Platin-C containing nanoparticles: a recipe for the delivery of curcumin–cisplatin combination chemotherapeutics to mitochondria†
Bhabatosh
Banik
 *abcd,
Akash
Ashokan
*abcd,
Akash
Ashokan
 ab,
Joshua H.
Choi
c,
Bapurao
Surnar
ab and
Shanta
Dhar
ab,
Joshua H.
Choi
c,
Bapurao
Surnar
ab and
Shanta
Dhar
 *abce
*abce
aNanoTherapeutics Research Laboratory, Department of Biochemistry and Molecular Biology, University of Miami Miller School of Medicine, Miami, FL 33136, USA. E-mail: bhabatosh.banik@cottonuniversity.ac.in; shantadhar@med.miami.edu
bSylvester Comprehensive Cancer Center, Miller School of Medicine, University of Miami, Miami, FL 33136, USA
cNano Therapeutics Research Laboratory, Department of Chemistry, University of Georgia, Athens, GA 30602, USA Web: https://www.shantadharlab.com
dDepartment of Chemistry, Cotton University, Panbazar, Guwahati-781001, Assam, India
eDepartment of Chemistry, University of Miami, Coral Gables, FL 33146, USA
First published on 13th January 2023
Abstract
The success story of cisplatin spans over six decades now and yet it continues to be the key player in most chemotherapeutic regimens. Numerous efforts have been made to improve its efficacy, address its shortcomings, and overcome drug resistance. One such strategy is to develop new platinum(IV)-based prodrugs with functionally active ligands to deliver combination therapeutics. This strategy not only enables the drug candidate to access multiple drug targets but also enhances the kinetic inertness of platinum complexes and thereby ensures greater accumulation of active drugs at the target site. We report the synthesis of Platin-C, a platinum(IV)-based cisplatin prodrug tethered to the active component of ancient herbal medicine, curcumin, as one of the axial ligands. This combination complex showed improved chemotherapeutic efficacy in cisplatin resistant A2780/CP70 cell lines compared with the individual components. An amine-terminated biodegradable polymer was suitably functionalized with the triphenylphosphonium (TPP) cation to obtain a mitochondria-directed drug delivery platform. Quantification of Platin-C loading into these NPs using complementary techniques employing curcumin optical properties in high-performance liquid chromatography and platinum-based inductively coupled plasma mass spectrometry evidenced efficacious payload incorporation resulting in functional activities of both the components. Stability studies for a period of one week indicated that the NPs remain stable, enabling substantial loading and controlled release of the prodrug. The targeting nanoparticle (NP) platform was utilized to deliver Platin-C primarily in the mitochondrial network of cancer cells as monitored using confocal microscopy employing the green fluorescence of the curcumin pendant. Our studies showed that amine terminated NPs were relatively less efficient in their ability to target mitochondria despite being positively charged. This re-validated the importance of lipophilic positively charged TPP surface functionalities to successfully target cellular mitochondria. We validated the capabilities of Platin-C and its mitochondria-targeting nanoparticles towards inflicting mitochondria-directed activity in cisplatin-sensitive and cisplatin-resistant cell lines. Furthermore, our studies also demonstrated the effectiveness of Platin-C incorporated targeting NPs in attenuating cellular inflammatory markers by utilizing the curcumin component. This study advances our understanding of the cisplatin prodrug approach to combine chemotherapeutic and inflammatory effects in accessing combinatory pathways.
Introduction
Even after 58 years of its discovery, cis-diamminedichloridoplatinum(II), or cisplatin, is still one of the most prescribed anticancer drugs available for the treatment of varied solid tumors.1–4 Despite the great success in treating cancer, the efficiency of cisplatin is compromised by its induction of several side effects and acquired resistance to its effectiveness.5–7 Inflammation, on the other hand, is closely related to tumorigenesis.8–10 Chronic inflammation plays a significant role in approximately 20% of human cancer cases.11 The inflammatory genes and cytokines associated with tumors contribute to the formation of an immunosuppressive microenvironment conducive for their growth and progression. Moreover, deletion or inhibition of inflammatory cytokines prevents tumor development. Nuclear factor kappa B (NF-κB) is one of the inflammatory genes that is a central regulator of the inflammatory response.12 Activation of NF-κB promotes cell proliferation while down regulation presents the opposite effect; thus NF-κB is one of the major factors in tumorigenesis.13–15 Curcumin, a major component of the spice turmeric, is an emissive compound16–18 that exhibits anti-cancer,19–21 anti-inflammatory,22–24 and anti-oxidative25,26 properties. Specifically, it plays a critical role in controlling the NF-κB signaling pathway by inhibiting the phosphorylation of the inhibitor of kappa B, thus causing down regulation of NF-κB.27,28 It also acts as a radical oxygen species scavenger via H-atom donation and electron transfer, thus exhibiting anti-oxidative properties.29 Although it exhibits multiple functions, its efficacy in preclinical and clinical studies is limited because of its poor water solubility and its low bioavailability which is a result of its short biological half-life.30,31 To circumvent these problems, delivery vehicles have been used to deliver curcumin to the target rapidly and accurately.32,33 A combination of curcumin and cisplatin can be a lucrative strategy for managing cancer.34–36 Administration of free-drug formulations may pose hurdles including achieving definite exposure of drugs at the targets of action, and differential pharmacokinetic and biodistribution parameters. These factors, though very difficult to control upon individual administration of drugs, can, however, be overcome via the construction of a single combination prodrug constituting the drugs of interest.37–42 Thus, we envisioned an alternative way to deliver a chemotherapeutic combination of curcumin and cisplatin by fabricating them into a platinum(IV) prodrug that, in a reductive intracellular environment, would give the respective active components. In addition to ascertaining specific stoichiometric compositions of the active components, a suitably designed platinum(IV) complex would enhance the circulation lifetime of the prodrug greatly owing to the higher kinetic inertness of such complexes.2 Our laboratory has made similar efforts in the past to successfully develop a combination therapeutic complex of cisplatin and aspirin packaged into a platinum(IV) prodrug called Platin-A and demonstrated that it was capable of diminishing chemotherapy associated inflammation.43–45 With previous experiences along with the associated challenges and opportunities in mind, we blended cisplatin and curcumin into a platinum(IV) complex, Platin-C, which can be reduced to yield the constituent active components and they can be directed to their respective biological action sites.Another added dimension that can provide tremendous additional advantage towards the activity of the prodrug is targeting the mitochondria of tumors.42,46–49 We have designed triphenyl phosphonium (TPP) cation functionalized polymeric nanoparticles (NPs) that, by virtue of their lipophilic delocalized positive charge, are selectively taken up by mitochondria.50–55 Surface modification of the FDA-approved polymer poly(lactic-co-glycolic acid)-block-poly(ethylene glycol) block copolymer (PLGA-b-PEG) with the TPP cation is a promising method of delivering payloads into mitochondria.53–55
Results and discussion
Synthesis and characterization of Platin-C
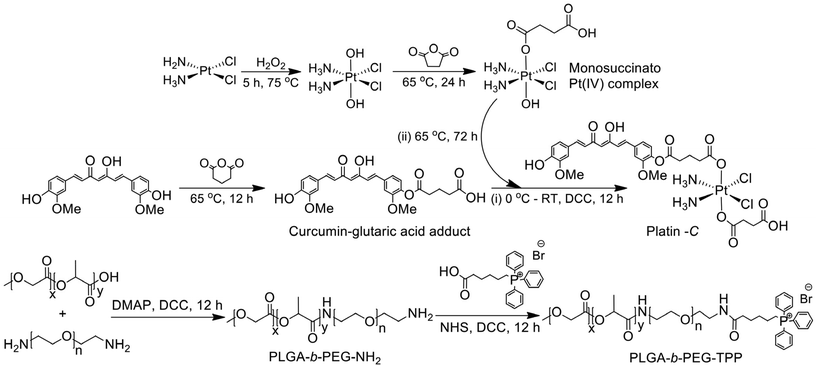
As described above, Platin-C is a Pt(IV) complex tethered to curcumin via a glutaric acid linker at one of the axial coordination sites. Curcumin was reacted with glutaric anhydride to yield the curcumin–glutaric acid adduct, which was then treated with dicyclohexylcarbodiimide (DCC) in the presence of a catalytic amount of 4-dimethylaminopyridine (DMAP) to produce the corresponding anhydride. On the other hand, cisplatin was oxidized using hydrogen peroxide following the well-known literature procedure to generate cis,cis,trans-diamminedichlorodihydroxoplatinum(IV). It was then allowed to react with an equivalent amount of succinic anhydride to generate cis,cis,trans,-diamminedichlorohydroxosuccinatoplatinum(IV). In the final step, this cis,cis,trans,-diamminedichlorohydroxosuccinatoplatinum(IV) was further reacted with the anhydride of the curcumin–glutaric acid adduct to yield the final product i.e., Platin-C. The curcumin–glutaric acid adduct was characterized using 1H and 13C NMR spectroscopy (ESI Fig. 1 and 2†), and its anhydride was prepared freshly and used for subsequent reactions without any purification. cis,cis,trans-Diamminedichlorodihydroxoplatinum(IV) and cis,cis,trans,-diamminedichlorohydroxosuccinatoplatinum(IV) were synthesized using a previously standardized protocol from our laboratory56 and the characteristic peaks in the 1H NMR spectrum confirmed the composition and purity of the desired complexes. The final product, Platin-C, was characterized using 1H NMR, gCOSY, 13C NMR, 195Pt NMR, and ESI-mass spectrometry techniques (Fig. 1 and ESI Fig. 3–5†). The results suggested that Platin-C was produced in analytically pure form. The purity of Platin-C was also ascertained using the HPLC technique (ESI Fig. 6†). The HPLC chromatogram showed distinctly separate peaks for Platin-C and curcumin and the retention time for Platin-C was less than that of curcumin, indicating an increase in the polarity of the resultant complex. The appearance of a peak at ∼1230 ppm in the 195Pt NMR spectrum confirmed the presence of Pt in its +4-oxidation state. The peak at 897.3 in mass spectral data also confirmed the formation of Platin-C. The isotopic peak patterns reconfirmed the neutral nature of the coordination complex as evidenced from the fact that one m/z unit separates the peaks. | ||
| Fig. 1 (A) 1H NMR spectrum of Platin-C recorded using a 400 MHz NMR spectrometer. Solvent residual peaks for dichloromethane, water and dimethyl sulphoxide in DMSO-d6 have been labeled using symbols (in red). (B) gCOSY NMR spectrum of Platin-C in DMSO-d6 recorded using a 400 MHz NMR spectrometer. Individual spectra have also been provided in ESI Fig. 3 and 4.† | ||
Synthesis and characterization of the polymers
Another aspect of this work is associated with the synthesis of the non-targeting PLGA-b-PEG-NH2 polymer and the mitochondria-targeting PLGA-b-PEG-TPP polymer. Since both these polymers yield nanoparticles with positive zeta potentials, with the help of these polymers we intended to validate the role of the lipophilic delocalized positive charge of the triphenylphosphonium (TPP) cation in delivering the drug cargo preferentially to the mitochondria of cells. The non-targeting polymer was synthesized employing DCC/DMAP assisted amide coupling between PLGA-COOH and NH2-PEG-NH2. Careful control of the stoichiometry and reaction conditions during this reaction afforded the formation of the desired polymer rather than the conjugation of PLGA at both ends of PEG. This polymer was further functionalized with TPP hexanoic acid to obtain the targeting polymer. Both the polymeric platforms were characterized using 1H NMR, 13C NMR, and gel permeation chromatography (GPC) techniques (ESI Fig. 7–11†). While NMR ascertained the purity of the synthesized polymers, molecular weights and polydispersity indices of the polymers were determined using GPC. The number average molecular weights (Mn) and polydispersity indices (PDI) of the non-targeting and targeting polymers as determined by GPC using DMF as an eluent were found to be 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1 (PDI = 1.61) and 22
200 g mol−1 (PDI = 1.61) and 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1 (PDI = 1.81), respectively.
600 g mol−1 (PDI = 1.81), respectively.
Synthesis and characterization of the targeting and non-targeting Platin-C nanoparticles
Targeting and non-targeting nanoparticles were loaded with increasing amounts of Platin-C using the nanoprecipitation technique (Fig. 2A). Dynamic light scattering (DLS) analyses revealed that the NPs were 40–80 nm in diameter. Both NT-NPs and T-NPs had a zeta potential of ∼40 mV when they are not loaded with Platin-C but it decreases to ∼30 mV for T-NPs and ∼20 mV for NT-NPs when they are loaded with the Platin-C complex (Fig. 2B and ESI Fig. 12† for NT-Platin-C-NPs). This could be due to the partial neutralization of the positive charges on the polymers by the carboxylate and phenolate groups present on Platin-C. Nonetheless, the polymeric nano-constructs were still found to have positive surface charge and the partial decrease in charge could be indicative of the successful encapsulation of Platin-C. We calculated the percentage loading and percentage encapsulation efficiency (EE) of Platin-C in T-NPs by using different percentage feeds of Platin-C with respect to the polymer used in the nanoprecipitation process. We utilized this library of NPs to quantify Platin-C in the NPs by using platinum-based inductively coupled plasma mass spectrometry (ICP-MS) (Fig. 2C) and employing curcumin optical properties in high-performance liquid chromatography (Fig. 2D). These data evidenced efficacious payload incorporation resulting in functional activities of both the components. On careful observation of the size of T-NPs with increasing Platin-C concentration, we observed that the size started to increase with a loading of 30% or more. So, we anticipated that the sizes of the NPs would go beyond 100 nm for 40% Platin-C feed in T-NPs, and therefore, we carried out all further investigations with NPs having 20% Platin-C feed. A 20% feed of Platin-C into these NPs resulted in ∼7% loading of the complex and this amounts to an encapsulation efficiency of ∼30%. This shows the ability of the polymeric platforms to encapsulate Platin-C successfully into NP formulations and may therefore prove to be efficient delivery vehicles capable of depositing the drug candidate at the site of action in substantially high concentrations. The percentage loading and %EE of Platin-C in NT-NPs at a percentage feed of 20% as determined by HPLC and ICP-MS indicated a similar Platin-C profile to that observed with T-NPs (Fig. 2E). Furthermore, transmission electron microscopy (TEM) images of the NPs showed spherical particles with a uniform size and shape (Fig. 2F).A time dependent release study of the prodrug was performed by dialyzing the T-Platin-C-NPs against 1× PBS at a physiologically relevant pH of 7.4 (Fig. 2G). It is evident that the NPs exhibit a controlled release profile as seen from the gradual release of Platin-C and it took 80 h to achieve a release of ∼70%. Stability studies conducted for 7 days demonstrated that there was no abrupt change in the size and zeta potential of the NPs as measured by DLS (Fig. 2H). So, the NPs prepared for all the subsequent studies were used within a week of their synthesis. As we moved further with the studies as described below, we were able to show that the cisplatin–curcumin combination therapeutic agent, Platin-C, was cytotoxic towards cisplatin resistant A2780/CP70 cells (Scheme 1). Moreover, it was successfully delivered preferentially into the mitochondria of these cells as observed using confocal microscopy and the toxicity of the nanoformulation enhanced by almost 4-fold compared to that of free Platin-C.
 | ||
| Scheme 1 Scheme depicting the strategy adopted for synthesis of the complexes, ligands and polymers. | ||
Cellular cytotoxicity in A2780/CP70 cells
Our primary objective for developing Platin-C is to enhance the cytotoxic potential of platinum drugs. In addition, we also aimed to modify cisplatin in a manner so as to overcome the resistance developed by certain cancer cell types. So, we chose to evaluate the cytotoxic activity of Platin-C in the cisplatin resistant human ovarian cancer cell line A2780/CP70. The results of this experiment appeared to be encouraging. With an IC50 value of ∼26 μM, Platin-C was found to be much less cytotoxic than either cisplatin or curcumin. While cisplatin showed an IC50 value of ∼12 μM, that of curcumin was ∼9 μM. When the cells were incubated with a mixture of both cisplatin and curcumin, the IC50 value decreased further to ∼8 μM. Nevertheless, the toxicity of Platin-C improved greatly when it was packaged into the polymeric nanoformulations. The IC50 value for NT-Platin-C-NPs was ∼11 μM, whereas that for the T-Platin-C-NPs was ∼5 μM. This could be due to the enhancement in the cellular uptake of the complex facilitated by nano-encapsulation. Furthermore, this observation reiterates the importance of drug accumulation inside the mitochondria of cells for the achievement of maximum toxicity. The higher toxicity of the targeting nanoparticles than that of the non-targeting ones could be attributed to the successful delivery of Platin-C into the mitochondria of cells utilizing the lipophilic positive charge on the TPP cation. Also, cisplatin, being one of the reduction products of Platin-C, upon accumulation inside the cellular mitochondria might have easier access to mitochondrial DNA (mt-DNA) thereby triggering cell death via mt-DNA damage.54 On the other hand, despite the positive surface charge on the NT-Platin-C-NPs, the absence of lipophilic character and effective charge delocalization on the amine terminus might have made them incapable of targeting mitochondria efficiently. A comparison of the IC50 values is presented in Fig. 3 and the representative MTT assay plots are presented in ESI Fig. 14.†Mitochondrial colocalization of Platin-C in A2780/CP70 cells
To ascertain the mitochondria targeting capabilities of the nanoparticles, we performed confocal microscopy experiments on A2780/CP70 cells incubated with the nanoparticles (Fig. 4). To our advantage, the green fluorescence of the curcumin ligand enabled monitoring of the intracellular localization pattern of Platin-C NPs. A DAPI containing stain, NucBlue®, was used to stain the nucleus and MitoTracker® Red FM was used to stain the mitochondria of cells. We found that both types of nanoparticles were internalized into the cells as evidenced from the green fluorescence inside the cells. Therefore, both the nanoparticle formulations exhibit enhancement in the cytotoxic potential of Platin-C upon encapsulation as evidenced from the IC50 values mentioned above. Both targeting and non-targeting nanoparticles localized primarily in the cytosol but the extent of colocalization of the T-Platin-C-NPs in the mitochondria was greater than that of the NT-Platin-C-NPs. This was confirmed by determining the Pearson correlation coefficients, which are 0.84 and 0.46 for the targeting and non-targeting NP samples, respectively. This observation confirms our hypothesis that the TPP containing T-Platin-C-NPs were able to target mitochondria more efficiently than the NT-Platin-C-NPs.Mitochondrial activity of T-Platin-C-NPs
The design of our targeting polymeric nano-platforms for the delivery of Platin-C was based on the premise that the drug payload is preferentially delivered to mitochondria. NT-Platin-C-NPs were designed to serve as appropriate controls. To determine the effect of these Platin-C loaded nanoparticles on the health of mitochondria in cancer cells, we performed the Mitostress test on A2780 (Fig. 5A) and A2780/CP70 (Fig. 5B) cells treated with the NP constructs. The Mitostress test monitors the oxygen consumption rate (OCR) of the cells under the influence of oligomycin, FCCP, antimycin-A and rotenone and thereby provides a measure of the mitochondrial respiration of these treated cells. A stark difference was observed between the mitochondrial behavior of the cisplatin sensitive A2780 cells and cisplatin resistant A2780/CP70 cells. While both the targeting and non-targeting NPs were equally effective in inhibiting the mitochondrial performance of both the cell lines, the targeting construct was found to be more effective in the cisplatin resistant cells. This pattern becomes more evident from the basal respiration and ATP production levels of the two treated cell lines. This could be indicative of the fact that in A2780 cells Platin-C induces cell death via damage to both genomic and mitochondrial DNA, while in A2780/CP70 cells, where genomic DNA is resistant to damage induced by platinum drugs, the T-Platin-C-NPs cause cell death by inflicting damage preferentially to the mitochondrial DNA.To further ascertain the effect of T-Platin-C-NPs on cellular mitochondria, we performed a citrate synthase activity assay. Citrate synthase is a mitochondrial enzyme and its activity is often used as a marker for functionally active mitochondria and mitochondrial content.57 In both A2780 (Fig. 5C) and A2780/CP70 (Fig. 5D) cells, treatment with T-Platin-C-NPs demonstrated significantly diminished levels of citrate synthase activity compared to that upon treatment with non-targeting NPs, curcumin, cisplatin or their physical mixture. It must also be noted that the citrate synthase activity was affected by Platin-C leading to comparable levels to those achieved by the targeting nanoparticles. This could mean that the mitochondria directed activity of the targeting nanoformulation is primarily because of the Platin-C prodrug. It is just that the targeting construct is able to direct it to the mitochondria while the non-targeting ones cannot.
Curbing tumor-associated inflammation in cells using Platin-C
Inflammation is intricately associated with tumor development and progression.11 Also, chemotherapeutic agents are known to generate inflammatory response and are therefore responsible for unwanted side effects.43 Platin-C was designed as a combination therapeutic agent that could simultaneously deliver a chemotherapeutic agent, cisplatin, and an anti-inflammatory agent, curcumin. We set out to explore the anti-inflammatory activity of Platin-C and its NPs using western blotting and real-time polymerase chain reaction (RT-PCR) techniques in PC-3 cells (Fig. 6). Bacterial lipopolysaccharides (LPS) engage toll-like receptors (TLRs) to induce immune responses and such TLR agonists have been shown to stimulate PC-3 cells towards the generation of pro-inflammatory cytokines and chemokines.58 We used bacterial LPS to induce inflammatory pathways in these cells. Western blot analyses evidenced the up regulation of TNF-α and NF-κB in these cells and T-Platin-C-NP treatment was able to reduce the elevated levels of these markers more efficiently than NT-Platin-C-NP treatment (Fig. 6A). Quantification of the protein levels further supported the conclusion (Fig. 6B). RT-PCR analyses of the mRNA expression of these markers indicated that both the targeting and non-targeting nanoparticles loaded with Platin-C were able to diminish the levels of pro-inflammatory cytokines, IL-6 and TNF-α, and down-regulate NF-κB and superoxide dismutase-2 (SOD-2) in LPS treated PC-3 cells (Fig. 6C). While NF-κB acts as a key regulator of tumor associated inflammation, such inflammation is also known to elicit SOD-2 upregulation.59 Among the reduction products of Platin-C, cisplatin is incapable of exhibiting any anti-inflammatory activity as reported earlier.43 Therefore the anti-inflammatory activity of Platin-C could be attributed to the curcumin conjugate.Conclusion
This work aimed at designing a Pt(IV)-based combination therapeutic agent and deliver it using suitably decorated polymeric nanoparticles for site-selective delivery and action. In our attempt we have been successful in synthesizing Platin-C by combining cisplatin and curcumin into a Pt(IV) coordination complex. The synthesis of this complex involved multiple step modification of cisplatin and curcumin, which were finally assembled into Platin-C. Positively charged nanoparticle platforms were also developed by suitably modifying PLGA-b-PEG polymers bearing NH2 and TPP termini. The purity of Platin-C, polymers, and the nanoparticles was ascertained by employing suitable characterization techniques. It was found that the nanoparticles were capable of encapsulating Platin-C efficiently into positively charged spherical nanoparticles with uniform sizes and shapes. The nanoparticles were able to deliver the encapsulated complex inside cells and the T-Platin-C-NPs showed enhanced selectivity towards the mitochondria of A2780/CP70 cells. The design of the complex together with the site-selectivity offered by the NPs endowed these nano-constructs with great potential towards eliciting cytotoxicity in these cisplatin-resistant cells. Our observations further emphasized the fact that mere positive charge on the nanoparticle surface may not be enough to evoke mitochondria-targeting properties unless it is protected inside a hydrophobic environment as is the case with the triphenylphosphonium (TPP) cation. Our findings show that Platin-C is capable of inducing mitochondria-directed toxicity as evident from its effect on the mitochondrial respiration pattern and the inhibition of mitochondrial enzymes, more so when it is packaged in mitochondria-targeting polymeric nanoparticles. Finally, we were also able to establish that in addition to exhibiting mitochondria-directed anti-cancer activity in cisplatin-resistant cells, Platin-C is capable of tackling associated inflammatory characteristics.Author contributions
S. D. and B. B. conceived the idea. S. D. provided resources and supervised the research. B. B., A. A., J. H. C., and B. S. performed the experiments. B. B., A. A., J. H. C., B. S., and S. D. analysed the data. B. B. and S. D. wrote the manuscript. All authors approved the final version of the manuscript.Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.Acknowledgements
This work was supported by the Department of Defense Prostate Cancer Idea Award (W81XWH-12-1-0406), and Bankhead Coley Cancer Research grant (8BC10) to S. D. We also thank the Sylvester Comprehensive Cancer Center and Florida Department of Health Bankhead Coley Cancer Research Award (8BC10) for financial support. BB acknowledges the DST-INSPIRE Faculty award (DST/INSPIRE/04/2017/001644; Faculty Registration No. IFA17-CH258) from the Department of Science and Technology (DST), India. We thank the Biomedical Microscopy Core at the University of Georgia for confocal microscopy facility.References
- S. Ghosh, Bioorg. Chem., 2019, 88, 102925 CrossRef CAS PubMed
.
- J. J. Wilson and S. J. Lippard, Chem. Rev., 2014, 114, 4470 CrossRef CAS PubMed
.
- U. Basu, B. Banik, R. Wen, R. K. Pathak and S. Dhar, Dalton Trans., 2016, 45, 12992 RSC
.
- S. Dasari and P. B. Tchounwou, Eur. J. Pharmacol., 2014, 740, 364 CrossRef CAS PubMed
.
- T. Makovec, Radiol. Oncol., 2019, 53, 148 CrossRef CAS PubMed
.
- I. W. Chkar, N. Abdulrahman, H. Al-Sulaiti, J. M. Joseph, S. Uddin and F. Mraiche, J. Transl. Med., 2018, 16, 96 CrossRef PubMed
.
- N. J. Wheate, S. Walker, G. E. Craig and R. Oun, Dalton Trans., 2010, 39, 8113 RSC
.
- S. I. Grivennikov, F. R. Greten and M. Karin, Cell, 2010, 140, 883 CrossRef CAS PubMed
.
- F. R. Greten and S. I. Grivennikov, Immunity, 2019, 51, 27 CrossRef CAS PubMed
.
- N. Singh, D. Baby, J. P. Rajguru, P. B. Patil, S. S. Thakkannavar and V. B. Pujari, Ann. Afr. Med., 2019, 18, 121 CrossRef PubMed
.
- H. Zhao, L. Wu, G. Yan, Y. Chen, M. Zhou, Y. Wu and Y. Li, Signal Transduction Targeted Ther., 2021, 6, 263 CrossRef CAS PubMed
.
- B. Hoesel and J. A. Schmid, Mol. Cancer, 2013, 12, 86 CrossRef CAS PubMed
.
- C. H. Yang, A. Murti, S. R. Pfeffer, L. Basu, J. G. Kim and L. M. Pfeffer, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 13631 CrossRef CAS PubMed
.
- F. Chen, V. Castranova and X. Shi, Am. J. Pathol., 2001, 159, 387 CrossRef CAS PubMed
.
- D. C. Guttridge, C. Albanese, J. Y. Reuther, R. G. Pestell and A. S. Baldwin, Mol. Cell. Biol., 1999, 19, 5785 CrossRef CAS PubMed
.
- E. Akbari, O. Akhavan, S. Hatamie and R. Rahighi, J. Drug Delivery Sci. Technol., 2018, 45, 422 CrossRef CAS
.
- A. Mukerjee, T. J. Sørensen, A. P. Ranjan, S. Raut, I. Gryczynski, J. K. Vishwanatha and Z. Gryczynski, J. Phys. Chem. B, 2010, 114, 12679 CrossRef CAS PubMed
.
- L. Nardo, A. Andreoni, M. Masson, T. Haukvik and H. H. Tønnesen, J. Fluoresc., 2011, 21, 627 CrossRef CAS PubMed
.
- M. A. Tomeh, R. Hadianamrei and X. Zhao, Int. J. Mol. Sci., 2019, 20, 1033 CrossRef CAS PubMed
.
- R. Wilken, M. S. Veena, M. B. Wang and E. S. Srivatsan, Mol. Cancer, 2011, 10, 12 CrossRef CAS PubMed
.
-
A. P. Gupta, S. Khan, M. M. Manzoor, A. K. Yadav, G. Sharma, R. Anand and S. Gupta, in Studies in Natural Products Chemistry, ed. R. Atta ur, Elsevier, 2017, vol. 54, pp. 355 Search PubMed
.
- V. P. Menon and A. R. Sudheer, Adv. Exp. Med. Biol., 2007, 595, 105 CrossRef PubMed
.
- S. J. Hewlings and D. S. Kalman, Foods, 2017, 6, 92 CrossRef PubMed
.
- M. C. Fadus, C. Lau, J. Bikhchandani and H. T. Lynch, J. Tradit. Complement. Med., 2017, 7, 339 CrossRef PubMed
.
- I. O. Alisi, A. Uzairu, S. E. Abechi and S. O. Idris, J. Adv. Res., 2018, 12, 47 CrossRef CAS PubMed
.
-
V. P. Menon and A. R. Sudheer, in The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease, ed. B. B. Aggarwal, Y.-J. Surh and S. Shishodia, Springer US, Boston, MA, 2007, pp. 105 Search PubMed
.
- B. van't Land, N. M. A. Blijlevens, J. Marteijn, S. Timal, J. P. Donnelly, T. J. M. de Witte and L. M'Rabet, Leukemia, 2004, 18, 276 CrossRef PubMed
.
- J. U. Marquardt, L. Gomez-Quiroz, L. O. A. Camacho, F. Pinna, Y.-H. Lee, M. Kitade, M. P. Domínguez, D. Castven, K. Breuhahn, E. A. Conner, P. R. Galle, J. B. Andersen, V. M. Factor and S. S. Thorgeirsson, J. Hepatol., 2015, 63, 661 CrossRef CAS PubMed
.
- S. V. Jovanovic, S. Steenken, C. W. Boone and M. G. Simic, J. Am. Chem. Soc., 1999, 121, 9677 CrossRef CAS
.
- A. Siviero, E. Gallo, V. Maggini, L. Gori, A. Mugelli, F. Firenzuoli and A. Vannacci, J. Herb. Med., 2015, 5, 57 CrossRef
.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Mol. Pharm., 2007, 4, 807 CrossRef CAS PubMed
.
- S. S. Bansal, M. Goel, F. Aqil, M. V. Vadhanam and R. C. Gupta, Cancer Prev. Res., 2011, 4, 1158 CrossRef CAS PubMed
.
- Z. Mirzaie, M. Barati and M. A. Tokmedash, Pharm. Chem. J., 2020, 54, 353 CrossRef CAS
.
- K. Mitra, S. Gautam, P. Kondaiah and A. R. Chakravarty, Angew. Chem., Int. Ed., 2015, 54, 13989 CrossRef CAS PubMed
.
- A. Upadhyay, S. Gautam, V. Ramu, P. Kondaiah and A. R. Chakravarty, Dalton Trans., 2019, 48, 17556 RSC
.
- K. Mitra, S. Gautam, P. Kondaiah and A. R. Chakravarty, Eur. J. Inorg. Chem., 2017, 2017, 1753 CrossRef CAS
.
- R. K. Pathak and S. Dhar, Chem. – Eur. J., 2016, 22, 3029 CrossRef CAS PubMed
.
- R. K. Pathak, C. D. McNitt, V. V. Popik and S. Dhar, Chem. – Eur. J., 2014, 20, 6861 CrossRef CAS PubMed
.
- T. Sarkar, S. Banerjee and A. Hussain, RSC Adv., 2015, 5, 16641 RSC
.
- R. K. Pathak, R. Wen, N. Kolishetti and S. Dhar, Mol. Cancer Ther., 2017, 16, 625 CrossRef CAS PubMed
.
- A. Garai, I. Pant, S. Banerjee, B. Banik, P. Kondaiah and A. R. Chakravarty, Inorg. Chem., 2016, 55, 6027 CrossRef CAS PubMed
.
- B. Banik, K. Somyajit, G. Nagaraju and A. R. Chakravarty, Dalton Trans., 2014, 43, 13358 RSC
.
- R. K. Pathak, S. Marrache, J. H. Choi, T. B. Berding and S. Dhar, Angew. Chem., Int. Ed., 2014, 53, 1963 CrossRef CAS PubMed
.
- R. K. Pathak, U. Basu, A. Ahmad, S. Sarkar, A. Kumar, B. Surnar, S. Ansari, K. Wilczek, M. E. Ivan, B. Marples, N. Kolishetti and S. Dhar, Biomaterials, 2018, 187, 117 CrossRef CAS PubMed
.
- B. Surnar, N. Kolishetti, U. Basu, A. Ahmad, E. Goka, B. Marples, D. Kolb, M. E. Lippman and S. Dhar, Biochemistry, 2018, 57, 6500 CrossRef CAS PubMed
.
- S. Kianamiri, A. Dinari, M. Sadeghizadeh, M. Rezaei, B. Daraei, N. E. Bahsoun and A. Nomani, Mol. Pharmaceutics, 2020, 17, 4483 CrossRef CAS PubMed
.
- C. Gao, Y. Wang, J. Sun, Y. Han, W. Gong, Y. Li, Y. Feng, H. Wang, M. Yang, Z. Li, Y. Yang and C. Gao, Acta Biomater., 2020, 108, 285 CrossRef CAS PubMed
.
- C. A. Reddy, V. Somepalli, T. Golakoti, A. K. Kanugula, S. Karnewar, K. Rajendiran, N. Vasagiri, S. Prabhakar, P. Kuppusamy, S. Kotamraju and V. K. Kutala, PLoS One, 2014, 9, e89351 CrossRef PubMed
.
- R. Wen, B. Banik, R. K. Pathak, A. Kumar, N. Kolishetti and S. Dhar, Adv. Drug Delivery Rev., 2016, 99, 52 CrossRef CAS PubMed
.
- M. P. Murphy, Biochim. Biophys. Acta, Bioenerg., 2008, 1777, 1028 CrossRef CAS PubMed
.
- M. P. Murphy, Trends Biotechnol., 1997, 15, 326 CrossRef CAS PubMed
.
- R. A. J. Smith, C. M. Porteous, A. M. Gane and M. P. Murphy, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 5407 CrossRef CAS PubMed
.
- S. Marrache and S. Dhar, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 16288 CrossRef CAS PubMed
.
- S. Marrache, R. K. Pathak and S. Dhar, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 10444 CrossRef CAS PubMed
.
- A. A. Kalathil, A. Kumar, B. Banik, T. A. Ruiter, R. K. Pathak and S. Dhar, Chem. Commun., 2016, 52, 140 RSC
.
- R. K. Pathak and S. Dhar, J. Am. Chem. Soc., 2015, 137, 8324 CrossRef CAS PubMed
.
- B. Banik, B. W. Askins and S. Dhar, Nanoscale, 2016, 8, 19581 RSC
.
- S. Mukherjee, M. A. Siddiqui, S. Dayal, Y. Z. Ayoub and K. Malathi, J. Inflammation Res., 2014, 7, 89 CAS
.
- L. Yi, H. Shen, M. Zhao, P. Shao, C. Liu, J. Cui, J. Wang, C. Wang, N. Guo, L. Kang, P. Lv, L. Xing and X. Zhang, Sci. Rep., 2017, 7, 7953 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2dt03149c |
| This journal is © The Royal Society of Chemistry 2023 |





