Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solid state luminescence of phosphine-EWO ligands with fluorinated chalcone skeletons and their PdX2 complexes: metal-promoted phosphorescence enhancement†
Jaime
Ponce-de-León
 a,
Marconi N.
Peñas-Defrutos
a,
Marconi N.
Peñas-Defrutos
 *a,
Andrea
Vélez
*a,
Andrea
Vélez
 a,
Gabriel
Aullón
a,
Gabriel
Aullón
 b and
Pablo
Espinet
b and
Pablo
Espinet
 *a
*a
aIU CINQUIMA/Química Inorgánica, Facultad de Ciencias, Universidad de Valladolid, 47071-Valladolid, Spain. E-mail: marconi_44@hotmail.com; espinet@qi.uva.es
bSecció de Química Inorgànica (Departament de Química Inorgànica i Orgànica), Institut de Química Teòrica i Computacional, Universitat de Barcelona, 08028 Barcelona, Spain
First published on 17th February 2023
Abstract
Complexes trans-[PdX2L2] (X = Cl and Br), where L is 1-(PR2),2-(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–C(O)Ph)-C6F4 (R = Ph, Cy, and iPr), display phosphorescent emission in the solid state, whereas due to their substantially lower lifetimes, the free ligands exhibit fluorescent behaviour. Alternatively, structurally identical derivatives with halide replaced by CN− or Pd replaced by Pt are non-emissive. DFT calculations explain this diverse behaviour, showing that the hybridization of orbitals of the MX2 moiety with those of the chalcone fragment of ligands is significant only for the LUMO of the emissive compounds. In other words, in our complexes, only MLMCT processes (LM = Metal-perturbed Ligand-centered orbital) lead to observable luminescence.
CH–C(O)Ph)-C6F4 (R = Ph, Cy, and iPr), display phosphorescent emission in the solid state, whereas due to their substantially lower lifetimes, the free ligands exhibit fluorescent behaviour. Alternatively, structurally identical derivatives with halide replaced by CN− or Pd replaced by Pt are non-emissive. DFT calculations explain this diverse behaviour, showing that the hybridization of orbitals of the MX2 moiety with those of the chalcone fragment of ligands is significant only for the LUMO of the emissive compounds. In other words, in our complexes, only MLMCT processes (LM = Metal-perturbed Ligand-centered orbital) lead to observable luminescence.
Chalcone derivatives (Ar–CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CO–Ar’) are mainly known for their biological activity and pharmacological applications,1 but some of them also display interesting photophysical properties.2 These compounds typically exhibit aggregation-induced emission due to lack of planarity that hinders π-stacking interactions and push–pull conjugation effects at the enone moiety,3 which are the subject of very recent studies. Two reviews3,4 and three relevant reports have been published in the last two years. Thus, the rare crystal jumping behaviour has been found for a highly luminescent chalcone derivative due to a reversible intermolecular [2 + 2] cycloaddition process in the solid state.5 Moreover, chemosensors with chalcone-based chromophores have been applied for the selective detection of Cu2+ in aqueous media.6 Besides, there is a report on the application of chalcone-derived liquid crystals for NH3(g) sensing.7
CH–CO–Ar’) are mainly known for their biological activity and pharmacological applications,1 but some of them also display interesting photophysical properties.2 These compounds typically exhibit aggregation-induced emission due to lack of planarity that hinders π-stacking interactions and push–pull conjugation effects at the enone moiety,3 which are the subject of very recent studies. Two reviews3,4 and three relevant reports have been published in the last two years. Thus, the rare crystal jumping behaviour has been found for a highly luminescent chalcone derivative due to a reversible intermolecular [2 + 2] cycloaddition process in the solid state.5 Moreover, chemosensors with chalcone-based chromophores have been applied for the selective detection of Cu2+ in aqueous media.6 Besides, there is a report on the application of chalcone-derived liquid crystals for NH3(g) sensing.7
Our pursuit of hemilabile ligands able to promote challenging Pd-catalysed C–C couplings (e.g. ArF–ArF’, ArF = fluorinated aryl) led us to develop the family of fluorinated PEWO ligands shown in Fig. 1A (PEWO = Phosphine bearing Electron-Withdrawing Olefins). The previously synthesized L1,8L2,9 and L3, reported herein for the first time, differ in the PR2 substituents (R = Ph, Cy, and iPr respectively), while the analogue of L1 labelled as L4, reported by Lei et al., (Fig. 1B), lacks fluorine substitution.10 All these PEWO ligands have a chalcone skeleton highlighted in red in Fig. 1. The hybrid chelating ligand L1 is extremely efficient in lowering the C–C reductive elimination barriers and allows for oxidative addition under catalytic conditions with either Ar–I or Ar–Br (Ar–Cl cannot be used).11,12 Particularly, the Z-chelated complex [PdCl2L1] (2L1) is an active catalyst for the Negishi heterocoupling of perhaloaryls.13
 | ||
| Fig. 1 Hybrid phosphine-EWO ligands used in this work with the chalcone group highlighted in red. A) Fluorinated derivatives (*L3 has not been previously reported). B) Non-fluorinated Ph analogue. | ||
The remarkably low activation barrier for E/Z olefin-isomerization (it occurs at room temperature) supports the extraordinary electronic delocalization of the double bond into both extremes (C6F4 and COPh) of our fluorinated chalcone phosphines (Scheme 1).9,14 The latter confirms the special pull–pull conjugation in our R-PEWO-F4 ligands, which is presumably related to the solid-state luminescence reported herein, displayed by the free ligands and some of their metal complexes, clearly enhanced by fluorination in the EWO.
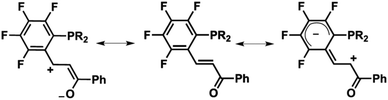 | ||
Scheme 1 Resonance forms contributing to the electron deficiency of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of the chalcone fragment. C double bond of the chalcone fragment. | ||
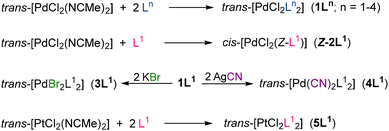
Complexes trans-[PdCl2(Ln)2] (1L1–4) show monodentate P-coordination and E configuration of the non-coordinated olefin, similar to the free ligands (Fig. 1). These compounds were selectively prepared by the reaction of trans-[PdCl2(NCMe)2] with the appropriate PEWO in a Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln = 1
Ln = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio (Scheme 2). Conversely, the reaction using a Pd
2 ratio (Scheme 2). Conversely, the reaction using a Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L1 = 1
L1 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio eventually leads to the formation of an E-chelated intermediate (E-2L1), which subsequently isomerizes to the Z-chelated complex cis-[PdCl2(L1)] (Z-2L1). The Z-configuration of Z-2L1 eventually triggers a C–F activation process, which opens the gate to new reactivity.15
1 ratio eventually leads to the formation of an E-chelated intermediate (E-2L1), which subsequently isomerizes to the Z-chelated complex cis-[PdCl2(L1)] (Z-2L1). The Z-configuration of Z-2L1 eventually triggers a C–F activation process, which opens the gate to new reactivity.15
Treatment of 1L1 with KBr or AgCN yields trans-[PdBr2(L1)2] (3L1) and trans-[Pd(CN)2(L1)2] (4L1), respectively. The platinum compound trans-[PtCl2(L1)2] (5L1) was synthesized analogously to 1L1 (Scheme 2). In all compounds (free ligands and trans-[MX2(Ln)2] complexes), the uncoordinated olefin group has the E-EWO configuration. The only exception is the chelate complex [PdCl2(Z-L1)] (Z-2L1), which is coordinated by the P atom and the double bond, which display a Z-EWO conformation. The X-ray structures of 1L1 (Fig. 2),8Z-2L1,8L2,9 and 1L4,16 are available in the literature, whereas those of ligand Ph-PEWO-F4 (L1) and complexes 1L2 and 1L3 (Fig. S1, ESI†) are first reported herein.
Neither the free ligands nor their complexes display emitting behaviour in solution,17 whereas Table 1 presents the experimental data (excitation and emission maxima, average lifetimes, and quantum yield percentages) measured for the crystalline solids, where some remarkably intense emissions are observed. The emission spectra as well as the decay profiles are given in the ESI (Fig. S4–S13†).
| Entry/Comp. | λ exc (nm)18 | λ emis (nm)a | Φ (%) | τ av (ns)b |
|---|---|---|---|---|
| a Very broad emission bands. b τ av = (A1τ12 + A2τ22 + ⋯)/(A1τ1 + A2τ2 + ⋯). | ||||
| 1/L1 | 413 | 606 | 10.1 | 2.5 |
| 2/L2 | 460 | 530 | 4.1 | 3.5 |
| 3/L4 | 472 | 552 | <3 | 3.0 |
| 4/1L1 | 405 | 649 | 28.0 | 1.3 × 103 |
| 5/1L2 | 412 | 676 | 21.8 | 1.6 × 103 |
| 6/1L3 | 405 | 643 | 15.6 | 1.4 × 103 |
| 7/1L4 | 469 | 680 | <3 | 6.4 × 102 |
| 8/Z-2L1 | Undetected emission | |||
| 9/3L1 | 415 | 666 | 15.7 | 5.1 × 103 |
| 10/4L1 | Undetected emission | |||
| 11/5L1 | Undetected emission | |||
The fluorinated ligands L1 and L2 show yellow emission in the solid state upon UV irradiation, while the E-complexes 1L1–3 and 3L1 show eye-catching orange luminescence under similar conditions (vide infra, Fig. 3). However, the chelated Z-complex 2L1, showing a drastic structural divergence, does not exhibit emissive properties. The lack of fluorination is clearly detrimental to the luminescence (see quantum yield percentages of L4 and 1L4 presented in Table 1), and more interestingly, both 4L1 and 5L1 complexes, which do not show any remarkable structural differences with 1L1, are not luminescent. Fig. 2 compares those three X-ray structures, including relevant distances within the chalcone ring (i.e. –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–) and involving the metal centres.
C–) and involving the metal centres.
 | ||
| Fig. 3 Emission spectra, with normalised intensity, of the fluorinated ligand L2 (yellow line) and its PdCl2 complex 1L2 (red line); dashed lines represent the maximum of excitation (λexc in Table 1).18 Photographs of a sample in a mortar, taken upon UV irradiation, are also shown. | ||
In the free phosphine ligands, the chalcone moiety is clearly affected by the orbital influence of the R substituents at the P atom, as supported by the variations in their λemis values (Table 1, entries 1–3). In the complexes, additional dependence on the MX2 metal fragment at which phosphine is coordinated is obvious (Table 1, entries 4–11).
Concerning the free ligands, the emissive transitions are necessarily intraligand. The effect of R substituents at P, with different electronic and steric features, is appreciable. The highest maximum wavelength (λemis = 606 nm) is found for the fluorinated L1 (R = Ph, entry 1). Note that the emission bands given in Table 1 are very broad and λemis is not enough to guess the colour (L1 is yellowish upon irradiation too, see Fig. S3, ESI†). For the fluorinated L2 (R = Cy, λemis = 530 nm, Fig. 3), the wavelength is closer to the value obtained for the non-fluorinated Ph-PEWO-H4 ligand L4 (entry 3) than for L1, as if the higher inductive effect of Cy acting as a σ-donor towards the P atom was compensating the electron-withdrawing effect of fluorination at the chalcone aryl group. Apparently, the two effects that should increase the σ-electron density at the chalcone ring (non-fluorination and inductive effect from R) diminish the quantum yield Φ values noticeably, to almost extinction in L4.
Remarkably, the colour change upon ligand coordination (yellow to orange) is linked to a substantially higher νexc − νemis difference (i.e. 9.5 × 103 cm−1vs. 2.9 × 103 cm−1 for L2 and 1L2 respectively) and longer lifetimes (in the order of μs for the complexes and ns for the ligands). Fig. 3 shows the comparison between 1L2 and L2. These observations suggest a luminescence switch upon coordination to PdCl2, from fluorescence in the free ligands to phosphorescence in the complexes.19
Considering now the metal compounds, a remarkable increase in the quantum yield percentages (Φ > 15%, up to 28% for 1L1), compared to the free ligands (Φ < 10%), is observed upon complexation to PdCl2 in 1L1–3 (Table 1, entries 4–6) or to PdBr2 in the case of 3L1 (Table 1, entry 9). In contrast, the non-fluorinated 1L4 complex (Table 1, entry 7) is scarcely luminescent. This observation supports the important effect of the fluorine substituents of the chalcone ring on the emissive properties.20
In the case of the non-luminescent complexes 4L1 and 5L1, obviously their structural similarity with 1L1 (Fig. 2) does not impede significant orbital differences when Pd or the halides are replaced by Pt or CN. The PdCl2 (1L1–3) and the PdBr2 (3L1) complexes have two medium-strength σ-donor and slightly π-donor halo ligands, and show similar λemis (nm) and Φ (%) values, as given in Table 1. Complex 4L1 (Table 1, entry 10) has more σ-donor and very strongly π-acceptor cyano ligands. However, 5L1 (Table 1, entry 11) has Pt replacing Pd, and the lanthanide contraction plus the relativistic effects in Pt produce large orbital energy differences between the Pd and Pt isoelectronic metal centres. Qualitatively, these two cases (4L1 and 5L1) are roughly similar, because both compounds are expected to have a metal centre greedier of electron density and, consequently, less prone to be polarized by π back-donation towards the phosphine ligand.
In order to identify the orbitals involved in the absorption and emission, as well as to support our qualitative hypotheses, DFT and TD-DFT calculations were carried out (see ESI for details†). We focused the analysis on the ligand L1 and the complexes 1L1, 4L1, and 5L1. As the emissions of L1 and 1L1 are only observed in the solid state, their X-ray structures are used as initial guesses for geometry optimizations in the gas phase, which led to minimal modifications of the solid structures.
The computed HOMO–LUMO gap fits well the experimental λexc measured for L1 (λcalc = 402 nm; λexp = 413 nm). The HOMO orbital is mainly located in the Ph2P moiety and the LUMO is a chalcone orbital with high participation of fluorinated C atoms and those of the double bond (Fig. S17, ESI†). However, the computed wavelengths of the deexcitations from triplet states are far from the experimentally observed emission (L1, λexp = 606 nm), discarding them to assign a phosphorescent behaviour. There is, however, a S1 → S0 transition from a singlet state, where slight structural bending of the chalcone group is suggestive of loss of sp2 character of the double bond, which predicts an emission from π*(EWO) at λcalc = 580 nm, much closer to the experimental value. Its fluorescent nature is strongly supported by the short lifetime of ca. 2 ns. In this transition, the participation of the R2P fragment in the orbitals involved is significant (Table S12†). This is consistent with the experimentally observed effect of the R substituents at P.
The simulation of the absorption spectra allows us to assign the electronic transition from HOMO-2 to the LUMO orbital as responsible for the excitation maxima observed for 1L1. The calculated transition (λcalc = 395 nm) matches reasonably well the experimentally observed λexc maximum (405 nm, Table 1, entry 4). The contribution of the different molecular moieties is 58% Ph2P + 22% PdCl2 + 20% EWO for HOMO-2 (Fig. S18†) and 42% PdCl2 + 38% EWO + 20% Ph2P for LUMO (Fig. 4). Both orbitals are symmetrical, with equal contribution of the two ligands. Remarkably, similar transitions (HOMO-2 to LUMO) are computed to be markedly more energetic either in the Pt analogue 5L1 (λcalc = 349 nm) or in the Pd(CN)2 complex 4L1 (λcalc = 333 nm), confirming the qualitative interpretation of similar behaviour of these two complexes (see Fig. S19 and S20 for MOs†).
Using TD-DFT calculations, the experimentally observed emission for 1L1 (λemis = 649 nm, Table 1, entry 4) is assigned to the computed monodeexcitation from the first triplet state (T1), with a much similar wavelength (λcalc = 667 nm, Table S9, ESI†). This emission band mainly involves the LUMO → HOMO phosphorescent transition. This is perfectly consistent with the high νexc − νemis difference and the long lifetime values provided in Table 1 and commented previously. It is worth remarking that geometry relaxation was discarded due to the very limited deformability allowed in the solid state.21 Moreover, the fluorescent emissions as well as other potentially phosphorescent ones were also computed and then discarded because of numerical results far from the experimental data (details in ESI†).
Fig. 4 gathers the frontier molecular orbitals (FMOs) of 1L1, 4L1 and 5L1 for comparison. Evidently, deexcitation from the LUMO (42% PdCl2, 38% EWO, 20% Ph2P) to the PdCl2 HOMO (Fig. 4, top), responsible for the orange-emitting behaviour of 1L1, is drastically affected either in the PtCl2 or in the Pd(CN)2 analogues. The computed phosphorescent emissions (decay from triplet states) become experimentally unobservable for 4L1 and 5L1 (Tables S10 and S11 respectively†), as a direct consequence of the changes in the FMOs. For 5L1 (Fig. 4, middle), the PtCl2 HOMO resembles its PdCl2 analogue but the contribution of the PtCl2 fragment in the LUMO drops to 7% (vs. 42% for 1L1) and the deexcitation from the first triplet is computed to be much more energetic (i.e. 555 nm). The effect of Cl by CN substitution in 4L1 FMOs is also clear (Fig. 4, bottom), with reduced participation of the Pd(CN)2 fragment in the LUMO of 4L1 (casually almost identical to the PtCl2 compound) and negligible in the HOMO.
After the identification of the key orbitals involved in the luminescent behaviour (with relevant participation of the PdCl2 fragment), the data provided in Table 1 can be better rationalized: (i) both the PdBr2 substitution in 3L1 (Br is electronically quite similar to Cl) or the R2P modification in 1L2 and 1L3 move in the range of variations of orbital polarization; (ii) totally different emission wavelengths must be expected from the Pd(CN)2 (4L1) or PtCl2 (5L1) congeners, with negligible contribution of the MX2 moieties in their LUMOs. In fact, these happen not to be experimentally detectable. While many examples of either MLCT (MLCT = Metal to Ligand Charge Transfer) or ILCT (IL = Intra-Ligand Charge Transfer) emitting metal compounds can be found in the literature; in our system, only MLMCT processes (LM = Metal-perturbed Ligand-centered LUMO)22 lead to observable phosphorescence.
In summary, we reported herein a family of yellow fluorescent 1-(PR2),2-(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–C(O)Ph)-C6F4 chalcone-derived phosphines (Ln) and some intensely orange phosphorescent trans-[PdX2Ln2] palladium(II) complexes (X = Cl and Br) with them displaying quantum yields (Φ) above 15%. The luminescence is only observed in the solid state and is enhanced by the fluorination in chalcone, both in the ligand and in the PdCl2 complex. Using TD-DFT calculations, the emission of the free Ph-PEWO-F4 ligand (L1) is assigned to the S1 → S0 transition presumably associated with a loss of sp2 character of the olefinic C atoms. Computational studies reproduce the excitation and emission data of the PdCl2 compound 1L1 satisfactorily and reveal the decisive involvement of the LUMO orbital, which features a clear metal/chalcone connection, in the luminescent behaviour. Conversely, the non-emitting Pd(CN)2 and PtCl2 analogues, structurally identical to 1L1, display LUMO orbitals with scarce participation of the metal centres, supporting the synergistic contributions of both the MX2 core and the chalcone skeleton in the emissive properties.
CH–C(O)Ph)-C6F4 chalcone-derived phosphines (Ln) and some intensely orange phosphorescent trans-[PdX2Ln2] palladium(II) complexes (X = Cl and Br) with them displaying quantum yields (Φ) above 15%. The luminescence is only observed in the solid state and is enhanced by the fluorination in chalcone, both in the ligand and in the PdCl2 complex. Using TD-DFT calculations, the emission of the free Ph-PEWO-F4 ligand (L1) is assigned to the S1 → S0 transition presumably associated with a loss of sp2 character of the olefinic C atoms. Computational studies reproduce the excitation and emission data of the PdCl2 compound 1L1 satisfactorily and reveal the decisive involvement of the LUMO orbital, which features a clear metal/chalcone connection, in the luminescent behaviour. Conversely, the non-emitting Pd(CN)2 and PtCl2 analogues, structurally identical to 1L1, display LUMO orbitals with scarce participation of the metal centres, supporting the synergistic contributions of both the MX2 core and the chalcone skeleton in the emissive properties.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Spanish MCINN (Projects PID2020-118547GB-I00 and PGC 2018-093863-B-C21) for the funding provided and both Eric Mates-Torres and Marta Mansilla (UBU) for help. J. P.-de-L. also acknowledges MCINN for a FPI studentship (BES-2017-080726). M. N. P-D. thanks the UVa for a Margarita Salas postdoctoral fellowship (ref. CONVREC-2021-221).References
- (a) C. Zhuang, W. Zhang, C. Sheng, W. Zhang, C. Xing and Z. Miao, Chem. Rev., 2017, 117, 7762–7810 CrossRef CAS PubMed; (b) N. A. A. Elkanzi, H. Hrichi, R. A. Alolayan, W. Derafa, F. M. Zahou and R. B. Bakr, ACS Omega, 2022, 7, 27769–27786 CrossRef CAS PubMed; (c) S. L. Gaonkar and U. N. Vignesh, Res. Chem. Intermed., 2017, 43, 6043–6077 CrossRef CAS.
- K. G. Komarova, S. N. Sakipov, V. G. Plotnikov and M. V. Alfimov, J. Lumin., 2015, 164, 57–63 CrossRef CAS.
- S. Kagatikar and D. Sunil, Chem. Pap., 2021, 75, 6147–6156 CrossRef CAS.
- P. Mahesha, N. S. Shetty and S. D. Kulkarni, J. Fluoresc., 2022, 32, 835–862 CrossRef CAS PubMed.
- X. Cheng, F. Yang, J. Zhao, J. Ni, X. He, C. Zhou, J. Z. Sun and B. Z. Tang, Mater. Chem. Front., 2020, 4, 651–660 RSC.
- L. J. Gomes, T. Moreira, L. Rodriguez and A. J. Moro, Dyes Pigm., 2022, 197, 109845 CrossRef CAS.
- A.-T. Mohammad and W. R. Abbas, RSC Adv., 2021, 11, 38444–38456 RSC.
- E. Gioria, J. M. Martinez-Ilarduya, D. Garcia-Cuadrado, J. A. Miguel, M. Genov and P. Espinet, Organometallics, 2013, 32, 4255–4261 CrossRef CAS.
- M. N. Peñas-Defrutos, A. Vélez, E. Gioria and P. Espinet, Organometallics, 2019, 38, 4701–4707 CrossRef.
- X. Luo, H. Zhang, H. Duan, Q. Liu, L. Zhu, T. Zhang and A. Lei, Org. Lett., 2007, 9, 4571–4574 CrossRef CAS PubMed.
- E. Gioria, J. del Pozo, J. M. Martínez-Ilarduya and P. Espinet, Angew. Chem., Int. Ed., 2016, 55, 13276–13280 CrossRef CAS PubMed.
- E. Gioria, J. del Pozo, A. Lledós and P. Espinet, Organometallics, 2021, 40, 2272–2282 CrossRef CAS.
- J. Ponce-de-León and P. Espinet, Chem. Commun., 2021, 57, 10875–10878 RSC.
- A. Roque, J. C. Lima, A. J. Parola and F. Pina, Photochem. Photobiol. Sci., 2007, 6, 381–385 CrossRef CAS PubMed.
- M. N. Peñas-Defrutos, A. Vélez and P. Espinet, Organometallics, 2020, 39, 841–847 CrossRef.
- H. Zhang, X. Luo, K. Wongkhan, H. Duan, Q. Li, L. Zhu, J. Wang, A. S. Batsanov, J. A. K. Howard, T. B. Marder and A. Lei, Chem. – Eur. J., 2009, 15, 3823–3829 CrossRef CAS PubMed.
- For an example of luminescent chalcones in the solid state but not in solution see: L. Zhang, J. Liu, J. Gao, R. Lu and F. Liu, RSC Adv., 2017, 7, 46354–46357 RSC.
- Excitation spectra of metal complexes are scarcely informative (Fig. S14 and S15†). λexc is assigned as the wavelength that maximizes the emission intensity.
- J. M. Forward, D. Bohmann, J. P. Fackler Jr. and R. J. Staples, Inorg. Chem., 1995, 34, 6330–6336 CrossRef CAS.
- For a different case see: J. Ponce-de-León, R. Infante and P. Espinet, Chem. Commun., 2021, 57, 5458–5461 RSC.
- Reoptimization of both S1 and T1 states led to profound structural change, presumably not accessible in the crystal.
- This notation has been recently used in: T. Theiss, S. Buss, I. Maisuls, R. López-Arteaga, D. Brünink, J. Kösters, A. Hepp, N. L. Doltsinis, E. A. Weiss and C. A. Strassert, J. Am. Chem. Soc., 2023, 145, 3937–3951 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of the complexes including NMR spectra, computational details and X-ray data. CCDC 2205013–2205017. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt00408b |
| This journal is © The Royal Society of Chemistry 2023 |