemical descriptors exist,48,49 but to our knowledge only provide generic classifications. Hence, better open data on the different uses and functionalities of chemicals in products would be helpful in the future to support chemical substitution.4.3 Collection and evaluation of hazard data
Most experimental data used in our study was obtained from REACH dossiers, and no, or only a few, experimental endpoints were found for substances not registered under REACH. Data contained in REACH dossiers, however, are not always compliant, and issues regarding REACH data reliability have been raised before.50,51 Future studies assessing safer alternatives using REACH data might therefore need to incorporate reliability assessments in order to communicate and deal with uncertainties, which will subsequently increase the transparency of the alternative assessment.52,53 Experimental data can furthermore be less reliable when only one study for an endpoint is available. In these cases, it might be better to use a set of QSAR predictions to define an endpoint instead of using this single experimental value.54 However, for many endpoints no experimental data were found and QSAR methods were used for all chemicals in order to fill in data gaps. Modelled results also need to be interpreted with caution, especially when results from different QSARs could not be combined as only one model for a specific endpoint was available. Thus, it is also important to generate confidence scores for QSAR results to reflect uncertainties in alternative assessments.55 Future studies need to better explore how both experimental and QSAR data can be combined in MCDAs and how confidence scores can be incorporated into these approaches. Furthermore, data gaps were found for all chemicals and included in the MCDA approaches by assigning standard scores. Methods on how to handle data gaps can influence the alternative assessment outcomes greatly.56 However, there is not yet an agreed method on how this should be done.21,57 Data gaps were mainly found for long-term exposure endpoints, emphasising the need of data generation and/or developments of in silico methods in these areas.4.4 Comparing and selecting safer alternatives
We were able to show that safer alternatives for Allura red, benzophenone-4 and climbazole exist, based on an assessment of hazards. However, none of the assessed alternatives fully satisfy the criteria for the set of 26 hazard endpoints, meaning that no ‘hazard-free’ alternatives are available. For the MCDA approaches, endpoints were selected based on OECD recommendations and weighed using an equal weighing approach. As the vast majority of low molecular weight substances that are neutral or weak to non-polar will be either bioaccumulative or mobile,58 many chemicals will receive similar scores for the combined bioaccumulation and mobility criteria. A few polar, ionisable or ionic substances can, on the other hand, be classified as both bioaccumulative and mobile, as is the case for some PFAS (e.g. perfluorooctanoic acid (PFOA)). Furthermore, a classification of a substance as bioaccumulative or mobile should not be reason for concern alone, but should always be combined with persistency; the higher the persistence of a substance, the higher the concern for potential long-lasting effects on human health and the environment.59 The MCDA method used in this study can thus be refined by applying higher weights to the most important endpoints, such as persistency.The relevance of other individual hazard endpoints varies according to the chemical and product type. A certain hazard might sometimes even be needed for the functionality of a chemical. For example, poorly degradable (i.e. persistent) UV-filters have a higher efficacy as they can protect the skin from UV radiation for a longer time.60 In addition, certain hazards are legally allowed for one use, but not for another. Category 1 and 2 CMR substances can, for example, be used in cosmetic products if the Scientific Committee on Consumer Safety deems the reasonably foreseen uses as safe (Art. 15 Regulation (EC) No. 1223/2009). Different stakeholders need to be involved in order to refine alternative assessments by e.g. selecting relevant endpoints, weighing endpoints in MCDAs and dealing with trade-offs.21,57,61,62
It is furthermore important to note that the present analysis solely focused on hazard assessments of the potential alternatives compared to the substances of concern. Further work is needed to characterise the relevant exposure routes for humans and the environment resulting from the use of the alternatives. This might change the level of concern for the assessed alternatives. Such an assessment could be performed qualitatively, as outlined in the OECD guidance.25
4.5 MCDA approaches for chemical alternative assessments
In this study, three different MCDA approaches were used to compare hazards and select a safer alternative to the case-study chemicals, which generally yielded similar results in identifying the top ranked alternatives. Even though the combined use of multiple MCDA approaches has been advised,63 it might not always be feasible to use multiple (complex) methods due to time and other resource constraints. Limitations and benefits of the heatmap, MAUT and ELECTRE approaches have previously been documented.34 We also found the heat map to be the most helpful in rapidly comparing burden-shifting of hazards across case-study chemicals and potential alternatives, and by applying the OECD scoring approach a hazard ranking is obtained by which the most preferred chemical is identified. This ranking, however, is for a large part determined by the toxicity to human health, relating to 12 out of 26 endpoints. This is circumvented by using aggregated scores for PBMT, as performed for the MAUT and ELECTRE ranking. Of these two methods, the MAUT method is the most user friendly. The ELECTRE method is an outranking technique where superior performance in some criteria can compensate for inferior performance in other criteria. Even though ELECTRE is best able to deal with data uncertainty, a potential downside, however, is that the method does not always reflect the magnitude of relative superior or under performance of individual criteria.21 Selection of the most suitable approach will depend greatly on the information needs of decision makers, but guidance is currently lacking to select and successfully implement MCDA approaches.64,654.6 Managing hazardous substances in cosmetic products
Following the combined application of the concepts of functional substitution and essential-use,19 none of the assessed uses of PMT/vPvM substances were found to be essential. Starting with considerations of the function, some uses of Allura red were found not to be needed for the performance of cosmetic products. For other uses of Allura red and all uses of benzophenone-4 and climbazole, as the chemical function was considered necessary for product technical performance, the essentiality depended on the availability of suitable alternatives. As safer alternatives are available, the use of the PMT/vPvM substances was non-essential and should be substituted.18 This also meant that the question whether the use of the chemical is necessary for health, safety or functioning of society did not need to be answered. It might, however, be argued that, based on essentiality criteria defined earlier,17,18 the use of hazardous substances in cosmetic products can always be classified as non-essential. Given that chemical risks are currently not adequately managed due to the many different chemicals and uses existing,6,66 discussions about the essentiality of chemical uses might help to decide whether the continued widespread use of chemicals for certain uses is desirable in the first place.4.7 Looking beyond chemical hazards
This work focussed mainly on the hazard assessment of chemical alternatives due to data limitations regarding the technical and economic feasibility. These other aspects should, however, be included in order to make an informed choice on the substitution of chemicals. The technical and economic feasibility of a specific alternative can depend highly on the type of actor performing the alternative assessment, and on the type of product in which the compound of concern is used. Similarly, due to lack of open data and resources, the availability of an alternative can be dependent on the actor who is attempting to phase-out a hazardous substance and could therefore not be evaluated with certainty in the context of this study.Human health and the environment might not sufficiently be protected when only hazards are considered,67,68 emphasising the need to include exposure assessments. In order to estimate emission and exposure, information on the different uses of chemicals and their volume in products is needed. However, to our knowledge this information is not yet openly available, and modelling approaches will be needed instead, e.g. to estimate chemical weight fractions.68,69 Furthermore, it is important to consider environmental impacts beyond chemical effects, as emphasised by e.g. the planetary boundaries concept and the ambition laid down in the CSS to transition towards safe and sustainable chemicals.12,70 Environmental impact assessments covering the whole product life-cycle can potentially be used to obtain a complete overview of all potential advantages and disadvantages of using a certain substance.67,71
As alternative assessments are a core element to phase out hazardous substances via the essential-use concept, it is key that these methods combine hazard-based considerations with broader life-cycle impacts to prevent burden shifting and assure that chemicals are used in a safe and sustainable manner. Such assessments might affect the ranking of most preferred alternatives found in this study. For example, beetroot red was in our analysis the best alternative to Allura red. However, the use of plant extracts in consumer products can result in a larger environmental footprint due to increased water depletion and CO2 emissions.72,73 Incorporation of sustainability assessments will add an additional layer of complexity to chemical assessment. Further methods are therefore required that combine safety and sustainability considerations in an accessible and transparent way.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is part of the Innovative Training Network ECORISK2050 and ZeroPM, and was supported by the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. [813124] and grant agreement [No. 101036756].References
-
Oxford Economics, The Global Chemical Industry: Catalyzing Growth and Addressing Our World's Sustainability Challenges, International Council of Chemical Associations (ICCA), Washington DC, US, 2019, available from: https://icca-chem.org/wp-content/uploads/2020/10/Catalyzing-Growth-and-Addressing-Our-Worlds-Sustainability-Challenges-Report.pdf Search PubMed
.
- Z. Wang, G. W. Walker, D. C. G. Muir and K. Nagatani-Yoshida, Toward a Global Understanding of Chemical Pollution: A First Comprehensive Analysis of National and Regional Chemical Inventories, Environ. Sci. Technol., 2020, 54(5), 2575–2584 CrossRef CAS PubMed
.
- R. Naidu, B. Biswas, I. R. Willett, J. Cribb, B. Kumar Singh and C. Paul Nathanail,
et al., Chemical pollution: A growing peril and potential catastrophic risk to humanity, Environ. Int., 2021, 156, 106616 CrossRef CAS PubMed
.
- L. Posthuma, M. C. Zijp, D. D. Zwart, D. V. d. Meent, L. Globevnik and M. Koprivsek,
et al., Chemical pollution imposes limitations to the ecological status of European surface waters, Sci. Rep., 2020, 10(1), 14825 CrossRef PubMed
.
-
IPBES, Summary for policymakers of the global assessment report on biodiversity and ecosystem services [internet], Zenodo, 2019, available from: https://zenodo.org/record/3553579#.X-MVdvlKg2w
.
- L. Persson, B. M. Carney Almroth, C. D. Collins, S. Cornell, C. A. de Wit and M. L. Diamond,
et al., Outside the Safe Operating Space of the Planetary Boundary for Novel Entities, Environ. Sci. Technol., 2022, 56(3), 1510–1521 CrossRef CAS PubMed
.
- W. Steffen, K. Richardson, J. Rockström, S. E. Cornell, I. Fetzer and E. M. Bennett,
et al., Planetary boundaries: Guiding human development on a changing planet, Science, 2015, 347(6223), 1259855 CrossRef PubMed
.
- S. E. Hale, H. P. H. Arp, I. Schliebner and M. Neumann, Persistent, mobile and toxic (PMT) and very persistent and very mobile (vPvM) substances pose an equivalent level of concern to persistent, bioaccumulative and toxic (PBT) and very persistent and very bioaccumulative (vPvB) substances under REACH, Environ. Sci. Eur., 2020, 32(1), 155 CrossRef CAS
.
- H. Rüdel, W. Körner, T. Letzel, M. Neumann, K. Nödler and T. Reemtsma, Persistent, mobile and toxic substances in the environment: a spotlight on current research and regulatory activities, Environ. Sci. Eur., 2020, 32(1), 5 CrossRef
.
- S. Schulze, D. Zahn, R. Montes, R. Rodil, J. B. Quintana and T. P. Knepper,
et al., Occurrence of emerging persistent and mobile organic contaminants in European water samples, Water Res., 2019, 80–90 CrossRef CAS PubMed
.
- European Commission. Proposal for a Regulation of the European Parliament and of the Council Amending Regulation (EC) No. 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures, COM(2022) 748 final. 2022/0432 (COD) [Internet], 2022. available from: https://environment.ec.europa.eu/publications/proposal-clp-revision_en.
- European Commission, COM(2020) 667 final, Communication from the Commission to the European Parliament, The Council, The European Economic and Social Committee and the Committee of the Regions, Chemicals Strategy for Sustainability Towards a Toxic-Free Environment [Internet]. Brussels; 2020 Oct, available from: https://ec.europa.eu/environment/pdf/chemicals/2020/10/Strategy.pdf.
-
K. Winkens Pütz, J. P. Benskin, S. Namazkar, M. Plassmann and K. Hansson, PFASs in Cosmetics [internet]. Stockholm, KEMI, 2021 [cited 2021 Nov 10]. Report No.: PM 9/21, available from: https://www.kemi.se/en/publications/pms/2021/pm-9-21-pfass-in-cosmetics Search PubMed
.
- M. Bilal, S. Mehmood and H. M. N. Iqbal, The Beast of Beauty: Environmental and Health Concerns of Toxic Components in Cosmetics, Cosmetics, 2020, 7(1), 13 CrossRef CAS
.
-
D. Kättström, A. Beronius, C. Rudén and M. Ågerstrand. Stricter Regulation Applies to Antimicrobial Substances when Used as Biocides Compared to Cosmetics under Current EU Legislation. Emerging Contaminants [internet], 2022, [cited 2022 May 2]; available from: https://www.sciencedirect.com/science/article/pii/S2405665022000154 Search PubMed
.
- European Commission, INCEPTION IMPACT ASSESSMENT: EU Chemicals Strategy for Sustainability – Revision of the Cosmetic Products Regulation. Ref. Ares(2021)6011962 [Internet]. 2021, available from: https://ec.europa.eu/info/law/better-regulation/.
- I. T. Cousins, J. C. D. Witt, J. Glüge, G. Goldenman, D. Herzke and R. Lohmann,
et al., Finding essentiality feasible: common questions and misinterpretations concerning the “essential-use” concept, Environ. Sci.: Processes Impacts, 2021, 23(8), 1079–1087 RSC
.
- I. T. Cousins, G. Goldenman, D. Herzke, R. Lohmann, M. Miller and C. A. Ng, The Concept of Essential Use for Determining when Uses of PFASs Can Be Phased Out, Environ. Sci.: Processes Impacts, 2019, 21(11), 1803–1815 RSC
.
- M. A. Roy, I. Cousins, E. Harriman, M. Scheringer, J. A. Tickner and Z. Wang, Combined Application of the Essential-Use and Functional Substitution Concepts: Accelerating Safer Alternatives, Environ. Sci. Technol., 2022, 56(14), 9842–9846 CrossRef CAS PubMed
.
- J. Glüge, R. London, I. T. Cousins, J. DeWitt, G. Goldenman and D. Herzke,
et al., Information Requirements under the Essential-Use Concept: PFAS Case Studies, Environ. Sci. Technol., 2021, 56(10), 6232–6242 CrossRef PubMed
.
- M. M. Jacobs, T. F. Malloy, J. A. Tickner and S. Edwards, Alternatives assessment frameworks: Research needs for the informed substitution of hazardous chemicals, Environ. Health Perspect., 2016, 124(3), 265–280 CrossRef PubMed
.
- K. Sackmann, T. Reemtsma, M. Rahmberg and D. Bunke, Impact of European chemicals regulation on the industrial use of plasticizers and patterns of substitution in Scandinavia, Environ. Int., 2018, 119, 346–352 CrossRef CAS PubMed
.
- J. A. Tickner, J. N. Schifano, A. Blake, C. Rudisill and M. J. Mulvihill, Advancing Safer Alternatives Through Functional Substitution, Environ. Sci. Technol., 2015, 49(2), 742–749 CrossRef CAS PubMed
.
- ECHA, Guidance On the Preparation of an Application for Authorisation, ECHA-20-G-03-EN, Helsinki, 2021.
- OECD, Guidance on Key Considerations for the Identification and Selection of Safer Chemical Alternatives, Series on Risk Management No. 60. Environment, Health and Safety, Environment Directorate,OECD, 2021 Search PubMed.
-
H. P. H. Arp and S. E. Hale, REACH: Improvement of guidance and methods for the identification and assessment of PMT/vPvM substances Final Report [Internet], Dessau-Roßlau, 2019. Available from: http://www.umweltbundesamt.de/publikationen Search PubMed
.
- European Commission, CosIng [Internet], [cited 2021 Jan 11]. Available from: https://ec.europa.eu/growth/tools-databases/cosing/.
- SpecialChem, Cosmetics Ingredients Selector [Internet], Available from: https://cosmetics.specialchem.com/ Search PubMed.
- BAuA, Subsportplus: Database of restricted and priority substances (List of lists) [Internet]. [cited 2022 Jun 17], available from: https://www.subsportplus.eu/subsportplus/EN/Substances/Database-of-restricted-and-priority-substances/restricted-priority-substances_node.html Search PubMed.
- ECHA. REACH—Registered Substances Factsheets [internet], available from: https://echa.europa.eu/information-on-chemicals/registered-substances Search PubMed.
- ECHA. Annex III Inventory [internet], [cited 2023 Jul 4], available from: https://echa.europa.eu/information-on-chemicals/annex-iii-inventory Search PubMed.
- S. Kovarich, L. Ceriani, A. Ciacci, R. Baldin, M. Perez Miguel, D. Gibin, et al., OpenFoodTox: EFSA’s chemical hazards database [internet], Zenodo, 2020 [cited 2022 Jun 21], available from: https://zenodo.org/record/3693783.
-
OECD, ECHA, OECD QSAR Toolbox v. 4.5 SP1 Search PubMed
.
- Z. Zheng, G. M. Peters, H. Peter and H. Arp, Combining In Silico Tools with Multicriteria Analysis for Alternatives Assessment of Hazardous Chemicals: A Case Study of Decabromodiphenyl Ether Alternatives, Environ. Sci. Technol., 2019, 53(11), 6341–6351 CrossRef CAS PubMed
.
- Z. Zheng, H. P. H. Arp, G. Peters and P. L. Andersson, Combining In Silico Tools with Multicriteria Analysis for Alternatives Assessment of Hazardous Chemicals: Accounting for the Transformation Products of decaBDE and Its Alternatives, Environ. Sci. Technol., 2021, 55(2), 1088–1098 CrossRef CAS PubMed
.
- IACM, Allura Red [Internet], Allura Red. [cited 2022 Oct 17], available from: https://iacmcolor.org/color-profile/allura-red-ac-fdc-red-no-40/ Search PubMed.
- A. R. Heurung, S. I. Raju and E. M. Warshaw, Benzophenones, Dermatitis, 2014, 25(1), 3–10 CrossRef CAS PubMed
.
-
S. Polati, F. Gosetti and M. Gennaro, Preservatives in Cosmetics. Analytical Methods, in: Analysis of Cosmetic Products, Elsevier Amsterdam, 2007, pp. 211–241 Search PubMed
.
- European Commission, Annex III to the Cosmetic Product Regulation. List of Substances which cosmetic products must not contain except subject to the restriction laid down. [Internet], 2023 [cited 2023 Jul 4], available from: https://ec.europa.eu/growth/tools-databases/cosing/pdf/COSING_AnnexIII_v2.pdf.
-
D. N. Brooke, J. S. Burns and M. J. Crookes, UV-Filters in Cosmetics – Prioritisation for Environmental Assessment. SCHO1008BPAY-E-P, Environment Agency, Rio House, Waterside Drive, Aztec West, Almondsbury, Bristol, BS32 4UD, 2008 Search PubMed
.
- A. Jesus, E. Sousa, M. T. Cruz, H. Cidade, J. M. S. Lobo and I. F. Almeida, UV Filters: Challenges and Prospects, Pharmaceuticals, 2022, 15(3), 263 CrossRef CAS PubMed
.
- European Commission, ANNEXES to the Commission Delegated Regulation Amending Regulation (EC) No 1272/2008 as Regards Hazard Classes and Criteria for the Classification, Labelling and Packaging of Substances and Mixtures. C(2022) 9383 final. ANNEXES 1 to 4 [Internet], 2022, available from: https://environment.ec.europa.eu/publications/clp-delegated-act_en.
- S. Schulze, D. Sättler, M. Neumann, H. P. H. Arp, T. Reemtsma and U. Berger, Using REACH registration data to rank the environmental emission potential of persistent and mobile organic chemicals, Sci Total Environ., 2018, 625, 1122–1128 CrossRef CAS PubMed
.
- E. Fries, T. Grewal and R. Sühring, Persistent, mobile, and toxic plastic additives in Canada: properties and prioritization, Environ. Sci.: Processes Impacts, 2022, 24(10), 1945–1956 RSC
.
- J. Coria, E. Kristiansson and M. Gustavsson, Economic interests cloud hazard reductions in the European regulation of substances of very high concern, Nat. Commun., 2022, 13(1), 6686 CrossRef CAS PubMed
.
- E. Kristiansson, J. Coria, L. Gunnarsson and M. Gustavsson, Does the scientific knowledge reflect the chemical diversity of environmental pollution? – A twenty-year perspective, Environ. Sci. Policy, 2021, 126, 90–98 CrossRef CAS
.
- A. Sobek, S. Bejgarn, C. Rudén and M. Breitholtz, The dilemma in prioritizing chemicals for environmental analysis: Known: versus unknown hazards, Environ. Sci.: Processes Impacts, 2016, 18(8), 1042–1049 RSC
.
- K. K. Isaacs, M. R. Goldsmith, P. Egeghy, K. Phillips, R. Brooks and T. Hong,
et al., Characterization and prediction of chemical functions and weight fractions in consumer products, Toxicol. Rep., 2016, 3, 723–732 CrossRef CAS PubMed
.
- K. A. Phillips, J. F. Wambaugh, C. M. Grulke, K. L. Dionisio and K. Isaacs, High-throughput screening of chemicals as functional substitutes using structure-based classification models, Green Chem., 2017, 19(4), 1063–1074 RSC
.
- E. Ingre-Khans, M. Ågerstrand, A. Beronius and C. Rudén, Reliability and relevance evaluations of REACH data, Toxicol. Res., 2019, 8(1), 46–56 CrossRef CAS PubMed
.
- UBA. REACH Compliance: Data Availability in REACH Registrations Part 2: Evaluation of Data Waiving and Adaptations for Chemicals ≥1000 tpa. Final Report. 2018; Available from: https://www.umweltbundesamt.de/sites/default/files/medien/1410/publikationen/2018-10-23_texte_64-<?pdb_no 2018?>2018<?pdb END?>_reach-compliance-data_ii.pdf.
- E. Ingre-Khans, M. Ågerstrand, C. Rudén and A. Beronius, Improving structure and transparency in reliability evaluations of data under REACH: suggestions for a systematic method, Hum. Ecol. Risk Assess., 2019, 1–30 Search PubMed
.
- C. T. A. Moermond, R. Kase, M. Korkaric and M. Ågerstrand, CRED: Criteria for reporting and evaluating ecotoxicity data, Environ. Toxicol. Chem., 2016, 35(5), 1297–1309 CrossRef CAS PubMed
.
- L. Li, Z. Zhang, Y. Men, S. Baskaran, A. Sangion and S. Wang,
et al., Retrieval, Selection, and Evaluation of Chemical Property Data for
Assessments of Chemical Emissions, Fate, Hazard, Exposure, and Risks, ACS Environ. Au, 2022, 2(5), 376–395 CrossRef CAS PubMed
.
- G. J. Myatt, A. Bassan, D. Bower, K. M. Crofton, K. P. Cross and J. C. Graham,
et al., Increasing the acceptance of in silico toxicology through development of protocols and position papers, Comput. Toxicol., 2022, 21, 100209 CrossRef CAS
.
- T. F. Malloy, P. J. Sinsheimer, A. Blake and I. Linkov, Use of multi-criteria decision analysis in regulatory alternatives analysis: A case study of lead free solder, Integr. Environ. Assess. Manage., 2013, 9(4), 652–664 CrossRef CAS PubMed
.
- J. Tickner, M. Jacobs, T. Malloy, T. Buck, A. Stone and A. Blake,
et al., Advancing Alternatives Assessment for Safer Chemical Substitution: A Research and Practice Agenda, Integr. Environ. Assess. Manage., 2019, 15(6), 855–866 CrossRef CAS PubMed
.
-
M. Neumann and I. Schliebner, Protecting the Sources of Our Drinking Water: the Criteria for Identifying Persistent, Mobile and Toxic (PMT) Substances and Very Persistent and Very Mobile (vPvM) Substances under EU Regulation REACH (EC) No 1907/2006, German Environment Agency, Dessau-Roßlau, 2019. Report No.: 127/2019 Search PubMed
.
- I. T. Cousins, C. A. Ng, Z. Wang and M. Scheringer, Why is high persistence alone a major cause of concern?, Environ. Sci.: Processes Impacts, 2019, 21(5), 781–792 RSC
.
- D. L. Giokas, A. Salvador and A. Chisvert, UV filters: From sunscreens to human body and the environment, TrAC, Trends Anal. Chem., 2007, 26(5), 360–374 CrossRef CAS
.
- K. A. Grant, L. Nakayama Wong, Q. Meng, H. Lee, D. Phelps and S. Davis,
et al., Informed substitution of hazardous chemicals through the lens of California's Safer Consumer Products Alternatives Analysis: Best practices, challenges, and opportunities, Integr. Environ. Assess. Manage., 2022, 18(4), 1007–1019 CrossRef PubMed
.
- J. Tickner, M. M. Jacobs and N. B. Mack, Alternatives assessment and informed substitution: A global landscape assessment of drivers, methods, policies and needs, Sustainable Chem. Pharm., 2019, 13, 100161 CrossRef
.
- B. I. Yatsalo, G. A. Kiker, J. Kim, T. S. Bridges, T. P. Seager and K. Gardner,
et al., Application of multicriteria decision analysis tools to two contaminated sediment case studies, Integr. Environ. Assess. Manage., 2007, 3(2), 223–233 CrossRef PubMed
.
- C. Beaudrie, C. J. Corbett, T. A. Lewandowski, T. Malloy and X. Zhou, Evaluating the Application of Decision Analysis Methods in Simulated Alternatives Assessment Case Studies: Potential Benefits and Challenges of Using MCDA, Integr. Environ. Assess. Manage., 2021, 17(1), 27–41 CrossRef PubMed
.
- H. V. Rowley, G. M. Peters, S. Lundie and S. J. Moore, Aggregating sustainability indicators: Beyond the weighted sum, J. Environ. Manage., 2012, 111, 24–33 CrossRef PubMed
.
- K. Fenner and M. Scheringer, The Need for Chemical Simplification As a Logical Consequence of Ever-Increasing Chemical Pollution, Environ. Sci. Technol., 2021, 55(21), 14470–14472 CrossRef CAS PubMed
.
- W. Greggs, T. Burns, P. Egeghy, M. R. Embry, P. Fantke and B. Gaborek,
et al., Qualitative Approach to Comparative Exposure in Alternatives Assessment, Integr. Environ. Assess. Manage., 2019, 15(6), 880–894 CrossRef PubMed
.
- O. Jolliet, L. Huang, P. Hou and P. Fantke, High Throughput Risk and Impact Screening of Chemicals in Consumer Products, Risk Anal., 2021, 41(4), 627–644 CrossRef PubMed
.
- K. K. Isaacs, K. A. Phillips, D. Biryol, K. L. Dionisio and P. S. Price, Consumer product chemical weight fractions from ingredient lists, J. Exposure Sci. Environ. Epidemiol., 2018, 28(3), 216–222 CrossRef CAS PubMed
.
- J. Rockström, W. Steffen, K. Noone, Å. Persson, F. S. I. Chapin and E. Lambin,
et al., Planetary Boundaries: Exploring the Safe Operating Space for Humanity, Ecol. Soc., 2009, 14(2), 32 CrossRef
, http://www.ecologyandsociety.org/vol14/iss2/art32/.
- P. Fantke, L. Huang, M. Overcash, E. Griffing and O. Jolliet, Life cycle based alternatives assessment (LCAA) for chemical substitution, Green Chem., 2020, 22(18), 6008–6024 RSC
.
- H. Holmquist, S. Roos, S. Schellenberger, C. Jönsson and G. Peters, What difference can drop-in substitution actually make? A life cycle assessment of alternative water repellent chemicals, J. Cleaner Prod., 2021, 329, 129661 CrossRef CAS
.
- M. Secchi, V. Castellani, E. Collina, N. Mirabella and S. Sala, Assessing eco-innovations in green chemistry: Life Cycle Assessment (LCA) of a cosmetic product with a bio-based ingredient, J. Cleaner Prod., 2016, 129, 269–281 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3em00025g |
| This journal is © The Royal Society of Chemistry 2023 |