Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
State of anion in ethylammonium nitrate enclosed between micrometer-spaced glass plates as studied by 17O and 15N NMR†
Andrei
Filippov
 * and
Oleg N.
Antzutkin
* and
Oleg N.
Antzutkin
Chemistry of Interfaces, Luleå University of Technology, SE-97187 Luleå, Sweden. E-mail: andrei.filippov@ltu.se
First published on 9th May 2023
Abstract
Some aprotic and protic ionic liquids (ILs) containing nitrate anion demonstrate unusual dynamic behavior of cations when these ILs are enclosed in micrometer-spaced layers between glass plates. We applied 17O and 15N NMR spectroscopy to discover the state and transformations of 17O and 15N isotopically enriched nitrate anion of ethylammonium nitrate (EAN) enclosed between glass plates. 15N NMR spectra demonstrated preferential orientation of the principal axes of the nitrate anions perpendicular to the normal of the glass surface. Therefore, isotropic ionic liquid EAN, when placed within a micrometer-spaced enclosure, forms an ordered phase, which is similar to a liquid crystal. The peculiarity of this phase is that the cations do not have a predominant orientation. Other features of this phase that are typical for liquid crystal phases are the changed local and translational dynamics in comparison with the isotropic state and slow transformation occurring under the action of an external magnetic field.
Introduction
Ionic liquids (ILs) are ionic compounds formed typically of organic cations and either organic or inorganic anions,1–3 which preserve the liquid state at temperatures below 100 °C. These compounds have attracted much interest as solvents for use as “green” replacements for organic liquids, media for chemical synthesis,1 components of lubricants4 and electrolytes for electrochemical devices (lithium batteries, supercapacitors and fuel cells).5 For many of their practical applications, for example, in mechanical devices, supercapacitors, batteries, and catalytic reactors, etc., understanding the properties of ILs close to a solid surface or in confinement has crucial importance. It is known that the structure and properties of molecular liquids as well as ILs at the liquid/surface interface and in confinement can be significantly different from those in bulk as they are affected by the size, shape, and topology of their geometric restrictions.6,7 This influences the rates of chemical reactions, electrical conductivity, thermal properties and viscosity. The growing application of ILs in various mechanical, chemical and electrochemical devices raises the challenge of understanding the properties of ILs near surfaces8–11 and in confining geometries.6,7,12Ethylammonium nitrate (EAN) (Fig. 1A and B), as well as other IL systems with the nitrate anion, when enclosed between micrometer-spaced glass or quartz plates, demonstrate unusual dynamics of cations that are different from that of ILs in bulk and in nano-confinement.13–15 The dynamics of the cations also reversibly changes during exposure of the ILs to a static magnetic field.15 These phenomena were analysed and interpreted as a result of intermolecular structure transformations occurring in the enclosed ILs.13,16 The conditions of these transformations were investigated by dynamic NMR methods, NMR-diffusometry and NMR-relaxometry,14–16 and their nature and mechanisms are under discussion. The mobility of the NO3− anion could be a key to further understanding this process. Indeed, the nitrate anion is always present in these systems, but was “invisible” in previous studies of the enclosed EAN because of the absence of protons. NO3− has a special anisotropic, plate-like structure,17 where one nitrogen atom is surrounded by three oxygen atoms in a trigonal planar arrangement (Fig. 1B). Potential “magnetic” isotopes, which can be used to study the structure of the nitrate anion of EAN by NMR, are 17O and 15N, however they are of low natural abundance. In this work, we studied the state of the nitrate anion in the layers between glass plates using NMR on 17O and 15N nuclei of nitrate anions of EAN isotopically enriched with 17O and 15N (15N,17O-EAN).
 | ||
| Fig. 1 Structures of ethylammonium cation (A) and nitrate anion (B).18A sample prepared from 37 polar glass plates 14 × 2.5 × 0.1 mm with enclosed EAN for NMR diffusion measurements placed in a square glass tube (C). The ruler below is scaled in cm units. Schematics showing orientation of normal to the glass plates of the sample (D). | ||
Experimental
Materials
H217O (90% 17O isotope enrichment) was purchased from Cambridge Isotope Laboratories, Inc. H15NO3 (98% 15N isotope enrichment) was purchased from Sigma-Aldrich. To prepare H15N17O3, H217O was mixed with H15NO3, sealed in the glass tube, and kept at 366 K for 24 h according to a previously described procedure.19 Then this aqueous solution of H15N17O3 was used for synthesis of EAN with 17O and 15N enriched NO3− (15N,17O-EAN). Syntheses of EAN using aqueous ethylamine and nitric acid has been described previously.13 After completion of the reaction, water was removed by rotary evaporation following by pumping at 2.3 × 10−3 mBar for 72 h. The quality of the final product was analyzed by 1H, 13C, 15N and 17O NMR spectroscopy. The degrees of enrichment were 38% for 17O and 98% for 15N.Enclosed EAN layers were prepared with glass plates arranged in a stack, as described previously.13 The plates (14 × 2.5 × 0.1 mm, Thermo Scientific Menzel-Gläser, Menzel GmbH, Germany) were carefully cleaned before sample preparation. A stack of glass plates was prepared in a glove box in a dry N2 atmosphere. Samples were prepared by adding 2 μL of an IL to the first glass plate, placing a new glass plate on top, adding 2 μL of the IL on top of this glass plate, etc. until the height of the stack reached 2.5 mm. IL that overflowed at the edges of the stack was removed by sponging. Finally, the sample consisting of a stack of ca. 37 glass plates was placed in a rectangular glass tube and sealed (Fig. 1C). The mean spacing between the glass plates was assessed by weighing the introduced IL, which yielded d ∼ 3.8–4.5 μm for EAN.13 The surface-to-volume ratio of the enclosed EAN layers was ∼5 × 105 m−1. Because most of the sample space is occupied by the glass plates, the filling factor of the coil for NMR measurements is decreased by a factor of ∼37 in comparison with the bulk EAN sample of the same geometry. A detailed report of the sample preparation and characterization has been described in our previous papers.13–16 To allow equilibration of ILs inside the samples, experiments were started a week after the sample preparation.
NMR spectroscopy
NMR measurements were executed on a Bruker Avance III (Bruker BioSpin AG) NMR spectrometer with the working frequency for protons 400.21 MHz (induction of the static magnetic field 9.4 T). For 17O and 15N NMR resonance frequencies were 54.28 MHz and 40.57 MHz, respectively. The NMR diffusion probe diff50 with 17O insert, 15N and 17O solenoid insert was used. With the solenoid insert, the normal n to the glass stack (Fig. 1D) can be oriented at an arbitrary angle relative to the vector of induction of the stationary magnetic field B0. 17O and 15N NMR spectra were obtained by a fast Fourier transformation (FFT) following the 90° radiofrequency pulse (90°-acq.). Durations of the 90° pulse were 5.7 μs and 5 μs for 15N and 17O, respectively.We also used 1H NMR spin-echo measurements to monitor water content and the state of the ethylammonium cation.13,151H NMR spectra were obtained by FFT of the descending half of the spin-echo (90°-τ-180°-τ-acq.). The duration of the 90° pulse for 1H was 7 μs. Pulse spacing in the 1H NMR spin-echo measurements τ was 5 ms. All measurements were performed at 295 K.
Results and discussion
1H NMR spectra
The 1H NMR spectra of the 15N,17O-EAN enclosed between glass plates are shown in Fig. 2. Only cations’ proton signals are present here. Just after placement of the sample in the probe of NMR spectrometer, the spectrum does not show a signal from –NH3 protons (bottom), which is related to their enhanced T2 NMR relaxation.15 After exposure to the magnetic field, the –NH3 proton signal is recovered. This behavior is typical for the enclosed EAN layers.15,1617O NMR spectra
17O NMR spectra of 15N,17O-EAN bulk and enclosed between glass plates are shown in Fig. 3. The peak position is at ∼419.6 ppm (against H217O) for both samples and for different conditions of measurements that agree with the previously reported value of 420 ppm for ammonium nitrate.20 All spectra present broad symmetric lines with widths at the half-height ∼1.0–1.4 kHz. For samples with layers of enclosed EAN, the line form slightly changes after placement of the sample in the static magnetic field. As is seen from Fig. 3, the 17O spectrum of confined 15N,17O-EAN just after placement of the sample in the magnetic field is narrower in comparison with that of the bulk sample and becomes broader with exposure to the magnetic field. The change of the spectrum width in time was analyzed and the result is presented in Fig. 4. | ||
| Fig. 3 17O NMR spectra of 15N,17O-EAN in bulk and enclosed between glass plates during the first five minutes and 20 hours after placement in a static magnetic field. H217O was used as a reference (0 ppm).21 | ||
As seen from Fig. 4, the 17O spectrum initial linewidth of the confined 15N,17O-EAN is a factor of 1.4 lower than that in the bulk. The linewidth begins to increase just after placement of the sample in the magnetic field. The rate of the change is comparable with the rate of the decrease in diffusivity of the ethylammonium cation of the enclosed EAN under the same conditions.15 With sufficient time, the process reaches saturation, with the 17O spectra linewidth close to that in bulk (dotted line). The time course of change of the linewidth is also similar to the kinetics of change of diffusivity and variation of 1H transverse NMR relaxation of –NH3 groups of the ethylammonium cation.15
17O has a spin 5/2 and is therefore quadrupolar. The nuclear quadrupolar moment Q = 2.558 × 10−30 m2.21 The average for the quadrupole constant is 4.2 ± 1.5 MHz for inorganic materials.21 This implies that for 17O the quadrupolar interaction is dominant,21 while other nuclear spin interactions such as dipolar interactions and chemical shift anisotropy play a role and cannot be neglected. Symmetric Lorentzian form of the line is characteristic for the fast motion regime, ω0τc ≪ 1.22 Additionally, rates of longitudinal and transverse quadrupole NMR relaxation processes as well as the line width of the NMR signal increase with increasing τc.22 In the case of the slow motion regime, ω0τc ≫ 1, the line width of the signal is inversely proportional to τc. We performed temperature measurements of 17O NMR linewidths ν for 15N,17O-EAN in bulk and confined in porous glasses34 with pore sizes 4 and 9.8 nm (Fig. S1 in the ESI†). Increasing temperature generally leads to a decrease τc. As is seen from the figure, ν is really decreasing. Therefore, the condition of the fast motion regime is fulfilled. In this motion regime, the linewidth is proportional to τc, the correlation time for reorientation of molecules.22 Therefore, our 17O NMR data (Fig. 4) demonstrate that the nitrate anion of the confined EAN has enhanced local mobility (τc is decreased) in comparison with that in bulk, which decreases with time as the sample is exposed to a strong, static magnetic field.
15N NMR spectra
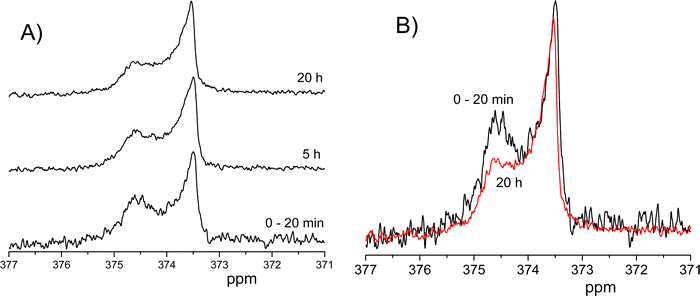
Na15NO3 in solution shows a 15N NMR spectrum with a narrow symmetric line at 374 ppm23 and linewidth of 4.5 Hz. We used this signal as a reference. In the case of bulk 15N,17O-EAN, the NMR spectrum also shows a symmetrical line with a chemical shift of 374 ppm and linewidth of 0.04 ppm (16.9 Hz), Fig. 5A. The line broadening in comparison with that in the Na15NO3 solution is conditioned by the higher viscosity of EAN.The 15N spectra of 15N,17O-EAN enclosed between glass plates show broad lines with chemical shifts and the form of the spectra depends on the orientation of the sample. Because the dynamics of the ethylammonium cation in the system varies with the duration of exposure to a static magnetic field,15 we first show the spectra for the enclosed EAN when the system reached equilibrium, after 20 h of exposure to the magnetic field. As is seen from the figure, for the sample orientation with normal perpendicular to the induction of the magnetic field, a sharp signal is displaced upfield (right) relative to the bulk EAN signal, and a shoulder is observed on the side of the higher chemical shifts the down field against the sharp peak (Fig. 5C). For the sample orientation normal along the induction of the magnetic field, a sharp signal is displaced downfield relative to the bulk EAN signal (left), and a “shoulder” is observed on the side of the lower chemical shifts the up field against the sharp peak (Fig. 5B). For the sample orientation close to 54.7° (at the “magic angle”,24), the chemical shift is close to that of the bulk sample (Fig. 5D).
Extended exposure of the sample to the magnetic field of the NMR spectrometer leads to a change in its 15N NMR spectrum, as shown in Fig. 6 where the orientation of the plates normal is perpendicular to the induction of the magnetic field. To describe the change, we tried to present the spectrum as a sum of two lines centered at 373.5 ppm and 374.5 ppm, respectively. Such a presentation seems reasonable, considering the change in the shape of the spectrum upon exposure to a magnetic field: the amplitude of the left spectrum line changes independently on the right spectrum line. The exact forms or even symmetries of each of the lines are not known because they depend on the degree of the ion ordering and intensity of molecular motion. However, for the spectrum at the beginning of the experiment (black line in Fig. 6B) the left line is resolved down to 0.4 and the right line down to 0.6 of the signal maxima. Therefore, we approximated these lines manually, as it is shown in Fig. S2 of the ESI.† Widths of the lines were estimated as ∼13 Hz (373.5 ppm) and ∼32 Hz (374.5 ppm).
From these figures, it is seen that the amplitude of the downfield (left) signal decreases with time when the sample is exposed to a static magnetic field.
Effect of water
Water is a medium for synthesis of EAN. On the other hand, EAN is a hygroscopic IL and water can be easily absorbed from the environment. The presence of water changes the physical properties of EAN, such as density, viscosity, and electrical conductivity,25 as well as diffusivity of cations.13,26 In the presence of water, the 1H NMR spectrum contains a signal from water protons (Fig. 7, top). At the same time, for the sample with enclosed EAN and the concentration of water higher than 2 wt% effects of confinement between the glass plates on the diffusivity of the ethylammonium cation and NMR relaxation of –NH3 protons minor.26The 17O NMR spectrum of 15N,17O-EAN enclosed between glass plates in the presence of water in the IL presents a broad symmetric line, as in the case of bulk EAN and water-free, enclosed EAN (Fig. 3). However, the spectrum linewidth is decreased down to 915 ± 5 Hz and does not depend on the sample orientation and does not change when exposed to the magnetic field of the NMR spectrometer. The narrowing of the line is not surprising, because the presence of water leads to a decrease in viscosity25 and an increase in the cation's mobility13,26 in EAN. Therefore, the motional contribution in linewidth of the 17O spectrum of the nitrate anion decreases in the presence of water. No effect of the magnetic field on this sample is not surprising because this was observed earlier for ethylammonium cation diffusion at a water concentration of 2 wt%.26
The 15N NMR spectrum of 15N,17O-EAN enclosed between glass plates in the presence of water is shown in Fig. 8, top. The spectrum line is broad, with a linewidth of 44 Hz, and does not demonstrate any special features compared to the water-free sample (bottom). The spectrum line does not depend on the orientation and the duration of exposure to the magnetic field of the spectrometer.
State and dynamics of EAN in the micrometer-spaced enclosure
15N NMR chemical shift is extremely sensitive even to minor changes in molecular structure; it can also vary depending on the orientation of the nucleus in the magnetic field.23,27,28 The nuclear spin interaction with the static magnetic field and motional averaging is described by secular Hamiltonian:24 | (1) |
A powder pattern for chemical shift anisotropy (CSA) of 15N is a superposition of many sharp peaks with different frequencies, generally resulting in a sharp, nonsymmetric 15N spectrum (Fig. 9).23,24
 | ||
| Fig. 9 Powder pattern lineshape for a single molecular site for uniaxial chemical shift tensor with negative chemical shift anisotropy.24 The dashed line shows an isotropic chemical shift due to motional averaging. | ||
Complete isotropic motional averaging characteristic for a liquid 15N spectrum in liquids gives a narrow line, which is observed for bulk 15N,17O-EAN (Fig. 5A). The form of the 15N NMR spectrum of 15N,17O-EAN in the presence of water (Fig. 8B) represents the powder pattern (Fig. 9). However, the line width of this spectrum is around 2 ppm which is much less than the 15N powder spectrum of 15N enriched NaNO3 (around 250 ppm).23 This means that the nitrate anion in the EAN like the powder has no preferential orientation; this agrees with the disordering effect of water for the enclosed EAN layers.26 Meanwhile, correlation times of the anion's reorientatonal mobility are not high enough to completely average CSA.
For the enclosed water-free EAN layers, broad lines of the spectra (Fig. 5B–D) are also conditioned by incomplete motional averaging of the chemical shift. Additionally, orientation dependencies of the chemical shifts and form of the spectra are typical for anisotropy of the rotational motion.24 The orientation dependence of the 15N spectrum demonstrates that for the case “n along B0” (Fig. 5B) the principal axis of the nitrate anion is preferentially oriented at 90° to B0, while for “n normal to B0” (Fig. 5C) the principal axis of the nitrate anion is preferentially oriented along B0. Therefore, the principal z-axes of the nitrate anions is preferentially oriented perpendicular to the normal of the glass plates, n. Previously, atomic force microscopy data revealed long-range (up to two nm) forces between solid surfaces and alkylammonium nitrates that appear to be electrostatic in origin.29,30 A number of solvation layers of EAN were observed on a smooth silica surface30 extending up to 4 nm from the surface. It was found that the typical self-assembling structure formed near the surface consists of alternating layers of cations and anions with a preferential orientation of ions relative to the surface.29 For our sample containing EAN layers enclosed between glass-plates separated by ∼4 μm, the contribution of surface layers with a thickness of 4 nm is only 0.1%. Therefore, our 15N NMR results demonstrate the ordering of the nitrate anion occurring not or not only in thin surface layers but in the whole volume of the sample. The usual method to detect liquid-crystalline anisotropy is the deutium NMR on 2H isotopically enriched samples.31 Our 2H NMR measurements of 2H isotopically enriched EAN did not show any quadrupolar splitting of NMR signals of –C2H3 and –C2H2– chemical groups specific to the liquid crystalline state.13 Also, there was no distinct self-diffusion anisotropy of cations.13 Therefore, while the rigid nitrate anion is in the ordered state, flexible chains of the ethylammonium nitrate remain in a disordered state. Wherein other properties characteristic of the liquid crystalline state of the studied systems were unexpectedly changed dynamics of cations relative to bulk13–16,26 and slow change of the dynamics under influence of the external magnetic field.14–16,26
The distinct two-component form of the 15N spectra of the enclosed water-free EAN, particularly just after the placement in the magnetic field (Fig. 6) means that alongside the one preferential orientation of the nitrate anion (normal to the plates), there are also another and even less ordered orientations of the anion principal z-axis relative to the glass plates. The last leads to the appearance of the broader 15N spectrum component. Taking this into account, the change of the 15N spectrum form at exposure to a static magnetic field (Fig. 6) corresponds to “enhancing” the orientation of the anion normal perpendicular to the glass plates and decreasing other orientations.
It is known that the orientation of rotating spins at the “magic angle” effectively averages their dipolar coupling as well as the chemical shift anisotropy.24 In our case, orientation of the sample at the angle close to the “magic angle” (Fig. 5D) leads to narrowing of the 15N spectrum line. At the same time, the broad background signal remains non-averaged. This might be due to the imperfect mutual orientations of spins and the relatively weak dipole–dipole coupling of 15N nuclei, which are rather far apart from each other.
What is the reason for orientation of the nitrate anions in the studied system? It is known that EAN has a three-dimensional hydrogen bond network like water.32,33 EAN is a highly polar ionic liquid, and it can interact with silanol groups on the polar surface of silica.30,31 We suggest that the interaction with silanol groups leads to the ordering of the nitrate anion. Changes in intermolecular interactions and electronic perturbations caused by variations in hydrogen bonds were observed by NMR chemical shift.34,35 Our vision of processes occurring with EAN and other similar ionic liquid systems with the nitrate anion after its enclosure between glass surfaces is that the nitrate anion ordering leads to rearrangement (weakening) of the hydrogen bonding network which further leads to the enhancing in molecular mobility of ions.
The effect of placement of EAN layers is stronger for transverse NMR relaxation of protons of –NH3 groups (decrease by a factor of ∼22) in comparison with protons of other groups (by a factor of ∼6).15,16 It is now evident that this is a result of electrostatic interaction of the positive charged –NH3 group with the nitrate anion undergoing anisotropic rotation.
Conclusions
We used NMR spectroscopy on 17O and 15N nuclei to study the state and dynamics of the nitrate anion of ethylammonium nitrate ionic liquids enclosed in micrometer-scaled layers between polar glass plates. 17O NMR reveals a broad, featureless spectrum with a linewidth change correlating with a change of the diffusivity of cations and transverse magnetic relaxation of –NH3 groups of the cation of EAN exposed to the static magnetic field. 15N NMR spectra are nonsymmetric and vary with the orientation of the layers in the magnetic field, which is due to preferential orientation of the principal z-axis of the nitrate anion normal to the surface of the glass plates. We suggested that this is conditioned by the interaction of anions with silanol groups on the surface and modification of the hydrogen bonding network in the whole EAN layer through NO3−–NH3+ interaction. Therefore, isotropic bulk EAN, when placed within a micrometer-spaced enclosure, forms an ordered phase, which is similar to a liquid crystal. The peculiarity of this phase is that the cations do not have a predominant orientation. Other features of this phase that are typical for liquid crystals are the changed local and translational dynamics in comparison with the isotropic state and slow transformation occurring under the action of an external magnetic field.Exposure of the sample to a static magnetic field leads to improved orientational ordering of the nitrate anions due to interaction with the magnetic field. The presence of water leads to a disordering system, making it isotropic.
Author contributions
Andrei Filippov: conceptualization, synthesis, methodology, characterization, NMR measurements, writing original draft. Oleg N. Antzutkin: conceptualization, methodology, editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the Foundation in memory of J. C. and Seth M. Kempe and the LTU laboratory fund, which provided grants that funded the purchase of the NMR equipment. The Swedish Foundation for Strategic Research (project EM16-0013) is gratefully acknowledged for financial support. We thank Scriptia Academic Editing for English correction and proofreading of this manuscript.References
- R. D. Rogers and K. R. Seddon, Ionic liquids-solvents of the future?, Science, 2003, 302, 792–793 CrossRef PubMed.
- T. L. Greaves and C. J. Drummond, Protic ionic liquids: properties and applications, Chem. Rev., 2008, 108, 206–237 CrossRef CAS PubMed.
- N. V. Plechkova and K. R. Seddon, Applications of ionic liquids in the chemical industry, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- F. U. Shah, S. Glavatskih and O. N. Antzutkin, Boron in tribology: from borates to ionic liquids, Tribol. Lett., 2013, 51, 281–301 CrossRef CAS.
- M. Yoshizava, W. Xu and C. A. Angell, Ionic liquids by proton transfer: Vapor pressure, conductivity, and the relevance of ΔpKα from aqueous solutions, J. Am. Chem. Soc., 2003, 125, 15411–15419 CrossRef PubMed.
- S. Zhang, J. Zhang, Y. Zhang and Y. Deng, Nanoconfined ionic liquids, Chem. Rev., 2017, 117, 6755–6833 CrossRef CAS PubMed.
- M. P. Singh, R. K. Singh and S. Chandra, Ionic liquids confined in porous matrices: physicochemical properties and applications, Prog. Mater. Sci., 2014, 64, 73–120 CrossRef CAS.
- E. Sloutskin, R. M. Lynden-Bell and S. Balasubramanian, The surface structure of ionic liquids: Comparing simulations with x-ray measurements, J. Chem. Phys., 2006, 125, 174715 CrossRef CAS PubMed.
- P. S. Gil, S. J. Jorgenson, A. R. Riet and D. J. Lacks, Relationship between molecular structure, interfacial structure, and dynamics of ionic liquids near neutral and charged surfaces, J. Phys. Chem. C, 2018, 122, 27462–27468 CrossRef CAS.
- M. Lexow, F. Maier and H.-P. Steinrück, Ultrathin ionic liquid films on metal surfaces: adsorption, growth, stability and exchange phenomena, Adv. Phys. X, 2020, 5, 1761266 CAS.
- A. Elbourne, K. Voïtchovsky, G. G. Warr and R. Atkin, Ion structure controls ionic liquid near-surface and interfacial nanostructure, Chem. Sci., 2015, 6, 527–536 RSC.
- J. M. Otero-Mato, H. Montes-Campos, O. Cabeza, L. J. Gallego and L. M. Varela, Nanoconfined ionic liquids: A computational study, J. Mol. Liq., 2020, 320, 114446 CrossRef CAS.
- A. Filippov, O. I. Gnezdilov, N. Hjalmarsson, O. N. Antzutkin, S. Glavatskih, I. Furó and M. W. Rutland, Acceleration of diffusion in ethylammonium nitrate ionic liquid confined between parallel glass plates, Phys. Chem. Chem. Phys., 2017, 19, 25853–25858 RSC.
- A. Filippov, O. N. Antzutkin, R. Gimatdinov and O. I. Gnezdilov, Self-diffusion in ionic liquids with nitrate anion: Effects of confinement between glass plates and static magnetic field, J. Mol. Liq., 2020, 312, 113404 CrossRef CAS.
- A. Filippov and O. N. Antzutkin, Magnetic field effects dynamics of ethylammonium nitrate ionic liquid confined between glass plates, Phys. Chem. Chem. Phys., 2018, 20, 6316–6320 RSC.
- A. Filippov, O. I. Gnezdilov and O. N. Antzutkin, Static magnetic field alters properties of confined alkylammonium nitrate ionic liquids, J. Mol. Liq., 2018, 268, 49–54 CrossRef CAS.
- W. Laue, M. Thiemann, E. Scheibler and K. W. Wiegand, Nitrates and Nitrites, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2006 Search PubMed.
- https://en.m.wikipedia.org/wiki/File:Nitrate-ion-with-partial-charges-2D.png .
- T. I. Taylor and J. C. Clarke, Exchange of nitric oxide with water in nitric acid solutions as a means of concentrating oxygen-18, J. Chem. Phys., 1959, 31, 277–278 CrossRef CAS.
- W. B. Moniz and C. F. Poranski, Semi-empirical chemical shift calculations: nitrogen and oxygen in the lower nitroalkanes, and the nitrate, nitronium, and nitrite ions, J. Magn. Reson., 1973, 11, 62–72 CAS.
- V. Lemaitre, M. E. Smith and A. Watts, A review of oxygen-17 solid-state NMR of organic materials - towards biological applications, Solid State Nucl. Magn. Reson., 2004, 26, 215–235 CrossRef CAS PubMed.
- G. Wu, 17O NMR studies of organic and biological molecules in aqueous solution and in the solid state, Prog. Nucl. Magn. Reson. Spectrosc., 2019, 114–115, 135–191 CrossRef CAS PubMed.
- X. Hou, R. J. Kirkpatrick, P. Yu, D. Moore and Y. Kim, 15N NMR study of nitrate ion structure and dynamics in hydrotalcite-like compounds, Am. Mineral., 2000, 85, 173–180 CrossRef CAS.
- M. Levitt, Spin dynamics, Basics of nuclear magnetic resonance, 2nd edn, Wiley & Sons, New York, 2008 Search PubMed.
- R. Zarrougui, M. Dhahbi and D. Lemordant, Transport and thermodynamic properties of ethylammonium nitrate–water binary mixtures: Effect of temperature and composition, J. Solution Chem., 2015, 44, 686–702 CrossRef CAS.
- A. Filippov, S. Kurakin, O. I. Gnezdilov and O. N. Antzutkin, Effect of magnetic field on diffusion of ethylammonium nitrate – water mixtures confined between polar glass plates, J. Mol. Liq., 2019, 274, 45–51 CrossRef CAS.
- K. L. Anderson-Altmann and D. M. Grant, A solid-state 15N NMR study of the phase transition in ammonium nitrate, J. Phys. Chem., 1993, 97, 11096–11102 CrossRef CAS.
- C. Wiedemann, D. Fushman and F. Bordusa, 15N NMR studies provide insight into physicochemical properties of room-temperature ionic liquids, Phys. Chem. Chem. Phys., 2021, 23, 12395–12407 RSC.
- M. A. Gebbie, A. M. Smith, H. A. Dobbs, A. A. Lee, G. G. Warr, X. Banquy, M. Valtiner, M. W. Rutland, J. N. Israelachvili, S. Perkin and R. Atkin, Long range electrostatic forces in ionic liquids, Chem. Commun., 2017, 53, 1214–1224 RSC.
- R. Atkin and G. G. Warr, Structure in confined room-temperature ionic liquids, J. Phys. Chem. C, 2007, 111, 5162–5168 CrossRef CAS.
- S. Kralj, A. Zidansek, G. Lahajnar, S. Zimer and R. Blinc, Phase behavior of liquid crystals confined to controlled porous glass studied by deuteron NMR, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1998, 57, 3021–3032 CrossRef CAS.
- P. A. Hunt, C. R. Ashworth and R. P. Matthews, Hydrogen bonding in ionic liquids, Chem. Soc. Rev., 2015, 44, 1257–1288 RSC.
- T. Zentel and O. Kühn, Properties of hydrogen bonds in the protic ionic liquid ethylammonium nitrate, Theor. Chem. Acc., 2017, 136, 87 Search PubMed.
- O. I. Gnezdilov, O. N. Antzutkin, R. Gimatdinov and A. Filippov, Temperature dependence of 1H NMR chemical shifts and diffusivity of confined ethylammonium nitrate ionic liquid, Magn. Reson. Imaging, 2020, 74, 84–89 CrossRef CAS PubMed.
- X. Tang, Y. Xu, X. Zhu and Y. Lu, Changes in microstructure of two ammonium-based protic ionic liquids proved by in situ variable-temperature 1H NMR spectroscopy: influence of anion, Magn. Reson. Chem., 2018, 56, 73–79 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cp01737k |
| This journal is © the Owner Societies 2023 |