Open Access Article
Open Access ArticleElectrochemically regulated luminescence of europium complexes with β-diketone in polyether matrices†
Ryoto
Yabuta
 ,
Norihisa
Kobayashi
,
Norihisa
Kobayashi
 and
Kazuki
Nakamura
and
Kazuki
Nakamura
 *
*
Graduate School of Engineering, Chiba University, 1-33, Yayoi-cho, Inage-ku, Chiba, 263-8522, Japan. E-mail: Nakamura.Kazuki@faculty.chiba-u.jp
First published on 25th July 2023
Abstract
This study investigates the electrochemical modulation of luminescence color, i.e., electrofluorochromism, of an Eu complex in a polyether solvent. The electrofluorochromic (EFC) reaction of the Eu complex occurred via a reversible redox reaction between Eu3+ and Eu2+. Initially, the intrinsically stable Eu3+ complex showed intense red photoluminescence (PL) induced by f–f transitions. After the electrochemical reduction of Eu3+ to Eu2+, broad blue PL was observed attributed to the d–f transitions in the Eu2+ complex. This distinct blue luminescence from the Eu2+ complex was attributed to the effective stabilization of the Eu2+ state by the polyether solvent. The dynamic EFC reaction that changes the valence state of the Eu ion can be potentially applied to novel chemical sensors, security devices, and display devices.
Introduction
Chromogenic materials exhibit optical properties including luminescence and absorption that can be altered using external stimuli such as light, heat, and electricity. They show potential applications in chemical sensors,1,2 biochemical labels,3 molecular memory,4 and display devices.5,6 Electrofluorochromic (EFC) materials display a change in luminescence color regulated via electrochemical redox reactions. They are innovative materials as they can convert electrical inputs into visual signals rapidly and repeatedly,4,7–10 and include molecules,11–15 conjugated polymers,16–20 inorganic compounds,21,22 and metal complexes.23–29Ln(III) complexes are composed of a luminescent core of Ln(III) ions surrounded by antenna ligands possessing a light-absorbing capability. The luminescence of Ln(III) complexes is enhanced via efficient intramolecular energy transfer from the antenna ligands to the Ln(III) center, resulting in brilliant luminescence.30–33 Furthermore, Ln(III) complexes show attractive photoluminescence (PL) properties such as characteristic and narrow emission bands in the visible-near infrared (vis-NIR) region attributed to Ln(III), long emission lifetimes, and high transparency in the visible region due to the large pseudo-Stokes shift.34 In particular, Eu complexes have excellent emission properties, and the stabilities of their trivalent (Eu3+) and divalent (Eu2+) states are higher than those of other Ln(III) complexes. Eu3+ is characterized by intense and long-lived red luminescence induced by f–f transitions and is extensively utilized in the development of biosensors, light-emitting materials, etc. Eu2+ is characterized by broad and blue luminescence induced by d–f transitions from the excited state of 4f6 5d1 to the ground level state of 8S7/2 (4f7),35 and the emission from Eu2+ in inorganic matrices could be applied to various potential luminescent devices.36–39 The reversible control of red luminescence from Eu3+ and blue luminescence from Eu2+via electrochemical redox reactions can contribute to the development of a novel display device. However, the observation of luminescence from Eu2+ is challenging because it is unstable in air and commonly used solutions. Therefore, previous studies reported high temperature and extended time requirements for Eu2+ formation; for instance, Eu2+-doped zeolite derivatives were synthesized via annealing of Eu-containing zeolites in a reducing atmosphere at 800 °C for 3 h.39 Additionally, the electrochemical control of trivalent and divalent ions is difficult, therefore, this study focuses on regulating the luminescence between Eu3+ and Eu2+via electrochemical redox reactions. It is well-known that the blue luminescence of Eu2+ can be stabilized by polyethers, such as crown ethers and polyethylene glycols.35,40 Upon stabilization, the Eu2+ state could be produced recurrently in a facile manner via the electrochemical reduction of Eu3+ in polyethylene glycol solutions. Considering the EFC reaction of Eu compounds, the β-diketonate Eu complex exhibiting superior red luminescence properties in the Eu3+ state was explored in the present study.
The electrochemical redox reactions of polyether solutions containing Eu ions and a β-diketonate Eu complex were investigated. The studies revealed that the electrochemically generated Eu2+ state was stable in both the solutions containing Eu ions and the Eu complex. A distinct change in luminescence color, i.e., electrofluorochromism, was observed via the electrochemical reduction of the Eu complex in the two-electrode device using luminescence spectroscopy and the naked eye.
Experimental
Materials
Europium nitrate hexahydrate (Eu(NO3)3·6H2O), tetra-n-butylammonium perchlorate (TBAP), silver nitrate (AgNO3), propylene carbonate (PC), and acetonitrile (MeCN) were purchased from Kanto Chemical Industry Co., Japan. Dimethyl sulfoxide (DMSO) was purchased from Sigma Aldrich Japan. Hexafluoro acetylacetone (hfa) was procured from Tokyo Chemical Industry Co., Japan. Lithium trifluoromethanesulfonate (CF3SO3Li), lithium nitrate (LiNO3), and polyethylene glycol 400 (PEG400) were purchased from FUJIFILM Wako Pure Chemical Co., Japan. CF3SO3Li was used as the supporting electrolyte without further purification. Indium tin oxide (ITO) (10 Ω sq−1; Yasuda, Japan) was used as an electrode after washing and ozonation.Synthesis of the luminescent Eu(III) complex
Tris (hexafluoro acetylacetonate) europium (Eu(hfa)3(H2O)2) was prepared according to a method previously reported in the literature.41 Europium acetate n-hydrate was dissolved in deionized water at room temperature. Subsequently, 3 equiv. of the liquid hfa-H2 was added dropwise into the solution. After stirring for 3 h, the obtained white precipitate was filtered and purified by recrystallization using methanol/water. Yield: 80%. Anal. Calcd for C15H7O8F18Eu: C, 22.48; H, 0.88%. Found: C, 21.52; H, 1.04%.Preparation of the electrolyte solutions
The electrochemical redox behaviour of Eu ions was investigated using a Eu3+ solution containing Eu(NO3)·6H2O (10 mmol L−1) and LiCF3SO3 (500 mmol L−1) in PEG400. For comparison, two types of electrolyte solutions without Eu ions, namely LiCF3SO3 (500 mmol L−1) in PEG400, and LiNO3 (60 mmol L−1) and LiCF3SO3 (500 mmol L−1) in PEG400, were prepared. The electrochemical redox behaviour of the β-diketonate Eu complex was investigated using a PEG400 solution containing Eu(hfa)3(H2O)2 (10 mmol L−1) and LiCF3SO3 (500 mmol L−1). For comparison, a solution composed of hfa (30 mmol L−1) and LiCF3SO3 (500 mmol L−1) in PEG400 was prepared. The solutions for optical measurements were purged with nitrogen gas for 20 min before each measurement.Construction of EFC devices
A two-electrode cell was constructed with ITO glass electrodes or carbon-modified ITO electrodes (CM electrodes) using a silicon spacer (300 μm; Mitsubishi Chemical, Japan). CM electrodes were prepared according to a previously reported method.42 Carbon paste was directly coated onto the ITO substrate via blade coating, and the gap between the blade and the ITO substrate was 180 μm. The obtained porous CM electrode was heated to 250 °C for 1 h on a hot plate. The Eu complex solution was sandwiched between the ITO electrodes (ITO–ITO device) or between the ITO and CM electrodes (ITO–CM device) to evaluate their electrochromic and PL properties.Electrochemical measurements
For the electrochemical measurements, a three-electrode cell comprising an ITO electrode as the working electrode, Pt wire (φ = 1 mm) or a CM electrode as the counter electrode, and an Ag/Ag+ electrode as the reference electrode were used. The reference electrode was prepared by injecting an Ag+ solution (10 mmol L−1 AgNO3 and 100 mmol L−1 TBAP in MeCN) into a sample holder with ion-permeable glass (BAS, Japan), and sealing with a cap containing an Ag wire (φ = 1 mm). Cyclic voltammetry (CV) measurements of the three-electrode cell were conducted using an electrochemical analyzer (ALS 440A, CH Instruments Inc., USA).Photophysical measurements
The PL spectra of Eu(NO3)3·6H2O and Eu(hfa)3(H2O)2 in the electrolyte solution were obtained using a JASCO FP-6600 spectrofluorometer. Quartz optical cells with an optical path length of 1 cm and the three-electrode electrochemical cell were used for the measurements. For the two-electrode EFC devices, the luminescence properties were characterized using an epifluorescence unit (JASCO EFA-133). Excitation wavelengths of 272 and 365 nm were used for the Eu(NO3)3·6H2O and Eu(hfa)3(H2O)2 solutions, respectively. The excitation spectra at a detection wavelength of 450 nm were obtained by subtracting the Raman scattering of PEG400.Electrode potential measurement
Electrode potential measurements were performed to characterize the redox behavior of the Eu(hfa)3(H2O)2 solution using two electrochemical analyzers, namely HZ-7000 (Hokuto Denko Co., Japan) and ALS 440A, as shown in Scheme 1, following the method in our previous report.43 An ITO electrode was used as the working electrode, ITO or CM electrodes as the counter electrodes (the distance between the electrodes was 1 cm), and an Ag/Ag+ electrode placed between the counter electrodes was used as the reference electrode. | ||
| Scheme 1 The structure of a two-electrode cell system for the measurement of electrode potential using two electrochemical analyzers. | ||
Results and discussion
Redox and luminescence properties of Eu ions and the Eu complex in PEG400
The CV curves of Eu(NO3)3·6H2O in PEG400 using the constructed three-electrode cell structure are shown in Fig. 1, displaying the redox current corresponding to the reduction and oxidation reactions of Eu ions dissolved in PEG400. | ||
| Fig. 1 Cyclic voltammograms of Eu(NO3)3·6H2O or LiNO3 in PEG400. ([LiCF3SO3] = 500 mM, [Eu(NO3)3·6H2O] = 10 mM, [LiNO3] = 60 mM). | ||
Scanning from the negative potential direction, a reduction current appeared from −0.75 V, reaching a maximum at −1.65 V. Scanning backward in the positive direction, an oxidation current corresponding to the reverse reaction was observed at −0.50 V. In contrast, no distinct redox current was observed for the electrolyte solution without Eu ions. Therefore, it is inferred that the redox current observed for the PEG400 solution containing Eu ions was due to the redox reaction of Eu3+/Eu2+. The ratio of the oxidation charge to the reduction charge of the Eu ion (Qa/Qc) in PEG400 was calculated to be approximately 54% whereas the ratios measured in common electrochemical solvents were relatively low (14% for DMSO, 16% for MeCN, and 13% for PC) (Fig. S1, ESI†). This indicates that the electrochemically generated Eu2+ is more stable in the PEG400 solution as compared to that in the other solvents. Fig. 2 shows the change in the emission spectrum of the Eu(NO3)3·6H2O solution. When a reduction potential at −1.65 V was applied for 60 min, a new emission band was observed at approximately 430 nm.
 | ||
| Fig. 2 Emission spectra of the Eu(NO3)3·6H2O solution in a three-electrode electrochemical cell with/without an applied reduction potential (−1.65 V). Excitation wavelength = 272 nm. | ||
It is proposed that Eu2+ is electrochemically generated and a broad emission band owing to the d–f transition in Eu2+ appeared. Simultaneously, the red emission intensity owing to f–f transitions in excited Eu3+ slightly decreased. Using the solvents, such as DMSO, MeCN, or PC, blue luminescence from Eu2+ was not observed (Fig. S2, ESI†), indicating that among all the solvents used in this study, the electrochemically generated blue luminescent Eu2+ is stable only in the PEG solution. However, the change in luminescence owing to both Eu3+ and Eu2+ in Eu(NO3)3·6H2O was weak and could not be detected with the naked eye because the excited state of the free Eu ions without ligands undergoes luminescence quenching owing to the organic solvent. Moreover, the excitation efficiency (i.e., the light absorption ability) of Eu ions was low due to the parity forbidden f–f transition.44 To improve the luminescence intensity of the developed EFC system, an Eu complex with β-diketonate ligands was used, which exhibited strong red luminescence in organic solutions. Thus, the redox and PL properties of the β-diketonate Eu complex were determined.
Fig. 3 shows the CV curves of Eu(hfa)3(H2O)2 in PEG400 using the constructed three-electrode cell. When the potential was scanned in the negative direction, a reduction current flowed from −0.85 V, and the corresponding oxidation reaction was observed when the potential was scanned back in the positive direction.
 | ||
| Fig. 3 Cyclic voltammograms of Eu(hfa)3(H2O)2 or hfa in PEG400. ([LiCF3SO3] = 500 mM, [Eu(hfa)3(H2O)2] = 10 mM, [hfa] = 30 mM). | ||
The redox reaction of Eu(hfa)3(H2O)2 occurred at approximately the same potential as that of Eu ions in PEG400 as depicted in Fig. 1. Moreover, the solution containing only the hfa ligand did not exhibit a significant redox reaction in this potential range, indicating that the Eu complex exhibited the same Eu3+/Eu2+ redox reaction as that of Eu ions in the PEG400 solution. Fig. 4 shows the change in the emission spectra of the Eu(hfa)3(H2O)2 solution. When a reduction potential of −1.80 V was applied for 30 min, a broad emission band of Eu2+ was observed at approximately 430 nm. As the Eu2+ emission increased, the red emission from Eu3+ at 615 nm slightly decreased, indicating that the emission shapes of the Eu complex can be electrochemically controlled between the Eu3+ and Eu2+ states for the β-diketonate Eu complex. This emission control was not observed for other solvents, such as MeCN (Fig. S3, ESI†), as depicted for Eu ions without ligands. Therefore, the Eu2+ state in the β-diketonate complex is stabilized in the PEG solution.
 | ||
| Fig. 4 Emission spectra of the Eu(hfa)3(H2O)2 solution in a three-electrode electrochemical cell with/without an applied reduction potential (−1.80 V). Excitation wavelength = 365 nm. | ||
To elucidate the luminescence mechanism of the Eu complex, the excitation spectra of the Eu(hfa)3(H2O)2 solution during the redox reaction were obtained by monitoring the Eu2+ emission at 450 nm and Eu3+ emission at 616 nm (Fig. 5).
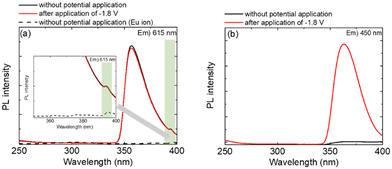 | ||
| Fig. 5 Excitation spectra of the Eu(hfa)3(H2O)2 solution before and after the application of a reduction voltage. ((a) excitation wavelength = 615 nm), (b) excitation wavelength = 450 nm). | ||
The luminescence from the Eu3+ state without potential application (Fig. 5(a), black line), resulted in a large excitation band and a small band with a maximum at 365 and 396 nm being observed. The large excitation band corresponds to light absorption by the hfa ligand, and the small excitation band is attributed to the f–f transition of Eu3+. Upon effective energy transfer from the ligand to the Eu ion, the light absorption of the hfa ligand enhanced the luminescence intensity of Eu3+ compared to that of Eu3+ without hfa ligands (Fig. 5(a), dotted line). When a reduction potential of −1.80 V was applied for 30 min (Fig. 5(a), red line), the intensity of the excitation band of the Eu3+ state was slightly decreased with decreasing Eu3+ emission (Fig. 4(b)). For the blue emission of the Eu2+ state (Fig. 5(b)), a broad excitation band with a maximum at 365 nm was observed as the reduction of Eu3+ proceeded. This indicates that Eu2+ in Eu(hfa)3(H2O)2 could be photo-excited via light absorption by the hfa ligands and subsequent energy transfer to the central Eu2+ ion.
However, in contrast to the increase in the Eu2+ luminescence intensity, a slight decrease in the Eu3+ luminescence intensity was observed. Eu2+ is generated at the electrode surface, whereas Eu3+ primarily occupies the solution in the cell (1 × 1 cm). To improve the contrast of the electrochemical control, two-electrode EFC devices were fabricated.
Electrofluorochromism of the Eu complex in the two-electrode devices
Fig. 6 shows the CV curves of Eu(hfa)3(H2O)2 in PEG400 using the constructed two-electrode devices. | ||
| Fig. 6 Cyclic voltammograms of the Eu(hfa)3(H2O)2 solution. (The black line represents the ITO–ITO device, and the red line represents the ITO–CM device). | ||
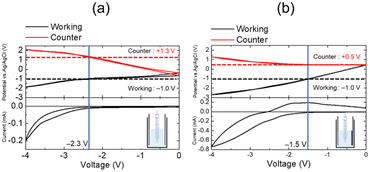
Two-electrode devices were prepared by sandwiching the Eu complex solution between two ITO electrodes (the ITO–ITO device) or between the ITO and carbon-modified ITO electrodes (ITO–CM device). When the potential is scanned in the negative direction, a redox current flowed from −2.3 and −1.5 V in the ITO–ITO and ITO–CM devices, respectively. Thus, the ITO–CM device exhibited better electrochemical properties with a lower redox voltage as compared to that of the ITO–ITO device. The difference in the redox behaviour of these two-electrode devices was investigated using electrode potential measurements. Fig. 7 shows the electrode potential (vs. Ag/Ag+) measurement results for the ITO–ITO and ITO–CM devices.
The electrode potentials of the working and counter electrodes in the EFC devices (vs. Ag/Ag+) were monitored during voltage scanning between the two electrodes. In both devices, the reduction current of the Eu complex flowed when the working electrode potential reached −1.0 V (vs. Ag/Ag+). For the ITO–ITO device, when a voltage of −2.3 V was applied, and the working electrode potential reached −1.0 V (vs. Ag/Ag+), a redox current began to flow. At this voltage, the counter electrode potential was +1.3 V (vs. Ag/Ag+). Alternatively, for the ITO–CM device, when a voltage of −1.5 V was applied, the working electrode potential was −1.0 V (vs. Ag/Ag+), but the counter electrode potential retained a low value of +0.5 V (vs. Ag/Ag+). Using the CM electrode, a significant decrease in the driving voltage of the EFC devices was observed owing to a decrease in the reaction potential of the counter electrode from +1.3 to +0.5 V because the surface area of the CM electrode is significantly larger than that of the ITO electrode.39 Using the CM as the counter electrode, only the working electrode potential was regulated, whereas the counter electrode potential remained the same.
Fig. 8 shows the emission spectra of the Eu(hfa)3(H2O)2 solution in the two-electrode devices under various applied bias voltages.
 | ||
| Fig. 8 Emission spectra of the Eu(hfa)3(H2O)2 solution in PEG400 using a two-electrode cell ((a)ITO–ITO or (b) ITO–CM) before and after the application of reducing and oxidizing voltages. | ||
When a reduction voltage was applied to the working electrode in both devices, the emission bands from the Eu2+ state (430 nm) increased significantly, and the red emission from the Eu3+ state (616 nm) decreased simultaneously as the electrochemical reduction proceeded (Fig. 8(a) and (b), red line). It is observed that the intensity ratio of Eu2+ emission (430 nm) to Eu3+ emission (616 nm) was significantly improved using the two-electrode devices; the I430 nm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I616 nm ratio was 1
I616 nm ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 for the three-electrode cell and 1
100 for the three-electrode cell and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5–3.0 for the two-electrode devices. Therefore, a change in the luminescence color could be detected by the naked eye after electrochemical reduction (Fig. 8 photos).
2.5–3.0 for the two-electrode devices. Therefore, a change in the luminescence color could be detected by the naked eye after electrochemical reduction (Fig. 8 photos).
Furthermore, a decrease in the degree of Eu3+ luminescence improves significantly for the two-electrode devices, whereas it remains unaltered in the three-electrode cell, during the electrochemical reaction.
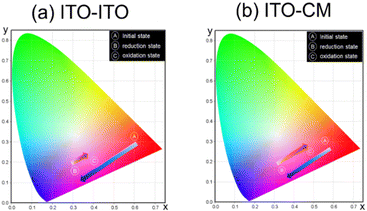
After the oxidation of Eu ions by applying an oxidation voltage for the working electrode, the emission band from the Eu2+ state (430 nm) slightly decreased and the emission band from the Eu3+ state (615 nm) slightly increased for the ITO–ITO device (Fig. 8(a)). In contrast, the change of luminescence intensity for each band during the redox reaction of Eu ions was enhanced for the ITO–CM device (Fig. 8(b)). This result was corroborated by the CIE chromaticity diagram (Fig. 9).
Before voltage application in the initial state, both devices exhibited bright red luminescence; however, after application of the reduction voltage for the working electrode, the color hue of the luminescence changed to bluish magenta. After an oxidation voltage was applied for the working electrode, the luminescence color of the ITO–CM device was similar to its initial state, indicating good luminescence reversibility.
Conclusions
This study focused on regulating the PL color from the β-diketonate Eu complex via electrochemical alteration of the valence state of the Eu ions between Eu3+ and Eu2+. The redox behaviour and changes in the luminescence properties of Eu ions and Eu complex in polyether solutions were investigated, revealing that the electrochemically generated Eu2+ was stable in both solutions. In the two-electrode device, a distinct change in the luminescence color via electrochemical reduction of the Eu complex, i.e., electrofluorochromism of the Eu complex, was observed using luminescence spectroscopy and the naked eye. Initially, intense red emission from the Eu3+ complex was observed, and subsequently, blue emission from the Eu2+ complex was generated, which increased owing to the electrochemical reduction of Eu3+. Furthermore, by changing the counter electrode from a flat ITO to a porous CM electrode, the reversibility of electrofluorochromism was improved due to a decreased driving voltage, which resulted in the suppression of undesired electrochemical side reactions. Therefore, this study successfully detected electrofluorochromism in the strongly luminescent β-diketonate Eu complex for the first time. These findings could be of great significance in the development of novel display devices and chemical and biological sensors.Author contributions
Ryoto Yabuta: investigation (Lead) and writing – original draft (Lead). Norihisa Kobayashi: writing – review & editing (Equal). Kazuki Nakamura: project administration (Lead) and writing – review & editing (Equal)Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was partly supported by JSPS KAKENHI (17H06377, 20K05641, 22H02154, and 23K04871).Notes and references
- D. Tyler McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537 CrossRef PubMed.
- R. Martínez-Máñez and F. Sancenón, Chem. Rev., 2003, 103, 4419 CrossRef PubMed.
- M. A. Rizzo, G. H. Springer, B. Granada and D. W. Piston, Nat. Biotechnol., 2004, 22, 445 CrossRef CAS PubMed.
- M. Irie, T. Fukaminato, T. Sasaki, N. Tamai and T. Kawai, Nature, 2002, 420, 19 CrossRef PubMed.
- C. Bechinger, S. Ferrere and A. Zaban, et al. , Nature, 1996, 383, 608 CrossRef CAS.
- A. A. Argun, P. H. Aubert, B. C. Thompson, I. Schwendeman, C. L. Gaupp, J. Hwang, N. J. Pinto, D. B. Tanner, A. G. MacDiarmid and J. R. Reynolds, Chem. Mater., 2004, 16, 4401 CrossRef CAS.
- P. Audebert and F. Miomandre, Chem. Sci., 2013, 4, 575 RSC.
- H. J. Yen and G. S. Liou, Chem. Commun., 2013, 49, 9797–9799 RSC.
- S. Seo, S. Pascal, C. Park, K. Shin, X. Yang, O. Maury, B. D. Sarwade, C. Andraud and E. Kim, Chem. Sci., 2014, 5, 1538 RSC.
- X. Yang, S. Seo, C. Park and E. Kim, Macromolecules, 2014, 47, 7043 CrossRef CAS.
- M. Chang, W. Chen, H. Xue, D. Liang, X. Lu and G. Zhou, J. Mater. Chem. C, 2020, 8, 16129 RSC.
- M. H. Chua, Q. Zhu, K. W. Shah and J. Xu, Polymers, 2019, 11, 98 CrossRef PubMed.
- H. Al-Kutubi, H. R. Zafarani, L. Rassaei and K. Mathwig, Eur. Polym. J., 2016, 83, 478 CrossRef CAS.
- F. Miomandre, Curr. Opin. Electrochem., 2020, 24, 56 CrossRef CAS.
- S. Kim and Y. You, Adv. Opt. Mater., 2019, 7, 1900201 CrossRef.
- K. Su, N. Sun, Z. Yan, S. Jin, X. Li, D. Wang, H. Zhou, J. Yao and C. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 22099 CrossRef CAS PubMed.
- C. P. Kuo, C. L. Chang, C. W. Hu, C. N. Chuang, K. C. Ho and M. K. Leung, ACS Appl. Mater. Interfaces, 2014, 6, 17402 CrossRef CAS PubMed.
- J. H. Wu and G. S. Liou, Adv. Funct. Mater., 2014, 24, 6422 CrossRef CAS.
- A. Beneduci, S. Cospito, M. La Deda and G. Chidichimo, Adv. Funct. Mater., 2015, 25, 1240 CrossRef CAS.
- C. P. Kuo and M. K. Leung, Phys. Chem. Chem. Phys., 2014, 16, 79 RSC.
- W. Gao, T. Yu, Y. Du, R. Wang, L. Wu and L. Bi, ACS Appl. Mater. Interfaces, 2016, 8, 11621 CrossRef CAS PubMed.
- B. Wang, L. H. Bi and L. X. Wu, J. Mater. Chem., 2011, 21, 69 RSC.
- Y. Kim, H. Ohmagari, A. Saso, N. Tamaoki and M. Hasegawa, ACS Appl. Mater. Interfaces, 2020, 12, 46390 CrossRef CAS PubMed.
- J. Lehr, M. Tropiano, P. D. Beer, S. Faulkner and J. J. Davis, Chem. Commun., 2015, 51, 6515 RSC.
- T. W. Ngan, C. C. Ko, N. Zhu and V. W. W. Yam, Inorg. Chem., 2007, 46, 1144 CrossRef CAS PubMed.
- F. Miomandre, R. B. Pansu, J. F. Audibert, A. Guerlin and C. R. Mayer, Electrochem. Commun., 2012, 20, 83 CrossRef CAS.
- M. Tropiano, N. L. Kilah, M. Morten, H. Rahman, J. J. Davis, P. D. Beer and S. Faulkner, J. Am. Chem. Soc., 2011, 133, 11847 CrossRef CAS PubMed.
- M. Yano, K. Matsuhira, M. Tatsumi, Y. Kashiwagi, M. Nakamoto, M. Oyama, K. Ohkubo, S. Fukuzumi, H. Misaki and H. Tsukube, Chem. Commun., 2012, 48, 4082 RSC.
- T. Sato and M. Higuchi, Tetrahedron Lett., 2019, 60, 940 CrossRef CAS.
- J. C. G. Bünzli, Acc. Chem. Res., 2006, 39, 53 CrossRef PubMed.
- J. C. G. Bünzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048 RSC.
- S. Faulkner, S. J. A. Pope and B. P. Burton-Pye, Appl. Spectrosc. Rev., 2005, 40, 1 CrossRef CAS.
- K. Miyata, Y. Konno, T. Nakanishi, A. Kobayashi, M. Kato, K. Fushimi and Y. Hasegawa, Angew. Chem., Int. Ed., 2013, 52, 6413 CrossRef CAS PubMed.
- A. Ishii and M. Hasegawa, Sci. Rep., 2015, 5, 11714 CrossRef PubMed.
- J. Jiang, N. Higashiyama, K.-I. Machida and G.-Y. Adachi, Coord. Chem. Rev., 1998, 170, 1 CrossRef CAS.
- W. Chen, Y. Ouyang, M. Mo, H. Zhang and Q. Su, J. Lumin., 2021, 229, 117672 CrossRef CAS.
- W. Liu, L. Liu, Y. Wang, L. Chen, J. A. McLeod, L. Yang, J. Zhao, Z. Liu, J. Diwu, Z. Chai, T. E. Albrecht-Schmitt, G. Liu and S. Wang, Chem. - Eur. J., 2016, 22, 11170 CrossRef CAS PubMed.
- A. Acharjya, B. A. Corbin, E. Prasad, M. J. Allen and S. Maity, J. Photochem. Photobiol., A, 2022, 429, 113892 CrossRef CAS.
- X. Yang, T. S. Tiam, X. Yu, H. V. Demir and X. W. Sun, ACS Appl. Mater. Interfaces, 2011, 3, 4431 CrossRef CAS PubMed.
- M. Mukaigawa and H. Ohno, J. Electroanal. Chem., 1998, 452, 141 CrossRef CAS.
- K. Nakamura, K. Kanazawa and N. Kobayashi, J. Photochem. Photobiol., C, 2022, 50, 100486 CrossRef CAS.
- Z. Liang, K. Nakamura and N. Kobayashi, Sol. Energy Mater. Sol. Cells, 2019, 200, 109914 CrossRef CAS.
- R. Ozawa, K. Nakamura, T. Tachikawa and N. Kobayashi, J. Imaging Soc. Jpn., 2022, 61, 562 CAS.
- Q. Dai, M. E. Foley, C. J. Breshike, A. Lita and G. F. Strouse, J. Am. Chem. Soc., 2011, 133, 15475 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cp02283h |
| This journal is © the Owner Societies 2023 |