Open Access Article
Open Access ArticleAssessing and managing environmental hazards of polymers: historical development, science advances and policy options†
Ksenia J.
Groh
 *a,
Hans Peter H.
Arp
*a,
Hans Peter H.
Arp
 bc,
Matthew
MacLeod
bc,
Matthew
MacLeod
 d and
Zhanyun
Wang
d and
Zhanyun
Wang
 *e
*e
aEawag, Swiss Federal Institute of Aquatic Science and Technology, 8600 Dübendorf, Switzerland. E-mail: ksenia.groh@eawag.ch
bDepartment of Environmental Engineering, Norwegian Geotechnical Institute, NO-0806 Oslo, Norway
cDepartment of Chemistry, Norwegian University of Science and Technology (NTNU), NO-7491 Trondheim, Norway
dDepartment of Environmental Science, Stockholm University, SE-106 91 Stockholm, Sweden
eEmpa – Swiss Federal Laboratories for Materials Science and Technology, Technology and Society Laboratory, 9014 St. Gallen, Switzerland. E-mail: zhanyun.wang@empa.ch
First published on 13th December 2022
Abstract
Polymers are the main constituents of many materials and products in our modern world. However, their environmental safety is not assessed with the same level of detail as done for non-polymeric chemical substances. Moreover, the fundamentals of contemporary regulatory approaches for polymers were developed in the early 1990s, with little change occurring since then. Currently, the European Commission is working on a proposal to initiate registration of polymers under the European Union's (EU) chemicals legislation REACH. This provides a unique opportunity for regulation to catch up on recent scientific advances. To inform this process, we here critically appraise the suggested regulatory approaches to the environmental assessment and management of polymers against the latest scientific findings regarding their environmental fate, exposure, and effects, and identify the remaining critical knowledge gaps. While we use the EU draft proposal as an example, our findings are broadly applicable to other polymer legislations worldwide, due to the similarity of polymer assessment criteria being used. We emphasize four major aspects that require more attention in the regulation of polymers: (i) increased transparency about chemical identities, physical characteristics and grouping approaches for in-use polymers; (ii) improved understanding of the environmental fate of polymers and materials composed of polymers across size and density categories and exposure profiles; (iii) comprehensive assessment of the environmental hazards of polymers, considering the effects of degradation and weathering and taking into account the actual uptake, long-term toxicity, and geophysical impacts; and (iv) consideration of the production volume and use/release patterns in determining regulatory data and testing requirements. Transitioning toward a toxic-free and sustainable circular economy will likely require additional policy instruments that will reduce the overall complexity and diversity of in-use polymers and polymeric materials.
Environmental significanceThough an enormous amount of research and policy attention has been dedicated to understanding and mitigating environmental impacts caused by plastics, our understanding of the environmental safety of polymers as a much broader group beyond just components of plastics is far from complete. Currently, the European Union is developing a regulatory proposal to initiate registration of selected polymers under REACH. This offers a unique opportunity to start collecting the missing information to close existing knowledge gaps; however, the draft REACH proposal falls short of that. We here highlight opportunities for improving contemporary approaches to regulatory assessment and management of polymers, based on a critical appraisal of current science on their environmental fate and effects. While using the REACH proposal as an example, our analysis is focused on the individual assessment criteria and is therefore broadly relevant for scientists, regulators and other stakeholders working on polymer assessment and management worldwide. |
1. Introduction
Polymers are the main constituents of plastics. Although plastic litter and its erosion products are rapidly accumulating in terrestrial and aquatic ecosystems,1 and despite widespread evidence of negative impacts on the environment,2 production volumes of plastics are expected to continue the strong increasing trend established since their introduction to the market.3 Consequently, it was estimated that by 2030 up to 53 million metric tons (Mt) of plastics per year could be emitted into our waterways,4 and that the volume of global plastic waste could nearly triple by 2060,5 if the upward trend is not curtailed by the forthcoming global plastics treaty.6 In addition to the insoluble, solid or semi-solid polymers in plastics that have received much public attention due to their “visibility” in the environment, many other polymers, including many that are water-soluble, are broadly used in different industrial applications and consumer products, including but not limited to detergents, household items, cosmetics and personal care products, wastewater treatment aids, agricultural soil conditioners, and fertilizer and pesticide formulations.7,8 Likely due to the analytical challenges of detecting soluble polymers in environmental samples, these polymer types have remained “invisible” and received little public attention so far.9–11A significant portion of chemicals in commerce are polymers: out of the over 235![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 chemical substances that are registered on the global market and have their Chemical Abstracts Service Registry Numbers (CASRNs) revealed, more than 37
000 chemical substances that are registered on the global market and have their Chemical Abstracts Service Registry Numbers (CASRNs) revealed, more than 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 have “poly” in their names, and many additional polymers may have been registered as “reaction products”.12 Furthermore, many of these CASRNs represent a group of polymers rather than unique structures.13
000 have “poly” in their names, and many additional polymers may have been registered as “reaction products”.12 Furthermore, many of these CASRNs represent a group of polymers rather than unique structures.13
Some polymers are known to have very large production volumes. For example, the global production of plastic polymers is estimated to have reached 380 Mt in 2015,3 and over 50 polymers were reported to be produced in, or imported into the US at levels above 450 t per year in 2015 (US EPA, 2016). But for many other polymers, public information on production volumes is scarce. Notably, some polymers may be subject to direct environmental releases when used in certain application areas, including water-soluble polymers used in wastewater treatment, oil and gas extraction, and agriculture.10 Thus, collectively, commercial production and widespread use of different polymers are certain to result in considerable exposure of people and the environment.
Despite their high exposure potential, polymers have historically been a low priority for chemical assessment and management programs worldwide, which commonly rely on polymer assessment schemes that were developed in the early 1990s. Under these assessment schemes, most polymers are assumed to be of “low hazard” due to their “high” molecular weight (MW), with a cut-off value typically set at 1000 dalton (Da) number-average molecular weight, designated as MWn. Since the 1990s, however, scientific understanding related to the human health and environmental impacts of polymers has progressed considerably. For example, the notion of “high”-MW polymers being generally inert and causing no biological effects due to the presumed lack of systemic uptake has recently been challenged based on a critical review of scientific evidence.14 Many recent studies have also demonstrated that weathering of plastics in the environment could result in significantly different toxicity profiles compared to the virgin materials.2 However, most of these insights have not yet been integrated into the regulatory assessment and management schemes for polymers.
A unique opportunity currently presents itself in the European Union (EU) to update and improve polymer assessment and management to reflect modern scientific knowledge. Polymers were exempted from registration in the first implementation phase of the EU's chemicals regulation, REACH, which has run from 2006 to date. The expectation was that polymers would be registered at a later stage, pending the development of a dedicated regulatory procedure. Following up on this commitment, the European Commission (EC) currently seeks to develop a set of criteria to distinguish polymers that are potentially harmful to human health or the environment and hence should be registered under REACH; such polymers are to be differentiated from those identified as “not of concern” and hence not requiring registration. This proposal for polymer registration under REACH is being developed as part of the ongoing REACH revision. The initial criteria set was outlined in a 2020 report by the Wood and PFA consultancies,15 and the EC is currently working, in consultation with industry and other stakeholders, to finalize the proposal by the end of 2023.
Unfortunately, as we show in Section 2, the unique opportunity that currently exists for the EC to modernize regulatory polymer assessment is on track to be squandered. All the criteria included in the draft of the EC's proposal as of April 2022 are largely based on a 1990s' era of scientific understanding of polymer safety. Recent scientific advancements have not been considered. The currently proposed criteria are similar to those used in other countries and thus will not place Europe in a global leadership position with regard to polymer safety assessment. Bearing in mind that registration is primarily a data-collection process, the current EC proposal is therefore quite radical in the sense that it would a priori exclude a large fraction of commercial polymers from data collection, based on manufacturers/importers asserting that they are “not of concern” according to outdated criteria developed using an incomplete knowledge base.
As a complementary measure, the EC has announced an intention to collect a defined set of data for all polymers in commerce in the frame of a mandatory notification/pre-registration process.16 While this represents a step in the right direction, the information planned to be collected does not go beyond the scope of the 1990's era criteria considered to be indicative of potential concerns.17
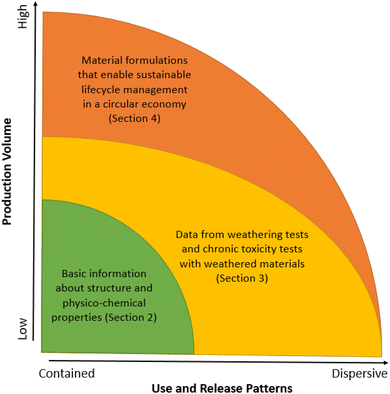
In this study, we critically appraise the current approaches to polymer assessment and management used worldwide, by considering them with reference to recent scientific advancements. Although we use the 2022 EC proposal for polymer registration under REACH as an example, our discussion is broadly relevant for other polymer legislations worldwide, due to the similarity of the majority of criteria currently being used across different jurisdictions. We first give a brief overview of the historical development and current approaches to polymer management used in different jurisdictions (Fig. 1, green area, and Section 2). We then analyze the gaps in current regulatory approaches given recent scientific advances and highlight several important aspects that need to be addressed in polymer assessment processes, which have received insufficient attention so far (Fig. 1, yellow area, and Section 3). We conclude with policy options on the way forward towards modernization of polymer registration under REACH and beyond (Fig. 1, yellow and orange areas, and Section 4).
2. A review of polymer assessment and management approaches worldwide
2.1 Historical overview
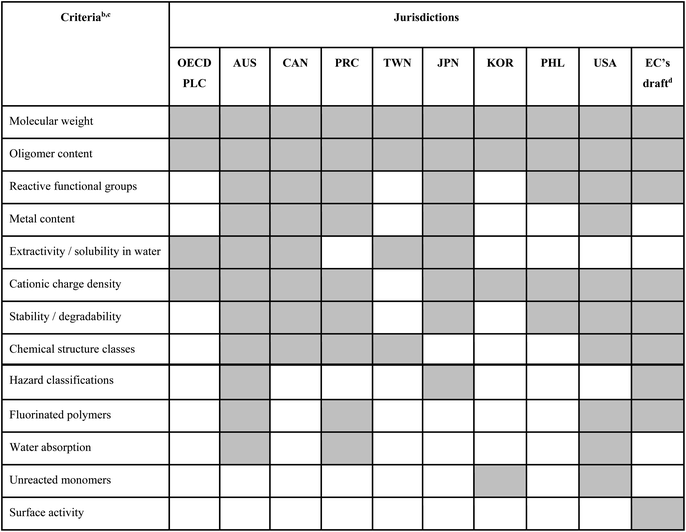
The OECD definition of polymer developed by the Expert Group on Polymer Definition in 1990–1991 (ref. 18) states that “a ‘POLYMER’ means a substance consisting of molecules characterized by the sequence of one or more types of monomer units and comprising a simple weight majority of molecules containing at least three monomer units which are covalently bound to at least one other monomer unit or other reactant and consists of less than a simple weight majority of molecules of the same molecular weight. Such molecules must be distributed over a range of molecular weights wherein differences in the molecular weight are primarily attributable to differences in the number of monomer units. In the context of this definition a ‘MONOMER UNIT’ means the reacted form of a monomer in a polymer”. To date, this OECD definition has been directly adopted by most jurisdictions worldwide, with the notable exception of Japan, where an additional qualifier requiring that the MWn is equal to or higher than 1000 Da is included (see ESI, Table S1†).During its third meeting in 1993, the OECD Expert Group clarified a few terms in the polymer definition, and, importantly, also agreed on a first set of criteria for identifying “polymers of concern”.19 These criteria later formed a basis in many jurisdictions for identifying the so-called “polymers of low concern” (PLCs), i.e., polymers that are deemed to have insignificant environmental and human health impacts and that are thus accepted as having reduced regulatory requirements.20 Different factors/criteria discussed by the OECD Expert Group for assessing a polymer's level of concern are summarized in Table 1, and more details can be found in the ESI, Table S2.†
| a A shaded cell indicates that metrics related to the respective criterion are part of the respective jurisdiction's approach to PLC identification; an unshaded cell indicates that the respective criterion is not being used. b All criteria apart from the last five below the bold line have been discussed by the OECD Expert Group already in the early 1990s. c Additional criteria that have been discussed by the OECD Expert Group but have so far not been included in the PLC criteria set adopted by any of the analyzed jurisdictions include: (i) lipophilicity (though high bioaccumulation potential is considered in Japan), (ii) particle size/respirability, (iii) production volume, (iv) intended uses. d In the current EC proposal (April 2022), polymer assessment criteria are not used to identify PLCs, but to identify polymers requiring registration (PRRs) instead. e Abbreviations: AUS, Australia; CAN, Canada; EC, European Commission; JPN, Japan; KOR, Republic of Korea; PHL, Philippines; PLC, polymer of low concern; PRC, People's Republic of China; PRR, polymer requiring registration; TWN, Taiwan; USA, United States of America. |
|---|
|
2.2 Current approaches to polymer registration and management in different jurisdictions
Table 1 summarizes our mapping of polymer assessment criteria that are included in the polymer legislations of different jurisdictions where polymers are assessed against a set of PLC identification criteria (for details, see the ESI Tables S2 and S3†). Many PLC identification criteria are common to several jurisdictions and were already suggested by the OECD Expert Group in the early 1990s, but the exact combinations of criteria may differ (Table 1). Polymers identified as PLCs are generally exempted from full registration requirements, but pre-notification or record keeping of polymer assessments may be required in some jurisdictions. For example, in Japan and the United States (US), manufacturers and importers are required to submit pre-defined datasets in order to obtain regulatory approval of their polymers as PLCs. In the EU, the latest proposal (status April 2022) does not foresee an inclusion of a PLC identification procedure, but instead proposes to use a set of similar criteria to identify a polymer as either a “polymer requiring registration” (PRR) or a “non-PRR” (note that the latter does not equate with a PLC).Several of the PLC criteria (or, respectively, the non-PRR criteria in the EC's proposal) are widely adapted across jurisdictions, while several other criteria are applied less often or even are unique to specific countries (Table 1). For example, bioaccumulation potential is included in the PLC criteria in Japan, but not in other regulatory frameworks. In addition, certain fluorinated polymers are excluded from being PLC in Australia, China and the US. By far the most widely adopted criterion across jurisdictions concerns the polymer's MW (usually referring to the number-average MW, designated as MWn), with a threshold for a polymer to be considered a PLC commonly set at >1000 Da (Table 1). This is because molecules with MWn above 1000 Da have been assumed to have “negligible” uptake into the organism, and hence be lacking biological effects.20 However, the assumptions of “negligible” uptake of substances with MWn > 1000 Da, as well as the general lack of biological effects in the absence of systemic uptake, have been challenged as science has evolved (see Section 3.3). Furthermore, other characteristics such as surface charge, especially cationicity, may result in marked toxicity, even for polymers with very high MWn.21
While PLC identification is a pragmatic approach to dealing with diverse sets of polymers, it has to be noted that many of the PLC criteria have been debated, and that some thresholds rely on seemingly arbitrary values rather than being backed by rigorous testing and scientific assessment.22 For some criteria, the cut-off values in some jurisdictions were based on internal documents, reviews or registration data, which were provided to the OECD Expert Group in the early 1990s for discussion;18,19 however, most of these documents are not publicly accessible. For other criteria, it is generally unknown how and based on which studies the criteria have been decided upon. Furthermore, while the OECD Expert Group considered a wider range of factors as (potentially) relevant for determining the hazards and risks of polymers, typically a much smaller set of factors has been selected by individual jurisdictions as part of their own PLC criteria selection (Table 1). The PLC criteria may also be integrated in different manners (e.g., several criteria being applied in parallel vs. in sequence), and these differences can affect the outcome of an assessment as well.
2.3 EC's proposal for polymer registration under REACH
The latest draft proposal of the EC includes a workflow where criteria are assembled in a stepwise manner to determine whether a given polymer is a “polymeric precursor” handled as an intermediate under strictly controlled conditions and therefore subject to reduced registration requirements,23 a PRR, or a non-PRR. These criteria (status April 2022, see ref. 24 and 25) are summarized in Table 2. In general, the current EC proposal for polymer registration under REACH adheres to the common approaches followed by many other jurisdictions. That is, although the identification of PLCs as such is currently not attempted in that proposal, most criteria proposed to be used to identify PRRs or not-PRRs are based on the common PLC-criteria options, the majority of which have their roots in the early 1990s. Table 2 also presents our assessment of deficiencies and knowledge gaps in relation to each step, and suggests an additional assessment criterion to be considered; selected aspects are discussed in more detail in Section 3. Note that, while using the EU proposal as an example, the discussion on the identified deficiencies and knowledge gaps is broadly applicable to other polymer legislations worldwide, where similar polymer assessment approaches and criteria are currently used.| Step # | Evaluation steps in the EC draft proposal (#1–9) and our additional suggestion (A) | Next step, if | Deficiencies and critical knowledge gaps |
|---|---|---|---|
| a The criterion C1 states that “cationic polymers or polymers that can be reasonably expected to become cationic in a natural environment are considered PRRs, except those whose cationic groups have a combined FGEW [functional group equivalent weight] of >5000 Da”. b The criteria state that “polymers with MWn of ≤1000 Da are considered as PRRs” (criterion MW1) and “polymers with MWn > 1000 Da are considered PRRs if containing >2% oligomer content of MWn < 500 Da or >5% oligomer content of MWn < 1000 Da”. c The criterion refers to following CLP hazard classes and categories: acute toxicity (category 1–4), mutagenicity (category 1A, 1B, 2), carcinogenicity (category 1A, 1B, 2), toxicity to reproduction (category 1A, 1B, 2 or disruption of lactation), aspiration toxicity (category 1), respiratory sensitization (category 1, 1A or 1B), skin sensitization (category 1, 1A or 1B), Specific Target Organ Toxicity upon Single Exposure (STOT SE, category 1–3), STOT upon Repeated Exposure (STOT RE, category 1, 2), eye damage (category 1), skin corrosion (category 1, 1A–C), aquatic acute toxicity (category 1), aquatic chronic toxicity (category 1–4), damage to ozone layer. Additional hazard classes that are currently being developed according to the EU's Chemicals Strategy for Sustainability will be added in the future, including Endocrine Disruption (ED), Persistent, Bioaccumulative and Toxic (PBT), very Persistent, very Bioaccumulative (vPvB), Persistent, Mobile and Toxic (PMT), and very Persistent, very Mobile (vPvM). d The criterion refers to the high-concern groups and the moderate-concern groups. The latter include: pendant acrylates and methacrylates; aziridines; carbodiimides; halosilanes; hydrosilanes; hydrazines; alpha or beta lactones; vinyl sulfones or analogous compounds; methoxy- and ethoxysilanes. The former include: conjugated olefinic groups not contained in naturally occurring fats, oils and carboxylic acid; alkoxysilanes with alkoxy groups > C2; acid anhydrides; acid halides; aldehydes, allyl ethers, epoxides, hemiacetals, imines; cyanates; iso(thio)cyanate; methylolamides; methylolamines; methylolureas; unsubstituted positions ortho or para to phenolic hydroxyl. e The surface activity criterion intends to cover anionic, non-ionic and amphoteric polymers and proposes a threshold of <45 mN m−1. | |||
| 1 | Is the polymer solely a precursor handled like intermediates under strictly controlled conditions to produce other polymers or articles? | YES: polymer is a polymeric precursor | It should not be assumed that being a precursor/intermediate guarantees the complete absence of exposure, because unreacted residuals remaining in the material could be released during the processing, use and disposal of the final products. For example, the substance GenX (CAS 62037-80-3) has been considered an intermediate for many years, but later included on the EU list of substances of very high concern when found to be widely present in the environment.26 |
| NO: step 2 | |||
| 2 | Is the polymer a polyester made from monomers on the EU list? | YES: polymer is not a polymer requiring registration (i.e., it is a non-PRR) | This exemption covers polyesters made from “low-hazard” monomers identified in the EU list, because polyesters are expected to degrade quickly and it is assumed that no hazardous degradation products are formed. However, degradation rates of polymer-containing articles depend on their thickness;27 sufficiently rapid degradation of polyesters has not been systematically confirmed for all environmental compartments;2 and comprehensive evaluation of all possible degradation products has not yet been performed though generation of degradation products other than monomers has already been demonstrated.28 Moreover, many polyesters contain high levels of oligomers, and particularly for cyclic oligomers, their toxicity profiles are not yet sufficiently characterized to allow comprehensive generalizations.29–32 Polyesters are also a large source of secondary microplastics.33–35 |
| NO: step 3 | |||
| 3 | Is the polymer fluorinated? | YES: polymer is a PRR | Different hazards are associated with fluorinated polymers along their life cycle.36 Thus, all fluorinated polymers should be considered PRRs. Moreover, in order to ensure adequate protection, this criterion should be assessed in the very beginning, i.e., before assessing whether a polymer is a “polymeric precursor”. |
| NO: step 4 | |||
| 4 | Is the polymer meeting cationic criterion C1a? | YES: polymer is a PRR | This criterion covers only cationic polymers, while other charged polymers (anionic and amphoteric) are expected to be covered with another criterion about surface activity (step 8). However, whether the surface activity is the only parameter affecting the toxicity of anionic/amphoteric polymers has not been systematically investigated. |
| NO: step 5 | |||
| 5 | Is the polymer meeting criteria MW1 or MW2b? | YES: polymer is a PRR | These criteria rely on the cut-off of ≤1000 Da for the number-average molecular weight (designated as MWn), as it is considered to indicate biological inertness because of a “negligible” uptake across biological membranes. This assumption has, however, been questioned14 because (a) increased uptake of substances >1000 Da is possible in some conditions, (b) some substances can cause adverse effects even at low uptake levels, and (c) biological effects without systemic uptake are possible at high local concentrations or upon chronic exposure to lower concentrations. For further discussion of these aspects, see Section 3.3. |
| NO: step 6 | |||
| 6 | Is the polymer classified as hazardous under the Classification, Labeling and Packaging (CLP) legislationc? | YES: polymer is a PRR | This criterion refers to the CLP classifications that are already present in the European Chemicals Agency's (ECHA) CLP database. However, while little testing data exists for polymers, CLP classifications of polymers are respectively rare. This criterion should be extended to cover monomers as well as stabilizing additives, i.e., additives that are added to virgin polymers to maintain their integrity. |
| NO: step 7 | |||
| 7 | Is the polymer having reactive functional groups (RFGs) of concernd? | YES: polymer is a PRR | This criterion is limited to assessment of polymers with 1000 < MWn < 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, however, this limitation is not scientifically justified and hence the criterion should be extended to cover polymers with higher MWn as well. Furthermore, it should be taken into account that weathering or degradation of a polymer can lead to the appearance of RFGs not present in the virgin material. 000 Da, however, this limitation is not scientifically justified and hence the criterion should be extended to cover polymers with higher MWn as well. Furthermore, it should be taken into account that weathering or degradation of a polymer can lead to the appearance of RFGs not present in the virgin material. |
| NO: step 8 | |||
| 8 | Is the polymer surface activee? | YES: polymer is a PRR | The background document notes that determination of surface activity “may not be possible or meaningful for some polymer classes” and that applicability will be specified in a future guidance by the ECHA. We also note that surface activity threshold was determined for detergents and hence its applicability to polymers in general is uncertain. |
| NO: step 9 | |||
| 9 | Is polymer suspected to degrade to substance(s) of concern? | YES: polymer is a PRR | As a “substance of concern”, this criterion understands only the substances with CLP classifications included under step 6c. Given the general scarcity of data on the hazard of polymer degradation products, toxicity tests should focus on testing mixtures of substances leaching from polymers, including not only virgin but also weathered polymers. It should be further considered whether microplastics produced by polymer-containing products could also be treated similarly to other substances of concern released by polymers.2 For further discussion, see Section 3.2. |
| NO: polymer is a non-PRR | |||
| A | Does the production volume of this polymer exceeds a defined number of tons per annum, in conjunction with single-use applications or other widely dispersive applications? | High production volumes and/or dispersive use patterns are indicative of high exposure potential. Such polymers require more extensive testing and assessment before being put on the market (Fig. 1), as high exposure potential in combination with high persistence is more likely to result in poorly reversible future impacts within a (relatively) short time period.37 Note that many polymers represent close variations of the same type and hence could result in additive toxicity.13 Such polymers should therefore be grouped together when determining their production volumes, use patterns and environmental hazards. For further discussion, see Section 3.4. | |
3. Scientific advances since the 1990s and open questions on the environmental fate and effects of polymers
This section presents relevant scientific developments since the 1990s on the environmental fate and effects of polymers, and highlights major lessons learned along with the remaining critical knowledge gaps. We then discuss how the criteria in the current EC proposal can be improved, including by adding one more criterion related to polymer production volumes and use patterns as proxies for exposure potential for humans and the environment.3.1 Chemical identity of polymers and grouping approaches
Up to 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 different polymers could be jointly produced or imported in the EU at substantial amounts.15 It is not feasible to test all of them. Many of these polymers are closely related variations of the same polymer types, differing in aspects such as the proportions of co-monomers, branching structures, functional groups, MW distribution, and/or oligomer content. Importantly, these same traits can also affect a polymer's toxicity, exposure and overall risk. Grouping polymers based on structural traits according to transparent rules is seen as an essential component for ensuring efficient registration and test data collection of polymers in use. In other words, one may reduce all polymers in use to different groups with the main representative(s) assigned in each group to undergo testing. However, given the dearth of data on polymers along with the largely outdated understanding of polymers in regulatory schemes in general, attaining sufficient certainty in defining the groups and identifying reliable group representatives for testing might be difficult at present. A useful approach here could be to gain an overview of structural features of all polymers in use, and then develop grouping strategies that take into account the chemical space defined by the main structural features. The collected data should then be used to identify the range and/or combinations of features that would result in that polymer requiring a regulatory oversight.
000 different polymers could be jointly produced or imported in the EU at substantial amounts.15 It is not feasible to test all of them. Many of these polymers are closely related variations of the same polymer types, differing in aspects such as the proportions of co-monomers, branching structures, functional groups, MW distribution, and/or oligomer content. Importantly, these same traits can also affect a polymer's toxicity, exposure and overall risk. Grouping polymers based on structural traits according to transparent rules is seen as an essential component for ensuring efficient registration and test data collection of polymers in use. In other words, one may reduce all polymers in use to different groups with the main representative(s) assigned in each group to undergo testing. However, given the dearth of data on polymers along with the largely outdated understanding of polymers in regulatory schemes in general, attaining sufficient certainty in defining the groups and identifying reliable group representatives for testing might be difficult at present. A useful approach here could be to gain an overview of structural features of all polymers in use, and then develop grouping strategies that take into account the chemical space defined by the main structural features. The collected data should then be used to identify the range and/or combinations of features that would result in that polymer requiring a regulatory oversight.
Unfortunately, for most in-use polymers, chemical identity information crucial for efficient grouping is generally not present in the public domain and may even not be known to the manufacturers/importers themselves. Moreover, no effective mechanism has been implemented for comprehensively communicating all crucial aspects of a polymer's chemical identity, with available options being able to only partially address this at best.38 As a result, many individual CASRNs and CAS names in fact represent multiple polymers differing by a small subset of individual structural properties. To reduce these ambiguities and enable more efficient polymer grouping, several steps should be taken, as recently outlined in ref. 13. Briefly, manufacturers and regulators should work together to produce and collect data on polymer identities and provide a comprehensive, global and publicly accessible overview of all polymers in commerce. These data should be openly shared through a central hub that hosts information from different submitters, including industry, academia and regulatory authorities. Adhering to FAIR data principles (FAIR standing for Findability, Accessibility, Interoperability, and Reusability39) would maximize efficiencies, enable a global overview, and ensure public accessibility. Furthermore, a greater efficiency for sharing compositional and structural information can be enabled by developing and widely integrating novel machine-readable identifiers into the existing systems.13 Our proposal is not consistent with current regulatory frameworks that are designed to protect data as “trade secrets” within government agencies that handle registrations. However, the challenge of chemical assessment of tens or hundreds of thousands of substances demands transparency and open data sharing to enable distribution of chemical and structural information on polymers (and indeed all chemicals) to all stakeholders.
While the chemical identity of polymers is already complex, they also present themselves in a variety of different physical states and forms, which may also change throughout their life cycle, particularly in the environment, and this will in turn impact their environmental fate and effects (Fig. 2). For the needs of hazard and risk assessment, Brunning and colleagues suggested three basic categories: bulk solid, dissolved, and low-MW polymers.40 While these three categories provide a useful starting point, dividing the “bulk solid” category into further subsets may prove useful when estimating the exposure potential (see Section 3.2). Furthermore, a significant subset of polymers may meet the criteria for two or three of these categories, as the proposed category boundaries are blurry (e.g., having a solubility or melting temperature near a threshold), because in the environment, these properties can be affected by specific environmental conditions such as presence of salts and dissolved organic matter. Thus, categorization of polymers' physical state or molecular size is not always unambiguous, particularly for nanoplastics, and it has to be expected that some polymers may fit into multiple categories. For instance, mixtures of octane, decane and dodecane could be considered a polymer based on the OECD definition of having more than three repeating ethane units (Section 2.1), and could even be considered a nanoplastic, since they are 1 nm or longer,41 but they clearly also fit the definition of a substance under REACH.
Overall, regardless of which structural-traits based grouping categories and physical-state categories are adopted for polymers, it has to be accepted that there will always be edge cases, as the categories are placed within a continuous spectrum of diverse properties.
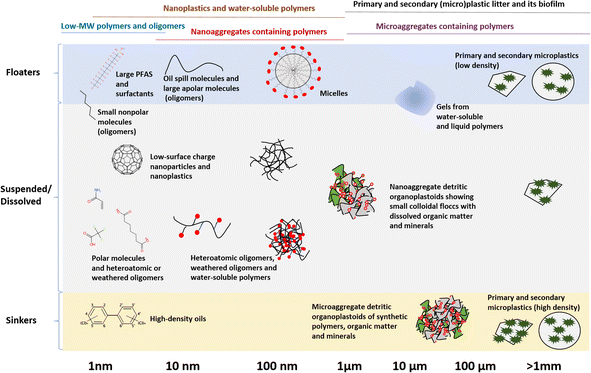
3.2 Understanding environmental fate of polymers
Understanding the environmental fate of different groups of polymers is a prerequisite for exposure and risk assessment and informed regulatory management. Once polymers enter the environment, they become distributed and weathered at varying rates due to abiotic and biotic processes as they interact with the natural world.2 During this distribution and weathering, polymers may be considered persistent in any of the following categories that differ in size and exposure profiles (Fig. 2):(i) Low-MW polymers and oligomers;
(ii) Nanoplastics and water-soluble polymers;
(iii) Nanoaggregates containing polymers;
(iv) Primary and secondary (micro)plastic litter and its biofilm;
(v) Microaggregates containing polymers.
Note that in these categories, “plastics” is only used in connection with a size range (nanoplastics, microplastics), otherwise the term “polymer” is used.
Fig. 2 presents an overview of how both the particle size and density distribution (PSDD), and the degree of surface aggregation, can affect the environmental fate and exposure pathways of polymers. Starting on the left of Fig. 2, the fate of the substances in the exposure group low-MW polymers and oligomers (<1 nm to ca. 5 nm) essentially follows those of chemicals and chemical spills. Here, all substances will partition between available environmental phases until their point of saturation, and once in excess will either float, sink or form an emulsion depending on the density. An exception here are the surfactants, which tend to accumulate and aggregate on the surface, eventually forming micelles at certain concentrations. Depending on degradation rates, these substances will either form new chemicals that may have a different fate, such as mineralization to carbon dioxide or ions with higher water solubility and lower adsorption onto organic matter, or remain unchanged in the environment as persistent organic contaminants. In principle, there are several chromatographic techniques to measure substances in this size range, provided the molecular structures are known. However, challenges remain to quantify all unknown chemical structures, including photo- and bio-degradation products, which requires non-targeted analysis and similar methods.42
The next category from left to right in Fig. 2 is nanoplastics and water-soluble polymers (1 nm to ca. 1000 nm). The main difference between nanoplastics and water-soluble polymers is surface activity, with water-soluble polymers having more positive or negative charges per unit molar volume compared to nanoplastics. Otherwise, these two groups have an overlapping size range with each other, and even large molecules and oligomers.10 Polymers sized in the nm range would show colloidal behavior. For instance, nanoplastics are colloidal, and therefore, capable of crossing cell membranes43,44 and being suspended in water regardless of their density, unless forming an aggregate.45–47 They therefore require specialized analytical approaches,48 some of which for water-soluble polymers in the environment are only emerging now.9,11 Chemical regulatory authorities, in collaboration with producers, should ensure the availability and accessibility of environmental analytical techniques for these substances, as without analytical methods and commercially available standards the environmental fate and effects of these substances cannot be tested and assessed.
A complicating aspect here is that both nanoplastics and especially the water-soluble polymers will tend to flocculate/aggregate with other colloidal particles, be they organic or mineral, through electrostatic interactions, leading to the formation of nanoaggregates containing polymers (ca. 10 to 1000 nm). Water-soluble polymers will be dissolved in water initially, but because of their high surface charge, will likely aggregate later to form composites with organic matter49 and minerals,50 and these composites can float, sink or be suspended in the water column.10 Being nanoaggregates, these particles will be colloidal in nature; their extremely complex compositions make quantifying them a formidable analytical challenge, considering that the current state-of-the-art analytical techniques are limited to non-aggregated nanoplastics and water-soluble polymers.51,52
Moving to a larger size category, the primary and secondary (micro)plastic litter and its biofilm (1 μm to >5 mm), the fate of its members is similar to the pure chemicals above the point of saturation; depending on their density, they will float, sink or be dispersed/suspended. The sinking and dispersion rates are very much dependent on fluid velocities and turbulences.45,46 Both microplastics and larger plastic debris will very rapidly form a surface for molecules and macromolecules from biological origin to sorb, which is often referred to as an eco-corona.53 From there, the surface can be colonized by bacteria, algae and a variety of larger organisms, which is often referred to as the plastisphere.54 This has two major exposure implications. The first is that the biofilm will affect the buoyancy of the particle, and this can affect whether a particle floats, sinks, or is suspended.55 The second is that the biological colonization of this debris will lead to weathering through enzymatic processes. This enzymatic weathering, together with abiotic processes such as photodegradation, hydrolysis and mechanical forces,2 can break the microplastics down into nanoplastics-sized weathering products and oligomers. Though there are now several techniques to measure microplastics and larger plastic debris, biofilm can complicate this analysis, notably the attenuated total reflectance-Fourier transform infrared (ATR-FTIR)-based approaches, which identify the polymers by their surface layer.56
The final category is the microaggregates containing polymers (1 μm to >5 mm), which would have a fate similar to primary and secondary microplastics, but are being much more complex in composition. The source of such microaggregates could be heavily degraded microplastics, nanoaggregates containing polymers that aggregated to a larger size, or both. Though such microaggregates are quite common, they remain difficult to quantify in their natural state because of their heterogeneity (Fig. 3). Most often, the organic components within such microaggregates would typically be broken or digested with acids, bases or oxidants during quantification,57 and thus, the scientific investigation of their presence and fate remains a significant challenge to date. Nevertheless, collectively, much of the polymers emitted into the environment, be they in the terrestrial and aquatic environments, will interact with organic matter, forming the detritic organoplastoid type of matter, occurring in both nano- and microaggregate size classes.58
 | ||
| Fig. 3 Examples of microaggregates containing polymers (photocredit: Elizabeth Ellenwood, used with permission). Scale bar = 2 mm. | ||
3.3 Understanding environmental hazards of polymers
To date, hazard assessment of polymers has been dominated by evaluating virgin substances for short-term (acute) toxicity. However, scientific studies increasingly show that this narrow focus is neither protective nor scientifically justified with regard to environmental fate as well as the uptake and chronic toxicity of polymers.2,14 Furthermore, it is important to keep in mind that the environmental effects of a polymer are determined by both the mechanical (e.g., PSSD, in vivo distribution and physical impacts) and the chemical (e.g., low-MW substances released by a polymer) aspects.This, however, does not apply just to solid plastic materials, since essentially all polymer types may be susceptible to degradation through both chemical (abiotic) and biological (biotic) processes.35 Apart from formation of shorter monomer-chains of the same polymer type (which may result in their increased uptake and bioavailability), degradation of polymers can also give rise to unknown and unexpected products resulting from, e.g., oxidative reactions on their side chains and functional substitutes.2,59 For example, UV-induced weathering of polyethylene was shown to produce a suite of low-MW, soluble degradation products, resulting in increased toxicity of corresponding leachates.60,61 The EC's proposal does mention that “a polymer that is designed, or can be expected, to substantially degrade, decompose or depolymerise into substances having one or more of the hazard classifications” is considered a PRR. However, limiting the current focus to the known (or classified) degradation products does not provide for a comprehensive assessment of environmental toxicity exerted by polymers, since some polymers could degrade into hazardous chemicals that are not necessarily known/classified or could be predicted beforehand. Moreover, the growing interest among industry and nonprofits to create and employ more biodegradable or compostable polymers further underscores the urgent need to better understand the potential hazards associated with biotransformation products of these polymers.
In addition to biological responses triggered by chronic exposure to low levels of high-MW substances crossing the membrane barriers, non-systemic toxicity manifestations, e.g., those occurring without organismal or cellular uptake, are also possible. Surface interactions produced by cationic (positively charged) polymers have received the most attention so far as being the most obvious.66 However, also uncharged polymers seem to be capable of producing biologically relevant responses based solely on surface (extracellular) interactions without intracellular uptake. These may occur, e.g., through interactions with outward-facing cellular receptors or impacts on the extracellular matrix structures. For example, PEG was shown to condense colonic mucus in mice both in vitro and in vivo, which in turn was suggested to negatively affect the organism's resistance to bacterial invasion.67 Similar effects were observed in response to carboxymethyl cellulose, a chemically modified natural polymer.68 In aquatic organisms, exposure of tadpoles to PEG was observed to disrupt development of neuromasts, which are the organs comprised of rosette-like formations of sensory cells at the skin surface and are responsible for maintenance of balance and sensing of the environment.63
Exposure to polymers can also affect both organisms and entire ecosystems through interaction with commensal and free-living microbiota. For example, water-soluble polymers were shown to exert specific toxicity on some bacteria and to inhibit oxygen consumption by nitrifying microorganisms, which could affect the proper functioning of nitrification processes in wastewater treatment plants and aquatic ecosystems alike.69 Based on these and other examples, several recent reviews emphasized that it cannot be excluded that water-soluble polymers, even those with high-MW, could produce a not yet characterized toxicity or other adverse effects upon interaction with organisms in the environment.9,10
In principle, low-MW polymers and oligomers can be (and some have been) assessed by standard tools existing to assess all other low-MW chemicals, e.g., hazard assessment tools outlined in the United Nations' Globally Harmonized System of Classification and Labeling of Chemicals (UN-GHS) or the REACH regulation. Assessing the hazard of the (often unknown) degradation products originating from polymers, which may include low-MW polymers, oligomers and other chemicals, can be more difficult. Here, testing of the whole chemical mixtures released by a polymer or a polymer-containing product has been applied to gain a better understanding of the overall toxicity.70–72 This approach could then be further enhanced by testing not only the virgin polymers/products, but also their counterparts subjected to weathering.2,60,61,73
One way to standardize the methods for testing the effects of weathering could be through employing and optimizing the available tests for assessing the durability of polymers under certain conditions. For instance, the ISO 4611:2010-12 standard allows measuring the changes in plastics' mass, size and color as a result of exposure to damp heat, water spray and salt mist, and similar ISO methods are available to look at weathering caused by exposure to sunlight (ISO 4892 part 1–3 (2013–2016), ISO 877-1:2009-06). For microbial degradation, tests such as those envisaged by Brunning et al. (2022)40 could be employed to benchmark the biodegradation of polymers, with the caveat that transformation products formed by primary biodegradation may not themselves be biodegradable.10 Importantly, rather than using such tests to simply record the changes occurring in a polymer, a possibility to couple them to diverse (eco)toxicity tests performed on the “end-products” of weathering should be actively explored and developed as a standardized addition. In this way, the influence of weathering on the toxicity hazard of the changed polymer can be directly investigated and, if necessary, further coupled to an effect-direct analysis to identify the causative substances.2
When testing chemical toxicity of polymers, it is further important to decouple the hazards triggered through polymer size, i.e., MW distributions, from those of leachates from compounded products which may contain individual additives (in the case of plastics) in addition to polymer degradation products. For this, standardized testing schemes should be designed to cover different stages of the product life cycle, such as tests on initial formulations, followed by tests on the degraded material obtained after exposure to standardized weathering conditions. Three things that should be considered here include (a) the rate of the change of PSDD, (b) the release of low-MW substances in the leachate, and (c) physical interactions with organic matter, which could lead to physical-mechanic effects in the individual organisms as well as geophysical impacts on the larger scale.
Regarding (a) and (b), the MW distribution and the toxicity of leachates can be quantified and compared.61 An important consideration for bulk-phase polymers is their PSDD, as it will ultimately determine the persistence and scale of degradation. For instance, large polyester bricks emitted to the ocean will be degrading to microplastics over a much longer time scales than the thin polyethylene plastic bags.27 Therefore, the results of such tests should be normalized toward the polymers' PSDD.
The ability of insoluble polymers to intertwine with organic matter and form micro- and macroaggregates of detritic organoplastoids (Fig. 3) has long been known, but its relevance and effects on the biosphere are only now starting to become understood. Plastics fragments littered in the environment could bring about changes in the structure of both sediments and soils, potentially resulting in ecological imbalance and yet unknown consequences.76 Therefore, much more information on geophysical impacts and ecosystem impacts of polymer exposures is needed than is available now, especially considering the fact that many polymers are poorly degradable and can be expected to persist and further accumulate in the environment in the years to come.2,10 A better understanding of the long-term geophysical impacts is also crucial for assessing the risks of polymer applications that involve direct manipulation of the environment, such as polymers used as flocculation aids, water retention agents in agricultural soils, or plastic films covering the farmland. Because these questions cannot yet be addressed through standardized testing, they should be rather tackled in the frame of dedicated research programs focused on understanding the geophysical changes caused by polymer exposure in the air (as recently discussed by ref. 77), sediment and soil.76 Research and monitoring data collected through different initiatives should be transparently shared to enable mutual progress and support long-term experiments.
3.4 Polymer assessment across their life cycle
Production volumes and use patterns have been discussed by the OECD Expert Group on polymers and in the context of general chemicals assessment frameworks as potential polymer assessment criteria (Table 1). While both parameters have generally been seen as part of exposure and risk assessments, here we recommend that they can also be used as exposure-related indicators to prioritize both the registration requirements and the scope of test data required for a given polymer (Fig. 1). This is because for polymers with higher production volumes and/or more dispersive use patterns, higher releases and higher exposure can be expected, and thus, the likelihood of causing significant harm to a larger population or ecosystem would be higher as well. The high persistence of many polymers and/or their degradation products further exacerbates the situation by making such exposure poorly reversible.37 Therefore, for polymers with high production volumes and/or dispersive use patterns, more comprehensive testing and assessment is warranted (Fig. 1). It should be noted that for new polymers that are to be put on the market, production volumes and use patterns are only best estimates, and follow-up monitoring on production volumes and use patterns needs to be considered.In consideration of the circular economy, while recovered polymers currently represent only a tiny fraction of the polymers on the global market (mainly related to plastic recycling3), the ongoing societal transition to a circular economy78 will make recovered polymers increasingly relevant. Unfortunately, current regulatory frameworks do not adequately address the circularity of polymers. For example, through the exemption of new registration of recovered substances that have already been registered and are being recovered in the European Community, REACH permits the unassessed use of (heavily) contaminated recovered polymers that may have up to 20% of unintentional impurities in comparison to the previously registered substances.79,80 In other words, REACH in its current form does not track polymers along their multiple use cycles, even when the recovered polymers are used in different applications and thus could have different exposure patterns from the virgin ones.79
To foster the transition to safe and sustainable circular uses of chemicals, including polymers, Wang and Hellweg (2021) proposed two changes in the current chemicals assessment paradigm: (1) introducing the consideration of multiple use cycles in the hazard and risk assessment stage, including different exposure patterns; and (2) introducing an additional “sustainable circularity” assessment stage requiring early consideration of the recoverability of polymers after each of their use cycle, and of the associated environmental impacts.81
4. Policy options for enabling safe and sustainable uses of polymers
Polymers are present in many materials and products that make up much of our modern world. They have been largely overlooked by regulators until now due to their complexity and because of the sheer diversity of polymers, material formulations and applications currently in use.82 Another, more logistical reason, stems from an extremely long lag time between the rapidly developing scientific understanding and its slow regulatory uptake.83 The work on developing a legal procedure for polymer registration under REACH, currently ongoing in the EU, provides a unique opportunity for regulatory management of polymers in the EU to catch up on the scientific advances.We emphasize the following major aspects to be better reflected in the REACH revision on polymers:
(i) The need for more transparency on the chemical identities and physical characteristics of polymers in use, including transparent and well-substantiated justification for grouping of polymers for testing (Section 3.1);
(ii) The need for improved understanding of environmental fate of polymers with regard to transitions across size categories and exposure profiles (Section 3.2);
(iii) The need for development of standard protocols and testing procedures that should deliver an improved understanding of environmental hazards of polymers, focusing on the effects of weathering as well as a comprehensive assessment of the uptake, long-term toxicity, physical-mechanic effects and geophysical impacts (Section 3.3); and
(iv) The suggestion to make the breadth of the data requirements for a given polymer dependent on the production volume and use/release patterns with regard to their dispersive potential. That is, the greater the production volume and/or the likelihood of environmental releases, the greater the data requirements should be over the entire lifecycle of a polymer, including the consideration of possible multiple use cycles (Fig. 1 and Section 3.4).
It has to be further considered that an assessment of polymer safety using approaches designed for other organic chemicals – even for high-production-volume chemicals – would not be able to effectively address the entirety of the challenge of managing polymers throughout their lifecycle of production, use and eventual disposal. In other words, chemicals regulations such as REACH may not be sufficient as the sole regulatory instrument to ensure proper management of polymers and the diverse formulations, materials and products that are made from them. Driving the transformation to a circular economy demands that producers be required to take responsibility for their products beyond their intended function. In particular, products and materials made from polymers must be readily reused and easily recyclable to close material flow cycles. Consequently, it is an inescapable reality of thermodynamics that transformation to a circular economy will require both chemical84 and material82 simplification.
The chemical and material simplification required to facilitate the transition to a circular economy cannot be achieved by relying on free-market forces, which favor diversity and product differentiation. Instead, it must be driven by standardization of material compositions and properties that will allow for ready reuse and easy recycling. Therefore, regulators require instruments that would allow blocking the introduction of “new” materials when a material with similar performance characteristics and simpler composition already exists, on the premise that the new material would unnecessarily complicate reuse/recycling. These considerations are beyond the scope of chemicals regulations such as REACH, but could be developed as a new class of standards and regulations whose aim would be to ensure that materials and products made from polymers conform to the requirements of a circular economy. Transparency and data sharing following FAIR principles are both necessary to facilitate transformation to a circular economy, while a desire to protect “trade secrets” must take a back-seat here. Transitioning to polymers and polymeric materials with simpler compositions known to be compliant with safe and sustainable requirements over product life cycles also has the benefit of eliminating costs associated with the safety and testing requirements of diverse new polymers.
While our analysis in this paper has focused primarily on the EU context, much is also transferrable to other jurisdictions. We hope that this analysis, informed by the legislative process for polymer registration being developed in the EU, will serve as a starting point to trigger further developments towards improved management of polymers worldwide. In particular, the OECD or the UN as global organizations could take a leading role in convening further expert meetings on polymers, aiming to evaluate the current state of science and to speed the integration of recent scientific advances into a revised, internationally harmonized framework for the assessment and management of polymers.
Author contributions
This paper was conceived in joint discussions among the co-authors. All co-authors wrote and edited parts of the paper, and all approved of the final draft.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
Elizabeth Ellenwood is thanked for providing the images used in the graphic abstract and Fig. 3; her photography was supported by the Fulbright Program. This project has received funding from the European Union's Horizon 2020 Research and Innovation Programme under the Grant Agreement No. 101036756, ZeroPM (H. P. H. A. & Z. W.) and the Research Council of Norway project SLUDGEFFECT (grant 302371/E10).References
- D. K. A. Barnes, et al., Accumulation and fragmentation of plastic debris in global environments, Philos. Trans. R. Soc., B, 2009, 364(1526), 1985–1998 CrossRef CAS PubMed.
- H. P. H. Arp, et al., Weathering Plastics as a Planetary Boundary Threat: Exposure, Fate, and Hazards, Environ. Sci. Technol., 2021, 55(11), 7246–7255 CrossRef CAS.
- R. Geyer, J. R. Jambeck and K. L. Law, Production, use, and fate of all plastics ever made, Sci. Adv., 2017, 3(7), e1700782 CrossRef PubMed.
- S. B. Borrelle, et al., Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution, Science, 2020, 369(6510), 1515–1518 CrossRef CAS PubMed.
- OECD, Global Plastics Outlook: Policy Scenarios to 2060, OECD, Paris, 2022, https://www.oecd-ilibrary.org/sites/aa1edf33-en/index.html?itemId=/content/publication/aa1edf33-en Search PubMed.
- M. Bergmann, et al., A global plastic treaty must cap production, Science, 2022, 376(6592), 469–470 CrossRef PubMed.
- K. Duis, T. Junker and A. Coors, Environmental fate and effects of water-soluble synthetic organic polymers used in cosmetic products, Environ. Sci. Eur., 2021, 33(1), 21 CrossRef CAS.
- A. Pecquet, et al., Polymers Used in US Household Cleaning Products: Assessment of Data Availability for Ecological Risk Assessment, Integr. Environ. Assess. Manage., 2019, 15(4), 621–632 CrossRef PubMed.
- S. Huppertsberg, et al., Making waves: water-soluble polymers in the aquatic environment: an overlooked class of synthetic polymers?, Water Res., 2020, 181, 115931 CrossRef CAS PubMed.
- H. P. H. Arp and H. Knutsen, Could We Spare a Moment of the Spotlight for Persistent, Water-Soluble Polymers?, Environ. Sci. Technol., 2020, 54(1), 3–5 CrossRef CAS.
- T. Mairinger, M. Loos and J. Hollender, Characterization of water-soluble synthetic polymeric substances in wastewater using LC-HRMS/MS, Water Res., 2021, 190, 116745 CrossRef CAS PubMed.
- Z. Wang, et al., Toward a Global Understanding of Chemical Pollution: A First Comprehensive Analysis of National and Regional Chemical Inventories, Environ. Sci. Technol., 2020, 54(5), 2575–2584 CrossRef CAS PubMed.
- Z. Wang, H. Wiesinger and K. Groh, Time to Reveal Chemical Identities of Polymers and UVCBs, Environ. Sci. Technol., 2021, 55(21), 14473–14476 CrossRef CAS.
- K. J. Groh, B. Geueke and J. Muncke, Food contact materials and gut health: Implications for toxicity assessment and relevance of high molecular weight migrants, Food Chem. Toxicol., 2017, 109, 1–18 CrossRef CAS PubMed.
- Wood and PFA-Brussels, Scientific and technical support for the development of criteria to identify and group polymers for registration/evaluation under REACH and their impact assessment, European Commission, Brussels, 2020, https://circabc.europa.eu/ui/group/a0b483a2-4c05-4058-addf-2a4de71b9a98/library/4acda744-777f-473e-b084-76e125430565/details Search PubMed.
- EC, Thought Starter on Information for Notification of Polymer Status (PLC/PRR/non-PRR/Precursor) and Identification and Naming of Polymers, Directorate-General for Environment, European Commission, Brussels, Belgium, p. 2022, https://circabc.europa.eu/ui/group/a0b483a2-4c05-4058-addf-2a4de71b9a98/library/6a2487f8-f8a9-485f-94b0-6ff5a35bf14d/details Search PubMed.
- Cefic, Expanded Cefic thoughts on naming of polymers and polymer groups, Cefic, 2022, https://circabc.europa.eu/ui/group/a0b483a2-4c05-4058-addf-2a4de71b9a98/library/801d4595-df8e-452b-af70-f2412f6bf302/details Search PubMed.
- OECD, Second meeting of the OECD Expert Group on polymer definition. Chairman's report, OECD, Paris, 1991, p. ENV/MC/CHEM(91)18 Search PubMed.
- OECD, Third meeting of OECD experts on polymers, Tokyo, 14th–16th April 1993, Chairman's report, OECD, 1993, ENV/MC/CHEM/RD(93)4 Search PubMed.
- OECD, Data analysis of the identification of correlations between polymer characteristics and potential for health or ecotoxicological concern, 2009, ENV/JM/MONO(2009)1 Search PubMed.
- J. M. Rawlings, et al., Understanding Ecotoxicological Responses of Fish Embryos and Gill Cells to Cationic Polymers, Environ. Toxicol. Chem., 2022, 41(9), 2259–2272 CrossRef CAS PubMed.
- B. Carney Almroth, K. Groh, et al., Statement on the registration of polymers under REACH and List of Signatures in Support, 2021 Search PubMed.
- ECHA, Guidance on intermediates, 2010, ECHA-2010-G-17-EN Search PubMed.
- EC, PRR criteria flowchart, 2022, https://circabc.europa.eu/ui/group/a0b483a2-4c05-4058-addf-2a4de71b9a98/library/8715513d-67f9-4886-8798-e153de035b42/details Search PubMed.
- EC, 8th Meeting of REACH and CLP Competent Authorities Sub-Group on Polymers, 2 May 2022, Webex, Concerns: Amended PRR-Identification Criteria, Directorate-General for Environment, Brussels, 2022, https://circabc.europa.eu/ui/group/a0b483a2-4c05-4058-addf-2a4de71b9a98/library/a550f048-78f3-4fab-aa45-9ea93a604f5e/details Search PubMed.
- S. E. Hale, et al., Persistent, mobile and toxic (PMT) and very persistent and very mobile (vPvM) substances pose an equivalent level of concern to persistent, bioaccumulative and toxic (PBT) and very persistent and very bioaccumulative (vPvB) substances under REACH, Environ. Sci. Eur., 2020, 32(1), 155 CrossRef CAS.
- A. Chamas, et al., Degradation Rates of Plastics in the Environment, ACS Sustainable Chem. Eng., 2020, 8(9), 3494–3511 CrossRef CAS.
- L. Sørensen, et al., UV degradation of natural and synthetic microfibers causes fragmentation and release of polymer degradation products and chemical additives, Sci. Total Environ., 2021, 755, 143170 CrossRef PubMed.
- N. Zhang, et al., Migration studies and chemical characterization of low molecular weight cyclic polyester oligomers from food packaging lamination adhesives, Packag. Technol. Sci., 2018, 31(4), 197–211 CrossRef CAS.
- S. Ubeda, M. Aznar and C. Nerín, Determination of oligomers in virgin and recycled polyethylene terephthalate (PET) samples by UPLC-MS-QTOF, Anal. Bioanal. Chem., 2018, 410(9), 2377–2384 CrossRef CAS PubMed.
- S. Ubeda, et al., Migration of oligomers from a food contact biopolymer based on polylactic acid (PLA) and polyester, Anal. Bioanal. Chem., 2019, 411(16), 3521–3532 CrossRef CAS PubMed.
- S. Ubeda, et al., Migration studies and toxicity evaluation of cyclic polyesters oligomers from food packaging adhesives, Food Chem., 2020, 311, 125918 CrossRef CAS.
- E. Hernandez, B. Nowack and D. M. Mitrano, Polyester Textiles as a Source of Microplastics from Households: A Mechanistic Study to Understand Microfiber Release During Washing, Environ. Sci. Technol., 2017, 51(12), 7036–7046 CrossRef CAS PubMed.
- B. M. Carney Almroth, et al., Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment, Environ. Sci. Pollut. Res., 2018, 25(2), 1191–1199 CrossRef CAS.
- K. Zhang, et al., Understanding plastic degradation and microplastic formation in the environment: a review, Environ. Pollut., 2021, 274, 116554 CrossRef CAS.
- R. Lohmann, et al., Are Fluoropolymers Really of Low Concern for Human and Environmental Health and Separate from Other PFAS?, Environ. Sci. Technol., 2020, 54(20), 12820–12828 CrossRef CAS PubMed.
- I. T. Cousins, et al., Why is high persistence alone a major cause of concern?, Environ. Sci.: Processes Impacts, 2019, 21(5), 781–792 RSC.
- P. Hodge, et al., A concise guide to polymer nomenclature for authors of papers and reports in polymer science and technology (IUPAC Technical Report), Pure Appl. Chem., 2020, 92(5), 797–813 CrossRef CAS.
- M. D. Wilkinson, et al., The FAIR Guiding Principles for scientific data management and stewardship, Sci. Data, 2016, 3(1), 160018 CrossRef PubMed.
- H. Brunning, et al., Toward a Framework for Environmental Fate and Exposure Assessment of Polymers, Environ. Toxicol. Chem., 2022, 41(3), 515–540 CrossRef CAS.
- J. Gigault, et al., Current opinion: what is a nanoplastic?, Environ. Pollut., 2018, 235, 1030–1034 CrossRef CAS.
- X. Gao, et al., Organophosphorus flame retardants and persistent, bioaccumulative, and toxic contaminants in Arctic seawaters: on-board passive sampling coupled with target and non-target analysis, Environ. Pollut., 2019, 253, 1–10 CrossRef CAS.
- Y. Ding, et al., Tissue distribution of polystyrene nanoplastics in mice and their entry, transport, and cytotoxicity to GES-1 cells, Environ. Pollut., 2021, 280, 116974 CrossRef CAS.
- N. J. Clark, et al., Demonstrating the translocation of nanoplastics across the fish intestine using palladium-doped polystyrene in a salmon gut-sac, Environ. Int., 2022, 159, 106994 CrossRef CAS PubMed.
- L. Khatmullina and I. Isachenko, Settling velocity of microplastic particles of regular shapes, Mar. Pollut. Bull., 2017, 114(2), 871–880 CrossRef CAS PubMed.
- I. Isachenko, Catching the variety: obtaining the distribution of terminal velocities of microplastics particles in a stagnant fluid by a stochastic simulation, Mar. Pollut. Bull., 2020, 159, 111464 CrossRef PubMed.
- L. Khatmullina and I. Chubarenko, Thin synthetic fibers sinking in still and convectively mixing water: laboratory experiments and projection to oceanic environment, Environ. Pollut., 2021, 288, 117714 CrossRef CAS.
- H. Cai, et al., Analysis of environmental nanoplastics: progress and challenges, Chem. Eng. J., 2021, 410, 128208 CrossRef CAS.
- G. J. Levy and W. P. Miller, Polyacrylamide adsorption and aggregate stability, Soil Tillage Res., 1999, 51(1), 121–128 CrossRef.
- J. F. Argillier, et al., Solution and adsorption properties of hydrophobically associating water-soluble polyacrylamides, Colloids Surf., A, 1996, 113(3), 247–257 CrossRef CAS.
- W. Zhang, et al., Direct Observation of the Release of Nanoplastics from Commercially Recycled Plastics with Correlative Raman Imaging and Scanning Electron Microscopy, ACS Nano, 2020, 14(7), 7920–7926 CrossRef CAS.
- L. N. Corbett and L. A. Amaral-Zettler, Nanoplastics and the Marine Environment, in Plastics and the Ocean, 2022, pp. 389–428 Search PubMed.
- F. Nasser and I. Lynch, Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna, J. Proteomics, 2016, 137, 45–51 CrossRef CAS PubMed.
- L. A. Amaral-Zettler, E. R. Zettler and T. J. Mincer, Ecology of the plastisphere, Nat. Rev. Microbiol., 2020, 18(3), 139–151 CrossRef CAS PubMed.
- C. D. Rummel, et al., Impacts of Biofilm Formation on the Fate and Potential Effects of Microplastic in the Aquatic Environment, Environ. Sci. Technol. Lett., 2017, 4(7), 258–267 CrossRef CAS.
- V. Morgado, et al., Validated spreadsheet for the identification of PE, PET, PP and PS microplastics by micro-ATR-FTIR spectra with known uncertainty, Talanta, 2021, 234, 122624 CrossRef CAS.
- L. M. B. Olsen, et al., Facilitating microplastic quantification through the introduction of a cellulose dissolution step prior to oxidation: proof-of-concept and demonstration using diverse samples from the Inner Oslofjord, Norway, Mar. Environ. Res., 2020, 161, 105080 CrossRef CAS PubMed.
- E. Ellenwood and H. P. H. Arp, The interweaving of the synthetic & the natural world, Unpublished book, 2022 Search PubMed.
- G. Biale, et al., A Systematic Study on the Degradation Products Generated from Artificially Aged Microplastics, Polymers, 2021, 13(12), 1997 CrossRef CAS.
- C. D. Rummel, et al., Effects of Leachates from UV-Weathered Microplastic in Cell-Based Bioassays, Environ. Sci. Technol., 2019, 53(15), 9214–9223 CrossRef CAS PubMed.
- C. D. Rummel, et al., Effects of leachates from UV-weathered microplastic on the microalgae Scenedesmus vacuolatus, Anal. Bioanal. Chem., 2022, 414(4), 1469–1479 CrossRef CAS.
- K. E. Pelka, et al., Size does matter – determination of the critical molecular size for the uptake of chemicals across the chorion of zebrafish (Danio rerio) embryos, Aquat. Toxicol., 2017, 185, 1–10 CrossRef CAS.
- Í. F. Nascimento, et al., Polyethylene glycol acute and sub-lethal toxicity in neotropical Physalaemus cuvieri tadpoles (Anura, Leptodactylidae), Environ. Pollut., 2021, 283, 117054 CrossRef.
- F. Freeling, et al., Occurrence and potential environmental risk of surfactants and their transformation products discharged by wastewater treatment plants, Sci. Total Environ., 2019, 681, 475–487 CrossRef CAS.
- Y. Jin, et al., Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish, Environ. Pollut., 2018, 235, 322–329 CrossRef CAS PubMed.
- K. A. Connors, et al., Environmental hazard of cationic polymers relevant in personal and consumer care products: a critical review, Integr. Environ. Assess. Manage., 2022, 1–14 Search PubMed.
- S. S. Datta, A. Preska Steinberg and R. F. Ismagilov, Polymers in the gut compress the colonic mucus hydrogel, Proc. Natl. Acad. Sci. U. S. A., 2016, 113(26), 7041–7046 CrossRef CAS PubMed.
- A. Preska Steinberg, Z.-G. Wang and R. F. Ismagilov, Food Polyelectrolytes Compress the Colonic Mucus Hydrogel by a Donnan Mechanism, Biomacromolecules, 2019, 20(7), 2675–2683 CrossRef CAS PubMed.
- U. Rozman and G. Kalčíková, The first comprehensive study evaluating the ecotoxicity and biodegradability of water-soluble polymers used in personal care products and cosmetics, Ecotoxicol. Environ. Saf., 2021, 228, 113016 CrossRef CAS.
- K. J. Groh and J. Muncke, In Vitro Toxicity Testing of Food Contact Materials: State-of-the-Art and Future Challenges, Compr. Rev. Food Sci. Food Saf., 2017, 16(5), 1123–1150 CrossRef PubMed.
- L. Zimmermann, et al., Benchmarking the In Vitro Toxicity and Chemical Composition of Plastic Consumer Products, Environ. Sci. Technol., 2019, 53(19), 11467–11477 CrossRef CAS PubMed.
- J. Völker, et al., Adipogenic Activity of Chemicals Used in Plastic Consumer Products, Environ. Sci. Technol., 2022, 56(4), 2487–2496 CrossRef.
- S. Coffin, et al., Comparisons of analytical chemistry and biological activities of extracts from North Pacific gyre plastics with UV-treated and untreated plastics using in vitro and in vivo models, Environ. Int., 2018, 121, 942–954 CrossRef CAS PubMed.
- M. Nendza, Hazard assessment of silicone oils (polydimethylsiloxanes, PDMS) used in antifouling-/foul-release-products in the marine environment, Mar. Pollut. Bull., 2007, 54(8), 1190–1196 CrossRef CAS.
- F. Faÿ, et al., Antifouling activity of marine paints: study of erosion, Prog. Org. Coat., 2007, 60(3), 194–206 CrossRef.
- M. MacLeod, et al., The global threat from plastic pollution, Science, 2021, 373(6550), 61–65 CrossRef CAS.
- M. Aeschlimann, et al., Potential impacts of atmospheric microplastics and nanoplastics on cloud formation processes, Nat. Geosci., 2022, 15, 967–975 CrossRef CAS.
- UNEP, Resolution adopted by the United Nations Environment Assembly on 2 March 2022: The Environmental Dimension of a Sustainable, Resilient and Inclusive Post COVID-19 Recovery, UNEP, 2022, https://wedocs.unep.org/handle/20.500.11822/39744 Search PubMed.
- ECHA, Guidance on waste and recovered substances, 2010, ECHA-10-G-07-EN Search PubMed.
- UBA, REACH and the recycling of plastics, Reference manual for an appropriate implementation of the REACH requirements for the operators of recycling plants, UBA-FB, 2012, p. 001522 Search PubMed.
- Z. Wang and S. Hellweg, First Steps Toward Sustainable Circular Uses of Chemicals: Advancing the Assessment and Management Paradigm, ACS Sustainable Chem. Eng., 2021, 9(20), 6939–6951 CrossRef CAS.
- V. G. Zuin and K. Kümmerer, Chemistry and materials science for a sustainable circular polymeric economy, Nat. Rev. Mater., 2022, 7(2), 76–78 CrossRef.
- T. Santos, et al., in The need for speed: why it takes the EU a decade to control harmful chemicals and how to secure more rapid protections, ed. J. Hunter, A. Anca and D. Godinho, EEB, Brussels, Belgium, 2022 Search PubMed.
- K. Fenner and M. Scheringer, The Need for Chemical Simplification As a Logical Consequence of Ever-Increasing Chemical Pollution, Environ. Sci. Technol., 2021, 55(21), 14470–14472 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2em00386d |
| This journal is © The Royal Society of Chemistry 2023 |