Open Access Article
Open Access ArticleIonic liquids revolutionizing biomedicine: recent advances and emerging opportunities
Yanhui
Hu†
abcd,
Yuyuan
Xing†
abc,
Hua
Yue†
 ac,
Tong
Chen
cd,
Yanyan
Diao
ac,
Tong
Chen
cd,
Yanyan
Diao
 *abc,
Wei
Wei
*abc,
Wei
Wei
 *ac and
Suojiang
Zhang
*ac and
Suojiang
Zhang
 *abc
*abc
aBeijing Key Laboratory of Ionic Liquids Clean Process, CAS Key Laboratory of Green Process and Engineering, State Key Laboratory of Multiphase Complex Systems, State Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China. E-mail: yydiao@ipe.ac.cn; weiwei@ipe.ac.cn; sjzhang@ipe.ac.cn
bInnovation Academy for Green Manufacture, Chinese Academy of Sciences, Beijing 100190, China
cCollege of Chemical and Engineering, University of Chinese Academy of Sciences, Beijing 100049, China
dChengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu 610041, China
First published on 26th September 2023
Abstract
Ionic liquids (ILs), due to their inherent structural tunability, outstanding miscibility behavior, and excellent electrochemical properties, have attracted significant research attention in the biomedical field. As the application of ILs in biomedicine is a rapidly emerging field, there is still a need for systematic analyses and summaries to further advance their development. This review presents a comprehensive survey on the utilization of ILs in the biomedical field. It specifically emphasizes the diverse structures and properties of ILs with their relevance in various biomedical applications. Subsequently, we summarize the mechanisms of ILs as potential drug candidates, exploring their effects on various organisms ranging from cell membranes to organelles, proteins, and nucleic acids. Furthermore, the application of ILs as extractants and catalysts in pharmaceutical engineering is introduced. In addition, we thoroughly review and analyze the applications of ILs in disease diagnosis and delivery systems. By offering an extensive analysis of recent research, our objective is to inspire new ideas and pathways for the design of innovative biomedical technologies based on ILs.
1 Introduction
Ionic liquids (ILs) are a class of molten salts, exhibiting a vast diversity with nearly 1018 potential combinations.1 ILs have a history of more than 100 years, starting with the synthesis of liquid ethylammonium by Walden in 1914.2 However, the first-generation ILs were unstable and sensitive to water and air, which limited their applications. The second-generation ILs emerged in 1992 with the synthesis of a stable 1-ethyl-3-methylimidazolium cation IL,3 in which the anions were replaced by weak coordination anions such as tetrafluoroborate and hexafluorophosphate ([BF4] and [PF6]). From then on, the research of ILs has progressed rapidly. After 2000, ILs can be functionally designed according to their physicochemical properties; so ILs have rapidly entered into the third-generation.4 Compared with the previous two generations, the third-generation ILs have been developing quickly. They are biodegradable and biocompatible (such as natural alkalis like choline, amino acids, and carboxylic acids). Throughout the development history of ILs, from unstable to stable states, and then to the designability of structures, ILs have gradually played an important role in many fields such as chemistry, energy, and machinery.As early as 2001, ILs were researched as antibacterial agents.5 Since then, the third-generation ILs have gradually been explored in multiple fields of biomedicine. ILs’ diverse ranges of structures allow for the customization of their physiochemical properties, making ILs highly advantageous in pharmaceutical applications. Specifically, ILs demonstrate potential in the development of drugs with antibacterial and anticancer properties by virtue of their ability to disrupt pathogen cell membranes and organelles.6,7 Another notable advantage is their miscibility, which can significantly improve the solubility of drugs and enhance the bioavailability of poorly soluble drugs. Furthermore, the tunability of ILs allows for the customization of their properties, including acidity, alkalinity, and H-bonds, which makes ILs highly versatile in pharmaceutical engineering, particularly as drug extractants and catalysts. Moreover, the modular nature of ILs enables the design of targeted drugs with responsive release capabilities. By tailoring the properties of ILs, drugs can be precisely released on-demand, enhancing their therapeutic efficacy. ILs can also form clusters, allowing for efficient loading and delivery of drugs, which can encapsulate drug molecules, protect drugs from degradation, and facilitate their controlled release at the desired site of action. Additionally, the high conductivity of ILs opens up exciting possibilities for their application in disease diagnosis. Overall, by selecting appropriate cations and anions, ILs can act as drug candidates, drug assistants,8 vaccine adjuvants,9 drug carriers,10,11 tissue engineering (TE)12 materials or biosensors.13 The extensive research conducted on ILs in the biological field reveals their immense potential for further development.
Compared with review papers on ILs in chemical engineering, there are far fewer review papers available on the applications of ILs in biomedicine. Nevertheless, the pilot summaries of the bio-application offer important insights into related designs of ILs. In 2017, Ananikov's group summarized the biological activities of ILs and their applications in pharmaceutics and medicine, focusing on the bioactivity of ILs and applications of ILs in drug synthesis and drug components.1 Subsequently, they further summarized the properties of ILs that could be employed as solvents and their auxiliary effects on drug bioavailability or delivery in 2018.14 In 2019, Gomes et al. summarized the ecotoxicology of biocompatible ILs, especially choline ILs, and their designs in pharmaceutical delivery and TE.15 Since then, there has been a rapid development of ILs in biomedicine with hundreds of papers published involving different types of ILs. However, there is a scarcity of systematic analyses and summaries, which are urgently needed to provide ideas for the designing and applying the next generation of ILs.
To tackle this gap, we conducted a comprehensive survey of over 190 papers published in the past five years on ILs in bio-related fields, focusing on the ILs’ tertiary structures (from molecular structures, to H-bonding networks, and to clusters) or other tunable physiochemical properties. Additionally, we concluded the experimental and simulation mechanisms of ILs as drug candidates from the level of cell membranes–organelles–protein-molecules, to provide ideas for the design of efficient and low-toxicity drugs. In particular, the application of ILs in new carrier/assistant systems, including drug delivery, imaging, and vaccine delivery, has been comprehensively reviewed and analyzed. By providing a comprehensive analysis of recent research in this area, we aim to inspire new ideas and avenues for the design of innovative IL-based biomedical technologies.
2 Structures and properties
2.1 Molecular composition of ILs
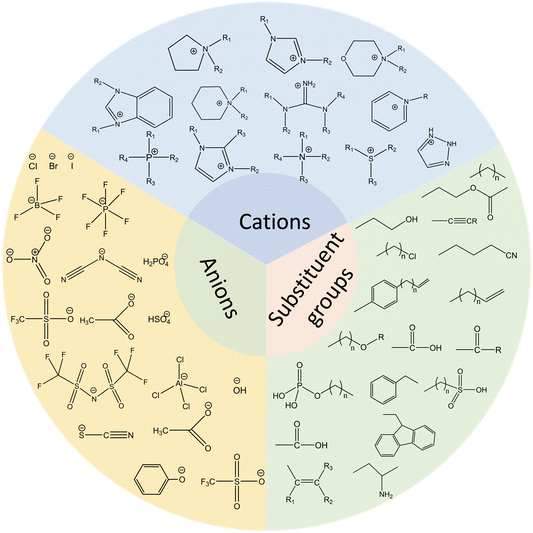
The widespread application of ILs, provided by the ability to easily tune anions and cations, has resulted in an overwhelming variety of ILs.16 Although there are many classifications for ILs according to the cation, proton or non-proton, or acid–base or neutral content, typical ILs consist of three parts: cations, anions, and substituent groups. Fig. 1 shows common ILs’ structures. In terms of cations, they are organic in nature and can be classified into imidazole, pyridine, piperidine, amine, pyrrole, morpholine, and phosphine. For anions, they can be organic (amino acid salt, benzene sulfonate, etc.) or inorganic (halide, tetrafluoroborate, hexafluorophosphate, etc.). Functional groups, such as cyano, hydroxyl, ether, amino, sulfonic, ester, and carboxyl, introduced into the structure can realize functionalized ILs with different properties.17–20 In general, the selection of anions and substituents can affect the acidity and alkalinity of ILs.A full understanding of the microstructures of ILs provides a research basis for the cognizance of the properties and possible applications. The diverse structures of ILs provide the ability to control the quantitative structure–activity relationship in terms of biological and chemical properties by precise changes in chemical structures, thereby increasing the range of applications and trials in the pharmaceutical industry. For example, the design and synthesis of targeted groups with pH (e.g., imine bond, carboxyl group), optical (e.g., azobenzene and cinnamic acid group), and magnetic (e.g., combined with magnetic nano) responses are based on the characteristics of ILs with many kinds and strong designability. By selecting the appropriate combination of cations and anions, scientists can construct different biomedical ILs.
2.2 Interaction forces in ILs
The unique properties of ILs are closely related to their structure and the interactions between ions. The structural parameters of ILs also have a significant impact on drug loading and release behavior.21 The properties of ILs are governed by various forces, including Coulombic, dispersion, H-bonding forces, van der Waals interactions (dipole-induced dipole, dispersion), and possible π–π or n–π stacking, which can be adjusted by changing the type of cation or anion and the length or the number of attached organic chains.22,23 H-bonds, which are electrostatic attractions between protons in one molecule and negatively charged atoms in another, are crucial for the structure and interaction modes of ILs.24H-bonds play a crucial role in the stability and miscibility of miscible systems,25,26 as well as determining the hydrophilic and hydrophobic properties of ILs.27 In general, ILs interact with water primarily through the anions in the aqueous solution.28 For example, the strength of H-bonds between water and anions follows the order [PF6]− < [SbF6]− < [BF4]− < [(CF3SO2)2N]− < [ClO4]− < [CF3SO3]− < [NO3]− < [CF3CO2]−.29 The existence of a small amount of water would also have a great impact on the H-bonding network of ILs.30 Water and ILs’ anions form new H-bonds, which could destroy the H-bond interaction between ion pairs in the original ILs. It should be noted that the cations of ILs also have a significant influence on the hydrophilicity and hydrophobicity of ILs.31 Given that hydrophilicity is a critical factor in the application of ILs in water-based systems and that water is the predominant substance in physiological environments, modular and interactive methods can be used to design ILs by understanding and predicting the impact of H-bonds. Specifically, the disadvantages of given anions with low water solubility can be mitigated by changing the counter cation with the ability to strengthen the H-bonds with water.31
H-bonding networks can be present in pure ILs. The strong interactions between anions and cations, as well as the large molecular volume and H-bond directions, allow ILs to self-assemble into various heterogeneous structures in a medium, such as vesicles, micelles, and microemulsions (Fig. 2A).32 These IL clusters are crucial for interpreting many physical phenomena of ILs, such as heterogeneous self-diffusion, surface layering, and surfactant-like micelles formed in IL–water mixtures.33 Vesicles are membrane-like self-assembled hollow structures formed by amphiphilic substances in solvents.34 Wang et al. first observed that 1-alkyl-3-methylimidazolium bromides [CnMIM][Br] (n = 10, 12, and 14) could be self-assembled to unilamellar vesicles in aqueous solutions without any additives.35 In this study, it was found that the clusters formed by ILs in water were larger than 200 nm. Micelles are amphiphilic structures, typically consisting of a hydrophilic periphery and a hydrophobic core, which can be used for encapsulating hydrophobic drugs. Goto et al. synthesized IL-based micelles, which incorporated a large number of drugs into the hydrophobic core through H-bonding interactions between ILs and the drug molecules.36 Microemulsion is usually defined as a stable, isotropic mixed solution composed of water, oil, a cosurfactant, and a surfactant, with three types: oil-in-water, water-in-oil, and bicontinuous.37,38 For example, the encapsulation of rifampicin was achieved through the use of a microemulsion system, which comprised the hydrophobic IL 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIM][PF6]), along with non-ionic surfactants (Brij35, TX100) and water. In this system, [BMIM][PF6] acted as the oil phase. The rifampicin was loaded into the microemulsion near the palisade layer/apolar side or towards the core of the microstructures.39 This method offers an enhanced approach for drug loading, stability, and controlled release properties.
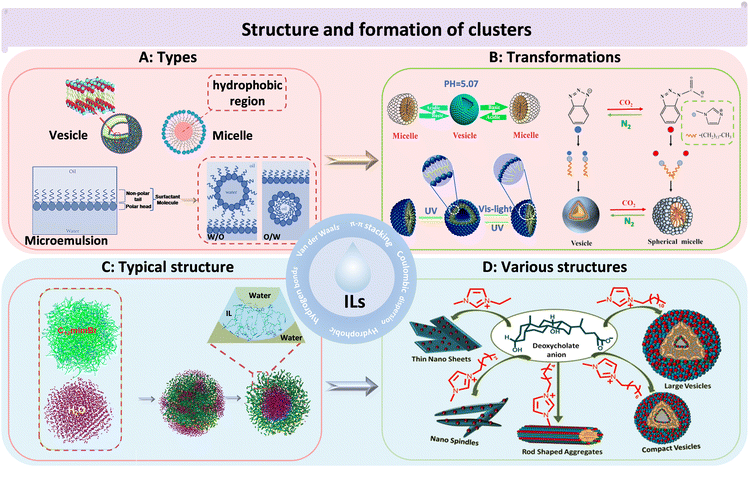 | ||
| Fig. 2 The structures and morphologies of ILs. (A) The categories of IL-based clusters. Vesicle (left), micelle (right), microemulsion (bottom). Reproduced with permission from ref. 34 and 38. Copyright 2015 Royal Society of Chemistry and 2017 Elsevier. (B) The transformations of IL clusters under different environmental conditions. pH-induced reversible transition of [C14MIM][H4COOKCOO] clusters in aqueous solution (left). UV/light-induced reversible transition of 4-butylazobenzene-4′-hexyloxytrimethyl-ammoniumtrifluoro-acetate clusters in aqueous solution (bottom). [C14MIM][1,2,3-Ben] cluster transition by bubbling CO2 (right). Reproduced with permission from ref. 40–42. Copyright 2014 American Chemical Society, 2015 Royal Society of Chemistry and 2022 Elsevier. (C) The clusters of [C12MIM][Br] in water. Green: [C12MIM]+, magenta: H2O.43 Reproduced with permission from ref. 43. Copyright 2015 American Chemical Society. (D) The cluster structure of [Cnmim][DC] changed with the length of the carbon chain.44 Reproduced with permission from ref. 44. Copyright 2018 American Chemical Society. | ||
The types of clusters formed by ILs could be transformed under different conditions (Fig. 2B). For instance, the vesicular and micellar structure of ILs could be altered by adjusting the pH.40 In another example, reversible transformations between micelles and vesicles of ILs (4-butylazobenzene-4′-hexyloxytrimethyl-ammoniumtrifluoro-acetate) were achieved through UV and visible-light irradiation, which could be exploited for light-responsive drug delivery.41 Similarly, 1-tetradecyl-3-methylimidazolium 1,2,3-benztriazole ([C14MIM][1, 2, 3-Ben]) exhibited a reversible conversion structure from vesicles to micelles in the presence of alternating CO2 and N2 bubbling.42 Chandrakar and Bhargava investigated the aggregation of a hydroxy functionalized IL (1-(n-hydroxyalkyl)-3-(n-hydroxyalkyl) imidazolium bromide ([HOCnCmOHIm][Br]) (n = 2, 6, 10, or 14) in aqueous solutions using atomistic molecular dynamics (MD) simulations.45 They found that the length of the hydroxyalkyl chains played a key role in determining the structures of the formed clusters. Specifically, the length of the substituent determined the ILs’ morphology. Small clusters could be formed when one of the substituents was a hydroxydecyl chain. However, when the substituent was hydroxyldecyl or hydroxyl tetradecyl chains, the solution structure was arranged as a thin film. Additionally, the concentration of ILs affected the types of clusters, with spherical micelles transitioning to rod-like micelles and then to vesicles with increasing concentration35 The morphology of ILs in water was typically spherical, with imidazole rings being mainly located near the interface between vesicles and water and dodecyl groups buried in two layers composed of imidazole rings (Fig. 2C).43 Besides being spherical, IL clusters can also take on other shapes, such as thin nanosheets, nanospindles, and rod shaped clusters (Fig. 2D).44 In addition to the diversity of morphology, for size, simulations and TEM studies have revealed that IL clusters, which were primarily focused on IL complexes could range in size from a few nanometers to several hundred nanometers.36,37,39,44
Currently, research on IL clusters has been widespread, focusing on their formation principles and compositional structures. IL clusters are highly influenced by cationic side chain lengths and anion types, which can lead to a diverse range of possible spatially organized structures. For example, Wang and Voth explored the effect of various cationic side chain lengths on ILs by a multiscale coarse graining. The simulation results showed that neutral tail groups of cations aggregated to form spatially heterogeneous domains of the tails with sufficient side chain length, while the charged head groups and anions distributed homogeneously due to the strong electrostatic interactions.46 Wang's team discovered that the hydrophobicity and aromaticity of IL anions played a crucial role in the formation of clusters. The influence of anions on the formation of IL clusters was attributed to the synergistic effect of hydrophobic and π–π interactions.42
A comprehensive understanding of the self-assembly mechanism of ILs can provide valuable theoretical guidance and assistance for designing IL clusters with various sizes, types, shapes, and properties for different applications. In particular, when combined with biomedical research, such as the mechanism of drug delivery and interaction with various organisms, ILs' aggregations hold great potential and merit further attention and in-depth investigation. Notably, the occurrence of experimental phenomena cannot be fully explained when ILs are completely composed of ions in the industrial field.2 Currently, molecular simulation is the primary method of predicting the formation process and structural characteristics of IL clusters. The behavior and morphology of IL clusters as biological carriers need to be further validated by experiments to fully uncover the true IL cluster characteristics. In addition, despite significant progress in the use of ILs in biomedicine, their underlying mechanisms remain unclear. Many structural factors related to ILs require investigation, e.g., their states in vivo and in vitro, their impacts on cells, and whether cluster formations affect metabolic processes in vivo.
2.3 Physicochemical properties of ILs
In addition to the IL cluster structure, other inherent properties of ILs, such as the melting point, electrochemical characteristics, and solubility, are closely related to their wide range of applications in various fields. ILs usually have a melting point below 100 °C and are liquid at room temperature, making them beneficial for use in medicine at physiological temperatures. The main reason for the low melting point of ILs is that the ion asymmetry forms a loose structure, which cannot be closely stacked. The melting point of ILs is also related to ionic size, delocalization of charge, and H-bonds.47–49 Deep eutectic solvents (DESs) are mixtures based on ILs that have a lower melting point than any of their individual components (e.g., choline chloride and urea).50 Generally, DESs can alter the physicochemical properties of ILs, such as the melting point, density, viscosity, and surface tension.51 Third-generation ILs and DESs have been gradually developed with continuously improved formulae in the fields of drug synthesis and drug delivery because of their liquidity at room temperature and good biocompatibility.ILs possess excellent conductivity, due to the wide electrochemical potential window and medium conductivity, typically between 10 and 20 mS cm−1.52 And, their liquid state allows for the free movement of anions and cations, which contributes to their inherent conductivity. The wide electrochemical potential window represents the stable electrochemical properties and mainly depends on the reduction resistance of cations and the oxidation resistance of anions.53 Conductivity is a measured value indicating the ability of a material to transmit electrons. Generally, high conductivity is accompanied by high liquid density, low viscosity, small ionic size, high mobility, etc.4,54 These factors are usually related to both external (environment) and internal (the structure of ILs) factors. For example, the viscosity of ILs is positively correlated with temperature. Thus, temperature is also a factor to be considered for conductivity. For internal factors, the types of cation and anion can affect the conductivity of ILs.55–57 The wide electrochemical window and superior electrochemical performance of ILs lead to their application in diagnostic medicine, such as using as an electrolyte and a modified electrode in the production and improvement of the performance of sensors.
Solubility is a crucial property to be considered in the process of drug development because it can directly affect the bioavailability of drugs in vivo. Inorganic and organic compounds have shown remarkable solubility in various ILs. The primary distinguishing characteristic between ILs and traditional organic solvents is solvent miscibility behavior, particularly with water. Generally, ILs that contain hydrophilic groups like hydroxyl or carboxyl groups can form a uniform mixed phase with water; while ILs with highly fluorinated and charged delocalized anions, such as bis(trifluoromethanesulfonyl)imide ([NTf2]) and [PF6] undergo liquid/liquid two-phase separation with water.58 This behavior can be attributed to the weak interaction between ILs and water.59 Moreover, the hydrophilicity of ILs continues to decrease with the length of the cation alkyl chain.60 The hydrophilicity and hydrophobicity of ILs can be regulated by adjusting anions, cations and substituents. For example, the hydrophobicity of [NTf2] can be mitigated by incorporating compensating cationic hydrophilic groups that enhance the IL's ability to accept and donate H-bonds to water.59 Therefore, most ILs have amphiphilic properties serving as a bridge between water and insoluble drugs and providing excellent solubility for many active pharmaceutical ingredients.
As neoteric solvents, ILs offer the potential to predict, adjust, and design properties, such as polarity, hydrophobicity, and solvent compatibility by imparting the different substituents, cations, and anions.61 Biocompatible ILs are often derived from natural or human metabolites, such as choline and phospholipid derivatives as cations and long-chain fatty acids, amino acids, and carboxylic acids as anions.61–64 In contrast, some research on toxicity of ILs suggests that it is possible for them to be used as active drug ingredients.65 Given the advantages of ILs, such as a low melting point, morphological diversity, and excellent miscibility, we should expand our horizons and make use of their toxicity and biocompatibility in biomedical applications.
3 Therapeutic components and mechanism
3.1 ILs as drug candidates
ILs have shown potential in the treatment of various diseases, including bacterial and fungal infections, inflammation, cancer, and other illnesses. The wide range of possible cation and anion combinations in ILs allows for the design of molecules with inherent resistance to specific diseases. Additionally, existing drugs can be modified into ILs to improve their therapeutic properties, such as solubility and absorption rates. In this section, we will discuss and summarize the application potential of ILs in antibacterial, antifungal, anti-inflammatory, anticancer and other diseases in recent years. Fig. 3 shows some examples of the application of ILs as drug candidates including as antibacterial, antifungal, anti-inflammatory, anticancer, and anti-oxidative agents.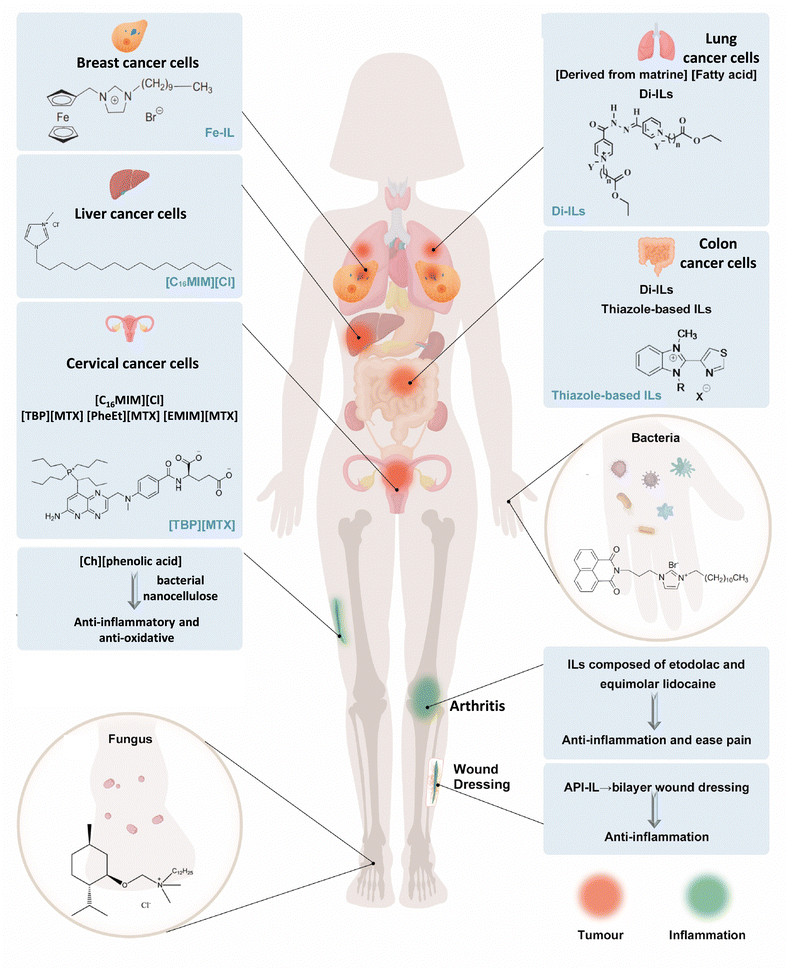 | ||
| Fig. 3 Schematic diagram of ILs as drug candidates including as antibacterial, antifungal, anti-inflammatory, anticancer, and anti-oxidative. The top human body represents the application exploration of ILs as anticancer agents.65–73 The bottom human body represents the application exploration of ILs as antibacterial, antifungal, anti-inflammatory, and anti-oxidative.74–78 The IL application in this picture does not represent in vivo experiments. | ||
Cations with an increasing number of alkyl side chains exhibit significant antimicrobial activity.84 This phenomenon, known as the ‘side-chain effect,’ refers to the heightened toxicity observed in longer alkyl side chains due to their increased lipophilicity.74,84–87 This higher lipophilicity contributes to the enhanced antimicrobial properties of the ILs. Recently, we found that the antibacterial activity of ILs increased with the increase of the cation side chain. Driven by hydrophobicity, the long cation side chain of ILs could penetrate the cell membrane of S. aureus and cause complete disruption (Fig. 4A).82 Chao et al. demonstrated that ILs with longer alkyl chains exhibited enhanced antibacterial effects against S. aureus and E. coli. These effects were attributed to the interplay of hydrophobic and electrostatic interactions between the ILs and bacterial membranes.83 However, there is a cut-off point beyond which the toxicity does not indefinitely increase.88 This phenomenon may be caused by the insufficient solubility of ILs, a decrease in perturbation, kinetic aspects, or an increase in spatial hindrance.89,90
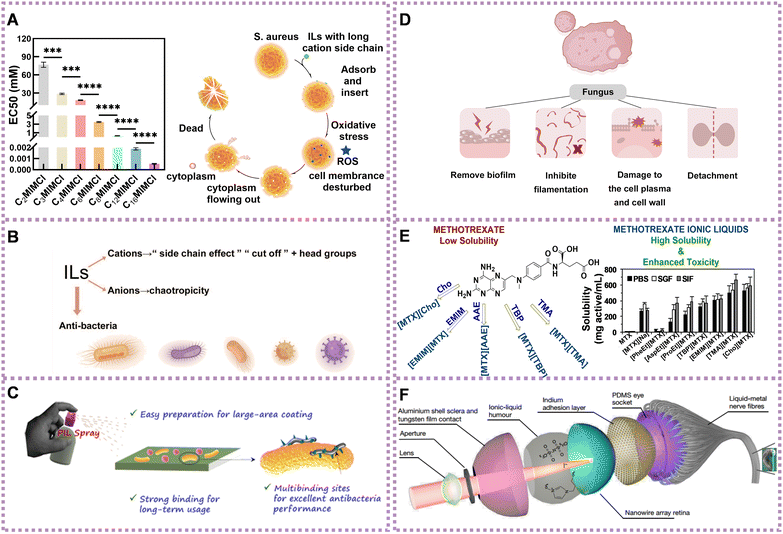 | ||
| Fig. 4 Application of ILs in the treatment of diseases. (A) The toxicity of ILs to S. aureus increasing with cation side chain (left) and the antibacterial mechanism of ILs with long cation side chain against S. aureus (right). Reproduced with permission from ref. 82. Copyright 2023 by the authors, licensee Frontiers Media S.A. (B) Summary diagram of antibacterial activity of ILs. (C) Schematic diagram of universal PIL-based antiseptic spray. Reproduced with permission from ref. 91. Copyright 2021 American Chemical Society. (D) Summary of the antifungal mechanism of ILs. (E) Structure (left) and anticancer activity of several ILs (right). Reproduced with permission from ref. 70. Copyright 2019 Elsevier. (F) Detailed structure of spherical biomimetic electrochemical eye. Reproduced with permission from ref. 92. Copyright 2020 Springer Nature. | ||
Moreover, the antibacterial effect of ILs can be tuned by changing the type of cation, or modifying the cation head group. When the alkyl chain length was fixed to 12, the antibacterial activities of the following cations decreased in the order: 1-methyl-imidazolium > piperidinium > pyrrolidinium > morpholinium for S. aureus, S. epidermidis, E. coli, and E. faecalis. When substituents such as alcohol, glucose, ether and terpene were introduced, the antibacterial activity of ILs decreased.93 When the tris(pentafluoroethyl)trifluorophosphate anion ILs encountered hydrophobic or bulky cations, they could form a highly correlated ion pair, leading to a significant reduction in ion-mediated toxicity. A possible explanation was that the ion pair was no longer permeable into the bacterial membrane.85
Compared with the cationic ILs, the influence of the anionic ILs has been less investigated. However, the antibacterial behavior of anions has been an important factor, especially for ILs with less toxic cations.94–96 When paired with chaotropic anions, ILs with cations exhibit enhanced antibacterial activity compared to ILs with non-chaotropic anions. The stronger chaotropic anions promote destabilization and disruption of bacterial cell membranes, increasing the susceptibility of bacteria to the ILs' antimicrobial effects.97 Based on the above, the antibacterial mechanism of ILs could be modulated and controlled through the mediation of side chains, head groups, and chaotropicity (Fig. 4B).
In addition, monomers with antimicrobial properties are often synthesized into IL polymers (PILs) with antibacterial effects. These PILs can be fabricated into various formulations, such as membranes and sprays, with unique advantages, such as long-term stability and effectiveness or ease of carrying, to fulfill different roles in different situations. Zhang et al. developed PIL-based microneedle (PIL-MN) patches loaded with salicylic acid to treat acne infections. The PIL-MN patches were synthesized by linking the 3-heptyl-1-vinylimidazolium IL, which exhibited high antimicrobial activity against Gram-positive S. aureus, Propionibacterium acnes, as well as Gram-negative E. coli.98 He et al. constructed a PIL brush-grafted biomimetic sharklet surface to prevent biofouling. The PIL brushes demonstrated good antibacterial properties against both S. aureus and E. coli and anti-bovine serum protein adhesion activity, resulting from the synergistic effect of the cationic imidazole and benzotriazole groups.99 Guo et al. found that tryptophan ions showed a synergistic effect with cations, leading to enhanced antibacterial activity compared to proline and bromine ions. They observed morphological changes in S. aureus and E. coli strains exposed to the surface of the intrinsically antibacterial poly IL membrane.100 As shown in Fig. 4C, Liu et al. prepared an efficient and robust antiseptic spray based on nonvolatile PILs, which indicated long-term antibacterial activity against both Gram-negative and Gram-positive bacteria on diverse materials, such as glass, PE, and cotton. The PIL with a longer side alkyl chain showed better antibacterial properties because of the stronger hydrophobic interactions with bacterial membranes caused by longer side chains, leading to membrane destruction.91
Compared with other antimicrobials, ILs exhibit multiple modes of action on bacterial cells. They can interact with and destroy bacterial membranes,83,101 disrupt proteins and enzymes,102,103 dysregulate bacterial metabolism,104,105 induce oxidative stress response,106,107 and cause DNA damage.108,109 The tunability of ILs, which stems from the free combination of ions, allows for the adjustment of alkyl chain length and the selection of different ions to regulate the strength of antibacterial properties. This flexibility and operability enable targeted inhibition of bacterial colony growth, pathogen killing, and biological compatibility. However, there is a lack of a comprehensive understanding of the principles that underlie the antibacterial properties of ILs. For example, more systematic research is needed on the effects of functional groups, the length of anion chain, and the atomic number of heterocycles on the antibacterial activity of ILs is lacking. The mechanisms involved also need further investigation, e.g., the different mechanisms of Gram-positive and Gram-negative bacteria with distinct structures. Moreover, existing solid antimicrobials, especially coated antimicrobials, have a short service life because of environmental factors, such as wear and wind. Innovative design, such as antibacterial spray, may overcome this limitation and has advantages in portability and ease of use, presenting a new avenue for the development of antimicrobial agents. Nevertheless, the long-term effectiveness, robustness, volatility, and biocompatibility of antibacterial agents require further exploration and design.
ILs can remove the biofilm of fungi, inhibit their filamentation, dissolve chitin (component of fungal cell walls),112,113 cause damage to the cell plasma and cell wall, and trigger detachment. For example, ILs have the capacity of damaging the A. nidulans’ cell wall of both filaments114 and fungal conidia.115 Diego et al. investigated through reverse transcription PCR at a molecular level the expression of A. nidulans genes after exposure to IL alkyl-tributyl phosphonium chlorides (TP-IL) in vivo. They found that TP-ILs were involved in the synthesis of saturated fatty acids and ergosterol (upregulation of fasA and HMGR1 genes), respectively. A. nidulans changed plasma membrane fluidity responsible for the membrane permeabilization evoked by TP-ILs. As an interrelationship between the cell membrane and the cell wall occurs, damage to the cell wall can also affect the organization of the plasma membrane (Fig. 4D).114
Various types of ILs have been found to have inhibitory effects on the growth of fungal colonies and biofilms. However, there were few research studies on the mechanism of ILs inhibiting the growth of fungi, and most lack a systematic and comparative approach. Most of the existing studies showed that a certain type of IL had resistance to the proliferation of certain bacteria or fungi; hence the resistance of ILs to bacteria and fungi varied with the species, yet the reasons had not been fully explained. Fungi are known to have more organelles than bacteria; therefore, is it better for them to overcome the inhibitory effects of ILs or does it increase the number of targets for ILs to attack and thus be susceptible to inhibition? In contrast, the main component of the bacterial cell wall is peptidoglycan, while the main component of fungal cell wall is chitin. Whether the ILs can be designed to target a structure (cell wall or organelle) to kill bacteria or fungi in a targeted way may be a research direction.
The use of ILs in anti-inflammatory applications has several implications, such as the significant improvement of water solubility, dual-function drugs, and multi-layer drug dressings, all providing opportunities for progress. As the drug effects of API-ILs have developed, their properties and cost-effectiveness have improved. However, the results of clinical trials have prompted researchers to be more cautious in selecting and designing IL-based drugs to ensure their effectiveness and safety. In addition, the mechanisms underlying the anti-inflammatory effects of ILs have been scarcely explored. It is unclear whether ILs cause ROS production or stimulate stress responses. There are few studies on the direct effect of ILs on internal anti-inflammation in the body. Several important questions must be addressed before implementing the use of anti-inflammatory ILs in clinical practice. One of the primary concerns is the potential damage to healthy tissues and the consequent side effects. In addition, it is essential to determine, which inflammatory ILs are effective and which require modification. While the ionization of approved drugs is a promising approach, the anti-inflammatory activity of ILs remains poorly understood and the potential for adverse reactions is unknown. Significant challenges require further investigation to fully realize the medical and economic potential of ILs in anti-inflammatory applications.
Some common ILs, such as imidazole-based ILs, also have antitumor activity. [C16MIM][Cl] can inhibit the growth of HeLa and human hepatocellular carcinoma (HepG2) cells, and reduce cell viability, damage to DNA, cause apoptosis, as well as alter the cell cycle.65,69 From a molecular point of view, [C16MIM][Cl] induced changes in the transcription of p53, Bax and Bcl-2, and genotoxicity, inhibited superoxide dismutase, imparted oxidative stress, decreased the glutathione content, and increased cellular malondialdehyde levels.65,69 Pyridinium, ammonium, and other ILs have also been reported to have anticancer activity.120–122 Aljuhani et al. showed anticancer activities of a series of pyridinium ILs on two lung cancer cell lines (A549 and H-1229), and the maximum rate of proliferation inhibition was 99.69%.123 Bourakadi et al. evaluated the antitumor activity of a series of 3-methyl-1-alkyl-2-(thiazol-4-yl) benzimidazol-3-ium ILs against four human tumor cell lines: HT29 (colon), K652 (Leukemia), MDA-MB231, and SKBR3 (breast) and established the antitumor prospect of ILs based on thiabendazole. Furthermore, the alkyl chain length of cationic substitution played an important role on the cytotoxicity of these ILs.73 Developing an IL-API that is non-toxic to normal cells remains a challenge. These results showed the broadly promising future for the application of ILs as possible anticancer agents in cancer treatment.
To achieve maximum efficacy with minimal side effects, one approach is to convert insoluble drugs that have already been approved into ILs. For example, methotrexate, choline, and amino acid ester have been transformed into ILs, namely tetrabutylphosphoniun methotrexate ([TBP][MTX]), L-phenylalanine ethyl ester methotrexate ([PheEt][MTX]), and 1-ethyl-3-methylimidazolium methotrexate ([EMIM][MTX]), as potential anticancer prodrugs. These ILs demonstrated more effective anticancer activity than free methotrexate against the human cervical carcinoma cell line (HeLa) cells (Fig. 4E).70 Matrinium-based ILs, composed of cations derived from the Chinese herbal medicine matrine and a series of fatty acid anions with long chains rooted in vegetable oils, have demonstrated improved anticancer activities compared with free matrine. They also exhibited lower cytotoxicity on normal cells (L929) compared with tumor cells (HeLa and A549). Additionally, IC50 of matrinium-based ILs to HeLa and lung cancer cells A549 decreased quickly with elongation of the anionic alkyl chain length. This suggests that long alkyl chains with a polar head could alter cell integrity, rapidly disturb the lipid layer, cause membrane rupture, and ultimately lead to cell death.68
Functionalized ILs could also have antitumor effects. Ferrocene has sparked great research for cancer therapeutics due to the remarkable properties, such as exceptional cylindrical structure, fascinating redox properties, favorable electrochemical behavior, and high stability.124–126 A series of ILs comprising ferrocene (Fe-ILs) by quaternization of 1-N-(ferrocenylmethyl) imidazole, 1-N-(ferrocenylmethyl) benzimidazole, and 1-N-(ferrocenylmethyl)-1,2,4-triazole with long alkyl chain bromides demonstrated significant anticancer activity.66 In particular, Fe-IL with the longest chain was found to be the most active with GI50 = 0.016 μM, which was less than that of the standard drug doxorubicin (GI50 = 0.018 μM) against human breast cancer cells MCF-7. Fe-ILs displayed moderate to good selectivity (against MCF-7 cells over normal Vero cells) and pharmacological parameters, including oral activity, effortless absorption or translocation.66 Hydrazones, existing in chemotherapeutic agents and many other drugs, attached to organic molecules could be applied to drug design relevant to anticancer activities.127–129 Al-Sodies et al. designed and synthesized novel dicationic ILs (Di-ILs), which contained hydrazone as a spacer linking double pyridines with alkyl functionalized esters to form cations. In their experiment, the two most active Di-ILs showed 99.99% and 99.86% compromised cell ability against human lung cancer cells (A549 and H1299, respectively).72
ILs with anticancer activity seem to be a viable treatment option in the context of other anticancer therapies with many side effects and poor drug availability. ILs ‘modified’ by existing drugs may be readily accepted for use but their properties, such as metabolism, may also differ from those of the original drug, necessitating further research. Common and functionalized ILs with anticancer activity themselves are also very competitive in future anticancer applications, because of advantages such as tunability, favorable combination abilities, and good solubility. In addition to using the inherent anticancer activity of ILs, the introduction of functional groups with targeted ligands, prodrug activators, enzyme inhibitors, etc., may allow for the integration of multiple therapeutic mechanisms to create a synergistic effect to enhance the overall treatment outcome.
In addition to good biocompatibility, ILs can also have anti-amyloidosis properties. Amyloidosis refers to the disease caused by the deposition of the abnormal protein amyloid in tissues or organs. The uniform and unstructured deposition of this protein leads to different degrees of dysfunction.131–133 1-Butyl-3-methylimidazolium bromide ([BMIM][Br]) has been proved to have anti-amyloidogenic activity. Atomic force microscopy (AFM) imaging unequivocally showed that the IL significantly attenuated fibrillogenesis in lysozymes.134 These results facilitated the development of more efficient therapeutics for amyloidosis.
The versatility of ILs has made them valuable in disease treatment by enhancing drug solubility and serving as active ingredients. ILs have been utilized in many therapeutic fields (e.g., antibacterial, antifungal, anti-inflammatory, and anticancer); yet there are still challenges to be addressed, such as ILs' stability, safety, and efficacy in vivo. As aforementioned, the ionization of approved drugs (e.g., lidocaine-diclofenac) can improve the solubility and bioavailability, which retain the potential for multiple therapeutic effects but avoid the de novo design. In this sense, a considerable amount of IL-based drugs is under developed. Given the ILs’ vast diversity structures with nearly 1018, it is also a complex and huge labor-intensive task to investigate the therapeutic potential of each IL via practical experiments. In order to rapidly and rationally design these ILs, the assistance of artificial intelligence (AI) is highly awaited. By machine learning and data mining of the existing experimental evidence, especially on the therapeutic effect and ILs’ components/physiochemical properties (anions, cations, side chains, functional groups, structures, etc.), the key parameters can be predicted and incorporated into IL-based drug formulations to achieve a desirable therapeutic effect.
3.2 Therapeutic mechanisms
To further advance the potential of ILs as promising drug candidates for disease treatment, a better understanding of the interaction mechanism between cells and ILs is essential. Due to the strong interaction among anions, cations, and other molecules, ILs have impacts on different degrees of life substances, from single proteins to intricate multicellular organisms.7,135–139 The interaction of ILs with biological systems has opened up a broad prospect for their application in biomedicine. In this section, we summarize the effects of ILs on cell membranes, organelles, nucleic acids and proteins.106,139–142 Simultaneously, a detailed analysis will be conducted on the relationship between the structure of ILs and their interaction with organisms.At the forefront of the interaction between ILs and cell membranes is the interaction between ILs and lipid bilayers. This is because lipid bilayers are the primary components and structures of cell membranes. The lipid membrane is comprised of phosphatidylcholine, which features a zwitterionic head group (net charge of zero) that facilitates electrostatic interactions and fatty acid tails in the inner region of the bilayer that provide hydrophobic interactions.143 ILs can control hydrophilic and hydrophobic characters by selecting the radius of their ionic moieties and size of their neutral parts to interact with bio-molecules. In fact, most ILs and cationic surfactants (in terms of structure, inherent charge, and function) have similar properties and can be adsorbed onto the surface of organisms, thereby increasing the permeability of the cell membrane.144,145
Previous studies have suggested that the interaction between ILs and cell membranes is influenced by the alkyl chain length of the ILs. The lipid bilayer is usually made of biomimetic membranes according to experiments, such as dioleoyl phosphatidylcholine (DOPC), which typically undergoes dissolution, drying, rehydration, vortex formation, and ultrasound treatment. Galluzzi et al. demonstrated that the length of the alkyl chain of the IL cation dominated the strength of the IL and lipid bilayer interaction and observed the interaction between lipid bilayers deposited on mica surfaces and ILs through atomic force microscopy.146 An experiment revealed that 1-hexyl-3-methylimidazolium acetate ([HMIM][OAc]) could be inserted into the lipid bilayer in the gel state, causing a more marked influence on the lipid bilayer than 1-butyl-3-methylimidazolium acetate ([BMIM][OAc]).147 The permeation of the IL cation into the lipid membrane led to the reorganization and softening of the bilayer, ultimately forming an IL/lipid complex. Furthermore, MD simulations revealed that the hydrophobicity of the IL cation, associated with its chain length, was the primary driver of its insertion into the lipid bilayer.145
Moreover, the interaction between ILs and lipid bilayers is also influenced by the ILs’ cationic head groups, number of substituents, and anions. ILs’ head-group structures have a strong dependence on the permeability of the lipid bilayer (Fig. 5A).148 Kaur et al. investigated the impact of six cationic head groups on lipid bilayers using solid-state NMR spectroscopy. Size, hydrophobicity, and delocalized and unshielded charges of the positively charged head-groups affected the degree of influence on lipid bilayers.148 In another study, they used double-chained 1-alkyl-3-octylimidazolium cations ([CnC8IM]+, n = 2, 4, 6, 8, 10, and 12) to evaluate membrane permeability through fluorescence-based dye leakage assays, which increased with the growth of the alkyl chain on N1 atoms, whereas double-chained ILs had lower permeability compared to single chain ILs with a similar carbon atomic number (Fig. 5B).149 Kumari et al. determined that anions were the main components causing the observed structural disturbances of lipid bilayer using MD simulations.150 A recent report discussed the relationship and mechanism between IL's molecular size and membrane damage. Xu's team found that ILs mainly interacted with negatively charged phosphate heads in lipid bilayers using antibacterial tests, fluorescent tracing, morphology observations, molecular biology, and MD simulations.151 The ability to insert into the bilayer membrane depended on the molecular size and disturbance of the membrane of ILs. Specifically, ILs with relatively short carbon chains could pass through the cell membrane causing membrane thinning, while ILs with long carbon chains remained in the cell membrane to disturb it. However, the medium-length carbon chain ILs were similar to the length of the bilayer membrane and had little impact on the cell membrane (Fig. 5C).151
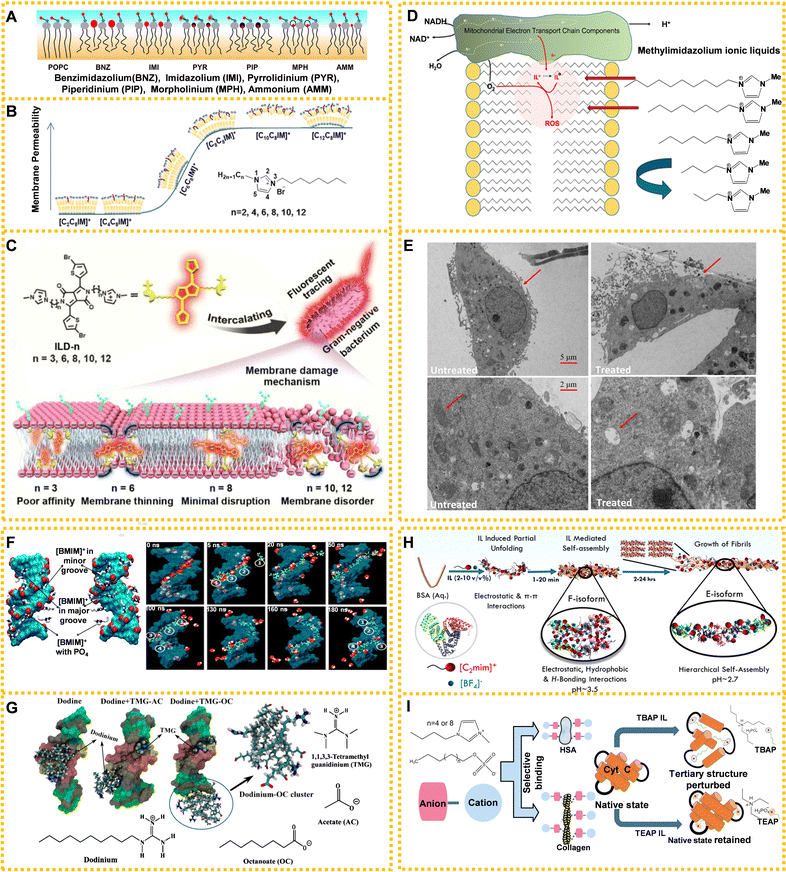 | ||
| Fig. 5 ILs acting on living organisms: (A) schematic models of different ILs' head-group size, hydrophobicity, and charge delocalization impacting the structure of 2-oleoyl-1-pamlitoyl-sn-glycero-3-phosphocholine (POPC) bilayer. Black wavy lines: alkyl chains in lipids and cations, grey filled circles: glycerol moieties in the lipid head-groups, blue and red filled circles connected with black lines: phosphocholine dipoles, and red circles: head-groups of cations intercalated in lipid molecules. Reproduced with permission from ref. 148. Copyright 2020 Elsevier. (B) The leakage kinetics of calcein dye from POPC and 2-oleoyl-1-pamlitoyl-sn-glycero-3-phosphoglycerol (POPG) large unilamellar vesicles and the regular model of membrane permeability associated with ILs’ cations. Reproduced with permission from ref. 149. Copyright 2021 American Chemical Society. (C) The membrane damage mechanism of ILs with different chain lengths on Gram-negative bacteria. Reproduced with permission from ref. 151. Copyright 2020 American Chemical Society. (D) Methylimidazolium ILs affecting the mitochondrial electron transport chain in mammalian cells. Reproduced with permission from ref. 152. Copyright 2020 Elsevier. (E) The effects of [C10MIM][Cl] on mitochondria of HeLa cells with the original TEM images (top) and zoomed TEM images (bottom). Reproduced with permission from ref. 141. Copyright 2021 Elsevier. (F) Distribution map of the IL interaction with DNA. Cyan: DNA, red: DNA phosphates, white: IL cations, and blue: ring nitrogen of IL cations. Reproduced with permission from ref. 153. Copyright 2012 American Chemical Society. (G) The mechanism for ILs’ long hydrocarbon chains of anions preventing a fungicide from destroying structural DNA. Reproduced with permission from ref. 154. Copyright 2020 American Chemical Society. (H) The formation mechanism for bovine serum albumin (BSA) amyloid fiber in the presence of [EMIM][BF4] (2–10 v/v%). Reproduced with permission from ref. 155. Copyright 2021 American Chemical Society. (I) The effect and relationship between the length of alkyl chain of anion (left) and cation (right) on proteins. Reproduced with permission from ref. 156 and 157. Copyright 2018 Royal Society of Chemistry and 2020 American Chemical Society. | ||
While many simulations have provided insights into the mechanism of cell membrane destruction by ILs, detailed experimental data are still lacking. Currently, the mainstream approach is to study how ILs' alkyl chain insertion, intercalation of the anionic rings, or disturbance of cationic head groups change the phospholipid bilayer structure and mechanical elastic properties. Currently, most of the studies on cell membrane toxicity of ILs focus on imidazole-ILs, limiting the study of cytotoxicity on cell membranes. In addition, biomimetic membranes are the main model structure for the study of IL's action on cell membranes. Although biomimetic membranes can partially mimic the structure of phospholipid bilayers, they fall short in replicating the full complexity and functionality of natural cell membranes. We speculate whether ILs as ionic compounds can also affect proteins and ion channels or the motion track of ions on cell membranes given the interaction between charged ions. Overall, studying the interactions between ILs and cell membranes in living cells is a complex area of research that requires careful consideration of experimental design and safety measures to ensure reliable and meaningful results.
At present, most studies on the toxicity of ILs to organelles focus on mitochondria.158 Methylimidazolium ILs can act as mitochondrial electron acceptors and longer chain ILs may act on the inner mitochondrial membrane affecting mitochondrial endometrial electron transmission and producing excess ROS (Fig. 5D).152 Research suggested that longer chain methylimidazolium liquids were toxic to sensitive liver progenitor cells because they both readily integrated within the inner mitochondrial membrane, accepting electrons from the electron chain and leading to oxidative stress. 1-Decyl-3-methylimidazolium chloride ([C10MIM][Cl]) caused mitochondrial swelling, vacuolation and cristae rupture, resulting in excessive ROS levels that ultimately damaged the mitochondrial membrane potential and induced cell apoptosis (Fig. 5E).141
Although our understanding only involves a few organelles like mitochondria and lysosomes, it shows the mode of action of ILs on cytotoxicity and highlights the lack of cytotoxic mechanism of ILs at this stage. Understanding ILs’ current mechanism of action on organelles is valuable for the development of targeted ILs that are tailored to a specific organelle. For example, lysosomes are known to have an acidic environment, making them attractive targets for drug delivery. In this regard, ILs can be designed into specific structures to neutralize the acidity of the lysosome and disrupt its membrane, thereby enabling the drug to escape and reach its target site.
Simulation methods are mainly used to study the interaction mechanism between ILs and nucleic acids. According to current reports, ILs mainly combine with nucleic acid grooves through H-bonds and hydrophobic interaction, giving origin to the groove binding mechanism. Although RNA and DNA are similar in structure, DNA is known for its greater stability, which has led to its prominence in investigations involving IL interactions. Rezki et al. demonstrated the interactions of imidazole ILs containing fluorine with DNA via H-bonds through modeling studies.160 Choline ions were stably combined with DNA atoms through multiple H-bonding networks from a microscopic viewpoint using MD simulations.161 Al-Sodies et al. found that ILs and DNA had good binding tendencies in the outer groove.72 The rod-like structure of imidazolium-based ILs exhibited a higher propensity to bind to the minor groove of calf thymus DNA, while the interaction of individual nucleic acid bases and imidazolium-based ILs confirmed the increasing tendency to bind to different bases in the following order: guanine, cytosine, thymine, uracil, and adenine.162 Nakano et al. demonstrated that multiple H-bonds played a significant role in the interaction between choline ions and DNA.161 Wang et al. found that the electrostatic interaction between the cationic head group of ILs and DNA and the hydrophobic interaction between the hydrocarbon chain of ILs and DNA predominated.163Fig. 5F shows the distribution map of IL's cations in DNA molecules.153
The interaction between ILs and nucleic acids has garnered significant attention in current research. Studies have demonstrated that while certain ILs can cause damage to nucleic acids, they also possess the capability to counteract the irreversible damage inflicted by toxic substances. For example, Sarkar and Singh prevented DNA damage from fungicide by adding ILs possessing anions with long hydrophobic chains (Fig. 5G).154 Long hydrophobic chain anions ILs combined with DNA through micelle-like structures prevented the combination of fungicide with DNA, and reversed the damage of DNA at the same time. Meanwhile, the application of ILs in nucleic acid delivery has developed rapidly, providing infinite possibilities for the application of ILs in biomedicine.
The interactions between ILs and proteins depend on H-bonds, van der Waals force, hydrophobic interactions, etc.155,165–168 For instance, anions of choline-based ILs choline iodide, choline bitartrate, and choline dihydrogen citrate ([Ch][I], [Ch][Bit] and [Ch][Dhc]) could spontaneously combine with the residues of bovine serum albumin (BSA) by H-bonds and van der Waals force, changing the natural structure of BSA significantly.165 Choline geranic acid ILs (CAGE) could inhibit human neutrophil elastase at low concentrations by disrupting the hydrophobic effect.169 Besides, the addition of ILs can change the pH of the environment and denature proteins. [EMIM][BF4] could cause conformational changes of BSA, resulting in the formation of amyloid fibers. The time-dependent hydrolysis of [EMIM][BF4] changes the pH of the solution. The synergism of pH and the electrostatic interaction of IL with BSA led to amyloid fibrils (Fig. 5H).155
The interactions between ILs and proteins depend on the structure of ILs. The ILs’ anionic hydrophobic structure can interact with proteins and lead to complex behaviors between ILs and proteins.157,170,171 For example, the interaction between choline-based ILs and BSA varied greatly due to different anions.165 Briefly, choline chloride ([Ch][Cl]) had no obvious effect on the conformation of BSA, while choline bromide [Ch][Br] changed its conformation slightly. In the presence of [Ch][I], [Ch][Bit] and [Ch][Dhc], the conformation of BSA changed significantly. The quenching mechanism of [Ch][Bit] and [Ch][Dhc] was a static quenching process, while [Ch][I] had a combined mechanism of charge transfer quenching and static quenching. Singh et al. demonstrated that the interaction of lysozyme (LYZ) with ILs was dependent on the molecular structure of the anionic counterpart of the ILs, with choline deoxycholate ([Ch][Doc]) showing greater involvement in bulk complexation compared with choline lauryl sarcosinate ([Ch][Sar]).171 [Ch][Doc] stabilized the secondary structure of LYZ via hydrophobic–hydrophilic interactions, while [Doc] adsorption onto LYZ provided stability through polar interactions. Conversely, [Sar] induced greater unfolding of LYZ, especially at higher concentrations, because of its flexibility and single-chain system with an amide moiety. What is more, the overall binding affinity between ILs and proteins increased with the length of the alkyl chain of the anion and cation of ILs. Fig. 5I shows the effect and relationship between the length of the alkyl chain of the anion and cation on proteins.156,157 Pabbathi and Samanta researched the effects of two ILs with different alkyl chain lengths on protein stability.156 The long chain ILs interacted with the protein hydrophobic region and destroyed the tertiary structure of Cyt-c. Conversely, the short chain ILs had a weaker effect on the Cyt-c structure because of reduced hydrophobicity compared with that of long chain ILs.
The inhibitory and disturbing effects of ILs on proteins make them promising antibacterial agents. Bacterial cell walls and membranes contain important proteins that can be denatured or deleted through reasonable design and addition of ILs. The current models and experimental conditions for investigating the interaction between ILs and proteins are often constrained and may not comprehensively capture the complexity of the system. Therefore, it is necessary to develop a research methodology that integrates both experimental and computational approaches, along with high-throughput screening methods.
Notably, although ILs have made substantial progress in exploration of ILs in biomedicine applications, further research is still needed to elucidate their underlying mechanisms. Firstly, investigations into the therapeutic mechanisms of ILs primarily concentrate on the alky side chains (e.g., long side chain-derived antibacterial effect). The exploration of other structures of ILs on therapeutic mechanisms, such as anions and cations, remains limited. It is crucial to broaden the scope of research to encompass the effects of various ILs’ structures on therapeutic mechanisms. Secondly, the absence of standardized protocols for assessing the relationship between the drug efficacy and the mechanisms might also hinder the comparability of different studies and limit the generalizability of their findings. Thirdly, in vivo research experiments mostly depend on the model organism or rodent animals, which could be expanded (to big animal beagles, pigs, etc.) to establish a multi-level and full chain biological evaluation system for the safety of ILs.
4 Biomedical aids
4.1 Application of ILs in pharmaceutical engineering
ILs are called ‘designer solvents’ in synthetic strategies because of their adjustable structure and ability to dissolve a wide range of compounds.172 ILs have ideal properties through rational design, such as broad solubility, unique H-bond system, negligible vapor pressure, and stable chemical properties, making them attractive alternatives to traditional extractants and catalysts in drug separation and synthesis. ILs also have high selectivity, making them an attractive option for separation and purification processes. In addition, they can be used as reaction catalysts in drug synthesis, offering advantages such as increased reaction rates, improved selectivity, and improved product purity. This section provides an overview of the use of ILs in drug separation as extractants and drug synthesis as catalysts.ILs can be used as direct extractants to extract active pharmaceutical components because of interactions between ILs and other substances. Compared with commonly used organic extractants, ILs offer clear advantages in terms of extraction efficiency, sample pretreatment, and environmental impact. For example, using ILs to extract and recover lipids from microalgae and yeast after fermentation could result in higher extraction efficiency, shorter processing time, lower energy consumption, and the ability to reuse the ILs, which could lead to significant improvements in economic efficiency.180 Gökdemir et al. used [BMIM][NTf2] as an alternative green solvent to extract curcumin from turmeric, with a maximum yield extraction of 2.94% under optimal conditions.181 Ji et al. showed how pure ILs could act as an efficient and selective extracting agent for minor anticancer prenylated flavonoids from a herbal medicine.182 Considering the H-bond interactions between [OMIM][BF4] and prenylated flavonoids, the extraction yield of prenylated flavonoids was as high as 78.92% using reversed-phase solid phase extraction from [OMIM][BF4].182
The interaction between ILs and substrates is a key consideration for extraction.183 Considering the ILs’ anion and cation tunability, specific properties of fluids can be designed to ensure that the target-active molecules are highly solvated to improve the extraction efficiency. In an IL-based aqueous two-phase system of separation of acteoside from Cistanche tubulosa, [BMIM][BF4] and (NH4)2SO4 had collaborative extraction roles. Specifically, the high polarity of [BMIM][BF4] provided multi-H-bond receptors for acteoside, whereas (NH4)2SO4 made acteoside stable by creating a weak acidic microenvironment and reducing solubility of acteoside in the salt phase.184 In another report, the IL-based surfactant-free microemulsion system consisting of [HMIM][BF4], 1,2-propanediol, and H2O was used to extract and separate hydrophilic (phenolic acids) and lipophilic compounds (alkaloids) from Camptotheca acuminata.176 van der Waals forces and H-bonds were supposed to be the main force between IL-based microemulsion and targeted compounds.
ILs are more environment friendly, with greater extraction efficiency and recycling advantages compared with traditional extractants. However, there are still many problems in the process of IL extraction. A key challenge in the extraction of bioactive compounds using ILs is their high viscosity, which can lead to lower extraction efficiency and slower mass transfer rates. To solve this issue, microwave-assisted extraction (MAE) and ultrasound-assisted extraction (UAE) have been developed. These methods employ high-frequency electromagnetic waves or sound waves to improve the mass transfer rate and extraction efficiency of IL-based extraction processes. Fig. 6A illustrates the extraction process for bioactive compounds using ILs assisted by either microwave or ultrasound.
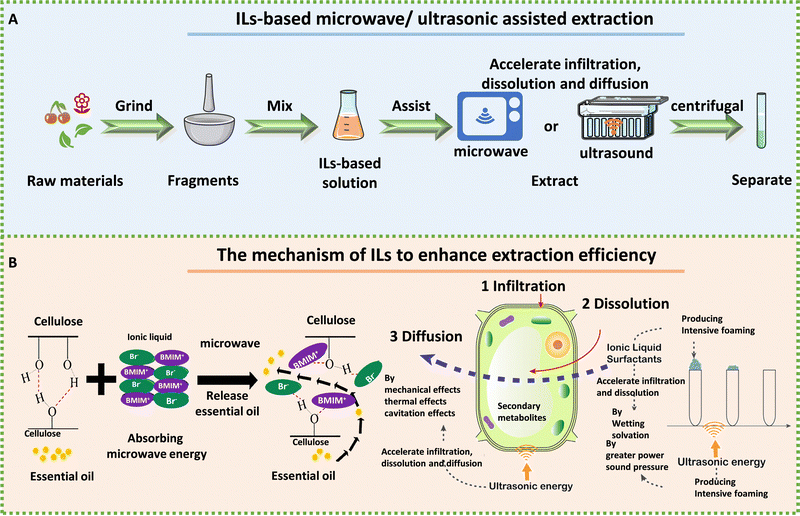 | ||
| Fig. 6 The process and mechanism of IL-based microwave- and ultrasonic-assisted extraction technology. A: Schematic extraction of biologically active ingredients by IL-assisted with microwave or ultrasound. B: Mechanism of ILs to enhance efficiency for extracting essential oil from Foeniculi fructus in IL-based microwave system (left) and psoralen and isopsoralen from Psoralea corylifolia seeds in IL-based ultrasonic extraction (right). Reproduced with permission from ref. 185 and 186. Copyright 2021 by the authors, licensee MDPI and 2020 Elsevier. | ||
ILs have emerged as a promising microextraction technology because of their unique ability to absorb microwave energy and convert it into heat. Additionally, because ILs are composed of cations and anions, high polar ILs can be reasonably designed to absorb and transfer microwave energy effectively.187 MAE can break the cytoderm and heat cells to accelerate the dissolution of intracellular effective components.135 Therefore, IL-MAE can depend on cell penetration and selective heating to help ILs in extracting drug components from plant raw materials. For example, Komaty et al. used ILs with MAE to extract atranorin, methyl-β-orcinol carboxylate, fumarprotocetraric acid and physodic acid from lichens more rapidly than when using conventional heating.188 Chen et al. successfully used magnetic 1-butyl-3-methylimidazolium tetrachloroferrate ([OMIM][FeCl4])-microwave to extract essential oil from lavender.189 Shi et al. employed ILs and MAE-assisted extraction to extract essential oil from Foeniculi fructus, which significantly improved the extraction efficiency. MAE had been shown to enhance the diffusion of essential oils from plants into ILs. The process of interaction between ILs and cellulose had also been emphasized, wherein non-covalent interactions (H-bonds and van der Waals forces) between the ILs and cellulose lead to the breakdown of the H-bonds’ cellulose structure.185
IL-based UAE extraction has been identified as an efficient technique to facilitate natural product extraction and increased yield. Compared with traditional extraction, UAE-assisted extraction has less solvent consumption and shorter extraction time.190 Ultrasound can damage the plant tissue and accelerate the penetration of solvents. Sui et al. utilized IL-based ultrasonic extraction of psoralen and isopsoralen from Psoralea corylifolia seeds through infiltration, dissolution, and defoaming; long alkyl-chain ILs such as [C10MIM][Br] had better extraction efficiency.186 UAE played three promoting roles in the system (vibration, thermal, and cavitation effects), improving the mass transfer efficiency, increasing the contact area, and enhancing the solubility of psoralen and isopsoralen. Sukor et al. found that the extraction efficiency of the UAE system was greatly improved after the addition of ILs.191 They believed that the cavitation effect after ultrasound and the special properties of 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([BMIM][NTf2]) synergistically improved the extraction efficiency. Sun et al. established a simple, rapid, and green system consisting of ultrasound-[BMIM][Br] solid–liquid extraction coupled with aqueous two-phase extraction high-performance liquid chromatography to extract naphthoquinone pigments in Arnebia euchroma (Royle) Johnst.192 Furthermore, Li et al. used an IL-based ultrasonic-assisted method to extract ganoderic acid A and D from Ganoderma lucidum, with an extraction yield of 3.31 mg g−1, which was much higher than that obtained using methanol as solvent.193 They also found that the extraction efficiency was related to the structure of the IL based on the comparison of five ILs, including [BMIM][Br], [BMIM][BF4], [BMIM][PF6], 1-hexyl-3-methylimidazolium bromide ([HMIM][Br]), and 1-octyl-3-methylimidazolium bromide ([OMIM][Br]). The extraction rate related to the alkyl chain length impacted the hydrophobicity, van der Waals force, and viscosity, while the anions interacted with the hydroxyl groups of the target compounds through the H-bonds, π–π conjugation, and ion/charge forces.193
Microwave and ultrasonic assisted-IL extractions improve the extraction efficiency through a fast and effectively enhanced process (Fig. 6B). In addition to the two IL-based external field assisted extraction methods, other IL-assisted extraction methods exist, such as IL-heating extraction,194 IL-based pulsed electric fields195 and IL-based pressurized liquid extraction.196 However, these methods may have drawbacks, such as increased energy requirements or the need for expensive and high-security equipment. What is more, most researchers believe that the combination of ILs and extracted substances is driven by the H-bonds, but there is limited detailed mechanisms explaining how microwave and ultrasound irradiation improve the extraction efficiency. Therefore, a better understanding of the extraction mechanisms for targeted biological complexes is needed to be explored. In addition, when separating the bioactive components extracted by ILs, organic solvents are often needed for back extraction to recover the target compounds in the treatment process, hindering the ‘green’ features of the separation process. The problems of IL residues in biologically active ingredients, and safety, also need to be further considered.
Researchers have reported IL applications in various chemical processes, e.g., as catalysts related to the production of drugs. These include antimicrobial, antiviral, antimalarial, antitumor, and other drug agents over the past few decades, e.g., [L-prolinium chloride][1-methylimidazolium-3-sulfonate] as the catalyst to synthesize antibacterial hydrazono-4-thiazolidinones.199 Sulfonic acid-functionalized ILs were utilized for catalytic synthesis of pyrano[3,2-c]coumarin derivatives and C3-substituted 4-hydroxycoumarins.200 IL cations had a strong influence on the catalytic activity in the synthesis of quinazoline-2,4(1H,3H)-diones, in which the H-bonds formed by the cations and the alkalinity of the cations affected the reaction.201 Wang et al. proposed IL catalytic mechanisms for the reactions of pyrano[3,2-c]coumarin derivatives and C3-substituted 4-hydroxycoumarins. They believed that the propargylic or allenic carbocation and H-bond effects between the catalysts and the substrates were the reason for the process of alkylation and cyclization.200Table 1 shows examples of ILs used as catalysts in drug synthesis.
| ILs | Drugs | Activities | Ref. |
|---|---|---|---|
| Piperidinium acetate | Spiro-piperidine derivatives | Antileishmanial | 202 |
| [DBU][Ac] | Triazole–tetrazole conjugates | Anthelmintic | 203 |
| [Trps][OTs] | Aspirin | Anti-inflammatory | 204 |
| [BMIM][Br] | 2-(1H-Benzimidazol-2-yl) phenol | Anticancer | 205 |
| 2-(Thiophen-2-yl)-1H-benzimidazole | |||
| [BMIM][BF4] | Pyrazolo quinoline derivatives | Anti-inflammatory and antioxidant | 206 |
| Hexamethylenetetramine based IL | Tetrazolo[1,5-a] pyrimidine-6-carbonitriles | Antiproliferation and antitumor | 207 |
| [MerDABCO-SO3H][Cl] | Pyrazolopyranopyrimidines | Antimicrobial and antioxidant | 208 |
| Brønsted acidic dicationic IL | 4,4′(Arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) | Antiviral, antibacterial, and antifungal | 209 |
ILs play an important role in drug synthesis as catalysts and offer numerous advantages over traditional synthesis techniques (e.g., excellent selectivity). However, several challenges require attention and resolution, such as the residual problem of ILs in drugs in the post-treatment process, environmental fate, and safety issues. Cross disciplinary collaborative research may be helpful in addressing these issues to elucidate the mechanisms underlying the interactions between ILs and biological systems, shedding light on their potential benefits or risks.
The emergence of green chemistry has significantly increased the interest in ILs within the domain of pharmaceutical engineering. ILs have gained recognition as extractants for drug separation and catalysts in drug synthesis and for their potential in synthetic biology. ILs offer unique properties, including negligible vapor pressure, high thermal stability, and adjustable solvation capabilities, making them highly attractive for applications in the field of synthetic biology. For instance, ILs can be employed as reaction media during the process of recombinant DNA and modular enzyme assembly. By deviating from conventional drug production systems, ILs have the potential to serve as extractants or catalysts for the design and synthesis of biological molecules, ultimately leading to the development of novel drugs.
4.2 Application of ILs in disease diagnosis
The unique ion-free composition of ILs consisting only of ions and their fascinating thermal, electrical, physical, and chemical properties aroused enormous interest in disease diagnosis. Thus far, there have been many researchers focusing on the application of ILs in diagnostics, including biosensing and imaging. A growing body of research demonstrates that IL can be used not only as sensors to improve performance (Fig. 7A), but also as electrodes, hence they are involved in the diagnosis of the disease and improve the diagnostic efficiency.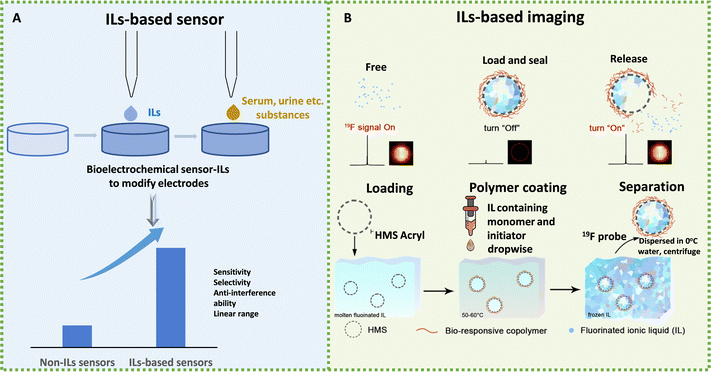 | ||
| Fig. 7 Schematic diagram of the application of ILs in disease diagnosis. (A) Schematic diagram of IL-based sensors to test substances. (B) Schematic diagram of the 19F MRI platform for stimuli-responsive 19F MRI. Reproduced with permission from ref. 210. Copyright 2020 Elsevier. | ||
IL-based sensors have shown potential in diagnosing kidney diseases by detecting the levels of biomarkers such as human serum albumin (HSA) and creatinine. Gao et al. developed a fluorescent sensor using a luminogen, decorated with tetraphenylethene (TPE) and 1-carboxymethyl-3-methyl-imidazolium bromide ([HOOCMIM][Br]) to detect HSA.211 TPE-IL molecules spontaneously docked with the hydrophobic subdomain of HSA because of the hydrophobic and H-bond interactions between TPE-IL and the amino acid residues of HAS, inducing fluorescence intensity enhancement. This sensor showed good sensitivity, selectivity, anti-interference ability, a linear range of 0.02–10 μg mL−1, and a detection limit of 0.007 μg mL−1, whose practicability was demonstrated by precise detection of HSA levels in human urine and serum samples.211 Boobphahom et al. developed a paper-based sensor decorated with 1-butyl-2,3-dimethylimidazolium tetrafluoroborate([BdMIM][BF4]), CuO, and reduced graphene for the non-enzymatic detection of creatinine. ILs acted as a charge transferring bridge, providing electro-catalytic ability and high ionic conductivity, resulting in an increased electron-transfer rate and a fast signal response. The linear detection range of the sensor was 0.01–2.0 mM with a limit of detection of 0.22 mM (S/N = 3) for creatinine detection.212 Teekayupak et al. fabricated a simple electrochemical sensor for non-enzymatic detection of creatinine, based on 3D-printed electrodes decorated by CuO-[BdMIM][BF4]/rGO (reduced graphene oxide).213 The modified electrodes could directly couple with a portable smartphone potentiostat controlled by an Android app. The linear detection range of the sensor was 0.5–35.0 mM with a limit of detection of 37.3 μM. These IL-based sensors show promise in the early detection and monitoring of kidney diseases.
IL-based sensors could be used to diagnose diabetes because ILs have shown specific achievements in the diagnosis of glucose and acetone, common indicators of diabetes. Graphene is a topical issue in electrochemical sensor research because of its unparalleled 2D honeycomb nanostructures, large specific surface area, as well as strong mechanical properties.214–216 The interaction between graphene and ILs can occur through electrostatic interaction, π–π stacking, H-bonds, and covalent bonds. The common ILs used for modifying graphene involve imidazolium-based ILs, ammonium-based ILs, phosphonium-based ILs, pyridinium-based ILs, and amine-terminated ILs (ILs-NH2). For instance, graphene, which contains a large number of π electrons, can interact with imidazole cations that are positively charged. Moreover, the aromatic rings present in imidazole cations can interact with graphene surfaces through π–π stacking interactions.217 ILs-NH2 can also react with epoxy groups on GO to form covalent bonds.218 The introduction of ILs not only produces IL-functionalized graphene with excellent conductivity, stability, and dispersibility, but also prevents the aggregation of graphene.219,220 Luan et al. constructed an enzyme-free glucose sensor by mixing Ni3S2 nanomaterials with 1-(3-aminopropyl)-3-methylimidazolium bromide ([A-MIM][Br])-functionalized graphene. This sensor possessed a satisfactory detection range with a wide linear range of 0–500 μM, strong anti-interference ability, excellent sensitivity, and good repeatability. The synergistic effect of electrochemical performance occurred between Ni3S2 and IL-functionalized graphene, which optimized conductivity and made the response more sensitive.221 Nishan et al. developed a colorimetric sensor for acetone detection based on the eosin dye, which reacted with acetone in the presence of protic IL 1-H-3-methylimidazolium ([H-MIM][Br])-coated TiO2 nanoparticles (NPs). The sensor exhibited a wide linear range from 1.6 × 10−6 to 6.1 × 10−5 mol L−1, a low limit of detection, only 5 min response time, and good selectivity and sensitivity.
IL-based sensors could be used to diagnose gout as they can enable more sensitive detection of uric acid (UA) in human blood and urine, which is highly beneficial for early disease diagnosis.222,223 Abbas et al. developed an electrochemical sensor for the real-time monitoring of UA using benzimidazolium-1-acetate to modify the electrode, which increased the intrinsic conductivity and thermal stability, due to the availability of high π-electron density from availability of carboxyl functional groups and aromatic rings. The sensor exhibited a highly sensitive and selective response, excellent anti-interference ability, a linear range of 2–1050 μM, as well as a detection limit of 1.27 μM, and was effective in monitoring UA from blood of gout patients and healthy individuals.224
IL-based sensors could be used to diagnose cancer and can exploit differences to detect cancer-specific biomolecules. Molecular imprinting is a commonly used technique to create recognition sites in a polymer matrix that are specific to a target molecule. In this context, the polymerized IL hydrogel could act as the matrix and cancer-specific biomolecules could serve as the template molecule for the molecular imprinting process. For instance, Wang et al. used polymerized 3-[((4-N,N-bis[(carbamoyl)ethylmethacrylate]butyl)((carbamoyl)amino)ethyl methacrylate)-propyl]-1-ethenyl-1himidazol-3-ium bromide ([BCCPEim][Br]) hydrogel as a matrix for the molecular imprinting of the target molecule carcinoembryonic antigen (CEA).225 The resulting molecularly imprinted polymer was then integrated into a photoelectrochemical sensor for CEA detection. The vinyl, amino and imidazolium cations in the polymerized [BCCPEim][Br] hydrogel interacted with CEA through hydrogen bonding and electrostatic attraction, providing complementary active sites that interact with CEA. The polymerized [BCCPEim][Br] hydrogel might have had a rigidity that helped maintain the structure of CEA and the intermolecular interaction between CEA and the imprinted active site, improving the sensing performance of CEA with high selectivity, sensitivity, and stability. The molecularly imprinted sensor displayed a linear response ranging from 0.05 to 5.0 ng mL−1 and a detection limit of 11.2 pg mL−1.225 Compared with the clinical application of chemiluminescence immunoassay, the polymerized [BCCPEim][Br] hydrogel-based sensor had excellent accuracy in detection results, with a relative deviation of less than 5%.
In addition, ILs have been utilized in the determination of other indicators, such as adrenaline and glutathione in human urine samples and whole blood.226–228 As shown in Table 2, ILs have been widely studied in the modification of electrodes as transfer bridges, functional agents, and linkers to improve electrochemical properties, so as to enhance the sensitivity, selectivity, and other properties of sensors and make it possible to diagnose diseases conveniently and accurately. Accurate monitoring of biomarkers in human blood or urine is crucial not only for early detection, but also for effective treatment of various diseases. However, it would be impossible for most people to go daily to the hospital for physical examination. Therefore, detecting these indicators using a portable and easy to use sensor based on ILs while maintaining accuracy is also an important goal for the development and improvement of sensors.
| Substance to be tested | Related disease | ILs | The action of ILs | Linear range | Detection limit | Ref. |
|---|---|---|---|---|---|---|
| HSA | Kidney diseases | [HOOCMIM][Br] | TPE-IL for fluorescence | 0.02–10 μg mL−1 | 0.007 μg mL−1 | 211 |
| Creatinine | Kidney disease | [BdMIM][BF4] | Modify electrodes | 0.01–2.0 mM | 0.22 μM | 212 |
| Creatinine | Kidney diseases | [BdMIM][BF4] | Modify electrodes | 0.5–35.0 mM | 37.3 μM | 213 |
| Glucose | Diabetes | [A-MIM][Br] | Functionalize graphene | 0–500 μM | 0.161 μM | 221 |
| Acetone | Diabetes | [H-MIM][Br] | Moderator for improved sensing properties of TiO2 | 1.6 × 10−6–6.1 × 10−5 M | 2.7023 × 10−5 mM | 229 |
| UA | Gout | BIL | Boost up the intrinsic conductivity and thermal stability of electrodes | 2–1050 μM | 1.27 μM | 224 |
| Ascorbic acid, DA, and UA | Gout, Parkinson, schizophrenia, etc. | [BMIM][PF6] | Modify electrodes | 25–400 μM, 0.2–10 μM, and 0.5–20 μM for ascorbic acid, DA, and UA | 6.64 μM, 0.06 μM, and 0.03 μM for ascorbic acid, DA, and UA | 230 |
| Guanine and UA | Gout | [BMIM][Cl] | Solvents for cellulose | 0.6–620 μM and 0.2–460 μM for UA and guanine | 0.077 and 0.144 for UA and guanine | 231 |
| HPV16 DNA | HPV16-positive head and neck cancer | [APHimi][Cl] | Functionalize graphene | From 8.5 nM to 10.7 μM | 1.3 nM | 232 |
| CEA | Cancer | [BMIM][PF6] | Functionalize graphene | From 0.001 fg mL−1 to 1 ng mL−1 | 0.0003 fg mL−1 | 233 |
| CEA | Cancer | [BCCPEim][Br] | Provide a more favorable environment | 0.05–5.0 ng mL−1 | 11.2 pg mL−1 | 225 |
| Glutathione | Diabetes and alcoholism, etc. | [BMIM]PF6] | Modify electrodes | 0.1–3.0 μM | 0.04 μM | 227 |
| Adrenaline | Parkinson's disease | [dMIM][BF4] | Modify electrodes | 0.1–400 μM | 0.07 μM | 228 |
| DA and UA | Depression, etc. | [BMIM][Br] | Modify electrodes | 0.1 to 300.0 μM for DA | 0.04 μM for DA | 234 |
| Choline and acetylcholine | AD, Parkinson diseases, etc. | [AMIM][NTf2] | GO-IL to provide a more favorable micro-environment | 5–1000 nM for choline and acetylcholine | 0.885 and 1.352 nM for choline and acetylcholine | 13 |
| O2˙− | AD etc. | IL polymer | Prevent enzyme leakage | 1.0–228.0 μM | 0.42 μM | 235 |
IL-based sensors have the potential to aid in the diagnosis of depression. ILs can enhance the sensitivity of electrode detection by improving the electron transfer between the electrode surface and target molecules, because of ILs’ high conductivity. The low sensitivity of dopamine (DA) detection in the brain, caused by its low concentration, could be effectively improved by the addition of ILs. Nagles et al. developed a novel sensor with an electrode modified by chitosan-single walled carbon nanotubes and [BMIM][BF4]. The sensitivity of the IL-modified electrodes improved compared with electrodes without IL modification under the same conditions. Specifically, the anodic peak current of DA detected using [BMIM][BF4]-modified electrodes increased by 17%.239 The neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) played a critical role in the emotional system and the involvement of ILs in 5-HT sensors could aid in the diagnosis of depression. Li et al. developed a highly sensitive nanocarbon 1-octylpyridinium hexafluorophosphate paste electrode for the detection of 5-HT. The electrode exhibited a linear range of 0.2–20 μM and a detection limit of 0.1 μM. The addition of highly conductive ILs was found to significantly enhance the electron transfer rate and increase the measured current, leading to improved sensitivity.240 Yang et al. also developed an electrochemical biosensor for the detection of glucocorticoid receptor alpha, a key biomarker of depression in the hippocampus and blood cells. The biosensor utilizing amino acid-coated gold NPs and amino-ion rGO (IL-rGO) markedly magnified the electrochemical signals.241
IL-based sensors could be used to diagnose AD. Choline and acetylcholine play crucial roles in various biological processes, for example, cognitive processes.242,243 Since some diseases are connected with choline and acetylcholine, which were used as biological media, the quantitative analyses of choline and acetylcholine are possibly helpful for diagnosis of AD. In the work of Albishri et al. sensitive and selective biosensors, composed of choline oxidase or acetylcholine esterase on glassy carbon electrode decorated GO-1-allyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([AMIM][NTf2]), were designed for the determination of choline and acetylcholine in human serum samples. ILs played an excellent role in the sensor, they could be used as modifiers to improve the performance of electrodes.244,245 Furthermore, the combination of GO and ILs could provide a more advantageous microenvironment for the stabilization and enhancement of catalytic performance for immobilized enzymes.13 The quantitative analysis of O2˙− is important in early diagnosis of AD induced by ROS.246–249 Peng et al. fabricated a carbon fiber microelectrode for acute and sensitive monitoring of O2˙− in living rat brains. The electrode was coated by a functionalized polymer carboxyl-rich IL onto Prussian blue NPs. Biocompatible polymer ILs prevented enzyme leakage by abundant points with superoxide dismutase, which was beneficial for the improvement of the sensitivity.235
ILs show excellent advantages for the production of sensors to diagnose diseases, which are mostly achieved by modifying electrodes. Due to the special structure of ILs, they can form H-bonds with a variety of substances, provide active sites for reactions, and have good electrochemical properties. These advantages enable ILs to modify the surface of a variety of substances to use in disease diagnosis. Currently, the primary approaches of mental disease diagnosis are through interview to identify the signs and symptoms. Therefore, it is crucial to develop IL-based sensors with low detection limits, high accuracy, sensitivity, and selectivity in the diagnosis of mental illnesses.
The use of fluorescent ILs probe for real-time monitoring is a novel approach. A fluorescent IL, N-methyl-6-hydroxyquinolinium bis(trifluoromethylsulfonyl)imide ([6MQc][NTf2]) probe had advantages of high fluorescence sensitivity to pH in the range of 6.0–7.5, selectivity, and biocompatibility for the quantitative imaging of intracellular pH. These were elucidated by Gao et al., who offered a crucial tool responsible for the early diagnosis of diseases.251 Dramatic fluctuations in intracellular pH can induce inappropriate cell growth, division, and function, and cause serious diseases.252–255 Hence, this real-time monitoring of intracellular pH was of significance for disease diagnosis and prevention.
Magnetic ILs composed of imidazolium cations based on perylene diimide (PDI) and anions of nitrate-chelated Gd(III) were established and the Gd(III) complex was used to enhance the MRI signals, while the PDI was applied in fluorescence imaging. The magnetic ILs were dispersed on the membrane in a formation with excellent luminous properties, making them valuable for fluorescence imaging of cells and tissues. In the aqueous environment, the magnetic ILs were aggregated by the π–π stacking interaction of cations, facilitating the enhancement of MRI signals on tumor tissues. The switching characteristic between the dispersed and aggregated formation, depending on changes in the physiological environment, makes this magnetic IL suitable for dual-mode imaging through fluorescence imaging and MRI.256
The structural tunability of ILs creates many possibilities for composition. ILs can be used as element donors and luminescence factors in imaging diagnosis, suggesting abundant applications in the diagnosis of disease influence. Moreover, the different states of ILs hold promise for imaging diagnosis. For example, the IL contrast agent is wrapped in the molten state, sealed in the solid state, and then followed by surface modification according to the particularity of the micro-environment or surface protein expression of disease lesions to achieve the purpose of targeted release for imaging. IL monomers with aromatic rings can be designed for fluorescence imaging by π–π stacking interactions after polymerization or aggregation in the micro-environment of disease foci. Until now, diagnosis and treatment have been mostly separated. ILs with dual characteristics of fluorescence/imaging and treatment can break the routine and combine diagnosis and treatment by introducing the 19F element into ILs with anticancer activity and then wrapping them in a shell that responds to the tumor site or attaching folic acid targeting to tumors. These design tasks can be tedious and complex and can be simplified by using the learning and prediction abilities of AI.
4.3 Application of ILs in delivery systems
Controlled drug release systems with enhanced drug efficacy and minimized side effects have garnered significant attention from researchers. To meet this demand, various organic and inorganic systems, such as hydrogels, micelles, and nano-controlled drug release delivery systems, have been developed.257,258 Considering the tunability of ILs’ structure, different response sensitive drug release systems could be designed under various conditions through the selection of compatible cationic and anionic moieties. In this section, we discuss current progress in IL drug delivery. | ||
| Fig. 8 Application of ILs in delivery systems. (A) Schematic diagram of ILs increasing drug solubility. (B) Schematic diagram of [Car][Tau] delivering insulin and dextran to tumor cells, inhibition of tumor growth (top), permeation of drugs across the skin with [Car][Tau] (left), and the content of IL delivery drugs in different layers of the skin (right). Reproduced with permission from ref. 263. Copyright 2021 American Chemical Society. (C) Topical delivery of siRNA-ILs through porcine skin in vitro (top) and gene knockdown by siRNA-IL formulation correlated with biological efficacy in vivo (bottom). Reproduced with permission from ref. 11. Copyright 2020 Elsevier. (D) Preparation of IL-based microemulsion and the proposed oral drug delivery system. Reproduced with permission from ref. 264. Copyright 2022 American Chemical Society. (E) Poly[P4,4,4,4][SS] as the pH- and thermally responsive receiver coated with CuCo2S4 nanoparticles to deliver drugs. Reproduced with permission from ref. 21. Copyright 2020 American Chemical Society. | ||
In addition to increasing solubility, ILs can increase the permeability of drugs.8 They can be used as chemical permeation enhancers to break down physiological barriers and deliver active ingredients to disease sites.10 Thus, application of ILs as a delivery system is feasible. Furthermore, combining cations or cations of biologically active pharmaceutical ingredients (APIs) with ILs can form IL-API drugs, which can promote the dissolution of poorly water-soluble drugs as innovative solutions, enabling reaching target cells through various barriers.265 For example, Lu et al. synthesized IL-based taurine with good biocompatibility, non-toxicity, and antitumor activity to deliver insulin and dextran.263 The results showed that taurine-based ILs could improve the drug permeability and efficacy (Fig. 8B).263 A biocompatible IL choline fatty acid [Ch][FA] was used as a skin permeation enhancer to mediate the dissolution of antigen peptides to treat tumor. Compared with the use of water solvent, the transdermal delivery flux of peptides increased 28-fold by using the IL. Furthermore, no skin irritation was caused and tumor growth in vivo was successfully inhibited.266
Mitragotri's team has been committed to the exploration of ILs in the field of biomedicine and made great advancements in using ILs as drug permeation enhancers in recent years. They presented siRNA robed with IL moiety (benzyl dimethyl octyl ammonium (BDOA)-siRNA) for the treatment of skin disease. The system displayed the necessary properties for dermal drug delivery, including biocompatibility, cell internalization, and skin permeability irrespective of the siRNA. The experiments demonstrated that therapeutic BDOA-siRNA could be viewed as a promising prodrug platform for treating skin diseases such as psoriasis, atopic dermatitis, and melasma.267 They developed complementary and synergistic strategies to deliver siRNA into the skin for a topical therapeutic administration (Fig. 8C).11 Demonstrating that BDOA-siRNA effectively permeated the labeled siRNA into the epidermis in vitro using the CAGE system for topical delivery. Furthermore, the administration of CAGE-BDOA siRNA to SKH1-E hairless mice resulted in a significant reduction of 44% in the expression of GAPDH protein compared with the group treated with CAGE-BDOA alone. This reduction was also 13-fold greater compared with the untreated group.11
ILs can effectively improve the water solubility of insoluble drugs and the permeability of drugs. They can promote absorption, break the physiological barrier, and improve drug bioavailability because of miscibility and ability to disrupt the lipid bilayer structure of biological membranes. However, the use of ILs as solution and permeation enhancers can have adverse effects, such as causing irritation or damage to the mucosal barriers or skin. Thus, ILs must be reasonably designed, and careful selection of their concentrations is needed to ensure the safety and efficacy of IL-based drug delivery systems.
ILs with good biocompatibility (e.g., based on original human metabolites) have been explored for the development of vaccine adjuvants. For example, choline dihydrogen phosphate IL has been used to enhance the therapeutic merit of vaccine candidate KMP-11 (a small protein) towards leishmaniasis under parenteral injection.269 Pancreatic ductal adenocarcinoma cells treated with CAGE enhanced immunogenicity and permeability of tumor cells.270 Mitragotri's team developed a new IL adjuvant synthesized using choline and lactic acid. It had good biocompatibility and a dispersion effect on ovalbumin antigen, induced effective antigen-presenting cell infiltration at the injection site, and generated a strong immune response to antigen.271 Zhang and colleagues were the first to develop an intranasal vaccine adjuvant system for influenza split virus antigen in the form of an oil-in-[Ch][Nic] nanoemulsion formulation with uniform size and excellent stability. This system promoted a robust mucosal immune response in the body, prolonged the nasal residence time (24-fold greater than in control participants), and enhanced the penetration of antigens into the nasal mucosal epithelial cells.272
At present, ILs used in research as vaccine adjuvants are generally limited to human metabolites. The application of biocompatible ILs in vaccine adjuvants could be expanded. The use of IL adjuvants through different vaccine delivery methods could be developed further. For example, oral vaccine adjuvants must overcome the adverse gastrointestinal tract environment with its strong acidity and digestive enzymes. Sufficient residence time in the gastrointestinal tract is needed for vaccine absorption to ensure that the vaccine can reach the induction site of mucosa-associated lymphoid tissue and trigger the immune response.273 The diversity of IL structures means that ILs can be designed to have acid resistance and sufficient retention time in the gastrointestinal tract based on the actual requirements of oral vaccine adjuvants.
ILs can form pH- or temperature-responsive drug delivery systems with nanoparticles. The hydrophobic alkyl chain and the hydroxyl group of the choline cation in choline acetate could establish a 3D network structure with the polymer chain of P123 through H-bonds and hydrophobic interactions. This would serve as a potential temperature-responsive drug carrier, allowing the drug to be transported in the gel network.277 The pH-sensitive microencapsulation, which consists of an IL core and a metal-phenolic network shell, has been proved to be effective for oral drug delivery. ILs effectively loaded drugs and increased the drug transport rate in endothelial cells to improve drug permeability (Fig. 8D).264 Zhou et al. used poly(lactide-co-glycolide) as the backbone, choline arginine for protein protection, and vitamin B12-chitosan for mucosal adhesion and pH responsiveness.278 Choline arginine proved effective in maintaining insulin stability throughout delivery system preparation, transportation, and storage.
ILs can modify nanoparticles to form a multi-stimuli-responsive delivery system. IL-based hydrogels loaded with Fe3O4 nanoparticles could deliver drugs efficiently with corresponding stimulation (pH and temperature), excellent biocompatibility, and self-healing properties.277 Fan et al. proposed a new drug release, which was composed of CuCo2S4 nanoparticles as the core and poly(tetrabutylphosphonium styrenesulfonate) (Poly[P4,4,4,4][SS]) as the pH- and thermally responsive receiver. The results showed that the combinatorial dual stimuli-triggered drug release behaviors can efficiently improve the cytotoxicity of cancer cells (Fig. 8E).21 Zhou et al. developed prochloraz IL-based microcapsules of pesticides.279 The prochloraz IL was used as nucleating agent to load CaCO3 onto the surface of micelles and wrap it with pectin. These IL-based microcapsules were pH/pectinase-responsive with excellent antibacterial performance and long-term efficacy against sclerotinia. Yu et al. used core–shell liquid-metal nanoparticles modified with 1-butyl-3-methylimidazolium (L)-lactate to combine chemodynamic therapy (CDT), microwave dynamic therapy (MDT), and MWTT.280
IL complexes, which are a class of ionic polymers can often bind responsive groups such as photo-, pH-, and thermally responsive groups. Cui et al. exploited microenvironmental changes in tumor cells to fabricate self-assembled nanoparticles with thermal and pH responses.281 Poly(IL-co-N-isopropylacrylamide) (PILNPM20) combined with deoxycholic acid was pH and thermal dual-responsive. PILNPM20 based on IL complexes was the polyelectrolyte backbone. The self-assembled nanoparticles can be used as drug carriers to effectively control drug release. An amphiphilic block polymer containing an IL complex, consisting of three parts: a target ligand, photo-response block, and pH-response block, could self-assemble into drug-loaded nanoparticles with a particle size of 80 nm in aqueous solutions, and the drug loading capacity of doxorubicin (DOX) was as high as 70%.282 Nanoparticle based IL complexes had no obvious side effects and good biocompatibility.
IL-response sensitizers are used as drug release switches and carriers for specific functions in biomedicine to maximize the effects. The relationship between nanoparticles and the environment can be reconciled by selecting appropriate ILs to modify the surface of nanoparticles. ILs provide new directions for nanomaterials and as pharmaceutical preparations, to treat diseases. However, the combination of nanomaterials and ILs requires comprehensive evaluation, including internal stability in vivo, biological distribution, and metabolic pathways, to assess drug safety and long-term biological behavior in vivo. At the same time, ILs often modify the surface of nanomaterials, which limits drug loading. Therefore, surface modification and drug activity should be balanced.
ILs have the unique ability to self-assemble into nanostructures in solutions. They can form different structures (introduced in Section 2.2), such as micelles, vesicles, and microemulsions. The diversity of IL structures also provides a broader space to increase drug solvation as drug carriers. IL-based drug carriers have good stability.284 They can change their structure by adjusting pH, temperature, light, enzyme and other physical or chemical stimuli for drug release. After receiving external stimulation, the structures of the carriers are often destroyed and the hydrophilicity or hydrophobicity changed, resulting in drug carriers becoming loose or shrinking to achieve the purpose of drug release. CAGE was combined with Tween 20 to form a microemulsion in water and loaded with methotrexate (MTX) for the treatment of psoriasis.285 The IL-based drug carrier system was thermo-responsive for release of MTX. When the temperature changed, the carrier transformed from a hydrophilic state to a hydrophobic state, leading to the contraction of the hydrogel network and the release of drugs. Further experiments on mice have proved excellent ability to deliver MTX through the skin, which could effectively treat skin inflammation without adverse side effects.
PILs have also made significant progress as drug carriers. PILs usually exhibit lower biological toxicity than IL monomers. Stimulation-responsive PILs have a wide range of applications and widely researched as drug carriers, suggesting that they are expected to become candidates for drug delivery. For example, 1-vinyl-3-butylimidazolium bromide ([VBIM][Br]) monomers were polymerized into hydrogels to coat the hydrophilic antibacterial drug tetracycline, and the ordered hydrogel molecular chains were disrupted into disordered molecular chains by temperature induction to release drugs.286 The drug release rate and cumulative release amount in this PIL-based hydrogel were profoundly biocompatible in vitro and could be precisely controlled by adjusting the temperature. Mukesh et al. reported a nanogel system, composed of choline acrylate-based PIL, which was able to release up to about 80% 5-fluorouracil, widely used in the treatment of cancer, at pH 1.2 (stomach pH) for 10 days at physiological human body temperature (37 °C). The system had a poor performance at pH 5.0 and 7.4.287
Apart from the aforementioned merits, ILs also can redirect the biodistribution and prolong their circulation by modifying the behavior of drug carriers. For example, Hamadani et al. developed a protein-avoidant IL strategy to modify nanoparticles for increasing bloodstream circulation and driving biodistribution.288,289 Compared with PEG modification with the risk of developing anti-PEG antibodies, the introduction of an IL (choline hexenoate) endowed the nanoparticles with negative charge and a larger hydrodynamic radius, resulting in diminished protein adsorption and reduced liver biodistribution.288 In addition, choline hexenoate could also be used to electrostatically solvate copolymers by direct dissolution, form stable drug carriers, reduce hemolysis, and imbue the ability of hitchhiking onto red blood cells.62 Furthermore, a library of choline carboxylate ILs assembled on polymeric nanoparticles was constructed to break biological barriers and prolong drug circulation for intravenous delivery.290 These findings and protocols highlighted the potential of ILs for advanced nanoparticle modification toward the development of more effective and precisely targeted therapies.
ILs have gained attention in drug delivery systems due to their ability to dissolve poorly soluble drugs and regulate the physiological properties of active drug ingredients. Challenges such as poor drug solubility, systemic toxicity, and short retention time remain significant hurdles in the field of biomedicine. The design of high drug-loading and stable supports based on ILs, by combining the diversity of IL cluster forms, is of great practical significance for the effective treatment of drugs with low bioavailability. The form of ILs plays a crucial role in their suitability for drug delivery applications. While there have been advancements in the investigation of IL clusters, more powerful and systematic research on their formation, morphology, and properties is needed to be explored. Additionally, a more thorough understanding of the transport principles governing the use of IL-based delivery systems in cellular uptake and tissue delivery is needed to be elucidated.
5 Conclusions and outlook
ILs offer remarkable advantages in biomedical development owing to their unique structures and biological and physicochemical properties. This review provides a detailed overview of the molecular composition, interaction forces of ILs, and the unique physicochemical properties, especially the ILs’ tertiary structures (from molecular structure, to H-bonding networks, and to clusters). Furthermore, the therapeutic potential of ILs and their interaction with cell structures are described, along with the underlying mechanisms. This review also highlights the potential applications of ILs in pharmaceutical engineering, disease diagnosis, and drug delivery systems, taking into account ILs’ unique tertiary structures or other tunable physiochemical properties.ILs have been used in many biomedical fields, yet there are still challenges to be addressed. Firstly, safety remains a significant challenge. Although approved drugs can be ionized into ILs, effectively improving their solubility and bioavailability, the efficacy and dosage still require further exploration. Second, the potential mechanism of ILs as biomedicine needs to be further elucidated in vitro. Several indications (e.g., side chain effect) have been identified, but they are insufficient to guide the design of biomedical ILs. Third, regarding the simple biological evaluation system of ILs in vivo, it is necessary to expand experimental animal models (beagles and other large animals) to establish a multi-level and full chain biological evaluation system for the safety of ILs. Fourth, the exploration of IL clusters has been insufficient to date. Their formulation, morphology, and properties should be explored using more powerful and systematic methodologies, given the rapid development of IL clusters for the application of drug delivery. Ultimately, more attention should be directed to addressing the residual ILs in the post-treatment processes of pharmaceutical engineering. Meanwhile, it is crucial to emphasize the impact of residual ILs on drug efficacy.
Based on the above discussions, ILs have potential to significantly impact the expansion of multiple drug formulations and routes of administration. Firstly, ILs can serve as protective additives and therapeutic drugs. For example, ILs have shown impressive inhibitory effects on pathogenic bacteria. Therefore, they have the potential to become protective additives to be added in clothing, masks, hand sanitizers, etc. Moreover, based on the side chain effect of ILs to break cell membranes, ILs might be used in antiviral activity against enveloped viruses, such as human immunodeficiency virus (HIV), Epstein–Barr virus (EBv), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). By leveraging the membrane-breaking effect of ILs, they might have the potential to inhibit the replication or entry of these enveloped viruses, contributing to the development of effective antiviral strategies. Secondly, utilizing AI to design IL drugs by incorporating parameters such as IL structures and therapeutic effectiveness results in a new shortened drug development time. Thirdly, ILs are potential biomedical aids for synthetic biology regarding the improvement of enzyme stabilization and activity, making them useful for biocatalytic reactions. ILs can also serve as efficient reaction media for critical processes like recombinant DNA and modular enzyme assembly. Fourthly, their miscibility, designability, and tertiary structures, especially clusters, make ILs attractive for drug delivery applications. Overall, the application of ILs in biomedicine is vast and seems borderless.
List of abbreviations
Cations
| [EMIM] | 1-Ethyl-3-methylimidazolium |
| [BMIM] | 1-Butyl-3-methylimidazolium |
| [HMIM] | 1-Hexyl-3-methylimidazolium |
| [OMIM] | 1-Octyl-3-methylimidazolium |
| [C10MIM] | 1-Decyl-3-methylimidazolium |
| [C12MIM] | 1-Dodecyl-3-methylimidazolium |
| [C14MIM] | 1-Tetradecyl-3-methylimidazolium |
| [C16MIM] | 1-Hexadecyl-3-methylimidazolium |
| [HOCnCmOHIm] | (1-(n-Hydroxyalkyl)-3-(n-hydroxyalkyl) imidazolium |
| [A-MIM] | 1-(3-Aminopropyl)-3-methylimidazolium |
| [APHimi] | 3-(2-Aminoethyl)-1-propyl-1H-imidazol-3-ium |
| [BdMIM] | 1-Butyl-2,3-dimethylimidazolium |
| [dMIM] | 1,3-Dipropylimidazolium |
| [H-MIM] | 1-H-3-methylimidazolium |
| [Ch] | Choline |
| [C2NH2MIm] | 1-Aminoethyl-3-methylimidazolium |
| [MOEMIm] | 1-Methoxyethyl-3-methylimidazolium |
| [HOEMIm] | 1-Hydroxyethyl-3-methylimidazolium |
| [6MQc] | N-Methyl-6-hydroxyquinolinium |
| [TBP] | Tetrabutylphosphoniun |
| [PheEt] | L-Phenylalanine ethyl ester |
| [BCCPEim] | 3-[((4-N,N-bis[(carbamoyl)ethyl methacrylate]butyl)((carbamoyl)amino)ethyl methacrylate)-propyl]-1-ethenyl-1H-imidazol-3-ium |
| [CnC8IM] | 1-Alkyl-3-octylimidazolium |
| [Trps] | N-(3-Propanesulfonic acid) tropine |
| [MerDABCO-SO3H] | 1,4-Diazabicyclo[2.2.2]octane-4,sulfonic acid |
| [HOOCMIM] | 1-Carboxymethyl-3-methyl-imidazolium |
| [P4,4,4,4] | Tetrabutylphosphonium |
| [VBIM] | 1-Vinyl-3-butylimidazolium |
Anions
| [BF4] | Tetrafluoroborate |
| [Cl] | Chloride |
| [Ac] | Acetyl |
| [Br] | Bromide |
| [PF6] | Hexafluorophosphate |
| [SbF6] | Hexafluoroantimonate |
| [(CF3SO2)2N] | Bis(trifluoromethyl)sulfonylamide |
| [ClO4] | Perchlorate |
| [CF3SO3] | Trifluoromethanesulfonates |
| [NO3] | Nitrate |
| [CF3CO2] | Trifluoracetate |
| [1,2,3-Ben] | 1,2,3-Benztriazole |
| [NTf2] | Bis(trifluoromethanesulfonyl)imide |
| [Caf] | Caffeate |
| [Gal] | Gallate |
| [Ell] | Ellagate |
| [lys] | Lysinate |
| [OTf] | Trifluoromethyl sulfonate |
| [OAc] | Acetate |
| [FeCl4] | Tetrachloroferrate |
| [OTs] | p-toluenesulfonic acid |
| [DHP] | Dihydrogen phosphate |
| [I] | Iodide |
| [Bit] | Bitartrate |
| [Dhc] | Dihydrogen citrate |
| [SS] | Styrene sulfonate |
| [MTX] | Methotrexate |
| [Nic] | Nicotinic acid |
| [DC] | Deoxycholate |
| [Doc] | Deoxycholate |
| [Sar] | Lauryl sarcosinate |
| [FAs] | Fatty acids |
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21978292, 21821005, T2225021, and 82341045), the Major Program of National Natural Science Foundation of China (21890762), the International Partnership Program of Chinese Academy of Sciences (Grant No. 122111KYSB20190060), IPE Project for Frontier Basic Research (Grant No. QYJC-2022-012), and National Major Scientific Instruments and Equipments Development Project of National Natural Science Foundation of China (22227811).References
- K. S. Egorova, E. G. Gordeev and V. P. Ananikov, Chem. Rev., 2017, 117, 7132–7189 CrossRef CAS PubMed.
- Y. Wang, H. He, C. Wang, Y. Lu, K. Dong, F. Huo and S. Zhang, JACS Au, 2022, 2, 543–561 CrossRef CAS PubMed.
- John S. Wilkes and M. J. Zaworotko, J. Chem. Soc., Chem. Commun., 1992, 13, 965–967 RSC.
- P. Dominguez de Maria and Z. Maugeri, Curr. Opin. Chem. Biol., 2011, 15, 220–225 CrossRef CAS PubMed.
- J. Pernak, J. Kalewska, H. Ksycin'ska and J. Cybulski, Eur. J. Med. Chem., 2001, 36, 899–907 CrossRef CAS PubMed.
- I. M. Marrucho, L. C. Branco and L. P. Rebelo, Annu. Rev. Chem. Biomol. Eng., 2014, 5, 527–546 CrossRef CAS PubMed.
- A. R. P. Goncalves, X. Paredes, A. F. Cristino, F. J. V. Santos and C. Queiros, Int. J. Mol. Sci., 2021, 22, 5612 CrossRef CAS PubMed.
- C. Liu, B. Chen, W. Shi, W. Huang and H. Qian, Mol. Pharmaceutics, 2022, 19, 1033–1046 CrossRef CAS PubMed.
- X. Lin, Y. Yang, S. Li, Z. Li, Y. Sheng, Z. Su and S. Zhang, Int. J. Pharm., 2022, 625, 122083 CrossRef CAS PubMed.
- Z. Sidat, T. Marimuthu, P. Kumar, L. C. du Toit, P. P. D. Kondiah, Y. E. Choonara and V. Pillay, Pharmaceutics, 2019, 11, 96 CrossRef CAS PubMed.
- V. Dharamdasani, A. Mandal, Q. M. Qi, I. Suzuki, M. Bentley and S. Mitragotri, J. Controlled Release, 2020, 323, 475–482 CrossRef CAS PubMed.
- R. M. Meira, D. M. Correia, S. Ribeiro, P. Costa, A. C. Gomes, F. M. Gama, S. Lanceros-Méndez and C. Ribeiro, ACS Appl. Polym. Mater., 2019, 1, 2649–2658 CrossRef CAS.
- H. M. Albishri and D. Abd El-Hady, Talanta, 2019, 200, 107–114 CrossRef CAS PubMed.
- V. A. Azov, K. S. Egorova, M. M. Seitkalieva, A. S. Kashin and V. P. Ananikov, Chem. Soc. Rev., 2018, 47, 1250–1284 RSC.
- J. M. Gomes, S. S. Silva and R. L. Reis, Chem. Soc. Rev., 2019, 48, 4317–4335 RSC.
- J. D. Holbrey and K. R. Seddon, Clean Products and Processes, 1999, 1, 223–236 Search PubMed.
- S. J. Zeng, X. P. Zhang, L. Bai, X. C. Zhang, H. Wang, J. J. Wang, D. Bao, M. D. Li, X. Y. Liu and S. J. Zhang, Chem. Rev., 2017, 117, 9625–9673 CrossRef CAS PubMed.
- J. Neumann, R. Ludwig and D. Paschek, J. Phys. Chem. B, 2021, 125, 5132–5144 CrossRef CAS PubMed.
- B. Yang, L. Bai, T. Li, L. Deng, L. Liu, S. Zeng, J. Han and X. Zhang, J. Membr. Sci., 2021, 628, 119264 CrossRef CAS.
- K. Wieszczycka, K. Filipowiak, I. Wojciechowska, T. Buchwald, K. Siwińska-Ciesielczyk, B. Strzemiecka, T. Jesionowski and A. Voelkel, Sep. Purif. Technol., 2021, 265, 118483 CrossRef CAS.
- S. Y. Fan, Y. N. Hao, W. X. Zhang, A. Kapasi, Y. Shu, J. H. Wang and W. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 9000–9007 CrossRef CAS PubMed.
- H. Li, T. Niemann, R. Ludwig and R. Atkin, J. Phys. Chem. Lett., 2020, 11, 3905–3910 CrossRef CAS PubMed.
- K. Dong, Y. T. Song, X. M. Liu, W. G. Cheng, X. Q. Yao and S. J. Zhang, J. Phys. Chem. B, 2012, 116, 1007–1017 CrossRef CAS PubMed.
- E. Arunan, G. R. Desiraju, R. A. Klein, J. Sadlej, S. Scheiner, I. Alkorta, D. C. Clary, R. H. Crabtree, J. J. Dannenberg, P. Hobza, H. G. Kjaergaard, A. C. Legon, B. Mennucci and D. J. Nesbitt, Pure Appl. Chem., 2011, 83, 1637–1641 CrossRef CAS.
- H. Y. He, H. Chen, Y. Z. Zheng, S. J. Zhang and Z. W. Yu, Chem. Eng. Sci., 2015, 121, 169–179 CrossRef CAS.
- A. Karton, M. Brunner, M. J. Howard, G. G. Warr and R. Atkin, ACS Sustainable Chem. Eng., 2018, 6, 4115–4121 CrossRef CAS.
- K. Dong and S. J. Zhang, Chemistry, 2012, 18, 2748–2761 CrossRef CAS PubMed.
- J. M. Vicent-Luna, D. Dubbeldam, P. Gomez-Alvarez and S. Calero, ChemPhysChem, 2016, 17, 380–386 CrossRef CAS PubMed.
- L. Cammarata, S. G. Kazarian, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2001, 3, 5192–5200 RSC.
- J. Zhou, X. M. Liu, S. J. Zhang, X. P. Zhang and G. R. Yu, AIChE J., 2017, 63, 2248–2256 CrossRef CAS.
- A. M. de Ferro, P. M. Reis, M. R. C. Soromenho, C. E. S. Bernardes, K. Shimizu, A. A. Freitas, J. Esperanca, J. N. Canongia Lopes and L. P. N. Rebelo, Phys. Chem. Chem. Phys., 2018, 20, 19307–19313 RSC.
- J. Dupont, Acc. Chem. Res., 2011, 44, 1223–1231 CrossRef CAS PubMed.
- S. M. Chen, S. J. Zhang, X. M. Liu, J. Q. Wang, J. J. Wang, K. Dong, J. Sun and B. H. Xu, Phys. Chem. Chem. Phys., 2014, 16, 5893–5906 RSC.
- L. Shi, F. Chen, N. Sun and L. Zheng, Soft Matter, 2015, 11, 4075–4080 RSC.
- H. Y. Wang, L. M. Zhang, J. J. Wang, Z. Y. Li and S. J. Zhang, Chem. Commun., 2013, 49, 5222–5224 RSC.
- M. K. Ali, R. M. Moshikur, R. Wakabayashi, M. Moniruzzaman and M. Goto, ACS Appl. Mater. Interfaces, 2021, 13, 19745–19755 CrossRef CAS PubMed.
- I. S. I. Al-Adham, N. Jaber, M. Al-Remawi, F. Al-Akayleh, E. Al-Kaissi, A. S. A. Ali Agha, L. B. Fitzsimmons and P. J. Collier, Lett. Appl. Microbiol., 2022, 75, 537–547 CrossRef CAS PubMed.
- S. P. Callender, J. A. Mathews, K. Kobernyk and S. D. Wettig, Int. J. Pharm., 2017, 526, 425–442 CrossRef CAS PubMed.
- A. R. Bhat, F. A. Wani, K. Behera, A. B. Khan and R. Patel, Colloids Surf., A, 2022, 645, 128846 CrossRef CAS.
- H. Wang, B. Tan, J. Wang, Z. Li and S. Zhang, Langmuir, 2014, 30, 3971–3978 CrossRef CAS PubMed.
- S. Shi, T. Yin, X. Tao and W. Shen, RSC Adv., 2015, 5, 75806–75809 RSC.
- X. Li, Y. Guo, H. Wang, Z. Li and J. Wang, J. Mol. Liq., 2022, 352, 118699 CrossRef CAS.
- X. M. Liu, G. H. Zhou, F. Huo, J. J. Wang and S. J. Zhang, J. Phys. Chem. C, 2015, 120, 659–667 CrossRef.
- G. Singh, K. M. Singh, O. Singh and T. S. Kang, J. Phys. Chem. B, 2018, 122, 12227–12239 CrossRef CAS PubMed.
- A. Chandrakar and B. L. Bhargava, J. Mol. Graphics Modell., 2020, 101, 107721 CrossRef CAS PubMed.
- Y. Wang and G. A. Voth, J. Am. Chem. Soc., 2005, 127, 12192–12193 CrossRef CAS PubMed.
- S. S. Urikhinbam and L. S. Shagolsem, Mater. Today: Proc., 2021, 46, 7044–7048 CrossRef CAS.
- A. M. Curreri, S. Mitragotri and E. E. L. Tanner, Adv. Sci., 2021, 8, e2004819 CrossRef PubMed.
- L. Lomba, A. Polo, J. Alejandre, N. Martínez and B. Giner, J. Drug Delivery Sci. Technol., 2023, 79, 104010 CrossRef CAS.
- X. Wu, Q. Zhu, Z. Chen, W. Wu, Y. Lu and J. Qi, J. Controlled Release, 2021, 338, 268–283 CrossRef CAS PubMed.
- O. Stolarska, A. Pawlowska-Zygarowicz, H. Rodríguez, E. Frydrych-Tomczak and M. Smiglak, J. Mol. Liq., 2021, 339, 116805 CrossRef CAS.
- A. Boruń, J. Mol. Liq., 2019, 276, 214–224 CrossRef.
- R. Jain, N. Jadon and K. Singh, J. Electrochem. Soc., 2015, 163, H159–H170 CrossRef.
- K. Dong, X. Liu, H. Dong, X. Zhang and S. Zhang, Chem. Rev., 2017, 117, 6636–6695 CrossRef CAS PubMed.
- H. Tokuda, K. Ishii, M. A. B. H. Susan, S. Tsuzuki, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2006, 110, 2833–2839 CrossRef CAS PubMed.
- M. Ahmed, S. S. Rao, A. Filippov, P. Johansson and F. U. Shah, Phys. Chem. Chem. Phys., 2023, 25, 3502–3512 RSC.
- M. Armand, P. Johansson, M. Bukowska, P. Szczeciński, L. Niedzicki, M. Marcinek, M. Dranka, J. Zachara, G. Żukowska, M. Marczewski, G. Schmidt and W. Wieczorek, J. Electrochem. Soc., 2020, 167, 070562 CrossRef.
- Y. Kohno and H. Ohno, Phys. Chem. Chem. Phys., 2012, 14, 5063–5070 RSC.
- A. A. Freitas, K. Shimizu and J. N. Canongia Lopes, J. Mol. Liq., 2020, 301, 112402 CrossRef CAS.
- X. Peng, X. Sui, J. Li, T. Liu and L. Yang, Ind. Crops Prod., 2021, 168, 113596 CrossRef CAS.
- S. Uddin, M. R. Chowdhury, R. Wakabayashi, N. Kamiya, M. Moniruzzaman and M. Goto, Chem. Commun., 2020, 56, 13756–13759 RSC.
- C. M. Hamadani, I. Chandrasiri, M. L. Yaddehige, G. S. Dasanayake, I. Owolabi, A. Flynt, M. Hossain, L. Liberman, T. P. Lodge, T. A. Werfel, D. L. Watkins and E. E. L. Tanner, Nanoscale, 2022, 14, 6021–6036 RSC.
- M. R. Chowdhury, R. M. Moshikur, R. Wakabayashi, M. Moniruzzaman and M. Goto, Int. J. Pharm., 2021, 601, 120582 CrossRef CAS PubMed.
- E. Judy and N. Kishore, Biochimie, 2022, 207, 20–32 CrossRef PubMed.
- R. Y. Wan, X. H. Xia, P. J. Wang, W. R. Huo, H. Dong and Z. J. Chang, Toxicol. In Vitro, 2018, 52, 1–7 CrossRef CAS PubMed.
- P. Bansode, P. Patil, P. Choudhari, M. Bhatia, A. Birajdar, I. Somasundaram and G. Rashinkar, J. Mol. Liq., 2019, 290, 111182 CrossRef CAS.
- X. Yang, Q. Y. Chen, M. Y. Kong, L. L. Qu, Z. R. Geng and Z. L. Wang, J. Mater. Chem. B, 2012, 22, 20299–20304 RSC.
- Z. Wang, J. Zhang, B. Lu, Y. Li, Y. Liang, J. Yuan, M. Zhao, B. Wang, C. Mai and J. Zhang, J. Mol. Liq., 2019, 296, 111822 CrossRef CAS.
- X. H. Xia, R. Y. Wan, P. J. Wang, W. R. Huo, H. Dong and Q. Y. Du, Ecotoxicol. Environ. Saf., 2018, 162, 408–414 CrossRef CAS PubMed.
- R. M. Moshikur, M. R. Chowdhury, R. Wakabayashi, Y. Tahara, M. Moniruzzaman and M. Goto, J. Mol. Liq., 2019, 278, 226–233 CrossRef CAS.
- X. Yang, Q. Y. Chen, X. Li and J. Gao, Colloids Surf., B, 2012, 98, 91–96 CrossRef CAS PubMed.
- S. A. Al Sodies, M. R. Aouad, S. Ihmaid, A. Aljuhani, M. Messali, I. Ali and N. Rezki, J. Mol. Struct., 2020, 1207, 127756 CrossRef.
- K. El Bourakadi, N. Merghoub, G. Hicham, M. E. M. Mekhzoum, E. M. Essassi, A. E. K. Qaiss and R. Bouhfid, J. Mol. Liq., 2019, 282, 63–69 CrossRef CAS.
- A. N. Duman, I. Ozturk, A. Tuncel, K. Ocakoglu, S. G. Colak, M. Hosgor-Limoncu and F. Yurt, Heliyon, 2019, 5, e02607 CrossRef PubMed.
- J. Suchodolski, J. Feder-Kubis and A. Krasowska, Microbiol. Res., 2017, 197, 56–64 CrossRef CAS PubMed.
- E. S. Morais, N. Silva, T. E. Sintra, S. A. O. Santos, B. M. Neves, I. F. Almeida, P. C. Costa, I. Correia-Sa, S. P. M. Ventura, A. J. D. Silvestre, M. G. Freire and C. S. R. Freire, Carbohydr. Polym., 2019, 206, 187–197 CrossRef CAS PubMed.
- Y. Miwa, H. Hamamoto and T. Ishida, Eur. J. Pharm. Biopharm., 2016, 102, 92–100 CrossRef CAS PubMed.
- A. Abednejad, A. Ghaee, E. S. Morais, M. Sharma, B. M. Neves, M. G. Freire, J. Nourmohammadi and A. A. Mehrizi, Acta Biomater., 2019, 100, 142–157 CrossRef CAS PubMed.
- Y. Yu, Z. Y. Yang, S. J. Ren, Y. N. Gao and L. Q. Zheng, J. Mol. Liq., 2020, 299, 112185 CrossRef CAS.
- J. Niu, G. Tang, J. Tang, J. Yang, Z. Zhou, Y. Gao, X. Chen, Y. Tian, Y. Li, J. Li and Y. Cao, J. Agric. Food Chem., 2021, 69, 6485–6494 CrossRef CAS PubMed.
- K. E. Eckhart, A. M. Arnold, F. A. Starvaggi and S. A. Sydlik, Biomater. Sci., 2021, 9, 2467–2479 RSC.
- Y. Hu, Y. Xing, P. Ye, H. Yu, X. Meng, Y. Song, G. Wang and Y. Diao, Front. Microbiol., 2023, 14, 1109972 CrossRef PubMed.
- X. Chao, C. Zhang, X. Li, H. Lv, G. Ling and P. Zhang, Biomater. Sci., 2022, 10, 1008–1017 RSC.
- T. Gundolf, R. Kalb, P. Rossmanith and P. Mester, Front. Microbiol., 2022, 13, 883931 CrossRef PubMed.
- N. Weyhing-Zerrer, R. Kalb, R. Ossmer, P. Rossmanith and P. Mester, Ecotoxicol. Environ. Saf., 2018, 148, 467–472 CrossRef CAS PubMed.
- J. Liao, X. Yu, Q. Chen, X. Gao, H. Ruan, J. Shen and C. Gao, J. Membr. Sci., 2020, 599, 117818 CrossRef CAS.
- O. Forero-Doria, C. Parra-Cid, W. Venturini, C. Espinoza, R. Araya-Maturana, F. Valenzuela-Riffo, C. Saldias, A. Leiva, Y. Duarte, J. Echeverria and L. Guzman, Bioorg. Chem., 2022, 126, 105914 CrossRef CAS PubMed.
- M. Wojcieszak, A. Lewandowska, A. Marcinkowska, Ł. Pałkowski, M. Karolak, A. Skrzypczak, A. Syguda and K. Materna, J. Mol. Liq., 2023, 374, 121300 CrossRef CAS.
- J. Łuczak, C. Jungnickel, I. Łącka, S. Stolte and J. Hupka, Green Chem., 2010, 12, 593–601 RSC.
- C. W. Cho, T. P. T. Pham, Y. Zhao, S. Stolte and Y. S. Yun, Sci. Total Environ., 2021, 786, 147309 CrossRef CAS PubMed.
- X. Liu, L. Chang, L. Peng, R. Bai, Y. Wei, C. Ma and H. Liu, ACS Appl. Mater. Interfaces, 2021, 13, 48358–48364 CrossRef CAS PubMed.
- L. L. Gu, S. Poddar, Y. J. Lin, Z. H. Long, D. Q. Zhang, Q. P. Zhang, L. Shu, X. Qiu, M. Kam, A. Javey and Z. Y. Fan, Nature, 2020, 581, 278–282 CrossRef CAS PubMed.
- W. Florio, S. Becherini, F. D'Andrea, A. Lupetti, C. Chiappe and L. Guazzelli, Mater. Sci. Eng., C, 2019, 104, 109907 CrossRef CAS PubMed.
- J. Sommer, S. Fister, T. Gundolf, B. Bromberger, P. J. Mester, A. K. Witte, R. Kalb and P. Rossmanith, Int. J. Mol. Sci., 2018, 19, 790 CrossRef PubMed.
- S. Marullo, G. Gallo, G. Infurna, N. T. Dintcheva and F. D'Anna, Green Chem., 2023, 25, 3692–3704 RSC.
- C. M. Chism, S. Plash, D. Zuckerman, G. S. Dasanayake, M. Bennett, S. K. Tripathi, S. D. Pedigo and E. E. L. Tanner, ACS Appl. Eng. Mater., 2022, 1, 23–31 CrossRef.
- P. Mester, M. Wagner and P. Rossmanith, Ecotoxicol. Environ. Saf., 2015, 111, 96–101 CrossRef CAS PubMed.
- T. Zhang, B. Sun, J. Guo, M. Wang, H. Cui, H. Mao, B. Wang and F. Yan, Acta Biomater., 2020, 115, 136–147 CrossRef CAS PubMed.
- B. He, Y. Du, B. Wang, X. Wang, Q. Ye and S. Liu, Prog. Org. Coat., 2021, 157, 106298 CrossRef CAS.
- J. Guo, Q. Xu, Z. Zheng, S. Zhou, H. Mao, B. Wang and F. Yan, ACS Macro Lett., 2015, 4, 1094–1098 CrossRef CAS PubMed.
- L. Guzman, C. Parra-Cid, E. Guerrero-Munoz, C. Pena-Varas, E. Polo-Cuadrado, Y. Duarte, R. I. Castro, L. S. Nerio, R. Araya-Maturana, T. Asefa, J. Echeverria, D. Ramirez and O. Forero-Doria, Bioorg. Chem., 2021, 115, 105289 CrossRef CAS PubMed.
- B. Bromberger, J. Sommer, C. Robben, C. Trautner, R. Kalb, P. Rossmanith and P.-J. Mester, Sep. Purif. Technol., 2020, 251, 117309 CrossRef CAS.
- A. Tarannum, R. R. Jonnalagadda and N. F. Nishter, Spectrochim. Acta, Part A, 2019, 212, 343–348 CrossRef CAS PubMed.
- T. Clapa, J. Michalski, A. Syguda, D. Narozna, P. van Oostrum and E. Reimhult, Res. Microbiol., 2021, 172, 103817 CrossRef CAS PubMed.
- K. S. Egorova and V. P. Ananikov, J. Mol. Liq., 2018, 272, 271–300 CrossRef CAS.
- J. Michalski, C. Odrzygóźdź, P. Mester, D. Narożna and T. Cłapa, J. Mol. Liq., 2023, 369, 120782 CrossRef CAS.
- H. Wang, H. Fan, H. Liu, M. Jin, S. Du, D. Li, P. Zhang, S. Ruan and J. Qiu, J. Hazard. Mater., 2020, 399, 122847 CrossRef CAS PubMed.
- J. Chen, M. Chen, Y. Cheng, C. Fang, J. Luo, X. Zhang and T. Qin, Carbohydr. Polym., 2022, 298, 120098 CrossRef CAS PubMed.
- P. Kowalczyk, A. Borkowski, G. Czerwonka, T. Cłapa, J. Cieśla, A. Misiewicz, M. Borowiec and M. Szala, J. Mol. Liq., 2018, 266, 540–547 CrossRef CAS.
- J. Suchodolski, J. F. Kubis and A. Krasowska, J. Biotechnol., 2015, 208, 91 CrossRef.
- G. K. K. Reddy and Y. V. Nancharaiah, Front. Microbiol., 2020, 11, 730 CrossRef PubMed.
- I. C. Ferreira, D. Araujo, P. Voisin, V. D. Alves, A. A. Rosatella, C. A. M. Afonso, F. Freitas and L. A. Neves, Carbohydr. Polym., 2020, 247, 116679 CrossRef CAS PubMed.
- J. L. Shamshina and P. Berton, Front. Bioeng. Biotechnol., 2020, 8, 11 CrossRef PubMed.
- D. O. Hartmann and C. Silva Pereira, New J. Chem., 2013, 37, 1569–1577 RSC.
- M. Petkovic, D. O. Hartmann, G. Adamová, K. R. Seddon, L. P. N. Rebelo and C. S. Pereira, New J. Chem., 2012, 36, 56–63 RSC.
- C. G. Guo and W. K. Leung, Gut Liver, 2020, 14, 179–189 CrossRef CAS PubMed.
- N. Klomjit and P. Ungprasert, Eur. J. Intern. Med., 2022, 101, 21–28 CrossRef CAS PubMed.
- Y. Kuwabara, H. Hamamoto, S. Hikake and Y. Miwa, J. Pain, 2013, 14, S73 CrossRef.
- S. N. Riduan and Y. Zhang, Chem. Soc. Rev., 2013, 42, 9055–9070 RSC.
- A. A. Nayl, W. A. A. Arafa, I. M. Ahmed, A. I. Abd-Elhamid, E. M. El-Fakharany, M. A. Abdelgawad, S. M. Gomha, H. M. Ibrahim, A. A. Aly, S. Brase and A. K. Mourad, Molecules, 2022, 27, 2940 CrossRef CAS PubMed.
- M. Kuczak, M. Musial, K. Malarz, P. Rurka, E. Zorebski, R. Musiol, M. Dzida and A. Mrozek-Wilczkiewicz, J. Hazard. Mater., 2022, 427, 128160 CrossRef CAS PubMed.
- T. H. Han, J. D. Lee, B. C. Seo, W. H. Jeon, H. A. Yang, S. Kim, K. Haam, M. K. Park, J. Park, T. S. Han and H. S. Ban, Ecotoxicol. Environ. Saf., 2022, 248, 114334 CrossRef CAS PubMed.
- A. Aljuhani, M. R. Aouad, N. Rezki, O. A. Aljaldy, S. A. Al-Sodies, M. Messali and I. Ali, J. Mol. Liq., 2019, 285, 790–802 CrossRef CAS.
- Z. Deng, C. Fang, X. Ma, X. Li, Y. J. Zeng and X. Peng, ACS Appl. Mater. Interfaces, 2020, 12, 20321–20330 CrossRef CAS PubMed.
- C. Ornelas, New J. Chem., 2011, 35, 1973–1985 RSC.
- S. Peter and B. A. Aderibigbe, Molecules, 2019, 24, 3604 CrossRef CAS PubMed.
- B. Aneja, N. S. Khan, P. Khan, A. Queen, A. Hussain, M. T. Rehman, M. F. Alajmi, H. R. El-Seedi, S. Ali, M. I. Hassan and M. Abid, Eur. J. Med. Chem., 2019, 163, 840–852 CrossRef CAS PubMed.
- G. Verma, A. Marella, M. Shaquiquzzaman, M. Akhtar, M. R. Ali and M. M. Alam, J. Pharm. BioAllied Sci., 2014, 6, 69–80 CrossRef PubMed.
- S. Pandey, V. K. Sharma, A. Biswas, M. Lahiri and S. Basu, RSC Med. Chem., 2021, 12, 1604–1611 RSC.
- B. Murugesan, M. Arumugam, N. Pandiyan, M. Veerasingam, J. Sonamuthu, S. Samayanan and S. Mahalingam, Mater. Sci. Eng., C, 2019, 98, 1122–1132 CrossRef CAS PubMed.
- C. M. Dobson, Nature, 2003, 426, 884–890 CrossRef CAS PubMed.
- D. Eisenberg and M. Jucker, Cell, 2012, 148, 1188–1203 CrossRef CAS PubMed.
- T. P. J. Knowles, M. Vendruscolo and C. M. Dobson, Nat. Rev. Mol. Cell Biol., 2014, 15, 384–396 CrossRef CAS PubMed.
- A. Basu, S. C. Bhattacharya and G. S. Kumar, Int. J. Biol. Macromol., 2018, 107, 2643–2649 CrossRef CAS PubMed.
- T. Raj, R. Morya, K. Chandrasekhar, D. Kumar, S. Soam, R. Kumar, A. K. Patel and S. H. Kim, Bioresour. Technol., 2023, 369, 128429 CrossRef CAS PubMed.
- Y. Zhao and Y. Zhen, J. Mol. Liq., 2022, 349, 118185 CrossRef CAS.
- R. M. Moshikur, M. K. Ali, R. Wakabayashi, M. Moniruzzaman and M. Goto, Mol. Pharmaceutics, 2021, 18, 3108–3115 CrossRef CAS PubMed.
- G. Jeremias, F. Jesus, S. P. M. Ventura, F. J. M. Goncalves, J. Asselman and J. L. Pereira, J. Hazard. Mater., 2021, 409, 124517 CrossRef CAS PubMed.
- P. Kumari, V. V. S. Pillai and A. Benedetto, Biophys. Rev., 2020, 12, 1187–1215 CrossRef CAS PubMed.
- A. Tarannum, J. R. Rao and N. N. Fathima, Int. J. Biol. Macromol., 2022, 209, 498–505 CrossRef CAS PubMed.
- L. X. Hu, Q. Xiong, W. J. Shi, G. Y. Huang, Y. S. Liu and G. G. Ying, Ecotoxicol. Environ. Saf., 2021, 208, 111629 CrossRef CAS PubMed.
- F. Fadaei, M. Tortora, A. Gessini, C. Masciovecchio, S. Catalini, J. Vigna, I. Mancini, A. Mele, J. Vacek, D. Reha, B. Minofar and B. Rossi, J. Mol. Liq., 2022, 347, 118350 CrossRef CAS.
- J. Dołżonek, C. W. Cho, P. Stepnowski, M. Markiewicz, J. Thöming and S. Stolte, Environ. Pollut., 2017, 228, 378–389 CrossRef PubMed.
- V. Tsarpali, A. Belavgeni and S. Dailianis, Aquat. Toxicol., 2015, 164, 72–80 CrossRef CAS PubMed.
- J. Liu, Y. Wang, C. Wang, J. Gao, W. Cui, B. Zhao, L. Zhang, H. He and S. Zhang, J. Phys. Chem. Lett., 2021, 12, 9926–9932 CrossRef CAS PubMed.
- M. Galluzzi, L. Marfori, S. Asperti, A. D. Vita, M. Giannangeli, A. Caselli, P. Milani and A. Podesta′, Phys. Chem. Chem. Phys., 2022, 24, 27328 RSC.
- L. H. Xiao, H. Y. Guo, B. Cao, G. Mo, Z. H. Li and Z. W. Yu, Phys. Chem. Chem. Phys., 2021, 23, 17888–17893 RSC.
- N. Kaur, M. Fischer, S. Kumar, G. K. Gahlay, H. A. Scheidt and V. S. Mithu, J. Colloid Interface Sci., 2020, 581, 954–963 CrossRef PubMed.
- N. Kaur, S. Kumar, Shiksha, G. K. Gahlay and V. S. Mithu, J. Phys. Chem. B, 2021, 125, 3613–3621 CrossRef CAS PubMed.
- M. Kumari, A. Gupta, Shobhna and H. K. Kashyap, J. Phys. Chem. B, 2020, 124, 6748–6762 CrossRef CAS PubMed.
- L. Zheng, J. Li, M. Yu, W. Jia, S. Duan, D. Cao, X. Ding, B. Yu, X. Zhang and F. J. Xu, J. Am. Chem. Soc., 2020, 142, 20257–20269 CrossRef CAS PubMed.
- T. M. Abdelghany, A. C. Leitch, I. Nevjestic, I. Ibrahim, S. Miwa, C. Wilson, S. Heutz and M. C. Wright, Food Chem. Toxicol., 2020, 145, 111593 CrossRef CAS PubMed.
- A. Chandran, D. Ghoshdastidar and S. Senapati, J. Am. Chem. Soc., 2012, 134, 20330–20339 CrossRef CAS PubMed.
- S. Sarkar and P. Chandra Singh, J. Phys. Chem. Lett., 2020, 11, 10150–10156 CrossRef CAS PubMed.
- G. Singh, M. Kaur, M. Singh, H. Kaur and T. S. Kang, Langmuir, 2021, 37, 10319–10329 CrossRef CAS PubMed.
- A. Pabbathi and A. Samanta, J. Phys. Chem. B, 2020, 124, 8132–8140 CrossRef CAS PubMed.
- R. R. Reddy, G. Shanmugam, B. Madhan and B. V. N. Phani Kumar, Phys. Chem. Chem. Phys., 2018, 20, 9256–9268 RSC.
- N. Nikfarjam, M. Ghomi, T. Agarwal, M. Hassanpour, E. Sharifi, D. Khorsandi, M. Ali Khan, F. Rossi, A. Rossetti, E. Nazarzadeh Zare, N. Rabiee, D. Afshar, M. Vosough, T. Kumar Maiti, V. Mattoli, E. Lichtfouse, F. R. Tay and P. Makvandi, Adv. Funct. Mater., 2021, 31, 2104148 CrossRef CAS.
- W. X. Li, L. Zhu, Z. K. Du, B. Li, J. H. Wang, J. Wang, C. Zhang and L. S. Zhu, Chemosphere, 2020, 249, 126119 CrossRef CAS PubMed.
- N. Rezki, F. F. Al-Blewi, S. A. Al-Sodies, A. K. Alnuzha, M. Messali, I. Ali and M. R. Aouad, ACS Omega, 2020, 5, 4807–4815 CrossRef CAS PubMed.
- M. Nakano, H. Tateishi-Karimata, S. Tanaka and N. Sugimoto, J. Phys. Chem. B, 2014, 118, 379–389 CrossRef CAS PubMed.
- K. Jumbri, M. A. Kassim, N. M. Yunus, M. B. Abdul Rahman, H. Ahmad and R. Abdul Wahab, Processes, 2019, 8, 13 CrossRef.
- H. Y. Wang, J. J. Wang and S. B. Zhang, Phys. Chem. Chem. Phys., 2011, 13, 3906–3910 RSC.
- A. M. O. Azevedo, S. A. P. Pereira, M. L. C. Passos, S. P. F. Costa, P. Pinto, A. Araujo and M. Saraiva, Chemosphere, 2017, 173, 351–358 CrossRef CAS PubMed.
- X. Wang, T. Guo, Y. Shu and J. Wang, Colloids Surf., B, 2021, 206, 111971 CrossRef CAS PubMed.
- A. Benedetto and P. Ballone, ACS Sustainable Chem. Eng., 2015, 4, 392–412 CrossRef.
- M. Mukherjee and J. Mondal, J. Phys. Chem. B, 2020, 124, 6565–6574 CrossRef CAS PubMed.
- A. A. Khachatrian, T. A. Mukhametzyanov, D. G. Yakhvarov, O. G. Sinyashin, B. F. Garifullin, I. T. Rakipov, D. A. Mironova, V. A. Burilov and B. N. Solomonov, J. Mol. Liq., 2022, 348, 118426 CrossRef CAS.
- N. Z. Laird, P. Phruttiwanichakun, M. Zhu, J. A. Banas, S. Elangovan and A. K. Salem, J. Biomed. Mater. Res., 2023, 111, 682–687 CrossRef CAS PubMed.
- X. Wang, T. Guo, Y. Shu and J. Wang, Colloids Surf., B, 2021, 206, 111971 CrossRef CAS PubMed.
- G. Singh, M. Kaur, D. Singh, A. K. Kesavan and T. S. Kang, J. Phys. Chem. B, 2020, 124, 3791–3800 CrossRef CAS PubMed.
- I. Pacheco-Fernández and V. Pino, Extraction With Ionic Liquids-Organic Compounds, in Liquid-Phase Extraction, Handbooks in Separation Science, Elsevier, 2020, pp. 499–537 Search PubMed.
- G. Katarzyna and P. Andrzej, Acta Pol. Pharm., 2010, 67, 3–12 Search PubMed.
- A. K. Ressmann, P. Gaertner and K. Bica, Green Chem., 2011, 13, 1442–1447 RSC.
- H. Passos, M. G. Freire and J. A. Coutinho, Green Chem., 2014, 16, 4786–4815 RSC.
- Z. Luo, M. Tian, N. Ahmad, W. Qiu, Y. Zhang, C. Li and C. Zhao, Colloids Surf., B, 2023, 222, 113067 CrossRef CAS PubMed.
- S.-C. Zhu, M.-Z. Shi, Y.-L. Yu and J. Cao, Ind. Crops Prod., 2022, 183, 114968 CrossRef CAS.
- X. Yang, R. Zhao, H. Wang, A. Ben, H. Lin, X. Zhang, C. Li and L. Yang, Ind. Crops Prod., 2022, 187, 115351 CrossRef CAS.
- R. Wang, Z. Yang, W. Lv, Z. Tan and H. Zhang, Ind. Crops Prod., 2022, 187, 115465 CrossRef CAS.
- G.-U. Kim, G.-S. Ha, M. B. Kurade, S. Saha, M. A. Khan, Y.-K. Park, W. Chung, S. W. Chang, K. K. Yadav and B.-H. Jeon, Chem. Eng. J., 2022, 446, 137285 CrossRef CAS.
- B. Gökdemir, N. Baylan and S. Çehreli, Anal. Lett., 2020, 133, 2111–2121 CrossRef.
- S. Ji, Y. J. Wang, S. K. Gao, X. Shao, W. Cui, Y. Du, M. Z. Guo and D. Q. Tang, J. Ind. Eng. Chem., 2019, 80, 352–360 CrossRef CAS.
- K. S. Khoo, X. Tan, C. W. Ooi, K. W. Chew, W. H. Leong, Y. H. Chai, S.-H. Ho and P. L. Show, J. Cleaner Prod., 2021, 284, 124772 CrossRef CAS.
- H. Xu, X. Li, Y. Hao, X. Zhao, Y. Cheng and J. Zhang, J. Mol. Liq., 2021, 333, 115982 CrossRef CAS.
- G. Shi, L. Lin, Y. Liu, G. Chen, A. Yang, Y. Wu, Y. Zhou and H. Li, Molecules, 2021, 26, 3169 CrossRef CAS PubMed.
- X. Sui, T. Liu, J. Liu, J. Zhang, H. Zhang, H. Wang and Y. Yang, Ultrason. Sonochem., 2020, 69, 105263 CrossRef CAS PubMed.
- G. Shi, L. Lin, Y. Liu, G. Chen, S. Fu, Y. Luo, A. Yang, Y. Zhou, Y. Wu and H. Li, Alexandria Eng. J., 2022, 61, 6897–6906 CrossRef.
- S. Komaty, A. Sauvager, J. P. Bazureau, S. Tomasi and L. Paquin, Phytochem. Anal., 2021, 32, 592–600 CrossRef CAS PubMed.
- P. Chen, X. Liu, L. Wang, C. Wang and J. Fu, J. Sep. Sci., 2021, 44, 585–599 CrossRef CAS PubMed.
- B. Albero, J. L. Tadeo and R. A. Pérez, TrAC, Trends Anal. Chem., 2019, 118, 739–750 CrossRef CAS.
- N. F. Sukor, R. Jusoh, N. S. Kamarudin, N. A. Abdul Halim, A. Z. Sulaiman and S. B. Abdullah, Ultrason. Sonochem., 2020, 62, 104876 CrossRef CAS PubMed.
- Q. Sun, B. Du, C. Wang, W. Xu, Z. Fu, Y. Yan, S. Li, Z. Wang and H. Zhang, Chromatographia, 2019, 82, 1777–1789 CrossRef CAS.
- C. Q. Li, Y. P. Cui, J. Lu, C. Y. Liu, S. T. Chen, C. Y. Ma, Z. H. Liu, J. M. Wang and W. Y. Kang, Molecules, 2020, 25, 1309 CrossRef CAS PubMed.
- R. Rodrigues, P. Lima, R. Santiago-Aguiar and M. Rocha, Algal Res., 2019, 38, 101391 CrossRef.
- A. Symes, A. Shavandi and A. E. D. A. Bekhit, Int. J. Food Sci. Technol., 2023, 58, 3935–3945 CrossRef CAS.
- R. F. da Silva, C. N. Carneiro, C. B. D. C. de Sousa, F. J. V. Gomez, M. Espino, J. Boiteux, M. de Los, Á. Fernández, M. F. Silva and F. D. S. Dias, Microchem. J., 2022, 175, 107184 CrossRef CAS.
- D. Gheidari, M. Mehrdad and S. Maleki, Appl. Organomet. Chem., 2022, 36, e6631 CrossRef CAS.
- T. M. Dhameliya, P. R. Nagar, K. A. Bhakhar, H. R. Jivani, B. J. Shah, K. M. Patel, V. S. Patel, A. H. Soni, L. P. Joshi and N. D. Gajjar, J. Mol. Liq., 2022, 348, 118329 CrossRef CAS.
- M. S. Mirakmahaleh, K. Rad-Moghadam, H. Kefayati and S. Falakro, Mol. Diversity, 2021, 25, 109–119 CrossRef CAS PubMed.
- N. Wang, Z. Li, Q. Hou, F. Han, Y. Yan, J. Zhang and C. Miao, J. Org. Chem., 2022, 87, 11669–11680 CrossRef CAS PubMed.
- G. Shi, K. Chen, Y. Wang, H. Li and C. Wang, ACS Sustainable Chem. Eng., 2018, 6, 5760–5765 CrossRef CAS.
- M. A. A. Mohamed, A. M. Kadry, S. A. Bekhit, M. A. S. Abourehab, K. Amagase, T. M. Ibrahim, A. M. M. El-Saghier and A. A. Bekhit, J. Enzyme Inhib. Med. Chem., 2023, 38, 330–342 CrossRef CAS PubMed.
- M. A. Siddiqui, M. H. Shaikh, A. A. Nagargoje, T. T. Shaikh, V. M. Khedkar, P. P. Deshpande and B. B. Shingate, Res. Chem. Intermed., 2022, 48, 5187–5208 CrossRef CAS.
- H. Ni, Y. Zhang, C. Zong, Z. Hou, H. Song, Y. Chen, X. Liu, T. Xu and Y. Luo, Int. J. Mol. Sci., 2022, 23, 12877 CrossRef CAS PubMed.
- K. Madgula, S. Dandu, S. Kasula and P. Halady, Inorg. Chem. Commun., 2022, 138, 109218 CrossRef CAS.
- D. D. Chudasama, M. S. Patel, J. N. Parekh, H. C. Patel, C. V. Rajput, N. P. Chikhaliya and K. R. Ram, Mol. Diversity, 2023, 27, 1409–1425 CrossRef CAS PubMed.
- M. H. Abdollahi-Basir, B. Mirhosseini-Eshkevari, F. Zamani and M. A. Ghasemzadeh, Sci. Rep., 2021, 11, 5109 CrossRef CAS PubMed.
- P. Patil, A. Yadav, L. Bavkar, N. B. Nippu, N. D. Satyanarayan, A. Mane, A. Gurav, S. Hangirgekar and S. Sankpal, J. Mol. Struct., 2021, 1242, 130672 CrossRef CAS.
- F. Rezaei, M. Ali Amrollahi and R. Khalifeh, ChemistrySelect, 2020, 5, 1760–1766 CrossRef CAS.
- X. L. Zhu, X. X. Tang, H. Y. Lin, S. G. Shi, H. H. Xiong, Q. J. Zhou, A. Li, Q. Y. Wang, X. Y. Chen and J. H. Gao, Chem, 2020, 6, 1134–1148 CAS.
- L. Gao, X. Lin and X. Chen, Talanta, 2020, 212, 120763 CrossRef CAS PubMed.
- S. Boobphahom, N. Ruecha, N. Rodthongkum, O. Chailapakul and V. T. Remcho, Anal. Chim. Acta, 2019, 1083, 110–118 CrossRef CAS PubMed.
- K. Teekayupak, C. Aumnate, A. Lomae, P. Preechakasedkit, C. S. Henry, O. Chailapakul and N. Ruecha, Talanta, 2023, 254, 124131 CrossRef CAS PubMed.
- S. Niyogi, E. Bekyarova, M. E. Itkis, J. L. McWilliams, M. A. Hamon and R. C. Haddon, J. Am. Chem. Soc., 2006, 218, 7720–7721 CrossRef PubMed.
- Y. V. M. Reddy, J. H. Shin, V. N. Palakollu, B. Sravani, C. H. Choi, K. Park, S. K. Kim, G. Madhavi, J. P. Park and N. P. Shetti, Adv. Colloid Interface Sci., 2022, 304, 102664 CrossRef CAS PubMed.
- M. A. Kanjwal and A. A. Ghaferi, Sensors, 2022, 22, 8661 CrossRef CAS PubMed.
- H. Zhou, S. Bai, Y. Zhang, D. Xu and M. Wang, Int. J. Environ. Res. Public Health, 2022, 19, 7584 CrossRef CAS PubMed.
- H. Yang, C. Shan, F. Li, D. Han, Q. Zhang and L. Niu, Chem. Commun., 2009, 3880–3882 RSC.
- D. Wei and A. Ivaska, Anal. Chim. Acta, 2008, 607, 126–135 CrossRef CAS PubMed.
- Q. Zhang, W. Cheng, D. Wu, Y. Yang, X. Feng, C. Gao, L. Meng, X. Shen, Y. Zhang and X. Tang, Food Chem., 2022, 367, 130727 CrossRef CAS PubMed.
- F. Luan, S. Zhang, D. D. Chen, F. M. Wei and X. M. Zhuang, Microchem. J., 2018, 143, 450–456 CrossRef CAS.
- H. Wang, C. Yan, Q. Wu, H. Zeng, Z. Zhang, W. Wang and X. Sun, J. Orthop. Surg. Res., 2023, 18, 61 CrossRef PubMed.
- T. J. Lin, K. T. Yen, C. F. Chen, S. T. Yan, K. W. Su and Y. L. Chiang, Sensors, 2022, 22, 3009 CrossRef CAS PubMed.
- Y. Abbas, S. Ali, M. Basharat, W. Zou, F. Yang, W. Liu, S. Zhang, Z. Wu, N. Akhtar and D. Wu, ACS Appl. Nano Mater., 2020, 3, 11383–11390 CrossRef CAS.
- C. Y. Wang, Y. Y. Wang, H. J. Zhang, H. P. Deng, X. X. Xiong, C. Y. Li and W. W. Li, Anal. Chim. Acta, 2019, 1090, 64–71 CrossRef CAS PubMed.
- F. H. Moghadam, M. A. Taher and H. Agheli, Top. Catal., 2022, 65, 677–683 CrossRef CAS.
- B. Z. Liu, M. Wang and B. Xiao, J. Electroanal. Chem., 2015, 757, 198–202 CrossRef CAS.
- R. Bavandpour, H. Karimi-Maleh, M. Asif, V. K. Gupta, N. Atar and M. Abbasghorbani, J. Mol. Liq., 2016, 213, 369–373 CrossRef CAS.
- U. Nishan, F. Bashir, N. Muhammad, N. Khan, A. Rahim, M. Shah, R. Nazir and M. Sayed, J. Mol. Liq., 2019, 294, 111681 CrossRef.
- K. Kunpatee, S. Traipop, O. Chailapakul and S. Chuanuwatanakul, Sens. Actuators, B, 2020, 314, 128059 CrossRef CAS.
- H. Liu, R. Jamal, T. Abdiryim, R. Simayi, L. Liu and Y. Liu, ACS Sustainable Chem. Eng., 2021, 9, 5860–5871 CrossRef CAS.
- L. Farzin, S. Sadjadi, M. Shamsipur and S. Sheibani, J. Pharm. Biomed. Anal., 2020, 179, 112989 CrossRef CAS PubMed.
- X. Wang, L. Shang, W. Zhang, L. P. Jia, R. N. Ma, W. L. Jia and H. S. Wang, Biosens. Bioelectron., 2019, 141, 111436 CrossRef CAS PubMed.
- M. Roostaee, H. Beitollahi and I. Sheikhshoaie, Micromachines, 2022, 13, 1834 CrossRef PubMed.
- Q. W. Peng, X. Y. Yan, X. R. Shi, S. S. Ou, H. Gu, X. X. Yin, G. Y. Shi and Y. Y. Yu, Biosens. Bioelectron., 2019, 144, 111665 CrossRef CAS PubMed.
- G. Vilagut, C. G. Forero, A. Pinto-Meza, J. M. Haro, R. de Graaf, R. Bruffaerts, V. Kovess, G. de Girolamo, H. Matschinger, M. Ferrer and J. Alonso, Value Health, 2013, 16, 564–573 CrossRef PubMed.
- M. W. Petersen, E. Ornbol, T. M. Dantoft and P. Fink, J. Psychosom. Res., 2021, 146, 110491 CrossRef PubMed.
- R. Koirala, E. G. Iyer Soegaard, Z. Kan, S. P. Ojha, E. Hauff and S. B. Thapa, J. Psychiatr. Res., 2021, 143, 23–29 CrossRef PubMed.
- E. Nagles, O. García-Beltrán and J. A. Calderón, Electrochim. Acta, 2017, 258, 512–523 CrossRef CAS.
- Y. H. Li, Y. Ji, B. B. Ren, L. N. Jia, G. D. Ma and X. S. Liu, Mater. Res. Bull., 2019, 109, 240–245 CrossRef CAS.
- S. Yang, W. Feng, L. Xue, M. Yin, B. Li, L. Lu, F. Dai, J. Jiao and Q. Chen, Biosens. Bioelectron., 2022, 201, 113972 CrossRef CAS PubMed.
- E. L. Grafe, C. J. Fontaine, J. D. Thomas and B. R. Christie, J. Neurophysiol., 2021, 126, 1622–1634 CrossRef CAS PubMed.
- A. Llorente-Ovejero, J. Martinez-Gardeazabal, M. Moreno-Rodriguez, L. Lombardero, E. Gonzalez de San Roman, I. Manuel, M. T. Giralt and R. Rodriguez-Puertas, ACS Chem. Neurosci., 2021, 12, 2167–2181 CrossRef CAS PubMed.
- D. A. El Hady and A. K. Youssef, Anal. Chim. Acta, 2013, 772, 68–74 CrossRef PubMed.
- D. A. El Hady, H. M. Albishri and H. Watzig, Electrophoresis, 2016, 37, 1609–1623 CrossRef CAS PubMed.
- M. I. Holubiec, M. Gellert and E. M. Hanschmann, Front. Aging Neurosci., 2022, 14, 1003721 CrossRef CAS PubMed.
- Z. R. Lou, P. Li and K. L. Han, Acc. Chem. Res., 2015, 48, 1358–1368 CrossRef CAS PubMed.
- Y. Yang, L. Wang, H. Q. Cao, Q. Li, Y. Li, M. J. Han, H. Wang and J. B. Li, Nano Lett., 2019, 19, 1821–1826 CrossRef CAS PubMed.
- W. Wu, C. Zhang, T. W. Rees, X. Liao, X. Yan, Y. Chen, L. Ji and H. Chao, Anal. Chem., 2020, 92, 6003–6009 CrossRef CAS PubMed.
- W. Gao, Y. Zhang, Y. Zheng, H. Zhang, X. Wang, L. Bai, Y. Wu and X. Ba, J. Nanopart. Res., 2020, 22, 342 CrossRef CAS.
- L. F. Gao, X. L. Lin, A. Q. Zheng, E. Shuang, J. H. Wang and X. W. Chen, Anal. Chim. Acta, 2020, 1111, 132–138 CrossRef CAS PubMed.
- P. Lesani, G. Singh, Z. Lu, M. Mirkhalaf, E. J. New and H. Zreiqat, Chem. Eng. J., 2022, 433, 133668 CrossRef CAS.
- A. Steinegger, O. S. Wolfbeis and S. M. Borisov, Chem. Rev., 2020, 120, 12357–12489 CrossRef CAS PubMed.
- X. Ye, Y. Xiang, Q. Wang, Z. Li and Z. Liu, Small, 2019, 15, e1901673 CrossRef PubMed.
- B. A. Webb, M. Chimenti, M. P. Jacobson and D. L. Barber, Nat. Rev. Cancer, 2011, 11, 671–677 CrossRef CAS PubMed.
- X. Yu, X. Yuan, Z. Huang, W. Zhang, F. Huang and L. Ren, ACS Biomater. Sci. Eng., 2020, 6, 6405–6414 CrossRef CAS PubMed.
- G. B. Yang, L. G. Xu, J. Xu, R. Zhang, G. S. Song, Y. Chao, L. Z. Feng, F. X. Han, Z. L. Dong, B. Li and Z. Liu, Nano Lett., 2018, 18, 2475–2484 CrossRef CAS PubMed.
- M. Amaral, A. B. Pereiro, M. M. Gaspar and C. P. Reis, Nanomedicine, 2021, 16, 63–80 CrossRef CAS PubMed.
- W.-X. Zhang, Y.-R. Gao, R. Xue, W. Nguyen, W. Chen, J.-H. Wang and Y. Shu, Mater. Today Phys., 2023, 30, 100925 CrossRef CAS.
- D. A. S. Agostinho, A. R. Jesus, A. B. P. Silva, J. Esperanca, A. Paiva, A. R. C. Duarte and P. M. Reis, J. Pharm. Sci., 2021, 110, 2489–2500 CrossRef CAS PubMed.
- J. Yuan, N. Zhou, J. Wu, T. Yin and Y. Jia, RSC Adv., 2022, 12, 3416–3422 RSC.
- Y. Shi, Z. Zhao, Y. Gao, D. C. Pan, A. K. Salinas, E. E. L. Tanner, J. Guo and S. Mitragotri, J. Controlled Release, 2020, 322, 602–609 CrossRef CAS PubMed.
- B. Lu, M. Yi, S. Hu, D. Wu, Z. Zhu, C. Wu, Z. Wang, Y. Li and J. Zhang, ACS Sustainable Chem. Eng., 2021, 9, 5991–6000 CrossRef CAS.
- L. Shen, Y. Zhang, J. Feng, W. Xu, Y. Chen, K. Li, X. Yang, Y. Zhao, S. Ge and J. Li, ACS Appl. Mater. Interfaces, 2022, 14, 45229–45239 CrossRef CAS PubMed.
- C. Agatemor, K. N. Ibsen, E. E. L. Tanner and S. Mitragotri, Bioeng. Transl. Med., 2018, 3, 7–25 CrossRef PubMed.
- Y. Tahara, K. Morita, R. Wakabayashi, N. Kamiya and M. Goto, Mol. Pharmaceutics, 2020, 17, 3845–3856 CrossRef CAS PubMed.
- M. Zakrewsky and S. Mitragotri, J. Controlled Release, 2016, 242, 80–88 CrossRef CAS PubMed.
- I. J. Lee, Y. H. Lan, P. Y. Wu, Y. W. Wu, Y. H. Chen, S. C. Tseng, T. J. Kuo, C. P. Sun, J. T. Jan, H. H. Ma, C. C. Liao, J. J. Liang, H. Y. Ko, C. S. Chang, W. C. Liu, Y. A. Ko, Y. H. Chen, Z. L. Sie, S. I. Tsung, Y. L. Lin, I. H. Wang and M. H. Tao, Emerging Microbes Infect., 2023, 12, 2149353 CrossRef PubMed.
- S. Saha, A. Sannigrahi, K. Chattopadhyay and J. Chowdhury, J. Mol. Liq., 2020, 301, 112475 CrossRef CAS.
- J. Huang, M. Wang, F. Zhang, S. Shao, Z. Yao, X. Zhao, Q. Hu and T. Liang, Adv. Sci., 2023, 10, e2206756 CrossRef PubMed.
- A. Ukidve, K. Cu, M. Goetz, P. Angsantikul, A. Curreri, E. E. L. Tanner, J. Lahann and S. Mitragotri, Adv. Mater., 2020, 32, e2002990 CrossRef PubMed.
- X. Lin, Y. Sheng, X. Zhang, Z. Li, Y. Yang, J. Wu, Z. Su, G. Ma and S. Zhang, J. Controlled Release, 2022, 346, 380–391 CrossRef CAS PubMed.
- B. Ou, Y. Yang, H. Lv, X. Lin and M. Zhang, BioDrugs, 2023, 37, 143–180 CrossRef PubMed.
- L. H. Su, Q. Wu, L. F. Tan, Z. B. Huang, C. H. Fu, X. L. Ren, N. Xia, Z. Z. Chen, X. Y. Ma, X. D. Lan, Q. Zhang and X. W. Meng, ACS Appl. Mater. Interfaces, 2019, 11, 10520–10531 CrossRef CAS PubMed.
- X. W. Chen, C. H. Fu, Y. Q. Wang, Q. R. Wu, X. W. Meng and K. Xu, Nanoscale, 2018, 10, 15677–15685 RSC.
- Q. Wu, N. Xia, D. Long, L. F. Tan, W. Rao, J. Yu, C. H. Fu, X. L. Ren, H. B. Li, L. Gou, P. Liang, J. Ren, L. F. Li and X. W. Meng, Nano Lett., 2019, 19, 5277–5286 CrossRef CAS PubMed.
- D. K. Pandey, M. Kuddushi, A. Kumar and D. K. Singh, Colloids Surf., A, 2022, 650, 129631 CrossRef CAS.
- J. Zhou, J. Zhang, Y. Sun, F. Luo, M. Guan, H. Ma, X. Dong and J. Feng, Int. J. Biol. Macromol., 2023, 244, 125263 CrossRef CAS PubMed.
- Z. Zhou, Y. Gao, G. Tang, Y. Tian, Y. Li, H. Wang, X. Li, X. Yu, Z. Zhang, Y. Li, Y. Liu and Y. Cao, Chem. Eng. J., 2022, 446, 137073 CrossRef.
- Y. Yu, Q. Wu, M. Niu, L. Gou, L. Tan, C. Fu, X. Ren, J. Ren, Y. Zheng and X. Meng, Biomater. Sci., 2022, 10, 3503–3513 RSC.
- W. Cui, X. M. Lu, K. Cui, L. Y. Niu, Y. Wei and Q. H. Lu, Langmuir, 2012, 28, 9413–9420 CrossRef CAS PubMed.
- B. B. Lu, G. X. Zhou, F. Xiao, Q. J. He and J. H. Zhang, J. Mater. Chem. B, 2020, 8, 7994–8001 RSC.
- H. Albadawi, Z. Zhang, I. Altun, J. Hu, L. Jamal, K. N. Ibsen, E. E. L. Tanner, S. Mitragotri and R. Oklu, Sci. Transl. Med., 2021, 13, eabe3889 CrossRef CAS PubMed.
- T. A. Shmool, A. P. Constantinou, A. Jirkas, C. Zhao, T. K. Georgiou and J. P. Hallett, Polym. Chem., 2022, 13, 2340–2350 RSC.
- Y. Shu, R. Xue, Y. Gao, W. Zhang and J. Wang, J. Mater. Chem. B, 2023, 11, 5494–5502 RSC.
- X. Qu, J. Liu, S. Wang, J. Shao, Q. Wang, W. Wang, L. Gan, L. Zhong, X. Dong and Y. Zhao, Chem. Eng. J., 2023, 453, 139785 CrossRef CAS.
- C. Mukesh, J. Bhatt and K. Prasad, Macromol. Chem. Phys., 2014, 215, 1498–1504 CrossRef CAS.
- C. M. Hamadani, M. J. Goetz, S. Mitragotri and E. E. L. Tanner, Sci. Adv., 2020, 6, eabd7563 CrossRef CAS PubMed.
- P. Khare, S. X. Edgecomb, C. M. Hamadani, E. E. L. Tanner and D. S. Manickam, Adv. Drug Delivery Rev., 2023, 197, 114861 CrossRef CAS PubMed.
- C. M. Hamadani, G. S. Dasanayake, M. E. Gorniak, M. C. Pride, W. Monroe, C. M. Chism, R. Heintz, E. Jarrett, G. Singh, S. X. Edgecomb and E. E. L. Tanner, Nat. Protoc., 2023, 18, 2509–2557 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally: Yanhui Hu, YuYuan Xing, Hua Yue. |
| This journal is © The Royal Society of Chemistry 2023 |