 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Comparative study on formic acid sensing properties of flame-made Zn2SnO4 nanoparticles and its parent metal oxides
Matawee
Punginsang
a,
Kanittha
Inyawilert
a,
Mameaseng
Siriwalai
bce,
Anurat
Wisitsoraat
d,
Adisorn
Tuantranont
d and
Chaikarn
Liewhiran
*ae
aDepartment of Physics and Materials Science, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand. E-mail: cliewhiran@gmail.com
bPhD Program in Nanoscience and Nanotechnology (International Program/Interdisciplinary), Faculty of Science, Chiang Mai University, Chiang Mai, 50200, Thailand
cGraduate School, Chiang Mai University, Chiang Mai, 50200, Thailand
dNational Security and Dual-Use Technology Center, National Science and Technology Development Agency (NSTDA), Klong Luang, Phathum Thani 12120, Thailand
eCenter of Excellence in Materials Science and Technology, Chiang Mai University, Chiang Mai 50200, Thailand
First published on 5th July 2023
Abstract
Correction for ‘Comparative study on formic acid sensing properties of flame-made Zn2SnO4 nanoparticles and its parent metal oxides’ by Matawee Punginsang et al., Phys. Chem. Chem. Phys., 2023, 25, 15407–15421, https://doi.org/10.1039/D3CP00845B.
Fig. 8–10 in the published version of the manuscript contained errors. Fig. 8(b) is partly overridden by another image, which is the correct image of Fig. 9. The image of Fig. 9 in the published version should have been Fig. 10. Fig. 10 in the published version of the manuscript is a copy of Fig. 11.
The correct images for Fig. 8–10 and the corresponding captions are given here.
 | ||
| Fig. 8 (a) Typical changes in resistance, and (b) sensor response (S) and response time (tres) of S-Zn2SnO4, S-SnO2 and S-ZnO with different CH2O2 concentrations at 300 °C. | ||
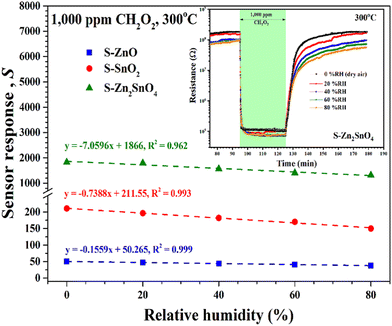 | ||
| Fig. 9 Sensor response of S-ZnO, S-SnO2, and S-Zn2SnO4 sensors towards 1000 ppm CH2O2 as a function of relative humidity (RH) at 0–80%. Inset: Corresponding change in resistance of S-Zn2SnO4 sensor. | ||
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © the Owner Societies 2023 |

