The geno-toxicological impacts of microplastic (MP) exposure on health: mechanistic pathways and research trends from a Chinese perspective
Lihui
Xuan
a,
Liang
Xiao
*b and
Ruixue
Huang
 *a
*a
aDepartment of Occupational and Environmental Health, Xiangya School of Public Health, Central South University, Changsha, Hunan Province 410078, China. E-mail: huangruixue@csu.edu.cn; 1179289247@qq.com; Tel: +86-731-84805460
bFaculty of Naval Medicine, Naval Medical University (Second Military Medical University), Shanghai, 200433, China. E-mail: hormat830713@hotmail.com
First published on 11th October 2022
Abstract
Due to their large-scale manufacture and widespread application, global concern regarding microplastics (MPs) has been increasing rapidly over the past decade, in particular their potential genotoxicity. The genome is constantly exposed to genotoxic insults that can lead to accumulation of reactive oxygen species (ROS), DNA damage, cell death, inflammation or genetic regulation which in turn can have consequences for health, such as the induction of carcinogenesis. In this review, we presented a comprehensive landscape of the effects of MPs on genotoxicity including the molecular mechanisms. Followed by the MP research trend analysis from a global viewpoint including the comparative research between China and USA and point out that scientists should continue to substantially contribute to the field of MPs through more extensive academic investigation, global cooperation, and the development of novel control methods. Challenges are also discussed. Overall, this review provides insights into the genotoxic effects of MPs on human health and related research trends in this field.
Environmental significanceMicroplastics (MPs), less than 5 mm in diameter, are main pollutants of air, water and soil. Due to their large-scale manufacture and widespread application, global concern regarding microplastics (MPs) has been increasing rapidly over the past decade, in particular their potential genotoxicity. The genome is constantly exposed to genotoxic insults that can lead to accumulation of reactive oxygen species (ROS), DNA damage, cell death, inflammation or genetic regulation which in turn can have consequences for health, such as the induction of carcinogenesis. |
1 Introduction
Microplastics (MPs) are plastic particles less than 5 mm in diameter,1 but there are also views that the size of MPs should be in the range of 1 μm to 1 mm.2 The sources of MPs are mainly divided into two categories: primary MPs and secondary MPs, both of which cause irreversible damage to the environment.3 Primary MPs refer to industrial products of plastic particles discharged into the water environment through rivers, sewage treatment plants, etc., including toothpaste, scrubs, synthetic protective clothing, and cosmetics, all of which contain plastic particles to varying degrees.4,5 Secondary MPs are plastics that are subjected to biotic and abiotic stresses, causing them to break down into small particles under 5 mm.6 Secondary MPs have a wide range of sources and complex components, and their pollution of the environment is more serious.7 MPs can absorb or release several harmful chemicals already present in water bodies, increasing their hazard. These substances include bisphenol A (BPA), phthalates (PAEs), polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCbs), polycyclic aromatic hydrocarbons (PAHs), and dichlorodiphenyltrichloroethane (DDT).8 With economic development and the continuous increase in the world population, people's demand for plastic products is also increasing day by day. Specifically during the new corona pandemic, people's demand for products containing plastics such as masks, disposable gloves and protective clothing has increased rapidly, making it necessary to find ways to face the serious consequences of plastic pollution in advance.9 In March 2022, the United Nations Environment Assembly adopted a resolution aimed at combating plastic pollution by 2024 through a legally binding global plastics treaty.10MPs have potential negative effects on human health,11 and they can enter the biota through direct or indirect routes,12 and human beings are exposed to them through food chains,13,14 air,15 and direct exposure.16 According to the current study, the toxicity of MPs is related to various factors such as their composition/polymer, size and shape.17 Microplastic exposure can lead to toxicological damage in humans or animals, such as MPs triggering inflammation and immune responses by destroying cells,18 acute reactions such as hypersensitivity and hemolysis,19etc. Studies have shown that microplastic particles of irregular shape and size may increase damage to cells due to metabolic difficulties in the organism.20,21 Microplastic exposure damages humans and animals mainly through toxicological processes such as induction of DNA damage, reactive oxygen species (ROS) accumulation, apoptosis, pyroptosis, and epigenetic alterations. Therefore, in this work, we review the evidence for the negative effects of MPs on humans and animals in vivo and in vitro, focusing on the above-mentioned aspects. In general, the search criteria for our topic were as follows: (i) search key words were “microplastic”, “toxicity”, and “genotoxiciy”; (ii) search language was limited to publications using English; (iii) search time was limited to 2010–2022 to obtain the newest findings in this field; (iv) search publication was limited to original articles and reviews rather than conference abstracts, letters to the editor or news reports. Moreover, in this review, we provided an analysis regarding relative research trends in China and highlighted the research projects and achievements in China to illustrate the roles of Chinese researchers in promoting study in this field.
2 MPs and geno-toxicology effects
2.1 MP–induced ROS production
Excess ROS accumulation induced by MPs has been identified to inhibit a large number of signaling pathways such as PI3K/Akt or cause apoptosis and inflammation reactions, which eventually result in regulating cellular physiological and biological functions.22 First, evidence indicated that MP-induced ROS tends to disrupt the cellular oxidative/antioxidative balance, leading to oxidative stress23 and alters redox status.15,24 Second, MP-indued ROS can act as a primary signaling cue for various signaling cascade events which can occur in the secondary metabolic products. The study by Li et al. showed that MP exposure may induce elevated intracellular ROS levels to activate AMPK-PGC-1α signaling and enhancing the glycolytic pathway.25 This is similar to the conclusions by Wang et al., who showed that MP exposure induces excess ROS production in myoblasts, modulating the expression of p38/MAPK and NF-κB pathways, and disturbs the balance between myoblast and adipocyte differentiation.26 Recent studies have shown that MPs induce an imbalance of mTORC1 and mTORC2 pathways in the mouse testis through ROS burst and alter the expression profile of actin-binding proteins, resulting in F-actin disassembly and reduced expression of connexins in the blood–testis barrier, ultimately leading to blood–testis barrier integrity disruption and spermatogenesis dysfunction.27 This is consistent with the study by Xie et al. that MP exposure induced activated JNK and p38/MAPK pathways, resulting in a significant decrease in sperm count and motility and a significant increase in sperm deformity rates in mice.28 Umamaheswari et al. showed that MPs induced a dose- and time-dependent ROS-mediated apoptotic response in zebrafish, and that MP exposure significantly upregulated the expression of the p53/GADD45BA/casp3b pathway and significantly upregulated the transcription of TNF-α and PTGS2a.29 Some studies further showed that MP-induced ROS is as an important factor for inhibiting cell growth.30 Since the over-accumulation of ROS can oxidize lipids leading to a damaged cell membrane, ROS formation may be the primary lethal mechanism of MPs to normal cells.2.2 MP–induced DNA damage
DNA damage is a permanent change in the DNA nucleotide sequence that occurs during replication and changes genetic characteristics.31 DNA damage can be caused even at a low MP level. As Josefa D. et al. report, it revealed that Coco-2 cells treated with only 0.26 μg cm−2 polystyrene plastics showed a significant increase in genotoxic damage compared with untreated cells.32 Other cell lines including lung cancer cells and macrophage cells have also shown genotoxic effects of DNA damage post MPs exposure.33 Cells deficient in these mechanisms generally exhibit increased sensitivity to DNA-damaging agents.34 Studies have shown that MPs can induce the accumulation of ROS in Oreochromis niloticus, leading to DNA damage.35 ROS is a recognized mediator of DNA damage, which can induce mitochondrial DNA damage, strand breaks, and mitochondrial DNA degradation, as well as other forms of DNA damage (e.g., 8-oxoguanine formation) directly by oxidizing nucleoside bases, and inhibit the expression of DNA glycosylase OGG1 to inhibit base excision repair. Different doses of MP exposure can lead to different degrees of DNA damage, as reported in grass carp (Ctenopharyngodon idella),36Mediterranean mussels, earthworms,37 and humans.38 MP exposure induces DNA damage in the nuclei and mitochondria of mouse livers, and activates the cGAS/STING pathway to translocate NF-κB into the nucleus and upregulate the expression of pro-inflammatory cytokines, ultimately promoting liver fibrosis.39 A. Araujo et al. speculate that DNA damage in zebrafish (Danio rerio) caused by MP exposure may be related to its oxidative stress toxicity,40 which is consistent with the findings of Anifowoshe41 and Kaloyianni42et al. In conclusion, DNA damage caused by microplastic exposure may be related to the excessive accumulation of ROS induced by it, but the molecular mechanism of DNA damage caused by MPs needs further study. The effects of MPs on DNA damage are associated with the particle chemical structure and the acute and long-term exposure. This indicates that further experimental data of various MP structures associated with DNA damage and genotoxic effects should be obtained to better reflect the potential functions under real-world environmental conditions.2.3 Microplastic–induced cell apoptosis
Apoptosis is an evolutionarily conserved form of programmed cell death that is critical for animal development and tissue homeostasis.43 Existing studies have shown that MPs can induce a variety of apoptosis through many signaling pathways. For example, MPs induce pyroptosis and apoptosis in rat ovarian cells through the NLRP3/Caspase-1 signaling pathway;44 MPs induce apoptosis in zebrafish through the ROS-mediated p53 signaling pathway.29 There is a potential risk of immune activation, which can upregulate the NF-κB signaling pathway and upregulate the expression of inflammatory factors, which can lead to apoptosis of microglia in mouse and human brains;45 nanoplastic (NP) exposure causes hematological damage and lipid metabolism disorders in mice, and induces apoptosis of mouse intestinal epithelial cells;46 MPs can also induce intracellular ROS accumulation and induce blood cell apoptosis in bivalve mollusks (Tegillarca granosa), and limit ATP synthesis in vivo.47 This result is consistent with the report by Li et al. that nanoplastics negatively affected cell viability, ion content, ion transport ATPase activity and ion transport-related gene expression in the gill cells of Macrobrachium japonicus.48 Although a large number of studies have confirmed that MPs can induce apoptosis in various ways, there is no conclusion to confirm the specific mechanism of MP-induced apoptosis. There is a guess here that the exposure of nano-MPs that can enter cells may cause apoptosis toxicity due to their specific surface areas, while the exposure of inaccessible MPs to cells may cause apoptosis toxicity. The area outside the cell comes into contact with the cell membrane. There are a large number of receptors on the cell membrane, and the presence of MPs may hinder the normal signal transmission between cells, thereby causing toxic effects on cells. The more complex the surface structure of the nano-MPs that can enter the cell, the more likely they are to combine with proteins or organelles in the cell, thereby destroying the cell and causing apoptosis. In conclusion, MPs can induce apoptosis by inducing ROS accumulation, up-regulating the NF-κB signaling pathway, and inhibiting the function of ATP and P53 signaling pathways in vivo. However, there is no conclusion to confirm the specific molecular mechanism of apoptosis caused by MPs, which may be related to energy metabolism pathways such as the AMPK signaling pathway and glycolysis pathway.2.4 Microplastic–induced cell pyroptosis
Pyroptosis was defined in 2015 as gasdermin-mediated programmed death.49 When the host is stimulated by a variety of exogenous or endogenous factors, gasdermins are cleaved by some caspases or granzymes, the N-terminal PFD dissociates from the C-terminal RD, and then the N-terminal PFD oligomerizes at the cell membrane. Pores are formed on the cells, leading to the release of inflammatory molecules and pyroptosis.50–52 Microplastic exposure may cause pyroptosis. Mu et al. suggest that the activation of pyroptosis and ferroptosis is related to the hepatotoxicity of polystyrene MPs in mice, and that MPs can induce NOD-like receptor protein 3 (NLRP3) inflammasome and apoptosis-associated speck-like protein (ASC) and caspase-1-dependent signaling pathways. Furthermore, their results suggest that MP-induced hepatic lipid peroxidation in mice can activate the expression of ferroptosis-related proteins.53 In addition, studies have shown that MPs induce rat ovarian granulosa cells44 and cardiomyocytes pyroptosis54 through the NLRP3/Caspase-1 signaling pathway. The above results show that MPs can induce pyroptosis in some cells of rats and mice, but this is only the conclusion of a small number of experiments. Whether MP exposure induces pyroptosis in cells in humans or other animals has not been definitively concluded.2.5 Microplastic and non-coding RNAs
Noncoding RNAs (including microRNAs, long noncoding RNAs (lncRNAs), and circular RNAs) are RNAs that do not encode proteins.55,56 A common feature of these noncoding RNAs is that they are all transcribed from the genome and can perform various biological functions at the RNA level.57 MPs may change the gene expression in the organism by regulating the transcription level of ncRNA in the organism. Studies have shown that MP exposure leads to differential expression of 269 circRNAs and 109 lncRNAs in rat lung tissue, and Fan et al. speculated that circRNAs and lncRNAs may play an important role in the development of PS-MP-induced lung inflammation.58 A study by Qu et al. showed that a certain number of miRNAs59 and lncRNAs60 play a crucial role in the response of nanopolystyrene after long-term and low-dose exposure in organisms. Existing research is not enough to prove that MP exposure causes harm to humans and animals by affecting the transcription level of ncRNA in the body, but it is an indisputable fact that MP exposure can affect the transcription of multiple ncRNAs in organisms. ncRNAs play key regulatory roles in shaping cellular activities, and they represent a diverse and ubiquitous group of RNAs with roles in carcinogenesis, tumor suppressor,61 immunity,62 apoptosis63 and autophagy64 and other biological phenomena. Therefore, research on the mechanisms by which MP exposure regulates ncRNAs in organisms is crucial. In summary, MP exposure can differentially express a variety of noncoding RNAs, but the molecular mechanism of this process is still unclear and requires further study (see Table 1).| Country | Size/treatment concentration/time | Damage caused | In vitro/in vivo | Mainly used experimental methods | Findings and references |
|---|---|---|---|---|---|
| Egypt | 80% > 100 nm/1 mg L−1 MPs, 10 mg L−1 MPs and 100 mg L−1 MPs/15 days | Oxidative stress and DNA damage | In vitro/Oreochromis niloticus | SDS-PAGE | MPs cause overproduction of reactive oxygen species in Oreochromis niloticus and alter antioxidant parameters, leading to oxidative stress and DNA damage.35 |
| Brazil | 23.03 ± 0.266 nm and 80% in the range from 20 to 26 nm)/0.04 ng L−1, 34 ng L−1 and 34 μg L−120 days | Oxidative stress and DNA damage | In vitro/Ctenopharyngodon idella juveniles | Comet assay | In the short term, exposure of larvae to small concentrations of PS NPs (i.e., environmentally relevant concentration) was able to induce DNA damage (as demonstrated by the comet assay), mutagenesis (as demonstrated by the micronucleus assay and other nuclear abnormalities) and cytotoxicity.36 |
| China | 100 nm and 1300 nm/0, 100 mg kg−1 and 1000 mg kg−1 14 days | Oxidative stress and DNA damage | In vitro/earthworm (Eisenia fetida) | Comet assay, ELISA kit, and hematoxylin and eosin (H&E) staining | Exposure to PS-MPs with a particle size larger than 100 nm caused DNA damage and oxidative stress in earthworms. Furthermore, 1300 nm PS-MPs were more toxic than 100 nm PS-MPs when exposed to 1000 mg kg−1 of 1300 nm particles.37 |
| China | 0.1 and 1 μm/1 mg L−1)/24 h | Liver damage and DNA damage | In vivo/HL7702 cells | Histological and immunostaining, qPCR, and western blot | Long-term accumulation of MPs can lead to liver damage and dysfunction, even at low concentrations. Mechanistically, MPs cause nuclear DNA and mitochondrial DNA damage, and the subsequently activated cGAS/STING signaling pathway is involved in the regulation of liver fibrosis.39 |
| Brazil | 35.46 μm ± 18.17 μm/2.7 × 108 PE-MPs particles per m3/15 days | Oxidative stress and DNA damage | In vivo/zebrafish (Danio rerio) | Micronucleus test, comet assay, and ELISA kit | MPS (alone or in admixture) have genotoxic and mutagenic effects on freshwater fish. On the other hand, the establishment of redox imbalance in this study was found to be closely related to the observed genotoxicity and mutagenicity.40 |
| Greece | Spherical PS-MPs (8 ± 3 μm)/food (10 mg PS-MPs g−1) | DNA damage, oxidative stress, ubiquitination and apoptosis | In vivo/zebrafish (Danio rerio) and Perca fluviatilis | Metabolomics analysis, comet assay, and SDS-PAGE | Cellular components and PS-MPs interactions exert toxic effects by producing oxidative stress in the liver and gills of the two fish species studied, as measured by lipid peroxidation, protein oxidation, and the extent of DNA damage.42 |
| China | PS-MPs < 5 μm/1 mg L−1/30 days | Oxidative stress, apoptosis, cell cycle arrest and Ca+ overload | In vitro and in vivo/C57BL/6 male mice and L02 cells | DCFH-DA, immunofluorescence, apoptosis and cell cycle detection by flow cytometry and western blot | Microplastic-induced calcium overload may be associated with ROS and/or its association with AMPK-PGC-1α signaling, suggesting that calcium signaling may be a therapeutic target for microplastic-induced hepatotoxicity.25 |
| China | PS MPs (0.5 μm)/0.015 mg kg−1 d−1 MPs group, 0.15 mg kg−1 d−1 MPs group, and 1.5 mg kg−1 d−1 MPs group/90 days | Pyroptosis, apoptosis and oxidative stress | In vitro/Wistar rats | Immunohistochemical staining, TUNEL, western blot and flow cytometry | Long-term exposure to PS MPs can trigger the NLRP3/Caspase-1 signaling pathway through oxidative stress, leading to granulosa cell pyroptosis and apoptosis, and a decreased ovarian reserve,44 |
| Korea | 0.2, 2 and 10 μm/0, 2.5, and 10 μg mL−1/7 days | Immune activation and apoptosis | In vitro and in vivo/C57BL/6 mice/human microglial HMC-3 cells | Detection of apoptosis and necrosis by flow cytometry, confocal imaging, western blot and qPCR | One week of oral administration of PS-MPS < 2 μm to mice was sufficient to achieve brain accumulation via microglial phagocytosis. This also provides evidence that PS-MP uptake induces microglial immune activation, ultimately leading to apoptosis.45 |
| Cell with 0.2, 2, or 10 μm-sized PS-MPs at concentrations of 1, 5, or 10 μg mL−1/24 h | |||||
| China | 5.0 μm/0.1, 0.5 and 1 mg mL−1/28 days | Inflammation, oxidative stress, pyroptosis and ferroptosis | In vivo/mice | ELISA kit, western blot and qPCR | MP-induced hepatic lipid peroxidation in mice can activate the expression of ferroptosis-related proteins, including iron metabolism, amino acid metabolism, and lipid metabolism, demonstrating that both pyroptosis and ferroptosis occur in MP-induced liver injury, and with intense oxidative stress and inflammation.53 |
| China | 0.5 mm/0.5, 5 and 50 mg L−1/90 days | Oxidative stress and pyroptosis | In vivo/Wistar rats | Hematoxylin and Eosin staining, ELISA kit, western blot and qPCR | MPs can induce cardiomyocyte pyroptosis through oxidative stress and inflammation and activate the NLRP3/Caspase-1 signaling pathway, ultimately leading to cardiac fibrosis and cardiac dysfunction.54 |
| China | 100 nm/10 mg mL−1, 100 μL/28 days | Apoptosis, inflammation and metabolic disorder | In vitro and in vivo/C57BL/6 mice/Caco-2 cells | Histopathological evaluation and immunohistochemistry assay, immunofluorescent staining, and western blotting | PS NPs can be taken up by intestinal epithelial Caco-2 cells via macropinocytosis and clathrin-mediated endocytosis and induce disruption of tight junctions between Caco-2 cells. Furthermore, the authors found that PS-NH2 and PS-COOH enter Caco-2 cells more easily.46 |
| China | 500 nm and 30 μm/25 mg mL−1/14 days | Apoptosis, oxidative stress and inhibition of energy supply | In vitro/adult blood clams T. granosa | Analysis of the total counts of haemocytes. DCFH-DA, qPCR and ELISA kit | MPs can induce intracellular ROS accumulation and induce blood cell apoptosis in bivalve mollusks (Tegillarca granosa), and limit ATP synthesis in vivo.47 |
| China | 100 nm/0, 5, 10, 20, 40 mg L−1/14 days | Apoptosis, decreased ATPase activity and impaired ion transport | In vivo/nippon prawns | Hematoxylin and eosin (H&E) staining and flow cytometry | Nanoplastics negatively affected cell viability, ion content, ion transport, ATPase activity and ion transport-related gene expression in the gill cells of Macrobrachium japonicus.48 |
| China | 5.0 μm/0.1, 0.5 and 1 mg mL−1/28 days | Oxidative damage, pyroptosis and ferroptosis | In vitro/C57BL/6 mice | Western blotting, qPCR and ELISA kit | MP-induced hepatic lipid peroxidation in mice can activate the expression of ferroptosis-related proteins.53 |
| China | 5 μm/50 μg L−1 and 500 μg L−1/21 days | Oxidative stress, inflammation and lipid metabolism | In vivo/Zebrafish | Gut microbiome and metabolomic analysis | MP exposure causes gut damage as well as alterations in the gut metabolome and microbiome.66 |
| China | 8 μm and 80 nm/10 μg L−1 and 1 mg L−1/21 days | Microbiota dysbiosis and intestinal inflammation | In vivo/Zebrafish | Transcriptome sequencing and metabolomic analysis | The gut-induced microbiota dysbiosis and intestinal inflammation in zebrafish may be more severe for NPs than for MPs.68 |
| China | 100 nm, 500 nm, 1 μm and 2.5 μm/0, 0.5, 1 and 2 mg/200 μL/3 days | Inflammation | In vivo/Sprague Dawley (SD) rats | Hematoxylin and eosin (H&E) staining, ELISA kit and Transcriptomic testing | circRNAs and lncRNAs may play an important role in the development of PS-MP-induced lung inflammation.58 |
2.6 Microplastic–induced inflammation effects
Inflammation is an adaptive response, triggered by noxious stimuli and conditions, such as infection and tissue damage.65 The study by Li et al. showed that mice exposed to high concentrations of MPs exhibited significant inflammation and higher expression of TLR4, AP-1, and IRF5 in their guts (colon and duodenum). Likewise, an inflammatory response to MP exposure was observed in the zebrafish gut,66,67 and the response was stronger with nanoplastics.68 The inflammatory response caused by the exposure of the human body to MPs is closely related to their roughness. When MPs are in direct contact with human fibroblasts and red blood cells, lactose dehydrogenase and erythrocytes occur due to cell membrane damaged by the physical stress. This phenomenon is amplified when the concentration and roughness of the microfragments increase.69 This further indicates that the toxic effects of MPs are mainly related to their shape and diameter.70 Numerous studies have shown that exposure to nanoplastics and MPs leads to impaired intestinal oxidative and inflammatory homeostasis and disruption of intestinal epithelial permeability. Furthermore, MPs contain additives that adsorb pollutants which may lead to further adverse effects.71 This is consistent with the study by Tong et al., that polyethylene MPs and H. pylori synergistically caused severe damage and inflammatory responses in the stomach of mice.72 Although MPs can cause severe inflammatory responses in animals, organisms seem unable to resist the damage caused by MPs on their own. Studies have shown that macrophages do not degrade MPs, and this also leads to increased intracellular glycolysis and reduced mitochondrial respiration.73 In conclusion, exposure to MPs can cause inflammatory responses in animals such as fish, mice, and humans, and MPs can provide a good growth environment for microorganisms to enhance their damage to the body. In addition, the inflammatory response caused by MPs is inextricably linked to its diameter, shape, and surface roughness. However, it has not been reported whether MP exposure induces an inflammatory response by regulating the expression of certain genes.3 Trends in MP research
3.1 Major Chinese funding mechanisms and schemes
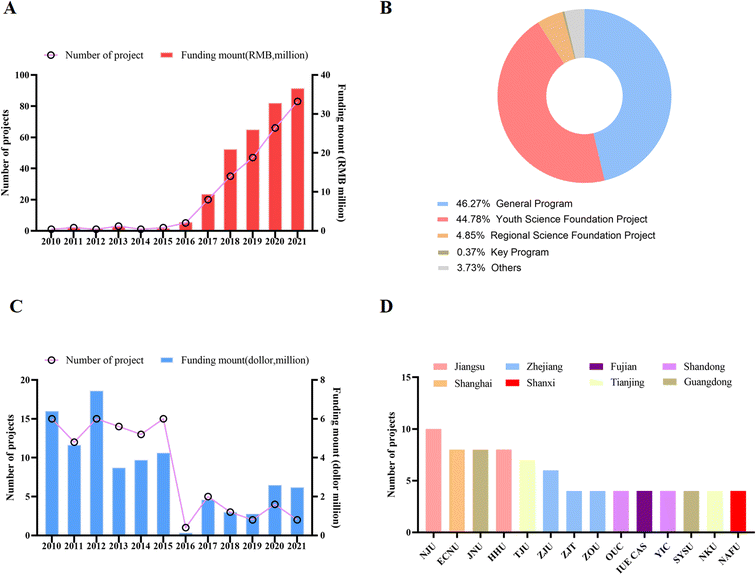
Since 2011, research on MPs in China has increased dramatically as the new era of China's economy begins to shift from high-production to high-quality development. The National Natural Science Foundation of China mainly invests in research on MPs, including pollution, physiological toxic effects, molecular mechanisms and prevention and control technologies. To explore funding information for MPs research in China, we conducted a search. As shown in Fig. 1A, a total of 226 projects were launched from 2010 to 2021, with a total amount of approximately RMB 133.96 million. The amount of funding increases with the year, and generally shows an upward trend. In addition to the downward trend in 2014, the number of funded projects has generally increased significantly year by year. Among these funded projects, general projects account for 46.63%, youth science fund projects account for 38.14%, regional science fund projects account for 4.68% (1.75% for key projects), and the rest are international cooperation and joint fund projects.The National Natural Science Foundation of China consists of eight scientific research funding departments including chemical science, life science, and medical science. The National Natural Science Foundation of China is mainly funded by General Projects, and researchers can choose to apply for topics according to the guidance of different departments. The age requirement has a limit for the Young Scientist Grant Program, accepting female scientists under 40 and male scientists under 35. Regional funding projects are limited to certain remote special areas, including some western provinces or low-income areas such as Guizhou Province. For nearly 20 years, the National Natural Science Foundation of China has been paying attention to and investing in these remote or low-income areas, aiming to promote scientific research and development and encourage scientists in these areas. In addition, to provide comparative information, we extracted research data on MPs from the US NIH and NSF websites. From 2010 to 2021, the US NIH and NSF have funded about 214 projects related to MP research, with an amount of about $85 million, much higher than that of the National Natural Science Foundation of China (Fig. 1B and C).
3.2 China's main research institutions in the direction of MPs
To explore Natural Science Foundation of China (NSFC)-funded institutions, we conducted analyses in the field of MPs. As shown in Fig. 1D, funded projects are mainly concentrated in developed regions such as Jiangsu, Shanghai, and Zhejiang or in coastal provinces. Compared with developed and high-income regions and provinces, the proportion of projects in remote areas and low-income provinces such as Heilongjiang, Inner Mongolia, Yunnan and Gansu is significantly lower. Colleges and universities, especially the national “985” and “211” educational colleges and universities receive a very large proportion of project funding. The top five universities receiving funding are Nanjing University, East China Normal University, Jinan University, Huohai University, and Tianjin University, all located in high-income provinces. With their strong research infrastructure and ability to compete for pooled funding, these universities have become veritable units of excellence in MP research in China. With strong scientific research teams and research equipment, as well as the ability to obtain research funds from the National Natural Science Foundation of China, these universities have become the focus of study in the field of MPs. These conditions, such as funding, research environment, and the prestige of the school, will make these universities attract more outstanding talents to join in the future, thus entering a positive cycle.3.3 Representative research projects and achievements
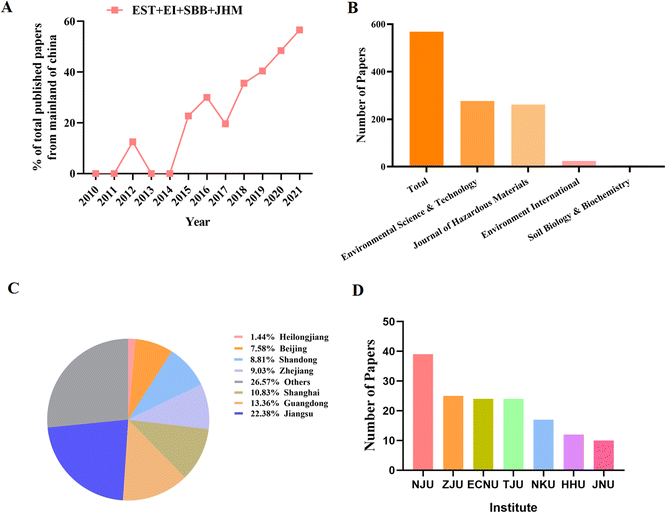
Research results are usually supported by representative published literature. In the scientific community, as shown in Fig. 2A, Chinese scholars have made significant contributions to the MP literature. Between 2010 and 2015, only 3 papers from China were published in internationally renowned high-quality journals such as EST, EI, SSB and JHM. However, in 2021, 173 studies from Chinese scholars will be published in JHM. From 2010 to 2021, we searched for papers published in the field of MP research. As shown in Fig. 2B, most were published in Environmental Science and Technology (277 out of 568; 48.7%), the Hazardous Substances Journal (262 out of 568; 46.1%), EI (24 out of 568; 4.2%), and Soil Biology and Biochemistry (9 out of 568; 1.6%) (Fig. 2B). It is worth mentioning that the publication volume of “Environmental Science and Technology” is growing faster than other journals, indicating that “Environmental Science and Technology” is valued by Chinese environmental scientists. We further investigated information on the publisher. Nearly 50% of the papers published in developed regions and high-income provinces such as Jiangsu, Guangdong, Shanghai and Zhejiang are published, of which about 22% of the papers published in Beijing in the past 10 years (Fig. 2C). We also found that the top five institutions are Nanjing University, Zhejiang University, East China Normal University, and Tianjin University (Fig. 2D), which are also located in developed regions.4 Current literature summary, future challenges and future perspectives
MP-induced genotoxic effects have garnered the attention of the public and scientific community, while intensive research has produced new fundamental insights into the mechanisms underlying MPs effects. In addition, novel technical strategies on eliminating environmental MPs are gradually being developed and studied. This review highlights the effects of MPs on genotoxic underlying mechanisms including ROS, DNA damage, apoptosis and non-coding RNA aspects as well as the research trends of MPs from a Chinese perspective. From the above illustration based on a current literature summary, the potential roles of MPs in regulation of geno-toxicity including the entry routes, and effects of ROS, DNA damage, and apoptosis have been intensively studied. However, the underlying mechanisms of MPs toxicology and the metabolic dynamics in the body remain incompletely illustrated. The plethora of MP genotoxic effects, along with their profound and complex interactions, have not yet been fully elucidated. Furthermore, the epidemiological data regarding MP genotoxicity are very insufficient. Thus, despite decades of extensive research and countless discoveries, much more work is needed.4.1 Challenge I
The first challenge for further study on MPs is the threshold criteria for better understanding the genotoxicity degree of MPs. This purpose could be promoted through a cell dose- and time-depended study, an animal model study and the risk assessment of MPs in humans.4.2 Challenge II
Despite the summary of MP-induced genotoxicity in recent reports, are there other MP-induced genotoxicity changes? For example, copper induced-cell death has been published as a new cell death mode.74 Then whether MPs can induce or promote copper cell death needs further investigation. In addition, whether there exists new cell death caused by MPs specifically remains unclear and is a very interesting point to discover in the future.4.3 Challenge III
As we demonstrated in this review, the genotoxicity of MPs is tightly associated with the dosage, exposure time, cell lines, and chemical structure. This informs that MPs may present a two-side effect at different backgrounds, resulting in some conflicting results in published reports. Thus, in the future, studies on MPs should pay more attention to the context requirement.4.4 Challenge IV
Early biomarkers for identifying DNA damage, cell death, inflammation reaction and regulation of non-coding RNA should be identified and used for analysis of MP-induced genotoxicity in the early damage period. Many early DNA damage sensors such as 53BP1 and γH2AX have been reported during the past few decades, but whether these early DNA damage biomarkers could be used to evaluate MP-induced DNA damage should be further tested. Moreover, some classic test methods such as comet assay and the Ames test have been widely used for testing the genotoxicity, and these methods could be modified to be more effective and sensitive. With the rapid development of molecular biological techniques, more novel methods for testing genotoxicity have the chance to be developed in the future.In conclusion, we believe that comprehensive research on the basic biology of genotoxicity, accompanied by rapid development of new technologies and further progress in MPs, will drive significant advances in the near future. Hopefully, the efforts by the scientific community would largely aid in the development of the MP industry and, ultimately, be of benefit to humans.
Author contributions
RXH contributed to the study concept and critical design of the study. LHX went through the literature and analyzed and interpreted the data. LHX wrote the initial manuscript. RXH critically reviewed and revised the final manuscript. All the authors read and approved the manuscript.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (grant no. 82073486 and U1803124).References
- X. Lim, Microplastics are everywhere–but are they harmful?, Nature, 2021, 593(7857), 22–25 CrossRef CAS PubMed.
- N. B. Hartmann, et al., Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris, Environ. Sci. Technol., 2019, 53(3), 1039–1047 CrossRef CAS PubMed.
- M. MacLeod, et al., The global threat from plastic pollution, Science, 2021, 373(6550), 61–65 CrossRef CAS.
- C. Alomar, F. Estarellas and S. Deudero, Microplastics in the Mediterranean Sea: Deposition in coastal shallow sediments, spatial variation and preferential grain size, Mar. Environ. Res., 2016, 115, 1–10 CrossRef CAS PubMed.
- S. M. Praveena, S. N. M. Shaifuddin and S. Akizuki, Exploration of microplastics from personal care and cosmetic products and its estimated emissions to marine environment: An evidence from Malaysia, Mar. Pollut. Bull., 2018, 136, 135–140 CrossRef CAS PubMed.
- R. C. Thompson, et al., Lost at sea: where is all the plastic?, Science, 2004, 304(5672), 838 CrossRef CAS.
- R. Bao, et al., Secondary microplastics formation and colonized microorganisms on the surface of conventional and degradable plastic granules during long-term UV aging in various environmental media, J. Hazard. Mater., 2022, 439, 129686 CrossRef CAS PubMed.
- S. Gündogdu, et al., The impact of nano/micro-plastics toxicity on seafood quality and human health: facts and gaps, Crit. Rev. Food Sci. Nutr., 2022, 1–19 CrossRef.
- G. Tagorti and B. Kaya, Genotoxic effect of microplastics and COVID-19: The hidden threat, Chemosphere, 2022, 286(Pt 3), 131898 CrossRef CAS PubMed.
- M. Bergmann, et al., A global plastic treaty must cap production, Science, 2022, 376(6592), 469–470 CrossRef PubMed.
- G. Chen, Q. Feng and J. Wang, Mini-review of microplastics in the atmosphere and their risks to humans, Sci. Total Environ., 2020, 703, 135504 CrossRef CAS PubMed.
- J. P. Desforges, M. Galbraith and P. S. Ross, Ingestion of Microplastics by Zooplankton in the Northeast Pacific Ocean, Arch. Environ. Contam. Toxicol., 2015, 69(3), 320–330 CrossRef CAS PubMed.
- D. Santillo, K. Miller and P. Johnston, Microplastics as contaminants in commercially important seafood species, Integr. Environ. Assess. Manage., 2017, 13(3), 516–521 CrossRef PubMed.
- S. A. Mason, V. G. Welch and J. Neratko, Synthetic Polymer Contamination in Bottled Water, Front. Chem., 2018, 6, 407 CrossRef.
- J. C. Prata, et al., Environmental exposure to microplastics: An overview on possible human health effects, Sci. Total Environ., 2020, 702, 134455 CrossRef CAS PubMed.
- Z. Liao, et al., Airborne microplastics in indoor and outdoor environments of a coastal city in Eastern China, J. Hazard. Mater., 2021, 417, 126007 CrossRef CAS.
- Z. Ji, et al., Effects of pristine microplastics and nanoplastics on soil invertebrates: A systematic review and meta-analysis of available data, Sci. Total Environ., 2021, 788, 147784 CrossRef CAS PubMed.
- A. D. Vethaak and J. Legler, Microplastics and human health, Science, 2021, 371(6530), 672–674 CrossRef CAS.
- J. Hwang, et al., An assessment of the toxicity of polypropylene microplastics in human derived cells, Sci. Total Environ., 2019, 684, 657–669 CrossRef CAS.
- C. G. Avio, et al., Pollutants bioavailability and toxicological risk from microplastics to marine mussels, Environ. Pollut., 2015, 198, 211–222 CrossRef CAS.
- P. Masiá, A. Ardura and E. García-Vázquez, Virgin Polystyrene Microparticles Exposure Leads to Changes in Gills DNA and Physical Condition in the Mediterranean Mussel Mytilus Galloprovincialis, Animals (Basel), 2021, 11(8), 2317 CrossRef.
- X. Lihui, et al., Albicanol inhibits the toxicity of profenofos to grass carp hepatocytes cells through the ROS/PTEN/PI3K/AKT axis, Fish Shellfish Immunol., 2022, 120, 325–336 CrossRef.
- D. Sacks, et al., Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke, Int. J. Stroke, 2018, 13(6), 612–632 Search PubMed.
- H. Yu, et al., Effects of microplastics and glyphosate on growth rate, morphological plasticity, photosynthesis, and oxidative stress in the aquatic species Salvinia cucullata, Environ. Pollut., 2021, 279, 116900 CrossRef CAS PubMed.
- S. Li, et al., Polystyrene microplastics trigger hepatocyte apoptosis and abnormal glycolytic flux via ROS-driven calcium overload, J. Hazard. Mater., 2021, 417, 126025 CrossRef CAS PubMed.
- W. Shengchen, et al., Polystyrene microplastics-induced ROS overproduction disrupts the skeletal muscle regeneration by converting myoblasts into adipocytes, J. Hazard. Mater., 2021, 417, 125962 CrossRef PubMed.
- Y. Wei, et al., Polystyrene microplastics disrupt the blood-testis barrier integrity through ROS-Mediated imbalance of mTORC1 and mTORC2, Environ. Pollut., 2021, 289, 117904 CrossRef CAS.
- X. Xie, et al., Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway, Ecotoxicol. Environ. Saf., 2020, 190, 110133 CrossRef CAS.
- S. Umamaheswari, et al., Polystyrene microplastics induce apoptosis via ROS-mediated p53 signaling pathway in Zebrafish, Chem. Biol. Interact., 2021, 345, 109550 CrossRef CAS.
- X. You, et al., Unraveling individual and combined toxicity of nano/microplastics and ciprofloxacin to Synechocystis sp. at the cellular and molecular levels, Environ. Int., 2021, 157, 106842 CrossRef CAS.
- X. Lihui, et al., Albicanol modulates oxidative stress and the p53 axis to suppress profenofos induced genotoxicity in grass carp hepatocytes, Fish Shellfish Immunol., 2022, 122, 325–333 CrossRef.
- J. Domenech, et al., Long-Term Effects of Polystyrene Nanoplastics in Human Intestinal Caco-2 Cells, Biomolecules, 2021, 11(10), 1442 CrossRef CAS.
- V. Paget, et al., Specific uptake and genotoxicity induced by polystyrene nanobeads with distinct surface chemistry on human lung epithelial cells and macrophages, PLoS One, 2015, 10(4), e0123297 CrossRef PubMed.
- S. P. Jackson and J. Bartek, The DNA-damage response in human biology and disease, Nature, 2009, 461(7267), 1071–1078 CrossRef CAS PubMed.
- M. Hamed, et al., Antioxidants and molecular damage in Nile Tilapia (Oreochromis niloticus) after exposure to microplastics, Environ. Sci. Pollut. Res. Int., 2020, 27(13), 14581–14588 CrossRef CAS PubMed.
- A. T. B. Guimarães, et al., Toxicity of polystyrene nanoplastics in Ctenopharyngodon idella juveniles: A genotoxic, mutagenic and cytotoxic perspective, Sci. Total Environ., 2021, 752, 141937 CrossRef.
- X. Jiang, et al., Toxicological effects of polystyrene microplastics on earthworm (Eisenia fetida), Environ. Pollut., 2020, 259, 113896 CrossRef CAS.
- R. Kumar, et al., Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans?, Chemosphere, 2022, 298, 134267 CrossRef CAS.
- R. Shen, et al., Accumulation of polystyrene microplastics induces liver fibrosis by activating cGAS/STING pathway, Environ. Pollut., 2022, 300, 118986 CrossRef CAS.
- A. Araújo, et al., Toxicity evaluation of the combination of emerging pollutants with polyethylene microplastics in Zebrafish: Perspective study of genotoxicity, mutagenicity, and redox unbalance, J. Hazard. Mater., 2022, 432, 128691 CrossRef.
- A. T. Anifowoshe, et al., Evaluation of cytogenotoxic potential and embryotoxicity of KRS-Cauvery River water in Zebrafish (Danio rerio), Ecotoxicol. Environ. Saf., 2022, 233, 113320 CrossRef CAS.
- M. Kaloyianni, et al., Toxicity and Functional Tissue Responses of Two Freshwater Fish after Exposure to Polystyrene Microplastics, Toxics, 2021, 9(11), 289 CrossRef CAS PubMed.
- X. Cheng and J. E. Ferrell Jr., Apoptosis propagates through the cytoplasm as trigger waves, Science, 2018, 361(6402), 607–612 CrossRef CAS.
- J. Hou, et al., Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/Caspase-1 signaling pathway in rats, Ecotoxicol. Environ. Saf., 2021, 212, 112012 CrossRef CAS.
- W. Kwon, et al., Microglial phagocytosis of polystyrene microplastics results in immune alteration and apoptosis in vitro and in vivo, Sci. Total Environ., 2022, 807(Pt 2), 150817 CrossRef CAS.
- D. Xu, et al., Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into Caco-2 cells, J. Hazard. Mater., 2021, 417, 126092 CrossRef CAS PubMed.
- W. Shi, et al., Immunotoxicities of microplastics and sertraline, alone and in combination, to a bivalve species: size-dependent interaction and potential toxication mechanism, J. Hazard. Mater., 2020, 396, 122603 CrossRef CAS.
- Y. Li, et al., Effects of nanoplastic on cell apoptosis and ion regulation in the gills of Macrobrachium nipponense, Environ. Pollut., 2022, 300, 118989 CrossRef CAS.
- J. Shi, et al., Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death, Nature, 2015, 526(7575), 660–665 CrossRef CAS.
- J. Ding, et al., Pore-forming activity and structural autoinhibition of the gasdermin family, Nature, 2016, 535(7610), 111–116 CrossRef CAS PubMed.
- X. Liu, et al., Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores, Nature, 2016, 535(7610), 153–158 CrossRef CAS.
- R. A. Aglietti and E. C. Dueber, Recent Insights into the Molecular Mechanisms Underlying Pyroptosis and Gasdermin Family Functions, Trends Immunol., 2017, 38(4), 261–271 CrossRef CAS PubMed.
- Y. Mu, et al., Activation of pyroptosis and ferroptosis is involved in the hepatotoxicity induced by polystyrene microplastics in mice, Chemosphere, 2022, 291(Pt 2), 132944 CrossRef CAS PubMed.
- J. Wei, et al., The impact of polystyrene microplastics on cardiomyocytes pyroptosis through NLRP3/Caspase-1 signaling pathway and oxidative stress in Wistar rats, Environ. Toxicol., 2021, 36(5), 935–944 CrossRef CAS PubMed.
- C. Zhou, et al., Genome-Wide Maps of m6A circRNAs Identify Widespread and Cell-Type-Specific Methylation Patterns that Are Distinct from mRNAs, Cell Rep, 2017, 20(9), 2262–2276 CrossRef CAS.
- B. Wu, et al., Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1, Nat. Commun., 2018, 9(1), 420 CrossRef PubMed.
- J. Li, et al., The Roles of Non-Coding RNAs in Radiotherapy of Gastrointestinal Carcinoma, Front. Cell Dev. Biol., 2022, 10, 862563 CrossRef PubMed.
- Z. Fan, et al., A study on the roles of long non-coding RNA and circular RNA in the pulmonary injuries induced by polystyrene microplastics, Environ. Int., 2022, 163, 107223 CrossRef.
- M. Qu, et al., Nanopolystyrene-induced microRNAs response in Caenorhabditis elegans after long-term and lose-dose exposure, Sci. Total Environ., 2019, 697, 134131 CrossRef CAS PubMed.
- M. Qu, et al., Identification of long non-coding RNAs in response to nanopolystyrene in Caenorhabditis elegans after long-term and low-dose exposure, Environ. Pollut., 2019, 255(Pt 1), 113137 CrossRef CAS PubMed.
- F. J. Slack and A. M. Chinnaiyan, The Role of Non-coding RNAs in Oncology, Cell, 2019, 179(5), 1033–1055 CrossRef CAS.
- J. K. Wang, Z. Wang and G. Li, MicroRNA-125 in immunity and cancer, Cancer Lett., 2019, 454, 134–145 CrossRef CAS PubMed.
- L. Zhang, C. Li and X. Su, Emerging impact of the long noncoding RNA MIR22HG on proliferation and apoptosis in multiple human cancers, J. Exp. Clin. Cancer Res., 2020, 39(1), 271 CrossRef CAS PubMed.
- L. Chen, et al., The Role of non-coding RNAs in colorectal cancer, with a focus on its autophagy, Pharmacol. Ther., 2021, 226, 107868 CrossRef CAS PubMed.
- R. Medzhitov, Origin and physiological roles of inflammation, Nature, 2008, 454(7203), 428–435 CrossRef CAS.
- R. Qiao, et al., Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in Zebrafish, Sci. Total Environ., 2019, 662, 246–253 CrossRef CAS PubMed.
- Y. Jin, et al., Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult Zebrafish, Environ. Pollut., 2018, 235, 322–329 CrossRef CAS.
- S. Xie, et al., Nanoplastics Induce More Serious Microbiota Dysbiosis and Inflammation in the Gut of Adult Zebrafish than Microplastics, Bull. Environ. Contam. Toxicol., 2021, 107(4), 640–650 CrossRef CAS PubMed.
- D. Choi, et al., In vitro chemical and physical toxicities of polystyrene microfragments in human-derived cells, J. Hazard. Mater., 2020, 400, 123308 CrossRef CAS PubMed.
- R. Qiao, et al., Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of Zebrafish, Chemosphere, 2019, 236, 124334 CrossRef CAS PubMed.
- N. Hirt and M. Body-Malapel, Immunotoxicity and intestinal effects of nano- and microplastics: a review of the literature, Part. Fibre Toxicol., 2020, 17(1), 57 CrossRef PubMed.
- X. Tong, et al., Polyethylene microplastics cooperate with Helicobacter pylori to promote gastric injury and inflammation in mice, Chemosphere, 2022, 288(Pt 2), 132579 CrossRef CAS PubMed.
- S. D. Merkley, et al., Polystyrene microplastics induce an immunometabolic active state in macrophages, Cell Biol. Toxicol., 2022, 38(1), 31–41 CrossRef CAS.
- P. Tsvetkov, et al., Copper induces cell death by targeting lipoylated TCA cycle proteins, Science, 2022, 375(6586), 1254–1261 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |