Open Access Article
Open Access ArticleInteraction of 2-propanol with predominantly SrO- and TiO2-terminated SrTiO3(100) surfaces studied by vibrational sum frequency spectroscopy†
Anupam
Bera‡
a,
Denise
Bullert
a,
Matthias
Linke
a,
Steffen
Franzka
b,
Ulrich
Hagemann
 b,
Nils
Hartmann§
b and
Eckart
Hasselbrink§
b,
Nils
Hartmann§
b and
Eckart
Hasselbrink§
 *a
*a
aFakultät für Chemie, Universität Duisburg-Essen, D-45117 Essen, Germany. E-mail: Eckart.Hasselbrink@Uni-Duisburg-Essen.de; Tel: +49 201 183 3055
bInterdisciplinary Center for Analytics on the Nanoscale (ICAN), Universität Duisburg-Essen, D-47057 Duisburg, Germany
First published on 10th August 2023
Abstract
The interaction of 2-propanol with SrTiO3(100) surfaces is studied with a focus on the role of different surface terminations in the deprotonation upon adsorption. Preferential SrO and TiO2-termination was corroborated by AFM, ARXPS and SIMS. Surface sensitive vibrational sum frequency spectroscopy (vSFS) reveals that 2-propanol predominantly adsorbs intact on SrO-terminated SrTiO3(100), but is to a large extent deprotonated on TiO2-terminated SrTiO3(100). The latter is in contrast with the lack of deprotonation of 2-propanol when interacting with thin film TiO2 as reported earlier.
1 Introduction
In the global effort to develop the faculties eventually allowing us a rational design of materials for heterogeneous catalysis, understanding the adsorption chemistry – including structure, composition and orientation of adspecies – is a key factor. This fundamental knowledge is a prerequisite for identifying the mechanistic steps underpinning how specific structural organisation leads to the reactivity and selectivity in the overall catalytic process.Titanium dioxide (TiO2)1–4 and strontium titanate (SrTiO3, henceforth abbreviated as STO)5,6 are among the most studied metal-oxides in catalysis research owing to their great potential for large scale applications. In particular, redox catalytic properties of STO – a perovskite – have been intensely studied and recently reviewed7,8 while the acid–base catalytic properties of this catalyst have been studied to a much lesser extent.7,9 Although oxidation of alcohol species is of high industrial significance, the interaction between 2-propanol and TiO2 and SrTiO3 has received little attention. Recently, Roy and coworkers experimentally and theoretically studied the interaction of 2-propanol with pristine STO(100).10 Furthermore, 2-propanol interacting with TiO2(110)11–13 and ceria(100) was reported.14
Complex surfaces such as the ones of mixed metal oxides exhibit a variety of surface terminations which differ in their acid–base character. Vibrational sum frequency spectroscopy (vSFS) is ideally suited to study the surface chemistry upon adsorption of a probe molecule under near ambient pressure conditions due to its surface specificity. Thus, our present effort aims to identify the role of acid–base properties in SrO and TiO2-terminated STO(100) surfaces by comparing the adsorption behaviour of 2-propanol between these. Specifically, TiO2 and SrO surface terminations (one aspect of the surface reconstruction) of SrTiO3(100) (see Fig. 1) were achieved with the help of thermal and chemical treatments in an acidic environment.
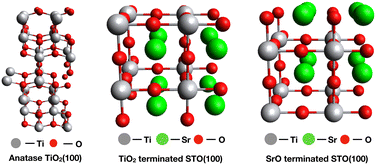 | ||
| Fig. 1 Ideal surface models of anatase TiO2(100) (left), TiO2-terminated STO(100) (middle) and SrO-terminated STO(100) (right) 2 × 2 × 2 supercell structures. | ||
Under real world catalytic conditions for alcohol oxidation – especially in liquid-phase catalysis – some water will always be present as it is formed as a reaction by-product. Hence, we aim in our study to replicate conditions characterized by an ambient atmosphere of alcohol close to its saturation vapour pressure and some residual water which sets this study apart from related ones carried out in air15 or in UHV.13 As a consequence, the sample surfaces will be at least partly hydroxylated because of the non-zero probability for dissociative interactions of H2O with transition metal oxide surfaces.16
We have recently reported on the adsorption chemistry of 1-propanol and 2-propanol on microcrystalline thin film TiO2.17 No indication of deprotonation upon adsorption was detected in contrast to the results reported for methanol18 and ethanol. In view of this background, a detailed comparative study of the adsorption properties on more complex TiO2- and SrO-terminated surfaces would be fundamentally important to gain further insight into the underlying role of the surface's acid–base properties. However, to the best of our knowledge, no comprehensive study on adsorption selectivity by controlling the surface termination of the SrTiO3(100) surface has been reported to date.
In this paper, we build on the preparation of STO(100) samples preferentially terminated by either TiO2 or SrO.19 We report that the propensity of deprotonation of 2-propanol when interacting with the two terminations is vastly different, with no observable deprotonation for the SrO termination and a substantial deprotonated coverage for the TiO2 one. The latter is in contrast with an earlier report by us where 2-propanol stays intact on microcrystalline TiO films.
2 Experimental methods
2.1 Sample preparation
TiO2 thin films of 150 nm thickness were prepared in a local optical workshop by evaporating Ti2O3 on glass slides. They are known to be microcrystalline and have a predominant anatase structure.20 STO(100) surfaces were prepared by following a well-established procedure19,21 consisting of three steps: cleaning, chemical etching and annealing. At first, the substrates which were received from MaTeck were cleaned in acetone, ethanol and 2-propanol using an ultrasonic bath at room temperature for 5 min in each solvent to remove any organic contaminants. In the 2nd step, a buffered hydrofluoric acid solution with pH 4.5 was utilized to remove any basic SrO by dipping into the solution for 30 s to obtain a TiO2-terminated surface. In the 3rd step, the etched samples were baked at 1000 °C in air for 90 min to obtain a TiO2-terminated surface with large flat terraces.22 Alternatively, the baking temperature was increased to 1200 °C and the duration extended to 72 h in order to obtain a SrO-terminated surface due to exdiffusion of Sr from the bulk. As a reference, a sample was also studied, which was prepared starting from the as-received crystal and skipping the annealing step, further referred to as the reference sample. Before recording any vSF spectra, the samples were further cleaned utilizing a two-stage cleaning process: i) exposure to an oxygen plasma (0.4 mbar, 15–30 min) and ii) after mounting in the spectroscopy cell, they were exposed to UV irradiation generated by a Xe lamp (Osram, XBO 150 W/1) in the presence of an oxygen atmosphere (100–200 mbar, 2–5 h).Anhydrous 2-propanol (99.5%) was obtained from Sigma Aldrich and used without further purification.
2.2 Secondary ion mass spectrometry
Time-of-flight secondary ion mass spectrometry (ToF-SIMS) was employed using a TOF.SIMS 5-100 (IONTOF). For surface analysis, the beam from a Bi+ primary ion gun operated in spectrometry mode at 30 kV was scanned in random mode at a field size of 500 × 500 μm2 and a digital raster of 256 × 256 pixels. Complementary measurements were also carried out at 15 kV. Charge compensation was ensured by employing a low energy electron flood gun. The analyzer was operated in positive polarity and corrected in order to compensate for surface potential shifts. The typical surface sensitivity at these parameters corresponds to an information depth of ≤1 nm. Parameters for dual beam depth profiling were chosen in order to ensure high sputter rate ratios, low transient widths, and high vertical resolution. For analysis, the Bi+ primary ion gun in this case was operated at 15 kV, a field size of 100 × 100 μm2, a digital raster of 128 × 128 pixels and otherwise unchanged parameters (cf. above, also considering charge compensation and analyzer settings). Noninterlaced sputtering was performed using a Xe+ source operated at 500 V and a sputter field size of 400 × 400 μm2. After each analysis cycle, a layer of the material was removed in a subsequent sputter cycle at a frame ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Some depth profiling measurements were also carried out operating the primary ion gun at 30 kV and employing a higher analysis and sputter frame ratio of 1
1. Some depth profiling measurements were also carried out operating the primary ion gun at 30 kV and employing a higher analysis and sputter frame ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. ToF-SIMS measurements generally were carried out at 3–5 different positions in order to check for surface heterogeneities. Depths of final sputter craters created with long sputter times were determined from profilometry measurements with a Dektak XT (Bruker) and a Sensofar S-neox (Sensofar). An apparent sputter rate of 0.037 nm s−1 was calculated taking these depth values into account assuming a constant erosion rate.
10. ToF-SIMS measurements generally were carried out at 3–5 different positions in order to check for surface heterogeneities. Depths of final sputter craters created with long sputter times were determined from profilometry measurements with a Dektak XT (Bruker) and a Sensofar S-neox (Sensofar). An apparent sputter rate of 0.037 nm s−1 was calculated taking these depth values into account assuming a constant erosion rate.
2.3 Atomic force microscopy
Surface topography was investigated by atomic force microscopy (AFM), performed in air (RH = 20–30%, T = 21 °C) using a Bruker Dimension Icon AFM in tapping mode. The typical scan rate was 1 Hz and 512 samples per line were acquired. RTESPA cantilevers (nom. resonant frequency: 300 kHz, nom. spring constant: 40 N m−1, nom. tip radius: 8 nm, Bruker) were used. Image analysis was performed using NanoScope Analysis 1.9 (Bruker). The images were flattened and eventually low-pass filtered to remove high frequency noise.2.4 Angle resolved X-ray photoelectron spectroscopy
For angle resolved X-ray photoelectron spectroscopy (ARXPS), a VersaProbe II (Physical Electronics) with a microfocused X-ray source (Mg Kα = 1486.6 eV) was used.2.5 Vibrational sum frequency spectroscopy
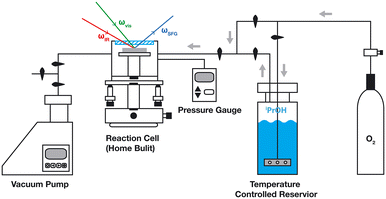
Vibrational sum frequency spectroscopy is based on a non-linear 3-wave mixing process, the details of which were discussed in detail by several authors.23,24 A scanning vSF spectrometer with approx. 12 cm−1 resolution was used (Ekspla PL2231 and PG501DFG) that utilizes wavelength tuneable IR pulses and 532 nm light pulses of 25 ps duration at a repetition rate of 25 Hz for upconversion. Pulse energies of 280 μJ (532 nm) and 10–30 μJ (IR) were utilized. For every data point, typically 300 laser pulses were sampled and averaged. To correct the daily variance in laser performance, the vSF signal from a Au surface was obtained as a calibration reference before recording a spectrum.The experimental set-up was described in detail elsewhere (Fig. 2).18 The samples were mounted in a home-built compact cell (63 mm inner diameter) which allowed for the preservation of a rough vacuum. 50 mbar of 2-propanol, which is close to its saturation pressure, was admitted, utilizing an evaporator. The laser beams enter and the signal leaves the cell through a MgF2 window. The IR and upconversion light beams were directed at the sample at incident angles of 53° and 62°, respectively. And the SF signal reflected from the top surface was recorded.
The spectra were fitted using the following, established expressions:25,26
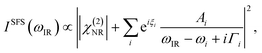 | (1) |
These parameters are real numbers and Ai has a positive value in our model. ξi is the relative phase of the response of each individual line with respect to the non-resonant background. For the ssp polarization combination, the responses of symmetric stretches and anti-symmetric ones of CH stretching modes have a phase difference of π. For the ppp polarization combination, that difference is treated as a fitting parameter.27 We choose to constrain the fitting such that all symmetric modes share a common phase, while in-plane and out-of-plane anti-symmetric modes share different ones in order to limit the number of free parameters.
The surface density of an adsorbed species Ni can be inferred from the Ai parameters derived from the fit to a spectrum as they are connected according to the following expression:28
| Ai = Ni〈ai〉f = Ni ∫ aif(Ω)dΩ, | (2) |
3 Results and discussion
3.1 Sample characterization
Experiments were conducted using i) a reference STO(100) substrate and ii) either preferentially TiO2- or SrO-terminated samples. Moreover, we refer to the vSF spectra obtained in an earlier study using iii) TiO2 nanocrystalline thin films (ca. 150 nm) of predominantly anatase structure deposited on glass substrates.17 The success of the preparation of one particular surface termination was verified by ToF-SIMS and XPS. Noteworthily, however, both ToF-SIMS and grazing-incidence XPS surface analysis sample the surface well beyond the very top layer. The surface morphology was studied using AFM.All three types of STO samples, referred to as the TiO2-terminated sample, SrO-terminated sample and reference sample, were analyzed by ToF-SIMS after carrying out the 2 or 3 initial preparation steps discussed above. Thereafter, the samples were handled in air and introduced into vacuum. As matrix effects are well known to complicate the ToF-SIMS analysis of oxidic materials, the analytical results obtained on the reference sample here are used as a point of reference. Following this approach, the comparison of respective ion intensity ratios, such as the Ti+/Sr+ intensity ratios presented in Fig. 3, is considered to semi-quantitatively reflect corresponding changes of the relative concentration ratios. As expected, the Ti+/Sr+ intensity ratio is significantly higher on the TiO2 terminated sample and the Ti+/Sr+ intensity ratio is significantly lower on the SrO terminated sample when compared with the values obtained on the reference sample. This suggests that the Ti+/Sr+ concentration ratio indeed is higher on the TiO2 terminated sample and lower on the SrO terminated sample.
Fig. 4 shows the corresponding data from ToF-SIMS depth profiling. Again we use the analytical data obtained on the reference sample, i.e., the Ti+Ref/Sr+Ref intensity ratio, as reference for normalization (cf. ESI† for data and discussion of Ti+/Sr+ depth profiles for all three types samples). Noteworthily, at the beginning of the measurements, sputter yields and ionization probabilities are stabilizing. Also, residual surface contaminants from sample storage and transport in ambient air are removed in the first few sputtering cycles. This impedes a detailed quantitative analysis in this transient region with a depth of <1 nm. Clearly, however, the normalized data here point to an increased Ti/Sr concentration ratio in the near-surface region of the TiO2 terminated sample before a constant ratio as in the bulk at a depth of 5–6 nm is reached. Also, even more pronounced, the data indicate a reduced Ti/Sr concentration ratio in the near-surface region of the SrO terminated sample, again approaching a constant ratio in the bulk at a depth of 5–6 nm.
Complementary AFM measurements (for a detailed discussion see ESI†) reveal a rearrangement and ordering of the sample surface structure upon annealing.
The ToF-SIMS data are corroborated by additional X-ray photoemission spectroscopy (XPS) measurements carried out at grazing incidence (Fig. 5). For the discussion, we use the ratio of the Ti signal (Ebind = 458.5 eV) to the sum of two Sr signals, namely the one from SrTiO3 and the one from SrO split by 0.6 eV and located at around 133 eV. The signals show a strong dependence on the emission angle. For the emission along the surface normal, the observed ratios are only a little different for the three samples. However, towards the grazing emission angle, the ratios progressively diverge as this technique progressively probes a smaller depth into the sample. The ratio decreases for the SrO-terminated sample and increases somewhat less pronounced for the other two, consistent with the expected predominance of Sr over Ti or vice versa in the near surface region. Notwithstanding the fact that at the smallest emission angle the data values only span the range from 1 to 0.5, it is worth noting that due to the extended information depth, these values could well point to a significantly larger enrichments of Ti or alternatively Sr at the surface. In view of this mismatch between the information depth at the smallest emission angle of 10° estimated as 1.5 nm and the size of the STO unit cell of 3.91 Å,29 the data are consistent with a near exclusive Sr termination in one case and strongly preferential Ti termination in the other. Noteworthily, the XPS data reveal a higher Ti/Sr ratio for the reference sample in comparison to the TiO2-terminated sample. This is in contrast with the ToF-SIMS results. The difference, however, is comparatively small and might well result from certain analytical complications and differences of the two techniques. The ToF-SIMS analysis could still be affected by matrix effects, for example. Also, a higher surface sensitivity of the ToF-SIMS analysis could explain the difference.
3.2 Surface layer vibrational spectroscopy
The vSF spectrum of 2-propanol in the C–H stretching region was discussed by us before when studying its interaction with TiO2 (Fig. 6).17 With increasing alkyl chain length, the spectra of alcohols become congested as a larger number of C–H modes are to be considered, which often overlap and for which multiple Fermi resonances are possible. The first assignment of the features observed in the vSF spectrum of 2-propanol was made by Wang et al.30,31 and later adopted by Kataoka.32 Yu et al. revised some of the earlier assignments based on a higher resolution Raman study of gas and liquid phase 2-propanol.33 At least four modes are to be expected: namely the CH3 symmetric-stretch, r+, and a related Fermi resonance with the overtone of the degenerated bending mode, r+FR, the CH3 anti-symmetric stretch, r−, and a methine CH stretching mode. The latter is split into two, due to differing interactions with the OH-group in the gauche- and trans-conformation of the methine CH-bond. Furthermore, a combination mode of the two was clearly identified by us when recording the spectra of CD3CHOHCD3.17 The sps spectrum allows us to clearly identify the r− mode at 2972 cm−1. More precisely, this is the out-of-plane mode. Meanwhile, the in-plane mode, located about 12 cm−1 higher in the wavenumber, carries less amplitude and we did not find it absolutely necessary to include it in every fit to a spectrum. In that case, we refrained from including it as the then larger number of parameters would only increase their arbitrariness. The modes at 2880 and 2940 cm−1 are predominant in the ssp spectrum and are assigned to the r+ mode and its Fermi resonance. Which of the two modes is the r+ one and which the Fermi resonance is debated in the literature. Yu et al. reported two further Fermi resonances, located at 2917 and 2933 cm−1 which we are not able to identify in our spectra. The conclusion is that the modes at 2880 and 2940 cm−1 overlap with the ones from the methine group. Hence, we treated them as one feature each in the fitting function, which should be possible as all these are symmetric modes which are anyway to some extent coupled. For this reason, we refrained from evaluating the amplitudes of these modes and only used a mode placed at 2916 cm−1 in the fit function as an indication of the contribution from the methine mode. | ||
| Fig. 6 vSF spectra of a microcrystalline TiO2 thin film surface exposed to 50 mbar of 2-propanol. Spectra were obtained for the ppp, ssp and sps polarization combinations. The ppp spectrum is severely influenced by a non-resonant background. The solid lines represent fits to the data. The dashed lines indicate the centre positions of the bands identified. Noteworthily, although the y-axis is scaled in arbitrary units, these are identical in all figures of the vSF spectra. Adopted from ref. 17. | ||
A detailed orientation analysis following the approach introduced by Kataoka and Cremer32 and adapted by Doughty et al.14 suggests that the symmetry axis of the 2-propyl group is directed nearly in-plane with the surface but that one of the two methyl groups points more upwards, while the other points more towards the surface.17 The C–O bond points then at an angle of 122° with respect to the normal towards the surface allowing the H in the OH group to come close to the surface. However, the determination of definite orientation angles in systems as the ones studied here has to be taken with a grain of salt, as recent ab initio molecular dynamics simulations by us34 demonstrated that the physisorbed molecule carries out large amplitude rotational motions which renders the notion of a preferred geometry at least questionable.
The spectrum of 2-propanol adsorbed on thin film TiO2 can very well be fitted without the need to assume that a fraction of the adsorbate is deprotonated. The deprotonated species generally exhibits a shift of all modes to smaller wavenumbers by about 20–30 cm−1 as was observed when studying adsorbed methanol and ethanol on TiO2.17,18,35 Hence, the lack of this spectral signature suggests that the extent of deprotonation for 2-propanol is too small to detect, pointing at a too low reactivity despite the fact that the O–H moiety is located close to the surface.
Surprisingly, the spectrum obtained for the predominantly TiO2-terminated STO(100) surface suggests a different chemistry (Fig. 7). For our purpose here, it needs to be noted that in particular the ppp spectrum can only be understood when assuming that a second species is present with similar bands but offset by about 21 cm−1. In particular, the feature at 2858 cm−1 is not present in the spectrum of the TiO2 sample. It is identified as the r+ mode of the deprotonated species, and thereby unambiguously indicating its presence in larger abundance. This interpretation is consistent with earlier studies on methanol18,35 and higher alcohol deprotonation.17 Apart from this finding, the coarse difference between the spectra is due to the non-resonant background that is by a factor 1.2 larger for the TiO2-terminated sample. While the relative phases between the modes are similar for TiO2 and TiO2–STO, they are offset by 0.6 rad with respect to the non-resonant background. This results in the r− mode appearing as a dip in the sps spectrum rather than a peak.
The spectrum obtained for the predominantly SrO-terminated surface differs substantially (Fig. 8). Due to the different dielectric response of the surface, the molecular modes exhibit a different phase with respect to the non-resonant background. However, the spectrum shows no indication of deprotonated 2-propanol. Note the lack of the feature at 2855 cm−1 that was indicative in the case of the spectrum of the TiO2-terminated sample.
Furthermore, it is worth noting that the spectra obtained from the reference sample (Fig. 9) also indicate the presence of the deprotonated species. Overall, its structure is rather similar to that of TiO2–STO but with less pronounced features from the deprotonated species, indicating a smaller abundance. This finding is perhaps not surprising as SIMS indicated that the additional Ti enrichment at the surface after the milder baking procedure is only about 10%.
Hence, we are left with the puzzling observation that 2-propanol is deprotonated on the reference sample and the predominantly TiO2-terminated one, but not on the TiO2 thin film nor the predominantly SrO-terminated STO(100) sample.
There are several lines of thought to address this question: this observation is consistent with Sr being a weaker Lewis acid than Ti as reported by Chapleski et al.10 According to their results, TiO2- and SrO-terminated surfaces differ in Lewis-acid character because Ti carries a higher partial charge than Sr with the consequence that 2-propanol binds more strongly by 0.49 eV on STO(100) terminated with TiO2 than on STO(100) terminated with SrO. In another recent work,9 adsorption microcalorimetry was utilized to characterize the surfaces of SrTiO3 with CO2 and NH3. In general, the result showed that SrO-terminated STO(100) has more basic sites and fewer acidic sites when compared with TiO2-termination, which is consistent with the higher Sr/Ti ratio on the SrO–STO sample. However, the strength of the basic or acid sites does not directly correlate with the density of the Ti sites. This finding might suggest that Sr–O or Ti–O sublayers work synergistically to tune the basic/acid properties of the surface.
Generally, the stronger binding goes along with a larger activation of the OH bond, as the strongest interaction of the intact molecule with the surface is via the oxygen atom. As a result, 2-propanol's O–H bond dissociation energies out of the molecularly bound state are higher on the SrO-terminated surfaces than on the TiO2-terminated surfaces. However, it is interesting to note that 2-propanol remains undissociated on thin-film TiO2. Thus, it is most likely an electronic effect that increases Ti′s Lewis acidity in the presence of Sr in STO(100). A more in-depth investigation is needed to confirm this hypothesis.
However, there is no justification for a monotonous relationship between the binding energy of the molecular species and the propensity to deprotonate. As much as the higher binding energy goes along with the weakening of the OH bond, i.e. an elongation of the bond, it stabilizes the system in this state. In other words, the larger binding energy may go along with a larger activation energy towards the transition state. It is at least conceivable that there exists an intermediate regime of binding energies in which the OH bond is elongated but at the same time the barrier towards deprotonation is not prohibitively high. In this speculative interpretation, the surface of STO(100) with predominant TiO2 termination might just have the right energetics, while the binding is too large in the case of the TiO2 films and the OH bond activation too small in the case of the SrO-terminated STO(100) surface.
A further aspect to address in search for the underlying reason is the surface morphology that was characterized by atomic force microscopy in this work (AFM). However, images show in either case large rather flat terraces with single unit cell height steps in the case of TiO2–STO and several cell height steps in the case of SrO–STO, and straight step-edges in either case (see the ESI†). Hence, the surface morphology may play some role but will not be the decisive factor explaining the different reactivity. However, it may be worth noting that Bondarchuk et al.12 reported that disrupting the long range order, e.g. by mild sputtering, inhibited 2-propanol reacting at low temperatures on TiO2(110). Nevertheless, the geometric difference between the lattices of TiO2 and TiO2-terminated STO may cause slight differences in the energetics of the reaction path for 2-propanol deprotonation. Moreover, it must be kept in mind that these experiments were deliberately carried out in a residual atmosphere containing substantial amounts of water vapour in order to resemble the conditions in a real reactor. As a consequence, the sample surface will be largely hydroxylated. No detailed information that would allow the differences of hydroxylation to be quantified for the differently terminated surfaces is available. However, a sufficient number of non-hydroxylated surface oxygen sites are a prerequisite for the conversion of physisorbed 2-propanol to the deprotonated adspecies. In this context, it may be noteworthy that we observed in SIMS a larger abundance of OH-containing species for the SrO-terminated substrate than for the other substrates (see the ESI† for details). It will take further efforts using theory and in situ surface characterisation to come up with answers to these open questions.
4 Conclusion
At room temperature, we found that 2-propanol's adsorption properties depend on the different terminations of STO(100) surfaces by surface sensitive vibrational sum frequency spectroscopy (vSFS). The extent of deprotonation of 2-propanol follows the earlier established surface acidity order of the perovskites STO(100).9,10 The Lewis acidity and extent of deprotonation are as follows: SrO–STO(100) (negligible Lewis acidity) < off-the-shelf STO(100) < TiO2–STO(100).9 A comparison of thin film TiO2 with TiO2-terminated STO(100) indicates that Sr in STO(100) increases Ti′s Lewis acidity.Data availability
The data from vSF spectroscopy shown in Fig. 6–9 are available from the Zenodo data depository.36Author contributions
Anupam Bera: conceptualization, methodology, investigation (vSFS), resources, validation, formal analysis, writing – original draft, and visualization. Denise Bullert: investigation (vSFS). Matthias Linke: formal analysis. Steffen Franzka: investigation (AFM) and formal analysis.: Ulrich Hagemann: investigation (ARXPS) and formal analysis. Nils Hartmann: investigation (SIMS) and formal analysis. Eckart Hasselbrink: conceptualization, writing – reviewing and editing, visualization, supervision, project administration, and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 388390466 – CRC/TRR 247 “Heterogeneous Oxidation Catalysis in the Liquid Phase”. Support from the Interdisciplinary Center for Analytics on the Nanoscale (ICAN) of the University of Duisburg-Essen, a DFG funded core facility (DFG resources reference: RI_00313, project no. 233512597 and 324659309), is gratefully acknowledged. We thank Martin Jerman for making the TiO2 substrates available to us. We have profited from valuable discussions with Sharani Roy.Notes and references
- A. Honda and K. Fujishima, Nature, 1972, 238, 37–38 CrossRef PubMed.
- Q. Guo, C. Zhou, Z. Ma, Z. Ren, H. Fan and X. Yang, Chem. Soc. Rev., 2016, 45, 3701–3730 RSC.
- A. L. Linsebigler, G. Lu and J. T. Yates Jr, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- H. Chen, C. E. Nanayakkara and V. H. Grassian, Chem. Rev., 2012, 112, 5919–5948 CrossRef CAS PubMed.
- J. M. P. Martirez, S. Kim, E. H. Morales, B. T. Diroll, M. Cargnello, T. R. Gordon, C. B. Murray, D. A. Bonnell and A. M. Rappe, J. Am. Chem. Soc., 2015, 137, 2939–2947 CrossRef CAS PubMed.
- J. Mavroides, J. Kafalas and D. Kolesar, Appl. Phys. Lett., 1976, 28, 241–243 CrossRef CAS.
- S. Royer, D. Duprez, F. Can, X. Courtois, C. Batiot-Dupeyrat, S. Laassiri and H. Alamdari, Chem. Rev., 2014, 114, 10292–10368 CrossRef CAS PubMed.
- P. Granger, V. I. Parvulescu, S. Kaliaguine and W. Prellier, Perovskites and related mixed oxides: concepts and applications, John Wiley & Sons, 2015 Search PubMed.
- F. Polo-Garzon, S.-Z. Yang, V. Fung, G. S. Foo, E. E. Bickel, M. F. Chisholm, D.-E. Jiang and Z. Wu, Angew. Chem., 2017, 129, 9952–9956 CrossRef.
- R. C. Chapleski, A. U. Chowdhury, K. R. Mason, R. L. Sacci, B. Doughty and S. Roy, Phys. Chem. Chem. Phys., 2021, 23, 23478–23485 RSC.
- Z. Zhang, O. Bondarchuk, J. White, B. Kay and Z. Dohnálek, J. Am. Chem. Soc., 2006, 128, 4198–4199 CrossRef CAS PubMed.
- O. Bondarchuk, Y. K. Kim, J. M. White, J. Kim, B. D. Kay and Z. Dohnalek, J. Phys. Chem. C, 2007, 111, 11059–11067 CrossRef CAS.
- J. Kräuter, L. Mohrhusen, F. Waidhas, O. Brummel, J. Libuda and K. Al-Shamery, J. Phys. Chem. C, 2021, 125, 3355–3367 CrossRef.
- B. Doughty, S. G. Srinivasan, V. S. Bryantsev, D. Lee, H. N. Lee, Y.-Z. Ma and D. A. Lutterman, J. Phys. Chem. C, 2017, 121, 14137–14146 CrossRef CAS.
- S. Tan, M. B. Gray, M. K. Kidder, Y. Cheng, L. L. Daemen, D. Lee, H. N. Lee, Y.-Z. Ma, B. Doughty and D. A. Lutterman, ACS Catal., 2017, 7, 8118–8129 CrossRef CAS.
- F. Fasulo, G. Piccini, A. B. Muñoz-García, M. Pavone and M. Parrinello, J. Phys. Chem. C, 2022, 126, 15752–15758 CrossRef CAS.
- A. Bera, D. Bullert, M. Linke and E. Hasselbrink, J. Phys. Chem. C, 2021, 125, 7721–7727 CrossRef CAS.
- A. Bera, D. Bullert and E. Hasselbrink, J. Phys. Chem. C, 2020, 124, 16069–16075 CrossRef CAS.
- R. Bachelet, F. Sánchez, F. J. Palomares, C. Ocal and J. Fontcuberta, Appl. Phys. Lett., 2009, 95, 141915 CrossRef.
- M. Jerman and D. Mergel, Thin Solid Films, 2007, 515, 6904–6908 CrossRef CAS.
- F. Gellé, R. Chirita, D. Mertz, M. V. Rastei, A. Dinia and S. Colis, Surf. Sci., 2018, 677, 39–45 CrossRef.
- R. Atif, Appl. Phys. A: Mater. Sci. Process., 2020, 127, 111 CrossRef.
- A. G. Lambert, P. B. Davies and D. J. Neivandt, Appl. Spectrosc. Rev., 2005, 40, 103–145 CrossRef CAS.
- C. S. Tian and Y. R. Shen, Surf. Sci. Rep., 2014, 69, 105–131 CrossRef CAS.
- M. Buck and M. Himmelhaus, J. Vac. Sci. Technol., A, 2001, 19, 2717–2736 CrossRef CAS.
- F. Vidal and A. Tadjeddine, Rep. Prog. Phys., 2005, 68, 1095–1127 CrossRef CAS.
- N. Ji, V. Ostroverkhov, C.-Y. Chen and Y.-R. Shen, J. Am. Chem. Soc., 2007, 129, 10056–10057 CrossRef CAS PubMed.
- X. Wei, X. Zhuang, S.-C. Hong, T. Goto and Y. R. Shen, Phys. Rev. Lett., 1999, 82, 4256–4259 CrossRef CAS.
- E. N. Maslen, N. Spadaccini, T. Ito, F. Marumo and Y. Satow, Acta Crystallogr., Sect. B: Struct. Sci., 1995, 51, 939–942 CrossRef.
- R. Lu, W. Gan, B.-H. Wu, Z. Zhang, Y. Guo and H.-F. Wang, J. Phys. Chem. B, 2005, 109, 14118–14129 CrossRef CAS PubMed.
- H.-F. Wang, W. Gan, R. Lu, Y. Rao and B.-H. Wu, Int. Rev. Phys. Chem., 2005, 24, 191–256 Search PubMed.
- S. Kataoka and P. S. Cremer, J. Am. Chem. Soc., 2006, 128, 5516–5522 CrossRef CAS PubMed.
- Y. Yu, Y. Wang, N. Hu, K. Lin, X. Zhou and S. Liu, J. Raman Spectrosc., 2014, 45, 259–265 CrossRef CAS.
- A. H. Omranpoor, A. Bera, D. Bullert, M. Linke, S. Salamon, S. Webers, H. Wende, E. Hasselbrink, E. Spohr and S. Kenmoe, J. Chem. Phys., 2023, 158, 164703 CrossRef CAS PubMed.
- C.-Y. Wang, H. Groenzin and M. J. Shultz, J. Phys. Chem. B, 2004, 108, 265–272 CrossRef CAS.
- A. Bera, D. Bullert and E. Hasselbrink, Zenodo data depository, 2023, DOI:10.5281/zenodo.7645699.
Footnotes |
| † Electronic supplementary information (ESI) available: (i) Characterization of the samples with AFM, SIMS and ARXPS. (ii) Obtained fitting parameters for the vSF spectra. See DOI: https://doi.org/10.1039/d3cy00310h |
| ‡ Present address: Institute of Physical Chemistry, Universität Jena, D-07743 Jena, Germany. |
| § Center for Nanointegration (CENIDE), Universität Duisburg-Essen. |
| This journal is © The Royal Society of Chemistry 2023 |