Open Access Article
Open Access ArticleEffect of metal complexation on the radiolytic stability of DOTA†
Ilyes
Mahti
 ,
Dominique
Guillaumont
,
Dominique
Guillaumont
 *,
Claude
Berthon
*,
Claude
Berthon
 ,
Georges
Saint-Louis
,
Xavier
Hérès
and
Laurence
Berthon
,
Georges
Saint-Louis
,
Xavier
Hérès
and
Laurence
Berthon
 *
*
CEA, DES, ISEC, DMRC, Univ Montpellier, Marcoule, France. E-mail: laurence.berthon@cea.fr; dominique.guillaumont@cea.fr
First published on 30th June 2023
Abstract
Radiometals are increasingly used in nuclear medicine for both diagnostic and therapeutic purposes. The DOTA ligand (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) is widely used as a chelating agent for various radionuclides, including 89Zr, with high thermodynamic stability constants and great in vivo stability. However, in contact with radioisotopes, chelating molecules are subjected to the effects of radiation, which can lead to structural degradation and induce alteration of their complexing properties. For the first time, the radiolytic stability of the Zr–DOTA complex in aqueous solution was studied and compared to the stability of the DOTA ligand. The identification of the major degradation products allows us to propose two different degradation schemes for the DOTA ligand and Zr–DOTA complex. DOTA is degraded preferentially by decarboxylation and cleavage of an acetate arm CH2–COOH, whereas in Zr–DOTA, DOTA tends to oxidize by the addition of the OH group in its structure. In addition, the degradation of the ligand, when involved in a Zr complex, is significantly less than when the ligand is free in solution, indicating that the metal protects the ligand from degradation. DFT calculations were performed to supplement the experimental data and give an improved understanding of the behaviour of DOTA and Zr–DOTA solutions after irradiation: the increase in stability upon complexation is attributed to the strengthening of the bonds in the presence of metal cations, which become less vulnerable to radical attack. Bond dissociation energies and Fukui indices are shown to be useful descriptors to estimate the most vulnerable sites of the ligand and to predict the protective effect of the complexation.
Introduction
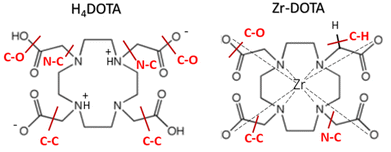
Radiometals (or radionuclides) play an important role in nuclear medicine through imaging techniques to visualize the distribution of radionuclides in the body or through therapy by specific irradiation of malignant cells. Whatever the application, a pharmaceutical agent (drug) is needed to act as a carrier molecule to deliver radionuclides to the target. Metal-based radiopharmaceuticals represent a dynamic and rapidly expanding field of research. SPECT (Single Photon Emission Computed Tomography) and PET (Positron Emission Tomography) have become increasingly popular cancer imaging techniques. The development of clinical PET imaging has significantly increased the efficiency of cancer diagnosis.1 For such PET applications, the ideal radionuclide emits β+ particles and must have a short half-life with rapid accumulation and clearance in tissue. These radiopharmaceuticals provide imaging within 24 hours of administration. In this context, 89Zr is currently being studied for PET2–12 applications and has received considerable attention in radioimmunotherapy applications because of its favourable decay characteristics and half-life period (t1/2 = 78.4 h) making it useful for labelling monoclonal antibodies.13 To deliver 89Zr to a given target, a powerful chelator must be bound to the tetravalent metal ion to prevent the release of the radionuclide into the human body.14–16 The coordination chemistry of zirconium(IV) suggests the use of a polydentate ligand with a denticity of 6–8 oxygen and nitrogen donor atoms. Several families of ligands based on hydroxamate, hydroxypyridone, catecholamide, hydroxyisophthalimide or carboxylate donor groups have been investigated.6,17 The desferrioxamine ligand (DFO) based on the hydroxamate donor group is the reference compound and is widely used in PET imaging.18,19 However, with this ligand, a slight release of 89Zr activity has been observed into the bones of mice.4,20 This instability may be related to the unsaturated coordination sphere of Zr when complexed with DFO. Summers et al. reported that the coordination sphere of Zr complexed to DFO is likely completed by two hydroxide ligands, Zr(DFO)(OH)2.19Polyaminocarboxylic ligands, known for their high affinity for metal ions and strong binding ability to a wide range of metal ions,21,22 represent a class of ligands that has been at the forefront of radiopharmaceutical development for nearly half a century.18,23–26 Pandya et al. used tetraazamacrocycles such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) (Fig. 1) as a Zr chelator.27 It was found that Zr–DOTA exhibited improved in vitro stability and in vivo behaviour compared to Zr–DFO. The molecular structure of Zr–DOTA was elucidated by single-crystal X-ray diffraction analysis and it was shown that Zr has an octodentate coordination with all four macrocyclic nitrogen atoms and acetate arms involved in the coordination.27
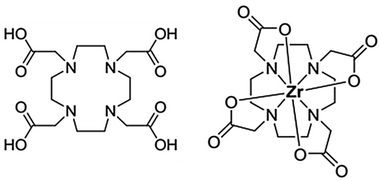 | ||
| Fig. 1 Schematic representation of the DOTA ligand (left) and Zr–DOTA complex (right). For simplicity's sake, DOTA is portrayed as neutral form regardless of the proton position. | ||
89Zr decays to metastable 89mY by a combination of β+ emission (23%) at low average energy (Eβ+ ≈ 396 keV) and electron capture (77%). 89mY undergoes an internal transition, which is accompanied by a high-energy (909 keV) gamma emission (99%) to give stable 89Y.
In contact with radiometals, the chelator can be subjected to radiolysis attacks, which affect its chemical structure and lead to radiolytic decomposition. This phenomenon is of major concern as it can lead to the release of radiometals into the body.
Some works have reported the impact of irradiation on polyaminocarboxylic acid molecules such as EDTA, HEDTA or DTPA.28–31 In the presence of ionizing radiation, these ligands in water are susceptible to indirect radiolysis and can be attacked by reactive species such as H˙ and HO˙ radicals.32–35 Very few studies are available on DOTA radiolysis.36,37 The alpha radiolysis of DOTA was investigated in aqueous solution under helium ion irradiation. This led to the formation of molecular hydrogen (H2) and carbon dioxide (CO2). It was observed that DOTA is degraded preferentially by decarboxylation (cleavage of CO2 moieties), loss of acetate arms (CH2COOH), and condensation of two carboxylic moieties with the elimination of a carbonic acid or glycolic acid group. In a recent study, Avraham et al. investigated the chemical reactions and changes that DOTA can undergo in response to radicals formed by the ionizing radiation induced by the radiometal.37 The reaction of DOTA with HO˙, H3C˙ and H3CO2˙ radicals was investigated. Hydroxyl radicals were shown to react significantly faster than methyl radicals.37 The authors have also studied the reactivity of lanthanide–DOTA complexes with methyl and hydroxyl radicals. It was shown that the methyl radical H3C˙ reacts more slowly with CeIII–DOTA− and DyIII–DOTA− complexes than with the free DOTA ligand. Such influence of metal ions on ligand radiolytic degradation, favouring or inhibiting certain reaction pathways, has already been observed in research work on solvent extraction for nuclear fuel reprocessing.38–40 For instance, the effect of metal complexation on the reaction kinetics of the dodecane radical cation toward nuclear fuel extraction ligands was reported. Uranyl complexation increases the reaction rate with some amide type extracting ligands, while it has a negligible effect with tri-butyl phosphate ligand.38 The complexation of americium and europium increases the reaction rate with the hexa-n-octylnitrilo-triacetamide ligand (HONTA).39 Kimberlin et al. characterized the effect of lanthanide complexation on the radiolysis of diglycolamide, finding that metal ions protect diglycolamide from degradation.40 Other studies have investigated the effect of metal complexation in aqueous solution.30,41–49 For example, in the case of DTPA solution, it was observed that the radiolytic yield of degradation was higher for uncomplexed DTPA than for the Sm–DTPA complex.50 These results underline the importance of considering the role of metals in radiolysis even though the effect of metal complexation on ligand radiolysis remains poorly understood. Recent work has shown that quantum chemistry tools can be very useful to complement experimental data in the study of radiolytic processes. In particular, Fukui functions and bond dissociation energies (BDEs) have been used to explain differences in the behaviour of ligands under irradiation.40,51–55 Fukui indices evaluate the chemical reactivity of a particular atom towards electrophilic, nucleophilic or radical attack, while BDEs assess bond strength. These tools allow the identification of the weak points of the molecules and thus the identification of the atoms susceptible to radiolysis attacks.
The goal of this work is to study the radiolytic stability of the DOTA ligand and Zr–DOTA complex in water to investigate the influence of complexation on the radiolytic stability of DOTA. Gamma irradiations were performed as the high-energy gamma emission of the 89mY decay is the major concern of 89Zr decay chain. The degradation products of DOTA and the Zr–DOTA complex have been identified and degradation schemes are proposed. Finally, bond stabilities and Fukui functions have been determined from DFT calculations in order to evaluate the most likely sites for radical attack on the ligand and the effect of complexation.
Experimental section
Chemicals
Solid DOTA (H4DOTA) was purchased from Chematech (Dijon, France) (purity > 98%, confirmed by HPLC). Solid zirconium(IV) acetylacetonate was obtained from Sigma-Aldrich (purity 97%). Anhydride methanol from Sigma-Aldrich (purity 99.8%) was utilized. All commercial products were used as received without further purification. The pH of the solutions was measured with a pH electrode (Metrohm) calibrated against standard buffers.Synthesis and characterization of Zr–DOTA
The Zr–DOTA complex was synthesized at room temperature and with a metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand ratio of 1
ligand ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 203 mg of solid DOTA (H4DOTA) was dissolved in methanol. DOTA did not dissolve completely after stirring. Zr(AcAc)4 was then slowly added to the solution. The resulting mixture was centrifuged and the supernatant was isolated in a separate microtube. After a few days, a precipitate appeared, which was separated and dried. Finally, a white powder corresponding to Zr–DOTA was obtained which was dissolved in pure water for characterization by mass spectrometry and NMR spectroscopy. The spectra are presented in the ESI.†
1. 203 mg of solid DOTA (H4DOTA) was dissolved in methanol. DOTA did not dissolve completely after stirring. Zr(AcAc)4 was then slowly added to the solution. The resulting mixture was centrifuged and the supernatant was isolated in a separate microtube. After a few days, a precipitate appeared, which was separated and dried. Finally, a white powder corresponding to Zr–DOTA was obtained which was dissolved in pure water for characterization by mass spectrometry and NMR spectroscopy. The spectra are presented in the ESI.†
Irradiation experiments
Aqueous solutions of DOTA and Zr–DOTA (5 × 10−3 mol L−1) were irradiated by gamma radiation using a GRS-D1 Gamma-Service-Medical-GmbH irradiation system at CEA Marcoule. It uses a calibrated 137Cs source with a dose rate of 0.8–0.9 kGy h−1. Dosimetry was performed by the Fricke method.56The concentration of 89Zr used for in vivo studies is approximately 1–2 × 10−7 M, which corresponds to a maximum dose rate of 0.3 kGy h−1 (without taking into account zirconium decay).27,57 Considering the position of the samples in the gamma irradiator, the dose rate delivered to the samples is three times higher. Under these conditions, degradation products are formed more rapidly than under usual in vivo conditions. However, to compare the effect of complexation on DOTA stability, all samples were irradiated under identical experimental conditions (DOTA concentration, irradiation dose and dose rate).
2 mL of each sample was prepared and irradiated for 12–24–36–120 hours corresponding to a dose of approximately 10–20–30–100 kGy. The doses, corresponding irradiation times and pH of the irradiated samples are given in Table 1.
| Sample | Dose rate (kGy h−1) | Irradiation time (h) | Dose (kGy) | pH |
|---|---|---|---|---|
| DOTA 0 kGy | 0 | 0 | 0 | 3.51 |
| DOTA 10 kGy | 0.87 | 9 h 25 | 8.2 | 3.82 |
| DOTA 20 kGy | 0.86 | 23 h | 20 | 4.22 |
| DOTA 30 kGy | 0.87 | 38 h 25 | 34 | 4.51 |
| DOTA 100 kGy | 0.87 | 120 h | 104 | — |
| Zr–DOTA 0 kGy | 0 | 0 | 0 | 5.7 |
| Zr–DOTA 10 kGy | 0.80 | 10 h 25 | 8.3 | 3.44 |
| Zr–DOTA 20 kGy | 0.80 | 25 h | 20 | 3.37 |
| Zr–DOTA 30 kGy | 0.80 | 38 h 25 | 31 | 3.39 |
| Zr–DOTA 100 kGy | 0.87 | 120 h | 104 | — |
Mass spectrometry
The samples were diluted in a concentration range of 10−4–10−3 mol L−1 in pure H2O or acidified water (H2O + 0.02%Vol HNO3) and analyzed with a micrOTOF-Q II (Bruker Daltonik GmbH, Bremen, Germany) electro-spray ionization (ESI) quadrupole time-of-flight (TOF) mass spectrometer calibrated daily using an Agilent (G1969-85000) ESI Low Concentration Tuning Solution. The addition of acid favours ionization of compounds and avoids the formation of sodium adducts. No other effects of the acid were observed. The samples were injected at a flow rate of 300 μL h−1 by using a syringe pump. The experimental conditions were as follows: positive ion mode, ion spray voltage of −4500 V, IsCID 0 eV, N2 as the drying and nebulizing gas, 4 L min−1, 0.3 bar, and 200 °C. A low mass tuning method was used, which allowed the analysis of DOTA and Zr–DOTA fragments. The Compass Data Analysis software (Bruker Daltonik) was used for data processing. Species were identified by comparing an experimental isotopic pattern with a simulated one using the DataAnalysis 4.2 software. MS/MS analysis of DOTA and major degradation products is given in the ESI.†Nuclear magnetic resonance
Analyses were performed at 25 °C with an Agilent DD2 400 MHz spectrometer equipped with a 5 mm OneNMR probe. To avoid dilution, an external tube containing the deuterated reference solvent (CDCl3 or acetoneD6) surrounded the tube containing the sample in pure H2O. All spectra were normalized with the reference solvent used as the external standard.The 1H NMR water signal was presaturated to enhance the signal intensity of our compounds. The OpenVnmrJ 4.2 software was used for data acquisition and the MestReNova 14.2.1 software for data processing.
Computational methods
The calculations were performed using density functional theory (DFT) with Gaussian 16.58 Optimized geometries were confirmed to be true minima by frequency calculations (no imaginary frequencies were found). The zero-point energies (ZPE) with the corresponding thermal correction at 298.15 K were computed by frequency calculations and added to the electronic energies. The 6-31G+(d,p) basis set was employed for H, C, N and O atoms. For zirconium, core electrons were represented by the MWB28 Stuttgart-Cologne quasi-relativistic effective core potential (ECP) with the associated basis set for valence electrons.59 The bond dissociation energy (BDE) is defined as the reaction enthalpy of homolytic bond dissociation according to the following reaction:| R–X → R˙ + X˙ | (1) |
The BDE of an R–X bond is calculated by using the difference in the enthalpies of each species involved in the homolytic reaction.
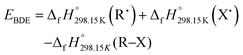 | (2) |
The hybrid density functional B3P86 (Becke's 3-parameter exchange functional with Perdew non-local correlation energy)60,61 was chosen to compute BDEs. Previous studies have shown good agreement with the experimental values for compounds similar to the acetate arm of DOTA, i.e. containing C–N, C–O or C–C bonds.62–64 It has been shown that B3P86 has an absolute mean error of 10.8 kJ mol−1, while B3LYP has an absolute mean error of 27 kJ mol−1 for such bonds. All BDE calculations were performed in the gas phase.
The Fukui function as defined by Parr and Yang65 describes the electron density at a given position r after adding or removing electrons. It can predict the sites of a molecule which are most likely to undergo a nucleophilic, electrophilic or radical attack. In this work, the Fukui function R for radical attack has been determined. It corresponds to:
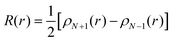 | (3) |
The Fukui indices have also been generated to obtain a quantitative description of the local reactivity.53,55 The radical Fukui index f0,α for an atom α is defined as follows:
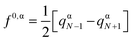 | (4) |
Finally, the condensed dual descriptor (CDD) proposed by Morell et al.66,67 was determined. It provides useful information on both stabilizing and destabilizing nucleophile/electrophile interactions and helps to identify the behaviour of a specific atom within a molecule. It corresponds to a linear combination of Fukui indices:
| CDDα = 2qαN − qαN+1 − qαN−1 | (5) |
The absolute value of CDD indicates the probability of the reaction with the site. The sign of CDD corresponds to the type of attack, and negative and positive values correspond, respectively, to nucleophilic and electrophilic attacks. For Fukui calculations, the geometries of DOTA and Zr–DOTA were optimized for the neutral reference using the B3LYP functional.68,69 The water was represented with the self-consistent reaction field (SCRF) method using the polarizable continuum model (IEFPCM).70 For Fukui indices and the condensed dual descriptor, the partial charges were derived from a natural population analysis (NBO).71
Results and discussion
Radiolytic stability of the free and complexed ligands
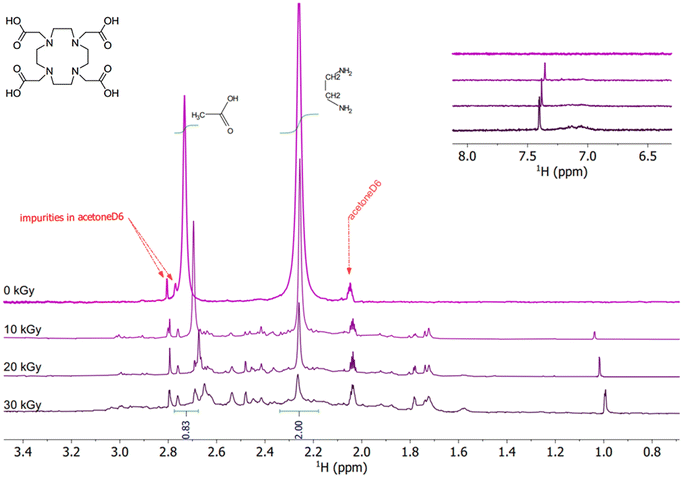
1H NMR analysis of 5 mM DOTA and Zr–DOTA samples in pure water was performed before and after radiolysis up to 100 kGy. 1H NMR spectra for the irradiated solutions up to 30 kGy are presented in Fig. 2 and 3. Their spectra were normalized using an external locking solvent so that the spectra can be compared with each other. An undeuterated residual signal from acetoneD6 for DOTA and a tetramethylsilane (TMS) signal in CDCl3 for Zr–DOTA were observed. The spectra of the solution irradiated at 100 kGy were not presented because they were hardly usable. At 100 kGy, the NMR signals of Zr–DOTA and DOTA are strongly reduced and in the case of Zr–DOTA, a white powder was observed in the bottom of the Pyrex flask, indicating that some zirconium has been hydrolysed.For the DOTA sample (pH of the solution before irradiation = 3.51), the characteristic signals of DOTA are observed at 2.26 ppm corresponding to the cyclen protons and at 2.74 ppm corresponding to the acetate protons (Fig. 2). From 10 kGy onwards, the signals of the acetate protons are shifted, indicating that the arms of DOTA are degraded, and some signals appear between 1 and 3 ppm. Another signal is also observed at 7.38 ppm. These signals can be attributed to the formation of degradation products. A decrease in signal intensity is observed upon irradiation, which was also reported by Fiegel et al.36 They observed that the higher the concentration of DOTA in solution, the higher the hydrogen yields and attributed the decrease in NMR signal intensity to the degradation of DOTA through hydrogen abstraction leading to the formation of molecular hydrogen H2.36 At 20 and 30 kGy, DOTA keeps degrading. This results in a decrease in intensity and an increasing shift of the NMR signals. On the other hand, the signal intensity of degradation products increases slightly. At 30 kGy, the increasing number of signals is causing a broadening of the baseline from 1.5 to 3.1 ppm.
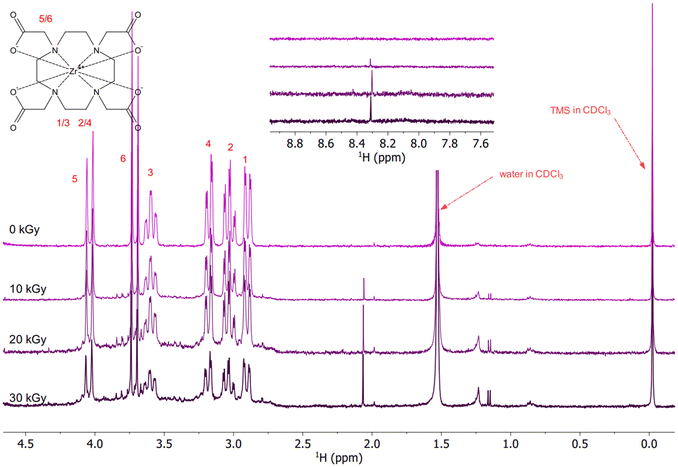
The 1H NMR spectra of the irradiated solutions of Zr–DOTA (pH of the solution before irradiation = 5.7) are shown in Fig. 3. The pH was not equalized between the ligand and the complex solutions to avoid the effects of acidic or basic species on the radiolysis of the samples. Moreover, previous studies have shown that within the pH range from 3 to 11, in the case of gamma irradiation, the radiolytic yields of the main radiolysis products of water (e−aq, HO˙, H˙, H2, H2O2 and HO2˙) barely change.32 The characteristic signals of the complex are visible and are assigned according to the literature.72 More details are provided in the ESI.† Zr–DOTA shows a different behaviour from that of the free ligand towards irradiation. As shown in Fig. 3, the signals of the complex are not shifted and their intensity hardly decreases. This indicates that the complex is more resistant to irradiation than the free ligand. Nonetheless, as for the free ligand, new signals appear upon irradiation but they are much less numerous.
The NMR signals of the ligand and complex were integrated between 0 and 30 kGy (see the ESI†). The average intensity of the signals decreases by 94% for DOTA and 42% for Zr–DOTA at 30 kGy. Considering the exponential decrease in the DOTA concentration with absorbed dose, which suggests pseudo-first-order kinetics, the dose constants d and G0 values can be calculated using eqn (6) and (7) from Mincher et al.73,74C0 and C are the initial and final concentrations (the C/C0 ratio was calculated using the NMR peak areas), ρ is the density of the solution and D is the absorbed dose. The results are given in Table 2. Notably, the values obtained for −G0 (radiolytic yields of disappearance) are much lower when the ligand is complexed with zirconium.
| C = C0edD | (6) |
| G0 = dC0/ρ | (7) |
| Sample | d (kGy−1) | −G0 (μmol J−1) |
|---|---|---|
| DOTA | −0.125 ± 0.003 | 0.625 ± 0.015 |
| Zr–DOTA | −0.0193 ± 0.003 | 0.096 ± 0.015 |
In summary, the degradation of the free ligand is much more pronounced than when complexed with Zr, considering the large decrease in intensity, −G0 and the shift of the ligand signals for the DOTA solutions. Most importantly, the lower occurrence of new signals after irradiation of the complex indicates that fewer degradation products are generated with Zr. The metal seems to protect the ligand from degradation.
The solutions were then analysed by ESI-MS to identify the degradation products. Fig. 4 shows the ESI-MS spectra of the DOTA aqueous solutions irradiated up to 30 kGy. At 0 kGy, one species is observed at m/z = 405 corresponding to the protonated DOTA ligand. From 10 to 30 kGy, peaks corresponding to the degradation products of DOTA are observed at m/z = 347, 317 and 361. The identifications were proposed based on the work of Fiegel et al.36 The major ion at m/z = 347 corresponds to DOTA with the loss of an acetate arm –CH2COOH. This explains the shift of the acetate arm signals observed in the NMR spectra. The two other ions are assigned to the loss of carbon dioxide groups, one molecule of CO2 at m/z = 361 and two molecules of CO2 at m/z = 317. The abundance of ions gradually decreases with the absorbed dose, causing ligand degradation as observed by NMR. An increase of the pH is also observed (Table 1), from 3.51 to 4.51, certainly due to the release of CO2.
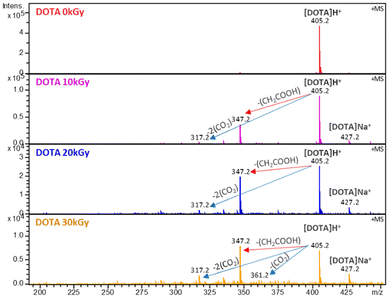 | ||
| Fig. 4 ESI-MS spectra of degraded solutions of 5 mM DOTA diluted 10 times in acidified water (H2O + 0.02%/Vol HNO3). Positive ionization mode. | ||
From the species identified by ESI-MS, a simplified degradation pathway of DOTA is proposed with the major degradation products (Fig. 5). This is in good agreement with the previous one proposed by Fiegel et al. However, fewer degradation products are detected, as the initial concentration is lower in the present study (5 mM vs. 100 mM in the study of Fiegel et al.).
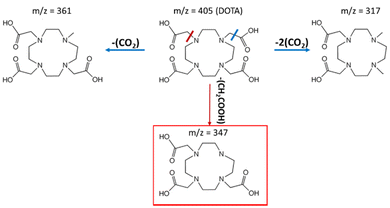 | ||
| Fig. 5 Simplified degradation pathway for DOTA radiolysis in pure water. The compound framed in red is the major degradation product observed by ESI-MS. | ||
The Zr–DOTA solutions were also analysed by ESI-MS (Fig. 6). A previous investigation has shown that ESI-MS is a valuable tool to identify metal–DOTA complexes in solution.75 At 0 kGy, a species is observed at m/z = 491 and corresponds to the protonated complex. In contrast to the free ligand, the abundance of degradation products is much lower. However, a degradation product is observed at a lower mass (m/z = 433). This compound results from the loss of a CH2COOH fragment, as observed for the free ligand. Nevertheless, its signal intensity is weak, suggesting that it is present in small amounts. This decarboxylation has been previously observed with the aged solution of Ce–DOTA complexes.76 It was attributed to the intramolecular and intermolecular redox processes. A heavier degradation product, not observed with free DOTA, is found at m/z = 507. The mass gain is 16 and may correspond to an addition of an oxygen atom to the complex. This can be attributed to C–H bond breaking, due to the reaction with the H˙ radical from the solution, leading to the release of H2 according to eqn (8). This could explain the small decrease in intensity of the NMR signals observed for the complex (Fig. 3). The dehydrogenated radical intermediate then reacts with a free HO˙ radical as described in reaction (9):
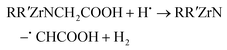 | (8) |
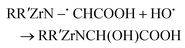 | (9) |
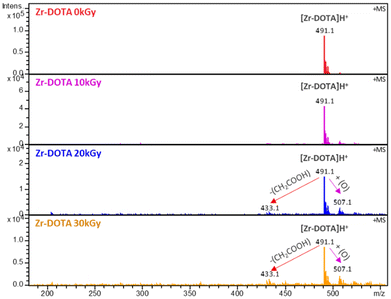 | ||
| Fig. 6 ESI-MS spectra of degraded solutions of 5 mM Zr–DOTA diluted 10 times in acidified water (H2O + 0.02%/Vol HNO3). Positive ionization mode. | ||
In contrast, the gamma radiolysis of diglycolamide ligands in aqueous nitrate solution has been shown to be driven by the hydroxyl ion which reacts rapidly with the ligands.77
Therefore, unlike the ligand, the Zr–DOTA complex tends to oxidize under irradiation. Oxidation reactions have already been observed under certain conditions with amide extractants in organic diluents.51,78,79 In summary, Zr–DOTA degrades differently from the free ligand. The results obtained by ESI-MS are in agreement with those obtained by 1H NMR: fewer degradation products are observed for the complex. An instant decrease of the pH is observed (Table 1) for the complex from 5.7 to 3.4. This is probably due to the release of acetic acid and zirconium hydrolysis.
Fig. 7 shows a simplified degradation pathway for Zr–DOTA with the main degradation products observed by ESI-MS. MS/MS experiments were conducted on the degradation product at m/z = 507 to obtain further structural information. However, due to the very low abundance of the ion, it was not possible to observe enough fragments to identify precisely the structure. Thus, two degradation products are proposed, one by hydroxylation in the acetate arms and the other in the cyclen.
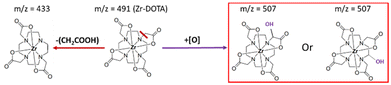 | ||
| Fig. 7 Simplified degradation pathway for Zr–DOTA radiolysis in water. Compounds framed in red are the major degradation products observed by ESI-MS. | ||
To summarize the results, the degradation products observed for DOTA and Zr–DOTA are shown in Table 3.
| m/z | Chemical formula | Species |
|---|---|---|
| DOTA | ||
| 405.2 | [C16H28O8N4]H+ | DOTA |
| 347.2 | [C14H26O6N4]H+ | Loss of CH2COOH |
| 317.2 | [C14H28O4N4]H+ | Loss of 2 CO2 |
| 361.2 | [C15H28O6N4]H+ | Loss of CO2 |
| Zr–DOTA complex | ||
| 491.2 | [ZrC16H24O8N4]H+ | Zr–DOTA |
| 507.1 | [ZrC16H24O9N4]H+ | Addition of O |
| 433.1 | [ZrC14H26O6N4]H+ | Loss of CH2COOH |
Quantum chemistry calculations
In order to evaluate the strength of the bonds in DOTA, free or engaged with Zr, bond dissociation energies (BDEs) were evaluated from DFT calculations. The speciation of DOTA in water depends on the pH of the solution. From the previously determined protonation constants and identified protonation sites,80–82 it is considered that the predominant species in aqueous solution at pH 3–4 has four protons attached to two trans nitrogen atoms of the ring and to two carboxylate groups as shown in Fig. 8. Calculations were performed for this species, which will be referred to as H4DOTA hereafter.Our experimental results and previous work36 show that cyclen bonds of DOTA are not subjected to radiolysis, while the acetate arms can be broken. Therefore, BDEs were calculated only for the bonds belonging to the acetate arms. The BDEs corresponding to the N–C, C–C, C–O and C–H bonds depicted in Fig. 8 were computed for H4DOTA and Zr–DOTA. The results are summarized in Table 4. The C–H BDE values are not discussed at first, as they are involved in degradation mechanisms as proved by previous authors for polyaminocarboxylic acids.28,36
| R–X bond | H4DOTA | Zr–DOTA | |
|---|---|---|---|
| Uncharged arm | Charged arm | ||
| C–C | 300 | 762 | 741 |
| N–C | 257 | 669 | 720 |
| C–O | 500 | 1078 | 1068 |
| C–H | 266 | 426 | 314 |
According to the calculated values for H4DOTA, the lower value and therefore the weakest bond for any arms of H4DOTA, with protonated and unprotonated nitrogen, corresponds to the N–C bond. Breaking this bond induces the loss of an acetate arm, CH2COOH. This is consistent with the experimental data that give DOTA with a loss of CH2COOH as the major degradation product. When comparing H4DOTA and Zr–DOTA, the weakest bonds are found in H4DOTA with BDE values ranging from 300 to 500 kJ mol−1 for the C–C, C–N and C–O bonds of uncharged arms. In Zr–DOTA, these values are significantly higher and vary from 720 to 1068 kJ mol−1. This difference indicates that the bond stabilities of the acetate arms are greatly enhanced when the ligand is complexed with zirconium. This increase in stability upon complexation is also consistent with what has been observed experimentally. It should be mentioned that the BDE values of the charged arms of H4DOTA are similar to those computed for Zr–DOTA (from 669 to 1078 kJ mol−1). This shows that the BDEs, and hence the bond stability, are strongly influenced by the presence of a cation (either Zr4+ or H+) attached to the ligand.
The calculated bond distances given in Table 5 indicate that there is no correlation between bond distances and BDE variations. For instance, the N–C bond distance is shorter in H4DOTA for the uncharged arm than that in Zr–DOTA: 1.473 Å vs. 1.485 Å, while the BDE is lower (257 and 720 kJ mol−1 respectively).
| R–X bond | H4DOTA | Zr–DOTA | |
|---|---|---|---|
| Uncharged arm | Charged arm | ||
| C–C | 1.536 | 1.554 | 1.528 |
| N–C | 1.473 | 1.502 | 1.485 |
| C–O | 1.306 | 1.278 | 1.309 |
| C–H | 1.095 | 1.092 | 1.093 |
The BDE value depends on the relative stability between the R–X molecule and the formed radicals R˙ and X˙ (eqn (2)). In the case of the HN+–C bond of H4DOTA, the cleavage of the bond leads to RR′HN˙+ and CH2COO˙− charged radicals. This bond is calculated to be more stable than the neutral N–C bond (669 vs. 257 kJ mol−1). Previous studies have shown similar results for N–H bonds.83–85 For example, Kaur et al. have found a BDE for protonated NH3 of 514 kJ mol−1 whereas for the neutral form they found a BDE of 433 kJ mol−1. According to our calculations, the protonation of RR′N˙ stabilizes the radical product by 1032 kJ mol−1 whereas the deprotonation of CH2COOH˙ destabilizes the radical product by 1444 kJ mol−1. This results in a decrease of the N–C BDE between the charged and the uncharged arm of 412 kJ mol−1. These results indicate that the destabilization of CH2COO˙− is responsible for the bond stabilization.
Finally, the lower BDE is found in Zr–DOTA for the C–H bonds in the acetate arms. This result corroborates the experimental degradation pathway observed for Zr–DOTA, i.e. oxidation to form Zr–DOTA[O] by replacing H˙ for HO˙ due to the rupture of a C–H bond. This phenomenon is not observed for H4DOTA because the cleavage of the C–H bond is immediately followed by the rupture of C–C bonds or N–C bonds through multiple reactions and rearrangement.28,36
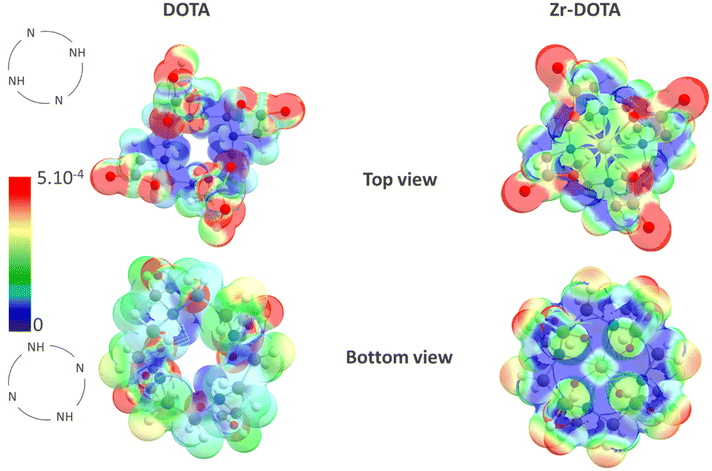
The Fukui function, Fukui indices and absolute values of the condensed dual descriptor were determined for H4DOTA and Zr–DOTA. The Fukui function describes the electron density after the addition or removal of the electron. It can predict the sites of a molecule that are most likely to undergo nucleophilic, electrophilic or radical attack. The Fukui indices determined for individual atoms give access to local reactivity (see the Computational methods section). Fig. 9 shows the values of the radical Fukui functions mapped onto the electron density isosurface for H4DOTA and Zr–DOTA. The higher the value, the more vulnerable the site is to radical attack (red dots).
For the ligand (H4DOTA) and the complex (Zr–DOTA), the higher values of the radical Fukui function are located on the carboxylate groups around the oxygen atoms. As expected, the smaller values are found on the carbon cyclen (blue in Fig. 9). Furthermore, for H4DOTA, the values of the radical Fukui function are higher for the unprotonated nitrogen atom (red dots) than for the protonated nitrogen atoms. For Zr–DOTA, no red dots are observed on nitrogen atoms. Other Fukui function values are also smaller on Zr–DOTA than those on DOTA, as shown by the predominant blue parts and fewer red dots in Fig. 9 (bottom view).
Afterwards, we calculated the radical Fukui indices (ƒ0) and absolute values of the condensed dual descriptor (|CDD|) for each atom to quantify the most reactive sites of the ligand and the complex. The ƒ0 and CDD values of the carbon atoms of H4DOTA and Zr–DOTA are shown in Table 6. For clarity, only carbon and nitrogen Fukui indices are given. The complete list of the calculated values is given in the ESI.†
| Atoms | C(COOH) | C(COO-) | C(CH2, N arm) | C(CH2, RN arm)a | C(CH2, cyclen)b | N | N(RN)a | |
|---|---|---|---|---|---|---|---|---|
| a R = H+ or Zr4+. b Average values of f0 and |CDD| for CH2 of the cyclen. | ||||||||
| H4DOTA | ƒ 0 | 0.079 | 0.003 | 0.012 | 0.002 | −0.004 | 0.049 | 0.003 |
| |CDD| | 0.16 | 0.006 | 0.042 | 0.005 | 0.013 | 0.085 | 0.001 | |
| Zr–DOTA | ƒ 0 | — | 0.011 | — | 0.002 | −0.005 | — | 0.001 |
| |CDD| | — | 0.017 | — | 0.01 | 0.004 | — | 0.023 | |
For Zr–DOTA, the lowest ƒ0 value is found for the cyclen carbon atoms (C(CH2, cyclen)) with a slightly negative average value of −0.004 indicating that they are not sensitive to radical attack. On the other hand, the highest values are obtained for carboxylate carbon atoms C(COO-), which can be assimilated to the release of CO2 due to the cleavage of the C–C bond. The values of the nitrogen and C(CH2, RN arm) atoms are positive and suggest that the N–C bond is also prone to radical attack. The CDD values give the same indications, with high values for acetate carbon and nitrogen atoms and low values for cyclen carbon atoms. This also suggests that the oxidation of the complex, observed experimentally, occurs preferentially on the carboxylate arm rather than on the cyclen.
In the case of H4DOTA, the highest values of ƒ0 are obtained for C(COOH), N and C(CH2, N arm) (0.079, 0.049 and 0.012e), similarly to Zr–DOTA, and correspond to the atoms belonging to the acetate arms. Interestingly, these ƒ0 values correspond to the acetate arms with unprotonated nitrogen. When the nitrogen is protonated, the values are much lower for the same atoms. The same trend is observed for the CDD values. As for Zr–DOTA, the smallest ƒ0 values are determined for the cyclen carbon atoms and they are the only negative ones (−0.004e).
Finally, if we compare the free ligand and Zr–DOTA, we find that the values obtained for the complex are much lower than those for the free ligand. The highest value for Zr–DOTA is 0.011e, while it reaches 0.079e for the free ligand.
According to the Fukui indices, Zr–DOTA is more resistant towards radical attack than free DOTA and they have both weaknesses in acetate arms and not in cyclen. Moreover, the Fukui indices ƒ0 and CDD values of the nitrogen atoms are lowered by the presence of H+ or Zr4+. This is all consistent with our BDE calculations and ESI-MS results showing that degradation is preferentially located on the DOTA arms for both molecules (the free ligand and ligand involved in the complex). In addition, this confirms that the binding of nitrogen to a cation (H+ or Zr4+) protects it from radical attack. Using Fukui descriptors, we can also conclude that Zr–DOTA is less prone to degradation than free DOTA.
Conclusion
In this work, we have investigated the radiolytic stability under gamma rays of the DOTA ligand and the Zr–DOTA complex in pure water. DFT calculations supplemented the experimental data and gave an improved understanding of the behaviour of the DOTA and Zr–DOTA solutions after irradiation.The major degradation products have been identified and the degradation products of the free ligand appear to differ from those of the complex. The free ligand is degraded preferentially by decarboxylation and cleavage of an acetate arm CH2–COOH, whereas the complex tends to oxidize to form Zr–DOTA[O]. Moreover, the degradation of the DOTA ligand, when complexed with Zr, is significantly less than when the ligand is free in solution, indicating that the metal protects the ligand from degradation. DFT calculations suggest that this increase in stability is due to the strengthening of the bonds in the presence of metal cations, which become less vulnerable to radical attack. Such a protective effect of the complexation can be expected for any cation if all the acetate arms of the ligands are involved in the cation complexation.
To evaluate the protective effect due to the complexation, further studies should be performed to quantify the DOTA concentration after irradiation. In this work, we investigated the effect of gamma radiation on the DOTA stability. To be more representative of the radiopharmaceutical solutions of 89Zr, this study could be extended to the investigation of the degradation of DOTA in the presence of 89Zr (being β+ and gamma emitters). Due to the difference in the linear energy transfer between beta and gamma rays, the same degradation products are likely to be observed with a slight difference in their distribution. Also, as DOTA is considered as a good chelator for many radiometals, including α emitters for targeted radiotherapy, it might be interesting to extend this study to the radiolytic stability of DOTA in the presence of α radionuclides such as 225Ac.86 Alpha emitters have a high linear energy transfer and deposit it within a short range. Moreover, for therapeutic applications, long-lived radionuclides are preferred (a half-life period up to 10 days). Consequently, in the presence of alpha emitters, the radiolytic stability of DOTA could be different.
The influence of a peptide carrier, bonded to DOTA, should also be studied (radiolytic and thermodynamic stabilities) as it is a subject of interest nowadays.87–89
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Bertrand Kuhnast and Charles Truillet from the Laboratoire d'Imagerie Biomédicale Multimodale Paris Saclay CEA for the fruitful discussions.References
- A. Kasbollah, P. Eu, S. Cowell and P. Deb, J. Nucl. Med. Technol., 2013, 41, 35–41 CrossRef PubMed.
- M. T. La, V. H. Tran and H.-K. Kim, Nucl. Med. Mol. Imaging, 2019, 53, 115–124 CrossRef CAS PubMed.
- E. C. Dijkers, T. H. O. Munnink, J. G. Kosterink, A. H. Brouwers, P. L. Jager, J. R. de Jong, G. A. van Dongen, C. P. Schroder, M. N. Lub-de Hooge and E. G. de Vries, Clin. Pharmacol. Ther., 2010, 87, 586–592 CrossRef CAS PubMed.
- J. P. Holland, V. Divilov, N. H. Bander, P. M. Smith-Jones, S. M. Larson and J. S. Lewis, J. Nucl. Med., 2010, 51, 1293–1300 CrossRef CAS PubMed.
- T. J. Wadas, E. H. Wong, G. R. Weisman and C. J. Anderson, Chem. Rev., 2010, 110, 2858–2902 CrossRef CAS PubMed.
- L. E. McInnes, S. E. Rudd and P. S. Donnelly, Coord. Chem. Rev., 2017, 352, 499–516 CrossRef CAS.
- H. A. Holik, F. M. Ibrahim, A. A. Elaine, B. D. Putra, A. Achmad and A. H. S. Kartamihardja, Molecules, 2022, 27, 3062 CrossRef CAS PubMed.
- M. A. Deri, B. M. Zeglis, L. C. Francesconi and J. S. Lewis, Nucl. Med. Biol., 2013, 40, 3–14 CrossRef CAS PubMed.
- R. Fu, L. Carroll, G. Yahioglu, E. O. Aboagye and P. W. Miller, ChemMedChem, 2018, 13, 2466–2478 CrossRef CAS PubMed.
- E. T. Sarcan, M. Silindir-Gunay, A. Y. Ozer and N. Hartman, J. Radioanal. Nucl. Chem., 2021, 330, 15–28 CrossRef CAS.
- G. W. Severin, J. W. Engle, R. J. Nickles and T. E. Barnhart, Med. Chem., 2011, 7, 389–394 CrossRef CAS PubMed.
- Y. Zhang, H. Hong and W. Cai, Curr. Radiopharm., 2011, 4, 131–139 CrossRef CAS PubMed.
- B. Altıparmak Güleç and F. Yurt, J. Radioanal. Nucl. Chem., 2021, 330, 1–13 CrossRef.
- E. W. Price and C. Orvig, Chem. Soc. Rev., 2014, 43, 260–290 RSC.
- N. Herrero Álvarez, D. Bauer, J. Hernández-Gil and J. S. Lewis, ChemMedChem, 2021, 16, 2909–2941 CrossRef PubMed.
- J. R. Dilworth and S. I. Pascu, Chem. Soc. Rev., 2018, 47, 2554–2571 RSC.
- E. Boros and A. B. Packard, Chem. Rev., 2019, 119, 870–901 CrossRef CAS PubMed.
- N. B. Bhatt, D. N. Pandya and T. J. Wadas, Molecules, 2018, 23, 638 CrossRef PubMed.
- K. L. Summers, E. K. Sarbisheh, A. Zimmerling, J. J. H. Cotelesage, I. J. Pickering, G. N. George and E. W. Price, Inorg. Chem., 2020, 59, 17443–17452 CrossRef CAS PubMed.
- T. K. Nayak, K. Garmestani, D. E. Milenic and M. W. Brechbiel, J. Nucl. Med., 2012, 53, 113–120 CrossRef CAS PubMed.
- M. Audras, L. Berthon, C. Berthon, D. Guillaumont, T. Dumas, M.-C. Illy, N. Martin, I. Zilbermann, Y. Moiseev, Y. Ben-Eliyahu, A. Bettelheim, S. Cammelli, C. Hennig and P. Moisy, Inorg. Chem., 2017, 56, 12248–12259 CrossRef CAS PubMed.
- C. Tamain, T. Dumas, C. Hennig and P. Guilbaud, Chem. – Eur. J., 2017, 23, 6864–6875 CrossRef CAS PubMed.
- Z. Baranyai, G. Tircsó and F. Rösch, Eur. J. Inorg. Chem., 2020, 2020, 36–56 CrossRef CAS.
- J. L. Domingo, Reprod. Toxicol., 1998, 12, 499–510 CrossRef CAS PubMed.
- S. Mathur, S. Flora, R. Mathur and S. Dasgupta, Hum. Exp. Toxicol., 1993, 12, 19–24 CrossRef CAS PubMed.
- D. S. Ma, F. Lu, T. Overstreet, D. E. Milenic and M. W. Brechbiel, Nucl. Med. Biol., 2002, 29, 91–105 CrossRef CAS PubMed.
- D. N. Pandya, N. Bhatt, H. Yuan, C. S. Day, B. M. Ehrmann, M. Wright, U. Bierbach and T. J. Wadas, Chem. Sci., 2017, 8, 2309–2314 RSC.
- S. N. Bhattacharyya and K. P. Kundu, Int. J. Radiat. Phys. Chem., 1972, 4, 31–41 CrossRef CAS.
- A. P. Toste, J. Radioanal. Nucl. Chem., 2004, 249, 283–288 CrossRef.
- N. E. Bibler, J. Inorg. Nucl. Chem., 1972, 34, 1417–1425 CrossRef CAS.
- C. A. Zarzana, G. S. Groenewold, B. J. Mincher, S. P. Mezyk, A. Wilden, H. Schmidt, G. Modolo, J. F. Wishart and A. R. Cook, Solvent Extr. Ion Exch., 2015, 33, 431–447 CrossRef CAS.
- S. Le Caër, Water, 2011, 3, 235–253 CrossRef.
- A. H. Samuel and J. L. Magee, J. Chem. Phys., 1953, 21, 1080–1087 CrossRef CAS.
- W. G. Burns and H. E. Sims, J. Chem. Soc., Faraday Trans. 1, 1981, 77, 2803–2813 RSC.
- B. Pastina and J. A. LaVerne, J. Phys. Chem. A, 1999, 103, 1592–1597 CrossRef CAS.
- V. Fiegel, C. Berthon, A. Costagliola, G. Blain, J. Vandenborre, J. Vermeulen, G. Saint-Louis, L. Guerin, T. Sauvage, M. Fattahi-Vanani, L. Venault and L. Berthon, Radiat. Phys. Chem., 2019, 165, 108409 CrossRef CAS.
- E. Avraham, D. Meyerstein, A. Lerner, G. Yardeni, S. Pevzner, I. Zilbermann, P. Moisy, E. Maimon and I. Popivker, Free Radicals Biol. Med., 2022, 180, 134–142 CrossRef CAS PubMed.
- C. C. Barros, C. D. Pilgrim, A. R. Cook, S. P. Mezyk, T. S. Grimes and G. P. Horne, Phys. Chem. Chem. Phys., 2021, 23, 24589–24597 RSC.
- T. Toigawa, D. R. Peterman, D. S. Meeker, T. S. Grimes, P. R. Zalupski, S. P. Mezyk, A. R. Cook, S. Yamashita, Y. Kumagai, T. Matsumura and G. P. Horne, Phys. Chem. Chem. Phys., 2021, 23, 1343–1351 RSC.
- A. Kimberlin, G. Saint-Louis, D. Guillaumont, B. Camès, P. Guilbaud and L. Berthon, Phys. Chem. Chem. Phys., 2022, 24, 9213–9228 RSC.
- B. K. Sharma and R. Gupta, Radiat. Eff., 1981, 57, 149–154 CrossRef.
- G. V. Buxton and R. M. Sellers, Coord. Chem. Rev., 1977, 22, 195–274 CrossRef CAS.
- S. N. Bhattacharyya and K. P. Kundu, Int. J. Radiat. Phys. Chem., 1971, 3, 1–10 CrossRef CAS.
- K. P. Kundu and N. Matsuura, Int. J. Radiat. Phys. Chem., 1975, 7, 565–571 CrossRef CAS.
- G. R. Buettner, T. P. Doherty and L. K. Patterson, FEBS Lett., 1983, 158, 143–146 CrossRef CAS PubMed.
- Y. A. Ilan and G. Czapski, Biochim. Biophys. Acta, Gen. Subj., 1977, 498, 386–394 CrossRef CAS PubMed.
- B. K. Sharma and R. Gupta, Radiat. Phys. Chem., 1984, 24, 233–237 CrossRef CAS.
- M. M. Khater, I. M. Kenawi, A. M. Atwa and M. B. Hafez, J. Radioanal. Nucl. Chem., 1987, 111, 17–26 CrossRef CAS.
- M. B. Hafez, H. Rouhdy and N. Hafez, J. Radioanal. Chem., 1978, 43, 121–129 CrossRef CAS.
- S. N. Bhattacharyya and E. V. Srisankar, Int. J. Radiat. Phys. Chem., 1976, 8, 667–671 CrossRef CAS.
- A. Kimberlin, D. Guillaumont, S. Arpigny, B. Camès, P. Guilbaud, G. Saint-Louis, H. Galán and L. Berthon, New J. Chem., 2021, 45, 12479–12493 RSC.
- P. I. Matveev, A. A. Mitrofanov, V. G. Petrov, S. S. Zhokhov, A. A. Smirnova, Y. A. Ustynyuk and S. N. Kalmykov, RSC Adv., 2017, 7, 55441–55449 RSC.
- A. Smirnova, A. Mitrofanov, P. Matveev, T. Baygildiev and V. Petrov, Phys. Chem. Chem. Phys., 2020, 22, 14992–14997 RSC.
- T. Koubský, J. Fojtíková and L. Kalvoda, Prog. Nucl. Energy, 2017, 94, 208–215 CrossRef.
- T. Koubský and J. Luštinec, J. Radioanal. Nucl. Chem., 2018, 318, 2407–2413 CrossRef.
- F. H. Attix, W. C. Roesch and E. Tochilin, Radiation dosimetry, Academic Press, New York [etc.], 1966 Search PubMed.
- C. Truillet, E. Thomas, F. Lux, L. T. Huynh, O. Tillement and M. J. Evans, Mol. Pharmaceutics, 2016, 13, 2596–2601 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson and H. Nakatsuji, Gaussian 16, Gaussian, Inc., Wallingford, CT, 2016 Search PubMed.
- D. Andrae, U. Häußermann, M. Dolg, H. Stoll and H. Preuß, Theor. Chim. Acta, 1990, 77, 123–141 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- J. P. Perdew, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 33, 8822–8824 CrossRef PubMed.
- Y. Feng, L. Liu, J.-T. Wang, H. Huang and Q.-X. Guo, J. Chem. Inf. Comput. Sci., 2003, 43, 2005–2013 CrossRef CAS PubMed.
- C. Qi, Q.-H. Lin, Y.-Y. Li, S.-P. Pang and R.-B. Zhang, J. Mol. Struct.: THEOCHEM, 2010, 961, 97–100 CrossRef CAS.
- H. Zeng, J. Zhao and X. Xiao, Chin. Phys. B, 2013, 22, 023301 CrossRef.
- R. G. Parr and W. Yang, J. Am. Chem. Soc., 1984, 106, 4049–4050 CrossRef CAS.
- C. Morell, A. Grand and A. Toro-Labbé, J. Phys. Chem. A, 2005, 109, 205–212 CrossRef CAS PubMed.
- C. Morell, A. Grand and A. Toro-Labbé, Chem. Phys. Lett., 2006, 425, 342–346 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 1372–1377 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3094 CrossRef CAS PubMed.
- E. Glendening, A. Reed, J. Carpenter and F. Weinhold, NBO 3.1 Search PubMed.
- D. Parker, K. Pulukkody, F. C. Smith, A. Batsanov and J. A. K. Howard, J. Chem. Soc., Dalton Trans., 1994, 689–693 RSC.
- B. J. Mincher, G. Modolo and S. P. Mezyk, Solvent Extr. Ion Exch., 2009, 27, 1–25 CrossRef CAS.
- B. J. Mincher and R. D. Curry, Appl. Radiat. Isot., 2000, 52, 189–193 CrossRef CAS PubMed.
- M. Audras, L. Berthon, N. Martin, N. Zorz and Ph. Moisy, J. Radioanal. Nucl. Chem., 2015, 303, 1897–1909 CAS.
- Y. Moiseev, Y. Ben-Eliyahu, M. Audras, L. Berthon, P. Moisy, A. Bettelheim and I. Zilbermann, J. Coord. Chem., 2016, 69, 2895–2907 CrossRef CAS.
- G. P. Horne, A. Wilden, S. P. Mezyk, L. Twight, M. Hupert, A. Stärk, W. Verboom, B. J. Mincher and G. Modolo, Dalton Trans., 2019, 48, 17005–17013 RSC.
- J. A. Drader, N. Boubals, B. Camès, D. Guillaumont, P. Guilbaud, G. Saint-Louis and L. Berthon, Dalton Trans., 2018, 47, 251–263 RSC.
- J. Drader, G. Saint-Louis, J. M. Muller, M.-C. Charbonnel, P. Guilbaud, L. Berthon, K. M. Roscioli-Johnson, C. A. Zarzana, C. Rae, G. S. Groenewold, B. J. Mincher, S. P. Mezyk, K. McCann, S. G. Boyes and J. Braley, Solvent Extr. Ion Exch., 2017, 35, 480–495 CrossRef CAS.
- J. Moreau, E. Guillon, J.-C. Pierrard, J. Rimbault, M. Port and M. Aplincourt, Chem. – Eur. J., 2004, 10, 5218–5232 CrossRef CAS PubMed.
- J. F. Desreux, E. Merciny and M. F. Loncin, Inorg. Chem., 1981, 20, 987–991 CrossRef CAS.
- L. Burai, I. Fábián, R. Király, E. Szilágyi and E. Brücher, J. Chem. Soc., Dalton Trans., 1998, 243–248 RSC.
- J. Hioe, D. Šakić, V. Vrček and H. Zipse, Org. Biomol. Chem., 2015, 13, 157–169 RSC.
- D. Kaur and R. P. Kaur, J. Mol. Struct.: THEOCHEM, 2008, 858, 94–100 CrossRef CAS.
- D. Šakić and H. Zipse, Adv. Synth. Catal., 2016, 358, 3983–3991 CrossRef.
- A. Morgenstern, C. Apostolidis, C. Kratochwil, M. Sathekge, L. Krolicki and F. Bruchertseifer, Curr. Radiopharm., 2018, 11, 200–208 CrossRef CAS PubMed.
- V.-L. Tran, F. Lux, N. Tournier, B. Jego, X. Maître, M. Anisorac, C. Comtat, S. Jan, K. Selmeczi, M. J. Evans, O. Tillement, B. Kuhnast and C. Truillet, Adv. Healthcare Mater., 2021, 10, 2100656 CrossRef CAS PubMed.
- F. Lux, V. L. Tran, E. Thomas, S. Dufort, F. Rossetti, M. Martini, C. Truillet, T. Doussineau, G. Bort, F. Denat, F. Boschetti, G. Angelovski, A. Detappe, Y. Crémillieux, N. Mignet, B.-T. Doan, B. Larrat, S. Meriaux, E. Barbier, S. Roux, P. Fries, A. Müller, M.-C. Abadjian, C. Anderson, E. Canet-Soulas, P. Bouziotis, M. Barberi-Heyob, C. Frochot, C. Verry, J. Balosso, M. Evans, J. Sidi-Boumedine, M. Janier, K. Butterworth, S. McMahon, K. Prise, M.-T. Aloy, D. Ardail, C. Rodriguez-Lafrasse, E. Porcel, S. Lacombe, R. Berbeco, A. Allouch, J.-L. Perfettini, C. Chargari, E. Deutsch, G. Le Duc and O. Tillement, Br. J. Radiol., 2018, 92, 20180365 CrossRef PubMed.
- P. Fries, D. Morr, A. Müller, F. Lux, O. Tillement, A. Massmann, R. Seidel, T. Schäfer, M. D. Menger, G. Schneider and A. Bücker, RoFo, 2015, 187, 1108–1115 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3dt00977g |
| This journal is © The Royal Society of Chemistry 2023 |