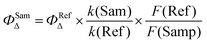Open Access Article
Open Access ArticleUnveiling the intersystem crossing dynamics in N-annulated perylene bisimides†
Jeswin
Sunny‡
 a,
Ebin
Sebastian‡
a,
Ebin
Sebastian‡
 a,
Suvarna
Sujilkumar‡
a,
Suvarna
Sujilkumar‡
 a,
Frank
Würthner
a,
Frank
Würthner
 b,
Bernd
Engels
b,
Bernd
Engels
 *c and
Mahesh
Hariharan
*c and
Mahesh
Hariharan
 *a
*a
aSchool of Chemistry, Indian Institute of Science Education and Research Thiruvananthapuram (IISER TVM), Vithura, Thiruvananthapuram, Kerala 695551, India. E-mail: mahesh@iisertvm.ac.in
bInstitut für Organische Chemie & Center for Nanosystems Chemistry, Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
cInstitut für Physikalische und Theoretische Chemie, Universität Würzburg, Emil-Fischer-Strasse 42, 97074 Würzburg, Germany
First published on 10th October 2023
Abstract
The efficient population of the triplet excited states in heavy metal-free organic chromophores has been one of the long-standing research problems to molecular photochemists. The negligible spin–orbit coupling matrix elements in the purely organic chromophores and the large singlet–triplet energy gap (ΔES–T) pose a hurdle for ultrafast intersystem crossing (ISC). Herein we report the unprecedented population of triplet manifold in a series of nitrogen-annulated perylene bisimide chromophores (NPBI and Br-NPBI). NPBI is found to have a moderate fluorescence quantum yield (Φf = 68 ± 5%), whereas Br-NPBI showcased a low fluorescence quantum yield (Φf = 2.0 ± 0.6%) in toluene. The femtosecond transient absorption measurements of Br-NPBI revealed ultrafast ISC (kISC = 1.97 × 1010 s−1) from the initially populated singlet excited state to the long-lived triplet excited states. The triplet quantum yields (ΦT = 95.2 ± 4.6% for Br-NPBI, ΦT = 18.7 ± 2.3% for NPBI) calculated from nanosecond transient absorption spectroscopy measurements showed the enhancement in triplet population upon bromine substitution. The quantum chemical calculations revealed the explicit role of nitrogen annulation in tuning the excited state energy levels to favor the ISC. The near degeneracy between the singlet and triplet excited states observed in NPBI and Br-NPBI (ΔES–T = −0.01 eV for NPBI, ΔES–T = 0.03 eV for Br-NPBI) facilitates the spin flipping in the molecules. Nitrogen annulation emerges as a design strategy to open up the ISC pathway and the rate of which can be further enhanced by the substitution of a heavier element.
Introduction
Intersystem crossing (ISC), a non-radiative transition involving spin flipping, is the quintessential process for populating the triplet manifold in chromophoric systems.1,2 The efficient population and harvest of triplet excitons find diverse applications in organic light-emitting diodes,3–5 organic photovoltaics,6–9 photodynamic therapy,10,11 photocatalysis,12,13etc. The long diffusion lengths of the triplet excitons have been exploited in optoelectronic devices to enhance the photocurrent as it increases the possibility of charge separation at donor–acceptor interfaces.8 The generation of reactive singlet oxygen via triplet sensitization from chromophores finds applications in photodynamic therapy.11,14 As a result, chromophores exhibiting efficient ISC are in great demand.Perylene bisimides (PBIs) are a class of highly sought-after n-type organic semiconductor materials for optoelectronic applications owing to their excellent electron affinity and mobility.15,16 The large molar absorption coefficient, high stability, easier tunability of electronic properties, etc., are attractive features of PBIs.16,17 A vital role in controlling the rate of ISC process in organic materials is played by spin–orbit coupling (SOC).1,18 In addition to SOC, the reduction in the singlet–triplet energy gap is important for enhancing the rate of intersystem crossing.2 Several strategies have been reported till date to generate triplet excitons, ranging from the conventional heavy atom effect,19 vibronic coupling,18,20 core twist,21–23 multiexciton generating singlet fission,24–28 and charge recombination.29–35 Seminal works by Castellano and co-workers36 and Würthner and co-workers37,38 have shown that incorporating transition metals to rylene bisimides enhances SOC-driven ISC. The large nuclear charge associated with transition metals imparts large SOC, thereby enhancing the rate of ISC.2 The cytotoxicity associated with chromophores encompassing heavy metals proposes the need for purely organic molecular materials exhibiting an enhanced triplet population. As an alternative strategy, non-metallic elements, including heavier halogens39–42 and chalcogens43–47 have been appended to chromophores to enhance the ISC rates. An unusual halogen bond-directed heavy atom effect was observed by Kim and co-workers in phosphorescent crystals of bromine-substituted aromatic carbonyls.48
The continuous efforts from our group in populating the triplet manifold have been fruitful in generating near unity triplet quantum yield in eight bromine atoms substituted PBI driven by the synergistic effect of core-twist and spin–orbit coupling.49 Herein, we report the observation of an ultrafast triplet population in a single bromine atom substituted nitrogen-annulated PBI (Br-NPBI) chromophore. Nitrogen-annulated PBI (NPBI) is found to have a moderately large fluorescence quantum yield (Φf = 68 ± 5%). Upon single bromine substitution at the bay position of NPBI, the fluorescence quantum yield remarkably decreases, rendering ultrafast ISC in Br-NPBI (Φf = 2.0 ± 0.6%). On the contrary, the unsubstituted PBI and mono-brominated Br-PBI exhibits strong fluorescence (Φf ∼ 100% for PBI and Φf = 97% for Br-PBI). The transient absorption measurements in the femtosecond and nano-second time regimes explicitly confirm the population of triplet excited states in Br-NPBI and NPBI. The quantum chemical calculations support the observation of triplet formation in Br-NPBI and NPBI, where efficient ISC occurs between the nearly degenerate S1 and T2 excited states (ΔES–T = −0.01 eV for NPBI, ΔES–T = 0.03 eV for Br-NPBI). The high singlet–triplet energy gap forbids the ISC pathway in PBI and Br-PBI. The present work sheds light on the research quest to achieve efficient triplet exciton formation in organic materials for advanced optoelectronic applications.
Experimental
Photophysical measurements
Steady-state and time-resolved optical measurements of the compounds were carried out in standard quartz cuvettes having path length of 1 cm for solutions in dried and distilled solvents. Electronic absorption spectra were recorded on Shimadzu UV-3600 UV-Vis-NIR, while emission and gated emission spectra were recorded on Horiba Jobin Yvon Fluorolog 3 spectrometer. The pulse width of the flash lamp for gated measurement is 3 μs.Estimating triplet quantum yield (ΦT)
To estimate the triplet quantum yield (ΦT) of Br-NPBI/NPBI, the process of triplet energy transfer to β-carotene was used with [Ru(bpy)3]2+ as the reference (ΦT ∼ 1) as reported in the literature.50–52 A known, equal volume of β-carotene in CHCl3 was added to the optically matched solutions (0.1–0.2 absorbance at 532 nm) of [Ru(bpy)3]2+ in methanol and Br-NPBI/NPBI in toluene. The energy transfer from [Ru(bpy)3]2+ or Br-NPBI/NPBI populates the β-carotene triplet, which is monitored at 530 nm. The ΦT values of the PBI derivatives were calculated according to the following equation where super-scripts “Sam” and “Ref” refers to the sample Br-NPBI/NPBI and reference [Ru(bpy)3]2+ respectively. kobs is the pseudo-first-order rate constant for the growth of the β-carotene triplet, and k0 is the rate constant for the decay of the donor triplets (in the absence of β-carotene) in solutions that contained [Ru(bpy)3]2+ or Br-NPBI/NPBI with same absorbance.Singlet oxygen (1O2) quantum yields (ΦΔ)
The singlet oxygen quantum yield (1O2) measurements were carried out using Rose Bengal (RB) in methanol as a standard having a 1O2 quantum yield of 0.80.53,54 1,3-Diphenylisobenzofuran (DPBF) in de-aerated dimethylformamide (DMF) solvent acts as the 1O2 scavenger, having absorbance around 1.0. The Br-NPBI (photosensitizer), was added into the cuvette (OD ∼ 0.2–0.3) containing DPBF. The cuvette was photo-irradiated using of 532 nm monochromatic light, and the absorption spectra was recorded in every 2 s interval. The slope of absorbance maxima of DPBF at 414 nm with irradiation time was plotted. Singlet oxygen quantum yield (ΦSamΔ) was calculated according to a modified equation.where k is the slope of the difference in the change in the absorbance of DPBF (at 414 nm) with irradiation time and F is the absorption correction factor, which is given by F = 1–10OD (OD at the irradiation wavelength).
Femtosecond transient absorption measurements
A Spectra-Physics Mai Tai SP mode-locked laser (800 nm, 86 MHz) was used as a seed for Spectra-Physics Spitfire Ace regenerative amplifier (1 kHz, 5.5 mJ). Using TOPAS, a portion of the amplified 800 nm output beam was converted into a 470 nm pump pulse. An 800 nm residual pulse passing through the ExciPro pump−probe spectrometer optical delay line (3.5 ns) was used in the generation of a white light continuum using calcium fluoride crystal. White light continuum passes through a beam splitter to produce the probe and the reference pulse. The femtosecond transient absorption spectra of the samples were recorded using a dual diode array detector, having a detection window and optical delay of 200 nm and 3.5 ns, respectively. Sample solutions were prepared in a 1.2 mm path length rotating cuvette. Determination of instrument response function (IRF) is essential for the accurate deconvolution of the spectra. IRF was determined by a two-photon absorption experiment using 10% benzene in a methanol solution and was found to be approximately 110 fs at 530 nm. Samples were excited at 470 nm with 100 fs pulses, and the use of a neutral density filter controlled the incident flux of the sample.Nanosecond transient absorption measurements
Nano-second laser transient absorption experiments of the samples were carried out in an Applied Photophysics Model LKS-60 laser kinetic spectrometer. Nitrogen-purged solution of samples were excited using the second harmonic (532 nm, pulse duration ∼8 ns) of a Quanta Ray INDI-40–10 series pulsed Nd:YAG laser. Oxygen-purged experiments confirm the presence of the triplet excited state through nanosecond transient absorption studies.Global analysis
Glotaran software is used for the global analysis of fsTA and nsTA data of the samples. The method evaluates the group velocity dispersion of the white light continuum and instrument response function (IRF), which allows accurate estimation of decay time constants and dispersion-compensated spectra. Global analysis of fsTA data carried out in a sequential model resulted in evolution-associated spectrum (EAS) with decay time constants.55Computational details
The ground state geometries of the Br-NPBI, NPBI, Br-PBI, and PBI were optimized using density functional theory (DFT) at CAM-B3LYP56/def2-SVP level of theory. Singlet excited states were computed using the time-dependent DFT (TD-DFT).56 Using the Tamm–Dancoff approximation (TDA) instead of TD-DFT the agreement to the experimental results deteriorates significantly (Table S6, ESI†). For triplet states, however, the Tamm–Dancoff approximation (TDA) were employed since it provides more reliable energies for such states.57 For all calculations the range-separated CAM-B3LYP functional in combination with def2-SVP and aug-cc-pVDZ58 basis sets were used. To obtain information about the shapes of the corresponding potential energy curves the S1 as well as the T2 equilibrium geometries were also determined. The spin–orbit coupling matrix elements between the spin singlet and triplet excited states were computed at the CAM-B3LYP/aug-cc-pVDZ levels of theory using the PySOC package.59,60 All the quantum chemical calculations were carried out using Gaussian 16 package.61Results and discussion
In this work, a series of perylene bisimide derivatives (PBI, Br-PBI, NPBI, and Br-NPBI) were synthesized and characterized following previously reported procedures (Fig. 1 and Scheme S1, ESI†).62,63 The geometries of the derivatives in the ground state were optimized using DFT at CAM-B3LYP/def2-SVP levels of theory (Fig. S1, ESI†). Unsubstituted PBI exhibited a planar structure in the ground state, whereas optimized geometry of Br-PBI revealed that the substitution of bromine atom on the bay position of PBI imposed a core twist in the chromophore with a twist angle of 20.6°. The optimized geometry of NPBI showcased a planar scaffold with a bowed geometry. The 5-membered heterocyclic ring formed, with a carbon-nitrogen bond length of 1.40 Å, imposed the inward bend in the core. The substitution of the bromine atom in the bay position of NPBI resulted in a negligible twist in the core, resulting in planar Br-NPBI. The insignificant core twist in Br-NPBI resulted from the reduced steric repulsion between the bromine and hydrogen atoms in the bay position supported by bowed geometry of the molecule (Fig. S1, ESI†). | ||
| Fig. 1 Molecular structures of (a) PBI, (b) Br-PBI, (c) NPBI, and (d) Br-NPBI. R in the chemical structure denotes n-hexyl chain. | ||
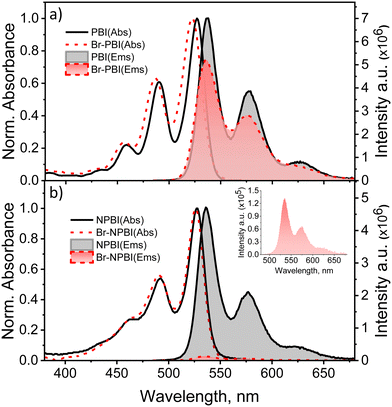
The steady-state optical measurements of PBI derivatives were performed in toluene at room temperature. The photophysical properties of PBI, Br-PBI, and NPBI, observed from the steady-state optical measurements, were consistent with the previous reports.22,63 The UV-Vis absorption spectra of PBI, Br-PBI, NPBI, and Br-NPBI show distinct vibronic features as a result of the strong coupling between the vinyl stretching modes of the PBI core (υC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C ∼ 1400 cm−1), and the S0 → S1 electronic transition (Fig. 2 and Fig. S2, ESI†). The UV-visible absorption spectrum of Br-NPBI showed two distinct absorption bands centered at 526 nm and 491 nm, corresponding to the 0-0, and 0-1 vibronic transitions, respectively. NPBI exhibited two distinct absorption bands at 527 nm and 492 nm corresponding to the 0-0, and 0-1 vibronic transitions, respectively. The absorption spectrum of Br-PBI showed three distinct vibronic bands at 522, 487, and 457 nm, whereas the unsubstituted PBI showcased vibronic bands centered at 527, 490, and 459 nm (Table 1).
C ∼ 1400 cm−1), and the S0 → S1 electronic transition (Fig. 2 and Fig. S2, ESI†). The UV-visible absorption spectrum of Br-NPBI showed two distinct absorption bands centered at 526 nm and 491 nm, corresponding to the 0-0, and 0-1 vibronic transitions, respectively. NPBI exhibited two distinct absorption bands at 527 nm and 492 nm corresponding to the 0-0, and 0-1 vibronic transitions, respectively. The absorption spectrum of Br-PBI showed three distinct vibronic bands at 522, 487, and 457 nm, whereas the unsubstituted PBI showcased vibronic bands centered at 527, 490, and 459 nm (Table 1).
The fluorescence emission spectra of Br-NPBI, NPBI, Br-PBI, and PBI revealed mirror images of the corresponding absorption spectra. Br-NPBI showed emission bands at 535 nm and 571 nm, whereas NPBI has emission bands centered at around 536 and 577 nm. The unsubstituted PBI has emission bands centered at 538, 577, and 627 nm, and Br-PBI has emission bands centered at 535, 575, and 628 nm (Fig. 2 and Fig. S3, ESI†). The fluorescence quantum yields (Φf) of the PBI derivatives were measured in toluene at room temperature. NPBI showed fluorescence quantum yield, Φf = 68 ± 5%, compared to Φf ∼ 100% for PBI and Φf = 97% for Br-PBI. Solvent-dependent fluorescence quantum yield measurements of Br-NPBI and NPBI have been carried out in acetone and acetonitrile solvents (Table S1, ESI†). Surprisingly, bromine substitution on NPBI reduced the fluorescence quantum yield to 2.0 ± 0.6% (Table 1). The observed reduction in the fluorescence quantum yield can be attributed to competitive non-radiative processes occurring from the S1 state.
The heavy atom effect induced by bromine atom substitution is known to enhance the ISC rate.49,64,65 However, a single bromine substitution on the PBI core has negligible effect on the triplet population.22 The SOC induced by the single bromine atom on PBI is inadequate for the enhancement of the ISC rate, as evident from the fluorescence quantum yield (Table 1). The reduced fluorescence in NPBI and the remarkably low fluorescence in its mono-brominated derivative, Br-NPBI, suggests the possibility of an enhanced triplet population in the molecules. Vauthey and coworkers reported ultrafast ISC in bromine and nitrogen-substituted naphthalene bisimide chromophore.42 The rate of the ISC can be theoretically determined using the Fermi golden rule where the rate constant of ISC depends on the SOC.66
To understand the competitive decay pathways from the singlet excited state, femtosecond transient absorption (fsTA) measurements were carried out in toluene at room temperature. The fsTA spectra of NPBI were adapted from ref. 63. The fsTA spectra of NPBI (Fig. 3a) after photoexcitation at 470 nm show negative ground state bleach (GSB) ranging from 480 nm to 557 nm. The band at 530 nm have contributions from both GSB and stimulated emission (SE). The band at 574 nm corresponds to higher vibronic bands of SE. The positive excited state absorption (ESA) lies in the region from 587 nm to 655 nm. In the initial delay times, NPBI shows ESA spectral features centered at 609 nm. As the delay time increases, the spectral signal decays with the slow evolution of a weak ESA feature centered at 570 nm. To elucidate the kinetics of the spectral evolution, a global analysis of the fsTA spectra was performed. The evolution-associated spectrum (EAS) of NPBI, was acquired by fitting using a two-component sequential kinetic model (A → B → GS). The two components can be attributed to two distinct excited state transitions, S1 → Sn and T1 → Tn. The initially populated singlet state spectral signature decays with the gradual evolution of spectral features corresponding to the triplet manifold. The ESA of the newly formed transient species centered at 570 nm can be attributed to the T1 → Tn transition. The fsTA spectra of Br-NPBI (Fig. 3b) after photoexcitation at 470 nm showed similar ESA and GSB compared to NPBI in the initial delay time. The positive spectral signature ranging from 613 nm to 627 nm attributed to the initially populated singlet state decay in ultrafast timescale (τA→B = 48.22 ± 0.13 ps), leading to the population of a new state with ESA centered at 570 nm. The decay of the newly formed species is not complete within the experimental time window. The de-convolution of the fsTA spectra was carried out through global analysis, extracting the EAS. The EAS of Br-NPBI was fitted into two components using a sequential kinetic model (A → B → GS). The first component (A) of the EAS is attributed to the absorption from the first singlet excited state to higher singlet states. The spectral signature associated with this transition decays in ultrafast timescale with (kISC) with the evolution of the second EAS component. The second component (B) can be attributed to the absorption from the first triplet excited state to higher triplet states.
 | ||
| Fig. 3 (Top) Femtosecond transient absorption spectra of (a) NPBI (adapted from ref. 63) and (b) Br-NPBI in toluene. Nanosecond transient absorption spectra of (c) Br-NPBI in toluene. (Middle) EAS reconstructed from global analysis. (Bottom) The relative population profiles of the excited states fitted using kinetic model. | ||
Nanosecond transient absorption (nsTA) measurements of NPBI and Br-NPBI in toluene at room temperature were carried out to understand the dynamics of the excited state in the longer delay times. Photoexcitation at 532 nm of nitrogen-purged solution of NPBI resulted in a positive peak centered at around 570 nm and a negative peak corresponding to the ground state bleach ranging from 470 nm to 545 nm (Fig. S4, ESI†). The positive spectral signature at 570 nm was quenched upon oxygen-purging of the NPBI solution, confirming the triplet population (Fig. S5, ESI†). The decay time constant of the triplet excited state of NPBI was found to be 1.42 ± 0.23 μs (Fig. S4, ESI†) in the degassed solution with a quantum yield (ΦT) of 18.7 ± 2.3% (Fig. S6, ESI†). nsTA measurements of nitrogen-purged Br-NPBI (Fig. 3c) solution in toluene showed an intense positive peak centered at around 570 nm after photoexcitation at 532 nm. The nsTA spectra showed ground state bleach in the region from 460 nm to 540 nm. The formation of a triplet excited state in Br-NPBI is confirmed by the depletion in the spectral peak intensity at 570 nm after purging the solution with oxygen (Fig. S7, ESI†). The decay time constant of the long-lived triplet excited state was found to be τT = 2.34 ± 0.08 μs. The triplet quantum yield of Br-NPBI in toluene was estimated to be ΦT = 95.2 ± 4.6% using a triplet–triplet energy transfer mechanism (Fig. S8, ESI†). The singlet oxygen quantum yield (ΦΔ) of Br-NPBI is estimated using Rose Bengal (RB) as a standard in methanol.54 Singlet oxygen quantum yield for Br-NPBI was found to be 86.0% using Rose Bengal as a standard (Fig. S9, ESI†). nsTA measurements of Br-PBI were carried out in toluene at room temperature. Photoexcitation at 532 nm of nitrogen-purged solution of Br-PBI showed no significant positive absorption, indicating the negligible intersystem crossing in the Br-PBI (Fig. S10, ESI†).
The gated emission measurements of Br-NPBI and NPBI were carried out exciting at 470 nm and 472 nm, respectively, at 77 K in toluene. For Br-NPBI (delay time of 0.05 ms, slit width 1.5 mm) the gated emission band was observed at 599 nm with a lifetime of 11.8 μs (Fig. S11, ESI†). Gated measurements of NPBI at 77 K exhibited a band at 597 nm (delay time of 0.05 ms, slit width 1.5 mm) with a lifetime of 7.5 μs (Fig. S12, ESI†).
To rationalize the role of bromine and nitrogen substitution on PBI molecule in enhancing the ISC rate, we performed quantum chemical calculations. The computed excitation energies with respect to the S0 ground state in its equilibrium geometry R(S0) using the range-separated CAM-B3LYP functional67 in combination with aug-cc-pVDZ basis sets58 are summarized in Table 2. The corresponding data using the smaller def2-SVP basis sets68,69 are given in Tables S2–S5 (ESI†). The data are also plotted in Fig. S14 and S15 (ESI†) for a better overview.
| Geometry | Energy relative to the ground state energy at R(S0) in eV | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBI | Br-PBI | NPBI | Br-NPBI | |||||||||||||
| S0 | S1 | T1 | T2 | S0 | S1 | T1 | T2 | S0 | S1 | T1 | T2 | S0 | S1 | T1 | T2 | |
| R(S0) | 0 | 2.70 | 1.64 | 2.93 | 0 | 2.72 | 1.69 | 2.92 | 0 | 2.82 | 1.82 | 2.66 | 0.0 | 2.83 | 1.86 | 2.64 |
| R(S1) | 0.18 | 2.45 | 1.37 | 2.92 | 0.21 | 2.49 | 1.39 | 2.91 | 0.15 | 2.64 | 1.60 | 2.65 | 0.15 | 2.66 | 1.63 | 2.63 |
| R(T2) | 0.29 | 2.61 | 1.46 | 2.88 | 0.29 | 2.60 | 1.47 | 2.84 | 0.11 | 2.74 | 1.71 | 2.54 | 0.11 | 2.74 | 1.74 | 2.53 |
The absorption spectra of the compounds (Fig. 2) show a three-band vibrational progression so that the most intense band given in Table 1 must be assigned to the transition between the lowest vibrational states of S0 and the S1 (0-0 transition). The other bands then result from excitations to higher vibrational states of the S1 (Fig. 4). The small difference between λabs and λf also indicates 0-0 transitions for absorption and emission. For all molecules, they differ only by about 0.05 eV, which most likely results from solvent effects. To model the vibrational progression and the exact energetic location of the 0-0 band, calculations that take into account the influence of nuclear motion are necessary, as we have recently shown in a benchmark.70 However, this benchmark also proved that adiabatic excitation energies (see Fig. 4 and Fig. S13, ESI†) approximate the 0-0 excitation energy to within about 0.1 eV, while simple vertical energies overestimate 0-0 transition energies by up to 0.3 eV.
 | ||
| Fig. 4 Schematic representation of potential energy surfaces of S0, S1, and T2 of (a) Br-PBI/PBI and (b) Br-NPBI/NPBI demonstrating the possibility of ISC. | ||
Table 2 shows similar effects for the molecules examined in this paper. For PBI, the vertical energy of 2.70 eV calculated with CAMB3LYP/aug-cc-pVDZ (Table 2: E(S1) at R(S0)) overestimates the experimentally measured 0-0 transition (Table 1: 527 nm = 2.35 eV) by about 0.35 eV. In contrast, the adiabatic excitation energy of 2.45 eV (Table 2: E(S1) at R(S1)) deviates by only 0.1 eV, which is similar to the inaccuracy found in our benchmark for adiabatic energies. If one uses the smaller def2-SVP basis (Tables S2–S5, ESI†), the deviations from the experimental data increase by about 0.1 eV. For Br-PBI, the picture is identical. For NPBI and Br-NPBI, however, the adiabatic energies deviate by about 0.3 eV from the experimental 0-0 transitions listed in Table 1. We attribute this somewhat stronger deviation to a more complex electronic structure, possibly resulting from the influence of the strain resulting from the ring formation.
However, more precise calculations are not necessary because the main difference between PBI/Br-PBI (high fluorescence quantum yield) and NPBI/Br-NPBI (low fluorescence quantum yield) results from the changes in the energetic sequence of S1 and T2 (Table 2 and Fig. S14, ESI†), which are clearly indicated by our calculations. For all four molecules, the T1 is well below the S1 (>1.0 eV), so an S1–T1 singlet–triplet transition as a reason for decreasing (fluorescence quantum yields) can be ruled out. For PBI and Br-PBI, the T2 for R(S1) is about 0.5 eV above the S1, so that an S1–T2 transition can also be excluded. For NPBI and Br-NPBI, however, the picture changes completely. For both molecules, our calculations predict that the S1 and the T2 are nearly degenerate (Table 2 and Fig. 4) inducing very efficient S1–T2 transitions even for the small spin–orbit couplings calculated for NPBI or Br-NPBI (Table S7, ESI†). The greatly reduced fluorescence yield of Br-NPBI compared to NPBI could result from larger SOC couplings, but this is not supported by the calculations (Table S7, ESI†) which predict a similar size of the couplings. Hence, we attribute this experimental finding to subtle different energetic positions of both states for Br-NPBI and NPBI being outside of the accuracy of the employed theoretical approach. The situation is sketched in Fig. 4. For PBI and Br-PBI, the T2 lies above the S1, so no ISC can happen. For NPBI and Br-NPBI, the T2 lies below the S1 for R(S0) but both are nearly energy degenerate so that after the excitation, an efficient S1–T2 ISC can take place.
Conclusions
In summary, we report the unprecedented triplet population in NPBI and Br-NPBI facilitated by a reduced singlet–triplet energy gap. The moderate and low fluorescence quantum yields were observed for NPBI and Br-NPBI, respectively. The concerted interplay of nitrogen annulation and single bromine substitution fosters efficient intersystem crossing in Br-NPBI with ISC rate, kISC = 1.97 × 1010 s−1 as observed from the fsTA measurements. The triplet quantum yield is enhanced from ΦT = 18.7 ± 2.3% in NPBI to ΦT = 95.2 ± 4.6% for Br-NPBI which could be rationalized as the effect of bromine substitution. The nsTA spectroscopy revealed the long decay times for the triplet states in NPBI and Br-NPBI. Theoretical investigations suggest the significant role of nitrogen annulation in diminishing the singlet–triplet energy gap in NPBI and Br-NPBI. The low ΔES–T value activates the ISC pathway in both NPBI and Br-NPBI, whereas the additional effect of bromine in Br-NPBI enhances the ISC rates to the ultrafast regime. The current work sheds light on the novel design strategies for triplet exciton generation in molecular materials, which have profound implications for developing organic light-emitting diodes and organic solar cells.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. H. acknowledges the Department of Science and Technology (DST) Nanomission [DST/NM/TUE/EE-01/2019] for the support of this work. We thank the support for the high-performance computing time at the Padmanabha cluster at IISER TVM. E. S. and S. S. acknowledge UGC and IISER TVM, respectively, for financial assistance.Notes and references
- N. J. Turro, V. Ramamurthy and J. C. Scaiano, Modern Molecular Photochemistry of Organic Molecules, University Science Books, 2010 Search PubMed.
- M. Hariharan, D. Sasikumar, A. T. John and J. Sunny, Access to the triplet excited states of organic chromophores, Chem. Soc. Rev., 2020, 49, 6122 RSC.
- J. Lee, H. F. Chen, T. Batagoda, C. Coburn, P. I. Djurovich, M. E. Thompson and S. R. Forrest, Deep blue phosphorescent organic light-emitting diodes with very high brightness and efficiency, Nat. Mater., 2016, 15, 92–98 CrossRef CAS PubMed.
- C. Adachi, M. A. Baldo, M. E. Thompson and S. R. Forrest, Nearly 100% internal phosphorescence efficiency in an organic light-emitting device, J. Appl. Phys., 2001, 90, 5048–5051 CrossRef CAS.
- Z. An, C. Zheng, Y. Tao, R. Chen, H. Shi, T. Chen, Z. Wang, H. Li, R. Deng, X. Liu and W. Huang, Stabilizing triplet excited states for ultralong organic phosphorescence, Nat. Mater., 2015, 14, 685–690 CrossRef CAS PubMed.
- M. Einzinger, T. Wu, J. F. Kompalla, H. L. Smith, C. F. Perkinson, L. Nienhaus, S. Wieghold, D. N. Congreve, A. Kahn, M. G. Bawendi and M. A. Baldo, Sensitization of silicon by singlet exciton fission in tetracene, Nature, 2019, 571, 90–94 CrossRef CAS PubMed.
- A. Rao and R. H. Friend, Harnessing singlet exciton fission to break the Shockley–Queisser limit, Nat. Rev. Mater., 2017, 2, 17063 CrossRef CAS.
- J. Lee, P. Jadhav, P. D. Reusswig, S. R. Yost, N. J. Thompson, D. N. Congreve, E. Hontz, T. Van Voorhis and M. A. Baldo, Singlet Exciton Fission Photovoltaics, Acc. Chem. Res., 2013, 46, 1300–1311 CrossRef CAS PubMed.
- W.-Y. Wong and C.-L. Ho, Organometallic Photovoltaics: A New and Versatile Approach for Harvesting Solar Energy Using Conjugated Polymetallaynes, Acc. Chem. Res., 2010, 43, 1246–1256 CrossRef CAS PubMed.
- V. N. Nguyen, Y. Yan, J. Zhao and J. Yoon, Heavy-Atom-Free Photosensitizers: From Molecular Design to Applications in the Photodynamic Therapy of Cancer, Acc. Chem. Res., 2021, 54, 207–220 CrossRef CAS PubMed.
- T. C. Pham, V.-N. Nguyen, Y. Choi, S. Lee and J. Yoon, Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy, Chem. Rev., 2021, 121, 13454–13619 CrossRef CAS PubMed.
- L. Schmid, F. Glaser, R. Schaer and O. S. Wenger, High Triplet Energy Iridium(III) Isocyanoborato Complex for Photochemical Upconversion, Photoredox and Energy Transfer Catalysis, J. Am. Chem. Soc., 2022, 144, 963–976 CrossRef CAS PubMed.
- F. Strieth-Kalthoff, M. J. James, M. Teders, L. Pitzer and F. Glorius, Energy transfer catalysis mediated by visible light: principles, applications, directions, Chem. Soc. Rev., 2018, 47, 7190–7202 RSC.
- L. A. Ortiz-Rodríguez, S. J. Hoehn, A. Loredo, L. Wang, H. Xiao and C. E. Crespo-Hernández, Electronic Relaxation Pathways in Heavy-Atom-Free Photosensitizers Absorbing Near-Infrared Radiation and Exhibiting High Yields of Singlet Oxygen Generation, J. Am. Chem. Soc., 2021, 143, 2676–2681 CrossRef PubMed.
- A. Nowak-Król, K. Shoyama, M. Stolte and F. Würthner, Naphthalene and perylene diimides – better alternatives to fullerenes for organic electronics?, Chem. Commun., 2018, 54, 13763–13772 RSC.
- X. Zhan, A. Facchetti, S. Barlow, T. J. Marks, M. A. Ratner, M. R. Wasielewski and S. R. Marder, Rylene and Related Diimides for Organic Electronics, Adv. Mater., 2011, 23, 268–284 CrossRef CAS PubMed.
- C. Li and H. Wonneberger, Perylene Imides for Organic Photovoltaics: Yesterday, Today, and Tomorrow, Adv. Mater., 2012, 24, 613–636 CrossRef CAS PubMed.
- T. J. Penfold, E. Gindensperger, C. Daniel and C. M. Marian, Spin-Vibronic Mechanism for Intersystem Crossing, Chem. Rev., 2018, 118, 6975–7025 CrossRef CAS PubMed.
- D. S. McClure, Triplet–Singlet Transitions in Organic Molecules. Lifetime Measurements of the Triplet State, J. Chem. Phys., 1949, 17, 905–913 CrossRef CAS.
- R. A. Vogt and C. E. Crespo-Hernández, Conformational Control in the Population of the Triplet State and Photoreactivity of Nitronaphthalene Derivatives, J. Phys. Chem. A, 2013, 117, 14100–14108 CrossRef CAS PubMed.
- P. Malakar, V. Borin, A. Bedi, I. Schapiro, O. Gidron and S. Ruhman, The impact of twisting on the intersystem crossing in acenes: an experimental and computational study, Phys. Chem. Chem. Phys., 2022, 24, 2357–2362 RSC.
- K. Nagarajan, A. R. Mallia, K. Muraleedharan and M. Hariharan, Enhanced intersystem crossing in core-twisted aromatics, Chem. Sci., 2017, 8, 1776–1782 RSC.
- C. A. M. Salla, G. Farias, M. Rouzières, P. Dechambenoit, F. Durola, H. Bock, B. de Souza and I. H. Bechtold, Persistent Solid-State Phosphorescence and Delayed Fluorescence at Room Temperature by a Twisted Hydrocarbon, Angew. Chem., Int. Ed., 2019, 58, 6982–6986 CrossRef CAS PubMed.
- M. B. Smith and J. Michl, Singlet Fission, Chem. Rev., 2010, 110, 6891–6936 CrossRef CAS PubMed.
- T. Ullrich, D. Munz and D. M. Guldi, Unconventional singlet fission materials, Chem. Soc. Rev., 2021, 50, 3485–3518 RSC.
- K. Miyata, F. S. Conrad-Burton, F. L. Geyer and X.-Y. Zhu, Triplet Pair States in Singlet Fission, Chem. Rev., 2019, 119, 4261–4292 CrossRef CAS PubMed.
- J. Xia, S. N. Sanders, W. Cheng, J. Z. Low, J. Liu, L. M. Campos and T. Sun, Singlet Fission: Progress and Prospects in Solar Cells, Adv. Mater., 2017, 29, 1601652 CrossRef PubMed.
- A. Mandal, M. Chen, E. D. Foszcz, J. D. Schultz, N. M. Kearns, R. M. Young, M. T. Zanni and M. R. Wasielewski, Two-Dimensional Electronic Spectroscopy Reveals Excitation Energy-Dependent State Mixing during Singlet Fission in a Terrylenediimide Dimer, J. Am. Chem. Soc., 2018, 140, 17907–17914 CrossRef CAS PubMed.
- M. L. Williams, I. Schlesinger, R. M. Jacobberger and M. R. Wasielewski, Mechanism of Ultrafast Triplet Exciton Formation in Single Cocrystals of π-Stacked Electron Donors and Acceptors, J. Am. Chem. Soc., 2022, 144, 18607–18618 CrossRef CAS PubMed.
- Z. E. X. Dance, S. M. Mickley, T. M. Wilson, A. B. Ricks, A. M. Scott, M. A. Ratner and M. R. Wasielewski, Intersystem Crossing Mediated by Photoinduced Intramolecular Charge Transfer: Julolidine−Anthracene Molecules with Perpendicular π Systems, J. Phys. Chem. A, 2008, 112, 4194–4201 CrossRef CAS PubMed.
- M. Lv, X. Lu, Y. Jiang, M. E. Sandoval-Salinas, D. Casanova, H. Sun, Z. Sun, J. Xu, Y. Yang and J. Chen, Near-Unity Triplet Generation Promoted via Spiro-Conjugation, Angew. Chem., Int. Ed., 2022, 61, e202113190 CrossRef CAS PubMed.
- X. Chen, A. A. Sukhanov, M. Taddei, B. Dick, J. Zhao, V. K. Voronkova and M. Di, Donato, Charge Separation/Recombination, Intersystem Crossing, and Unusually Slow Intramolecular Triplet–Triplet Energy Transfer in Naphthalenediimide–Anthracene Compact Energy Donor–Acceptor Dyads, J. Phys. Chem. Lett., 2022, 13, 8740–8748 CrossRef CAS PubMed.
- X. Xiao, I. Kurganskii, P. Maity, J. Zhao, X. Jiang, O. F. Mohammed and M. Fedin, A long-lived charge-separated state of spiro compact electron donor–acceptor dyads based on rhodamine and naphthalenediimide chromophores, Chem. Sci., 2022, 13, 13426–13441 RSC.
- X. Wang, L. Martínez-Fernández, Y. Zhang, K. Zhang, R. Improta, B. Kohler, J. Xu and J. Chen, Solvent-Dependent Stabilization of a Charge Transfer State is the Key to Ultrafast Triplet State Formation in an Epigenetic DNA Nucleoside, Chem. – Eur. J., 2021, 27, 10932–10940 CrossRef CAS PubMed.
- Z. Wang, L. Ma, H. Zhao, Y. Wan, X.-F. Zhang, Y. Li, Z. Kuang and A. Xia, Spin–orbit charge-transfer intersystem crossing in heavy-atom-free orthogonal covalent boron-dipyrromethene heterodimers, Phys. Chem. Chem. Phys., 2023, 25, 24386–24394 RSC.
- E. O. Danilov, A. A. Rachford, S. Goeb and F. N. Castellano, Evolution of the triplet excited state in Pt II perylenediimides, J. Phys. Chem. A, 2009, 113, 5763–5768 CrossRef CAS PubMed.
- M. J. Sun, O. Anhalt, M. B. Sárosi, M. Stolte and F. Würthner, Activating Organic Phosphorescence via Heavy Metal–π Interaction Induced Intersystem Crossing, Adv. Mater., 2022, 34, 2207331 CrossRef CAS PubMed.
- M. Schulze, A. Steffen and F. Würthner, Near-IR phosphorescent ruthenium(II) and iridium(III) perylene bisimide metal complexes, Angew. Chem., Int. Ed., 2015, 54, 1570–1573 CrossRef CAS PubMed.
- G. Farias, C. A. M. Salla, M. Aydemir, L. Sturm, P. Dechambenoit, F. Durola, B. de Souza, H. Bock, A. P. Monkman and I. H. Bechtold, Halogenation of a twisted non-polar π-system as a tool to modulate phosphorescence at room temperature, Chem. Sci., 2021, 12, 15116–15127 RSC.
- E. Pomarico, P. Pospíšil, M. E. F. Bouduban, J. Vestfrid, Z. Gross, S. Záliš, M. Chergui and A. Vlček, Photophysical Heavy-Atom Effect in Iodinated Metallocorroles: Spin–Orbit Coupling and Density of States, J. Phys. Chem. A, 2018, 122, 7256–7266 CrossRef CAS PubMed.
- J. Vestfrid, M. Botoshansky, J. H. Palmer, A. C. Durrell, H. B. Gray and Z. Gross, Iodinated Aluminum(III) Corroles with Long-Lived Triplet Excited States, J. Am. Chem. Soc., 2011, 133, 12899–12901 CrossRef CAS PubMed.
- O. Yushchenko, G. Licari, S. Mosquera-Vazquez, N. Sakai, S. Matile and E. Vauthey, Ultrafast Intersystem-Crossing Dynamics and Breakdown of the Kasha–Vavilov's Rule of Naphthalenediimides, J. Phys. Chem. Lett., 2015, 6, 2096–2100 CrossRef CAS PubMed.
- J. Sivanarayanan, E. Sebastian, K. Vinod and M. Hariharan, Ultrafast Intersystem Crossing in Selenium-Annulated Perylene Bisimide, J. Phys. Chem. C, 2022, 2022, 126–13319 Search PubMed.
- P. Mentzel, M. Holzapfel, A. Schmiedel, I. Krummenacher, H. Braunschweig, A. Wodyński, M. Kaupp, F. Würthner and C. Lambert, Excited states and spin–orbit coupling in chalcogen substituted perylene diimides and their radical anions, Phys. Chem. Chem. Phys., 2022, 24, 26254–26268 RSC.
- R. D. Pensack, Y. Song, T. M. McCormick, A. A. Jahnke, J. Hollinger, D. S. Seferos and G. D. Scholes, Evidence for the Rapid Conversion of Primary Photoexcitations to Triplet States in Seleno- and Telluro- Analogues of Poly(3-hexylthiophene), J. Phys. Chem. B, 2014, 118, 2589–2597 CrossRef CAS PubMed.
- F. Sadiq, Z. Wang, Y. Hou, J. Zhao, A. Elmali, D. Escudero and A. Karatay, Thienyl/phenyl bay-substituted perylenebisimides: Intersystem crossing and application as heavy atom-free triplet photosensitizers, Dyes Pigm., 2021, 184, 108708 CrossRef CAS.
- S. Mai, M. Pollum, L. Martínez-Fernández, N. Dunn, P. Marquetand, I. Corral, C. E. Crespo-Hernández and L. González, The origin of efficient triplet state population in sulfur-substituted nucleobases, Nat. Commun., 2016, 7, 13077 CrossRef CAS PubMed.
- O. Bolton, K. Lee, H.-J. Kim, K. Y. Lin and J. Kim, Activating efficient phosphorescence from purely organic materials by crystal design, Nat. Chem., 2011, 3, 205–210 CrossRef CAS PubMed.
- A. Mohan, E. Sebastian, M. Gudem and M. Hariharan, Near-Quantitative Triplet State Population via Ultrafast Intersystem Crossing in Perbromoperylenediimide, J. Phys. Chem. B, 2023, 2020, 37 Search PubMed.
- S. K. Rajagopal, K. Nagaraj, S. Deb, V. Bhat, D. Sasikumar, E. Sebastian and M. Hariharan, Extending the scope of the carbonyl facilitated triplet excited state towards visible light excitation, Phys. Chem. Chem. Phys., 2018, 20, 19120–19128 RSC.
- W. E. Ford and P. V. Kamat, Photochemistry of 3,4,9,10-perylenetetracarboxylic dianhydride dyes. 3. Singlet and triplet excited-state properties of the bis(2,5-di-tert-butylphenyl)imide derivative, J. Phys. Chem., 1987, 91, 6373–6380 CrossRef CAS.
- V. Karunakaran, D. D. Prabhu and S. Das, Optical Investigation of Self-Aggregation of a Tetrazole-Substituted Diphenylacetylene Derivative: Steady and Excited State Dynamics in Solid and Solution State, J. Phys. Chem. C, 2013, 117, 9404–9415 CrossRef CAS.
- A. A. Buglak, A. Charisiadis, A. Sheehan, C. J. Kingsbury, M. O. Senge and M. A. Filatov, Quantitative Structure–Property Relationship Modelling for the Prediction of Singlet Oxygen Generation by Heavy-Atom-Free BODIPY Photosensitizers, Chem. – Eur. J., 2021, 27, 9934–9947 CrossRef CAS PubMed.
- L. Huang, Z. Li, Y. Zhao, Y. Zhang, S. Wu, J. Zhao and G. Han, Ultralow-Power Near Infrared Lamp Light Operable Targeted Organic Nanoparticle Photodynamic Therapy, J. Am. Chem. Soc., 2016, 138, 14586–14591 CrossRef CAS PubMed.
- I. H. M. van Stokkum, D. S. Larsen and R. van Grondelle, Global and target analysis of time-resolved spectra, Biochim. Biophys. Acta, Bioenerg., 2004, 1657, 82–104 CrossRef CAS PubMed.
- C. Adamo and D. Jacquemin, The calculations of excited-state properties with Time-Dependent Density Functional Theory, Chem. Soc. Rev., 2013, 42, 845–856 RSC.
- C. Brückner and B. Engels, Benchmarking singlet and triplet excitation energies of molecular semiconductors for singlet fission: tuning the amount of HF exchange and adjusting local correlation to obtain accurate functionals for singlet–triplet gaps, Chem. Phys., 2017, 482, 319–338 CrossRef.
- D. E. Woon and T. H. Dunning, Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon, J. Chem. Phys, 1993, 98, 1358–1371 CrossRef CAS.
- X. Gao, S. Bai, D. Fazzi, T. Niehaus, M. Barbatti and W. Thiel, Evaluation of Spin–Orbit Couplings with Linear-Response Time-Dependent Density Functional Methods, J. Chem. Theory Comput., 2017, 13, 515–524 CrossRef CAS PubMed.
- R. Ahmed and A. K. Manna, Origins of Molecular-Twist-Triggered Intersystem Crossing in Functional Perylenediimides: Singlet–Triplet Gap versus Spin–Orbit Coupling, J. Phys. Chem. A, 2022, 126, 6594–6603 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- A. D. Hendsbee, J.-P. Sun, W. K. Law, H. Yan, I. G. Hill, D. M. Spasyuk and G. C. Welch, Synthesis, Self-Assembly, and Solar Cell Performance of N-Annulated Perylene Diimide Non-Fullerene Acceptors, Chem. Mater., 2016, 28, 7098–7109 CrossRef CAS.
- E. Sebastian, J. Sunny and M. Hariharan, Excimer evolution hampers symmetry-broken charge-separated states, Chem. Sci., 2022, 13, 10824–10835 RSC.
- K. Nagarajan, A. R. Mallia, V. S. Reddy and M. Hariharan, Access to Triplet Excited State in Core-Twisted Perylenediimide, J. Phys. Chem. C, 2016, 120, 8443–8450 CrossRef CAS.
- S. Sarkar, H. P. Hendrickson, D. Lee, F. DeVine, J. Jung, E. Geva, J. Kim and B. D. Dunietz, Phosphorescence in Bromobenzaldehyde Can Be Enhanced through Intramolecular Heavy Atom Effect, J. Phys. Chem. C, 2017, 121, 3771–3777 CrossRef CAS.
- C. M. Marian, Understanding and Controlling Intersystem Crossing in Molecules, Annu. Rev. Phys. Chem., 2021, 72, 617–640 CrossRef CAS PubMed.
- T. Yanai, D. P. Tew and N. C. Handy, A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP), Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS.
- F. Weigend and R. Ahlrichs, Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy, Phys. Chem. Chem. Phys., 2005, 7, 3297 RSC.
- A. Schäfer, H. Horn and R. Ahlrichs, Fully optimized contracted Gaussian basis sets for atoms Li to Kr, J. Chem. Phys., 1992, 97, 2571–2577 CrossRef.
- M. Deutsch, S. Wirsing, D. Kaiser, R. F. Fink, P. Tegeder and B. Engels, Geometry relaxation-mediated localization and delocalization of excitons in organic semiconductors: a quantum chemical study, J. Chem. Phys., 2020, 153, 224104 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Adiabatic energies and plots, nsTA features, triplet and singlet oxygen quantum yield. See DOI: https://doi.org/10.1039/d3cp03888b |
| ‡ These authors contributed equally. |
| This journal is © the Owner Societies 2023 |