 Open Access Article
Open Access ArticleRate constant and branching ratio of the reaction of ethyl peroxy radicals with methyl peroxy radicals
Cuihong
Zhang
abc,
Chuanliang
Li
cd,
Weijun
Zhang
a,
Xiaofeng
Tang
 a,
Laure
Pillier
c,
Coralie
Schoemaecker
a,
Laure
Pillier
c,
Coralie
Schoemaecker
 c and
Christa
Fittschen
c and
Christa
Fittschen
 *c
*c
aAnhui Institute of Optics and Fine Mechanics, Hefei Institutes of Physical Science, Chinese Academy of Sciences, Hefei 230031, Anhui, China
bScience Island Branch, Graduate School, University of Science and Technology of China, Hefei 230026, Anhui, China
cUniversité Lille, CNRS, UMR 8522-PC2A-Physicochimie des Processus de Combustion et de l’Atmosphère, F-59000 Lille, France. E-mail: christa.fittschen@univ-lille.fr
dShanxi Engineering Research Center of Precision Measurement and Online Detection Equipment and School of Applied Science, Taiyuan University of Science and Technology, Taiyuan 030024, China
First published on 23rd June 2023
Abstract
The cross-reaction of ethyl peroxy radicals (C2H5O2) with methyl peroxy radicals (CH3O2) (R1) has been studied using laser photolysis coupled to time resolved detection of the two different peroxy radicals by continuous wave cavity ring down spectroscopy (cw-CRDS) in their AÖ![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) electronic transition in the near-infrared region, C2H5O2 at 7602.25 cm−1, and CH3O2 at 7488.13 cm−1. This detection scheme is not completely selective for both radicals, but it is demonstrated that it has great advantages compared to the widely used, but unselective UV absorption spectroscopy. Peroxy radicals were generated from the reaction of Cl-atoms with the appropriate hydrocarbon (CH4 and C2H6) in the presence of O2, whereby Cl-atoms were generated by 351 nm photolysis of Cl2. For different reasons detailed in the manuscript, all experiments were carried out under excess of C2H5O2 over CH3O2. The experimental results were best reproduced by an appropriate chemical model with a rate constant for the cross-reaction of k = (3.8 ± 1.0) × 10−13 cm3 s−1 and a yield for the radical channel, leading to CH3O and C2H5O, of (ϕ1a = 0.40 ± 0.20).
electronic transition in the near-infrared region, C2H5O2 at 7602.25 cm−1, and CH3O2 at 7488.13 cm−1. This detection scheme is not completely selective for both radicals, but it is demonstrated that it has great advantages compared to the widely used, but unselective UV absorption spectroscopy. Peroxy radicals were generated from the reaction of Cl-atoms with the appropriate hydrocarbon (CH4 and C2H6) in the presence of O2, whereby Cl-atoms were generated by 351 nm photolysis of Cl2. For different reasons detailed in the manuscript, all experiments were carried out under excess of C2H5O2 over CH3O2. The experimental results were best reproduced by an appropriate chemical model with a rate constant for the cross-reaction of k = (3.8 ± 1.0) × 10−13 cm3 s−1 and a yield for the radical channel, leading to CH3O and C2H5O, of (ϕ1a = 0.40 ± 0.20).
Introduction
The oxidation of volatile organic compounds (VOCs) in the troposphere is mainly driven by hydroxyl radicals (OH) and leads, after addition of O2, to the formation of organic peroxy radicals (RO2). The fate of these RO2 radicals depends on the chemical composition of the environment and a detailed review on their chemistry has been given by G. Tyndall and collegues.1,2 Briefly, in a polluted atmosphere they mainly react with nitric oxide (NO) to form alkoxy radicals or react with nitrogen dioxide (NO2) to form peroxynitrates (RO2NO2). Subsequent to the reaction with NO, alkoxy radicals can react with O2 to form hydroperoxy radicals (HO2) together with carbonyl compounds. HO2 further oxidises NO into NO2 and thus regenerates OH, closing the quasi-catalytic cycle. The photolysis of the produced NO2 leads subsequently to the formation of ozone (O3) and is the only relevant formation path of tropospheric ozone. In clean environments with low NOx (NOx = NO + NO2) concentrations, the fate of RO2 change and their dominant loss becomes the reaction with HO2 forming hydroperoxides ROOH and terminating the radical reaction chain. Other reaction pathways under low NOx conditions for RO2 radicals are either self-reaction (RO2 + RO2) or cross-reaction with other RO2 (RO2 + R′O2)1 or with OH radicals (RO2 + OH).3Methane and ethane are amongst the most abundant hydrocarbons, and their atmospheric oxidation leads to the formation of methyl peroxy (CH3O2) and ethyl peroxy (C2H5O2) radicals. For both radicals, the kinetic and product distribution for the self-reaction has been studied numerous times (for CH3O24–12 and for C2H5O213–26), the same is true for their reaction with HO2 (for CH3O25,9,27–32 and for C2H5O213,14,20,26,30,33–35). Their reaction with OH radicals has been the subject of a few studies (for CH3O23,36–41 and for C2H5O242–44). The cross-reaction between both peroxy radicals has only been measured once using UV absorption spectroscopy45 whereby the experimental details given in that paper are sparse and it is not clear how the rate constant was extracted from the absorption time profiles measured at only one wavelength where the cross sections of both radicals are very similar. As for the product distribution of this cross reaction, three pathways can be expected:
| C2H5O2 + CH3O2 → C2H5O + CH3O + O2 | (R1a) |
| →C2H5OH/CH3OH + CH2O/CH3CHO + O2 | (R1b) |
| →C2H5O2CH3 + O2 | (R1c) |
The investigation of this reaction is not straightforward, because secondary chemistry cannot be avoided. Both radicals will react in self-reactions, leading to analogous reaction products. The measurements are complicated, because the product of the reaction path (R1a) leads, after rapid reaction with O2, to the formation of HO2 radicals
| CH3O + O2→ CH2O + HO2 | (R2) |
| C2H5O + O2→ CH3CHO + HO2 | (R3) |
| CH3O2 + HO2→ CH3O2H + O2 | (R4) |
| C2H5O2 + HO2 → C2H5O2H + O2 | (R5) |
In this work we present a more direct measurement of the rate constant of (R1). Measurements have been carried out under an excess of C2H5O2 radicals over CH3O2, and both radicals have been followed in their Ö![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) electronic transition using two different wavelengths. HO2 concentration time profiles have been measured simultaneously in a highly selective way in the 2ν1 vibrational overtone at 6638.21 cm−1.
electronic transition using two different wavelengths. HO2 concentration time profiles have been measured simultaneously in a highly selective way in the 2ν1 vibrational overtone at 6638.21 cm−1.
Experimental
Experimental setup
The setup has been described in detail before46–49 and is only briefly discussed here. The setup mainly consists of a 0.79 m long flow reactor made of stainless steel. The beam of a pulsed excimer laser (Lambda Physik LPX 202i), running at 351 nm, passed the reactor longitudinally. The flow reactor contained two identical continuous wave cavity ring-down spectroscopy (cw-CRDS) absorption paths, which were installed in a small angle with respect to the photolysis path. An overlap of the near IR-path with the photolysis beam of 0.288 m is achieved with an excimer beam width delimited to 2 cm. Both beam paths were tested for a uniform overlap with the photolysis beam before experiments. For this purpose, both cw-CRDS instruments were operated to simultaneously measure HO2 concentrations. Deviations between HO2 concentrations were less than 5%, demonstrating that the photolysis laser was well aligned, i.e. both light paths probed a very similar photolysed volume in the reactor. A small helium purge flow prevented the mirrors from being contaminated. Three different DFB lasers are used for the detection of the three species: HO2: NEL NLK1E5GAAA, 6629 ± 17 cm−1, on CRDS path 1, CH3O2: NEL NLK1B5EAAA, 7480 ± 20 cm−1 on CRDS path 2, C2H5O2: AOI-1312-BF-20-CW-F1-H2-N127, 7622 ± 15 cm−1 on CRDS path 2. They are coupled into one of the cavities by systems of lenses and mirrors. Each probe beam passed an acousto-optic modulator (AOM, AAoptoelectronic) to rapidly turn off the 1st order beam once a threshold for light intensity at the exit of the cavity was reached, in order to measure the ring-down event. Then, the decay of light intensity was recorded and an exponential fit is applied to retrieve the ring-down time. The absorption coefficient α is derived from eqn (1). | (1) |
Ethyl- and methylperoxy radicals were generated by pulsed 351 nm photolysis of C2H6/CH4/Cl2/O2 mixtures inducing the following reactions:
Cl2 + hν351![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm → 2 Cl nm → 2 Cl | (R7) |
| CH4 + Cl → CH3 + HCl | (R8) |
| C2H6 + Cl → C2H5 + HCl | (R9) |
| CH3 + O2 (+ M) → CH3O2 (+ M) | (R10) |
| C2H5 + O2 (+ M) → C2H5O2 (+ M) | (R11a) |
| C2H5 + O2 → C2H4 + HO2 | (R11b) |
C2H6 (Air Liquide, N35), CH4 (Air Liquide, N45) and Cl2 (Air Liquide, 5% in Helium) were used directly from the cylinder: a small flow was added to the mixture through a calibrated flow meter (Bronkhorst, Tylan). All experiments were carried out at 298 K.
Results and discussion
Determination of the absorption cross sections
Detecting peroxy radicals in the Ö![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) electronic transition in the near IR region has the potential of a more selective detection for peroxy radicals compared to UV absorption spectroscopy. In order to demonstrate this, we have carried out measurements for the determination of the rate constant of the cross reaction between CH3O2 and C2H5O2 radicals. The rate constant of this reaction was measured only once using UV absorption spectroscopy45 whereby the experimental details given in that paper were sparse. It is not clear how the rate constant was extracted from the absorption time profiles measured only at one wavelength where the cross sections of both radicals are very similar.
electronic transition in the near IR region has the potential of a more selective detection for peroxy radicals compared to UV absorption spectroscopy. In order to demonstrate this, we have carried out measurements for the determination of the rate constant of the cross reaction between CH3O2 and C2H5O2 radicals. The rate constant of this reaction was measured only once using UV absorption spectroscopy45 whereby the experimental details given in that paper were sparse. It is not clear how the rate constant was extracted from the absorption time profiles measured only at one wavelength where the cross sections of both radicals are very similar.
The Ö![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) transitions of peroxy radicals consist generally of peaks with a few cm−1 FWHM on a rather broad background.50 To check for the mutual selectivity of the detection for both radicals, the absorption cross sections for both radicals have been measured at three different wavelengths: at one “peak” of the Ö
transitions of peroxy radicals consist generally of peaks with a few cm−1 FWHM on a rather broad background.50 To check for the mutual selectivity of the detection for both radicals, the absorption cross sections for both radicals have been measured at three different wavelengths: at one “peak” of the Ö![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) transitions of the CH3O2 radical at 7488.14 cm−1 (named in the following M1, green symbols in Fig. 1), at the maximum of the transition of C2H5O2 at 7596.47 cm−1 (named E1, red symbols in Fig. 1) and at a “plateau” at 7602.25 cm−1 (named E2, blue symbols in Fig. 1).
transitions of the CH3O2 radical at 7488.14 cm−1 (named in the following M1, green symbols in Fig. 1), at the maximum of the transition of C2H5O2 at 7596.47 cm−1 (named E1, red symbols in Fig. 1) and at a “plateau” at 7602.25 cm−1 (named E2, blue symbols in Fig. 1).
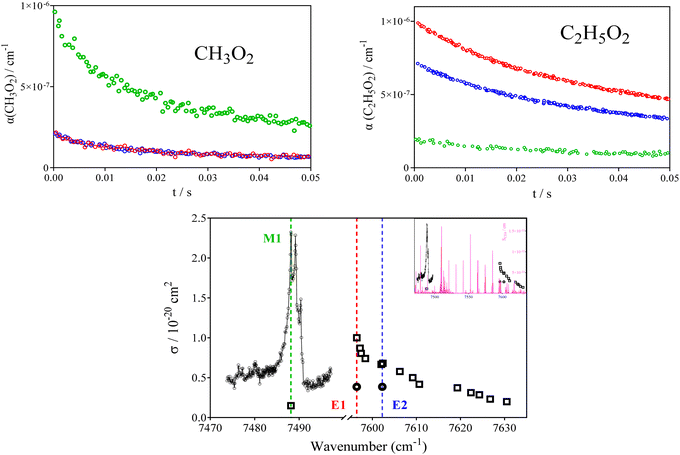 | ||
| Fig. 1 CH3O2 (upper left graph, [Cl]0 = 4.2 × 1013 cm−3, [CH4] = 1.9 × 1017 cm−3) and C2H5O2 (upper right graph: [Cl]0 = 1.0 × 1014 cm−3, [C2H6] = 4.4 × 1016 cm−3) profiles obtained at the three different wavelengths represented by colored vertical lines in the lower graph. Lower graph shows spectrum for both species (CH3O2 as circles, adapted from Farago et al.51 and C2H5O2 as square adapted from Zhang et al.52), main graph shows zoom on both sections with x-axis interrupted, insert shows continuous wavelength scale. Magenta lines in insert represent CH4 spectrum from HITRAN database.53 | ||
The upper graphs of Fig. 1 show for one Cl-concentration the absorption time profiles for both radicals (left: CH3O2, right: C2H5O2) at all three wavelengths. It can be seen that both radicals still absorb at the wavelength corresponding to the transition of the counterpart radical: for both radicals the absorption at its peak is around 4 times larger than at the peak of the counterpart radical (second column Table 1). The absorption cross sections at the peak wavelengths are known from earlier works3,51,52 and have been used here to obtain the absorption cross sections at the peak wavelength of the counterpart radical from the relative intensities in Fig. 1 type experiments (experiments using 3 different Cl-atom concentrations have been carried out). The results are summarized in Table 1 and illustrated in the lower graph of Fig. 1.
| Ratio (σpeak/σoff) | σ (M1)/cm2 | σ (E1)/cm2 | σ (E2)/cm2 | |
|---|---|---|---|---|
| 7488.13 cm−1 | 7596.47 cm−1 | 7602.25 cm−1 | ||
| CH3O2 | 4.0 | 2.2 × 10−20 | 5.5 × 10 −21 | 5.5 × 10 −21 |
| C2H5O2 | 6.6/5.0 | 1.5 × 10 −21 | 1.0 × 10−20 | 7.6 × 10−21 |
| CH453 | 1.2 × 10−24 | 1.1 × 10−23 | 5.0 × 10−25 | |

|
14.6 | 0.55 (= 1/1.81) | 0.72 (= 1/1.38) |
It can be seen that the absorption cross sections for both radicals at the “counterpart wavelengths” (in italic in Table 1) are small (1.5 and 5.5 × 10−21 cm−2), but not zero, and thus complete selectivity cannot be obtained.
Determination of the rate constant
To get best selectivity for investigating the cross reaction between both radicals, C2H5O2 was used for all experiments in excess over CH3O2 for different reasons:• To limit the reaction of Cl-atoms with peroxy radicals: the reaction of Cl-atoms with CH4 is much slower than the reaction of Cl-atoms with C2H6 (0.01 and 5.9 × 10−11 cm3 s−1 for CH4 and C2H6, respectively).54 Therefore, to even obtain identical CH3O2 and C2H5O2 concentrations, already 580 times more CH4 than C2H6 is needed. And because CH4 is absorbing in the near IR region (the absorption cross sections for CH4 at the three wavelengths are given in Table 1 and is shown as magenta stick spectrum53 in Fig. 1), the amount of CH4 that can be added in our experiments is limited to a few 1017 cm−3. If an excess of CH3O2 would have been chosen, only a few 1013 cm−3 C2H6 would need to be added to obtain comparable C2H5O2 concentrations. Such low hydrocarbon concentrations would lead to Cl-atom decays too slow to avoid major complications due to the reaction of Cl-atoms with CH3O2 or C2H5O2.
• To limit absorption of the “counterpart” radical and thus increase selectivity: the ratio of the absorption cross sections between both radicals at a given wavelength (last row of Table 1) is higher at the methyl peroxy transition: σ(CH3O2) is 14.6 times higher compared to σ(C2H5O2) at (M1), while the inverse ratio is only 1.81 and 1.38 at (E1) and (E2), respectively. Therefore, in the example of a 10-fold (5-fold) excess of CH3O2 over C2H5O2, the absorbance at (M1) would be more than 99% (98%) due to CH3O2 (i.e. excellent selectivity), but at (E1) only 15% (27%) and at (E2) only 12% (22%) of the absorbance would be due to C2H5O2, respectively. In the example of a 10-fold (5-fold) excess of C2H5O2 over CH3O2, the absorbance at (E1) would be around 95% (90%) and at (E2) 93% (87%) due to C2H5O2 (i.e. still good selectivity), but now at (M1) around 59% (75%) of the signal is due to CH3O2 absorption.
• To maximize the importance of the cross-reaction: the self-reaction of C2H5O2 is 3.5 times slower than that of CH3O2 (or 2 times, taking the very recent determination of the CH3O2 self-reaction rate constant by Onel et al.12), making the loss through self-reaction less important in a reaction system with excess C2H5O2 compared to excess CH3O2.
Therefore, experiments with a 5- to 10-fold excess of C2H5O2 over CH3O2 should lead to a good sensitivity towards the rate constant of the cross-reaction: decays at (E1) or (E2) represent nearly pure C2H5O2 decays mostly governed by the self-reaction, the correction of these profiles due to CH3O2 absorption is very minor. Simultaneously measured profiles obtained at (M1) can now be corrected for C2H5O2 absorption, and the remaining CH3O2 decay is mostly due to the cross reaction with C2H5O2: the rate constant of the cross reaction can be extracted with good sensitivity.
Even though the absorption cross section for C2H5O2 is higher on (E1) compared to (E2), all experiments have been carried out at (M1) and (E2) due to the much lower CH4 absorption cross sections at (E2) compared to (E1): even though C2H5O2 is used in excess, high CH4 concentrations (up to 3 × 1017 cm−3) were still added and absorbed too much light at (E1).
Three series of experiments have been carried out, and the experimental conditions are summarized in Table 2. The initial Cl-atom concentrations (column 1) have been measured before each experiment through measuring and fitting HO2 decays from the reaction of Cl-atoms with excess CH3OH. C2H6 and CH4 concentrations (column 2 and 3) have been obtained from flow and pressure measurements, and the initial peroxy radical concentrations (column 4 and 5) and their ratio (column 6) have then been calculated using the literature values of the rate constants for (R8) and (R9), as given in Table 3. To demonstrate the relatively good selectivity towards both radicals, the percentage of the absorbances at M1 and E2, that are due to the searched-after radical, have then been calculated using the radical concentrations and the absorption cross sections from Table 1 (column 7 and 8).
| [Cl]/1013 cm−3 | [C2H6]/1015 cm−3 | [CH4]/1017 cm−3 | [C2H5O2]0/1013 cm−3 | [CH3O2]0/1013 cm−3 | [C2H5O2]0/[CH3O2]0 | α C2H5O2 at E2 (%) | α CH3O2 at M1 (%) |
|---|---|---|---|---|---|---|---|
| 8.1 | 2.90 | 2.00 | 7.25 | 0.85 | 8.56 | 92.2 | 63.2 |
| 11.0 | 9.85 | 1.15 | |||||
| 13.8 | 12.4 | 1.44 | |||||
| 7.4 | 2.08 | 2.98 | 5.95 | 1.45 | 4.12 | 85.1 | 78.1 |
| 10.4 | 8.37 | 2.03 | |||||
| 12.5 | 10.1 | 2.44 | |||||
| 7.1 | 1.25 | 2.98 | 5.05 | 2.05 | 2.46 | 77.3 | 85.6 |
| 9.2 | 6.54 | 2.66 | |||||
| 11.8 | 8.39 | 3.41 |
| Reaction | k cm3 s−1 | Ref. | |
|---|---|---|---|
| Initiation reactions | |||
| 8 | Cl + CH4 → CH3 + HCl | 1.0 × 10−13 | 54 |
| 9 | Cl + C2H6 → C2H5 + HCl | 5.9 × 10−11 | 54 |
| 10 | CH3 + O2 + M → CH3O2 + M | 1.4 × 10−13 | 55 |
| 11a | C2H5 + O2 + M → C2H5O2 + M | 4.8 × 10−12 | 56 |
| 11b | C2H5 + O2 → C2H4 + HO2 | 3.5 × 10−14 | This work |
| Peroxy radical self- and cross-reactions | |||
| 1a | C2H5O2 + CH3O2 → C2H5O + CH3O + O2 | 1.5 × 10−13 | This work |
| 1b | C2H5O2 + CH3O2 → stable products | 2.3 × 10−13 | This work |
| 2 | CH3O + O2 → CH2O + HO2 | 1.92 × 10−15 | 54 |
| 3 | C2H5O + O2 → CH3CHO + HO2 | 8 × 10−15 | 57 |
| 4 | CH3O2 + HO2 → CH3OOH + O2 | 5.2 × 10−12 | 52 |
| 5 | C2H5O2 + HO2 → C2H5OOH + O2 | 6.2 × 10−12 | 52 |
| 12a | 2 C2H5O2 → 2 C2H5O + O2 | 3.2 × 10−14 | 24 |
| 12b | 2 C2H5O2 → stable products | 7.0 × 10−14 | 24 |
| 13a | 2 CH3O2 → 2 CH3O + O2 | 1.3× 10−13 | 54 |
| 13b | 2 CH3O2 → stable products | 2.2 × 10−13 | 54 |
| 14 | CH3O + HO2 → products | 1.1 × 10−10 | 58 |
| 15 | 2 HO2 → H2O2 + O2 | 1.7 × 10−12 | 59 |
| Secondary Cl-atom reactions | |||
| 6a | Cl + C2H5O2 → ClO + C2H5O | 5–8 × 10−11 | See text |
| 6b | Cl + C2H5O2 → Products | 5–8 × 10−11 | See text |
| 16 | Cl + CH3O2 → ClO + CH3O | 7.5 × 10−11 | 60 |
| 17 | Cl + CH3O2 → Products | 7.5 × 10−11 | 60 |
| 18 | Cl + CH2O + O2 → HCl + HO2 + CO | 7.32 × 10−11 | 61 |
| 19 | C2H5O2/CH3O2 + ClO → C2H5O/CH3O + ClOO | 1.6 × 10−12 | 54 |
| 20 | HO2 + ClO → O2 + HOCl | 6.9 × 10−12 | 62 |
| 21 | ClOO (+ M) → Cl + O2 (+ M) | 6.2 × 10−13 | 62 |
| 22 | Cl + O2 (+ M) → ClO2 (+ M) | 1.6 × 10−33 | 62 |
| Other secondary chemistry | |||
| 23 | C2H5O + C2H5O2 → products | 7 × 10−12 | This work |
| 24 | C2H5O + HO2 → products | 1 × 10−10 | 63 |
| 25 | C2H5O2/CH3O2 → diffusion | 2 s−1 | This work |
| 26 | HO2 → diffusion | 3 s−1 | This work |
Fig. 2 shows the experimental absorption time-profiles obtained at M1 and E2 for the 3 series (highest C2H5O2 excess upper graph, note the different y-axis for both wavelengths, and lowest C2H5O2 excess bottom graphs) as colored dots: the absorption time-profiles obtained at M1, the wavelength mostly selective to CH3O2, are shown in the left column, the profiles obtained at E2, mostly selective to C2H5O2, are shown in the right column.
 | ||
| Fig. 2 Absorption-time profiles at M1 (left graphs) and E2 (right graphs) for all three series with conditions such as given in Table 2. Full lines present the simulated absorption-time profiles using the model from Table 3 and are presented as sum of absorbance due to CH3O2 and C2H5O2, dotted lines represent the part of the absorbance due to major radical: CH3O2 in the left column, C2H5O2 in the right column. | ||
The profiles at both wavelengths have been simulated simultaneously using the model from Table 3, by best reproducing the signals at M1 as
| αM1 = σCH3O2,M1 × [CH3O2] + σC2H5O2,M1 × [C2H5O2] | (2) |
| αE2 = σCH3O2,E2 × [CH3O2] + σC2H5O2,E2 × [C2H5O2] | (3) |
The model contains, next to peroxy self-and cross reactions, also some secondary chemistry of Cl-atoms: these reactions could not completely be avoided, even though their impact is minor. Preliminary results in our laboratory indicate that the reaction of Cl-atoms with C2H5O2 leads with a rate constant of around 1 × 10−10 cm3 s−1 and a yield of 50% to formation of C2H5O and ClO, while no clear statement can currently be made for the fate of the other 50%. The rate constant of this reaction has also been determined by Maricq et al.64 to be 1.6 × 10−10 cm3 s−1, and therefore this reaction has been included into the mechanism (see Table 3) and tests have been run with the rate constant being varied between 1.0–1.6 × 10−10 cm3 s−1, but the impact on simulated profiles and thus on the sought-after rate constant was within the noise of the experimental profiles.
Fig. 3 shows for the example of the highest C2H5O2 excess (upper graphs of Fig. 2) the breakdown of the fate of the 2 peroxy radicals into the different possible reaction paths: the left graphs represent CH3O2, the right graphs C2H5O2. The red symbols represent the fraction of the peroxy radical, which has reacted in the cross reaction (R1): it can be seen that for CH3O2, this reaction is the major fate for all initial radical concentrations (upper graph represent blue symbols from Fig. 2, lower graph represent green symbols from Fig. 2), while for C2H5O2 this reaction is a minor loss. The major reaction path for C2H5O2 is its self-reaction (black symbols), with the cross-reaction with HO2 being the secondary contributor (blue symbols). These two pathways are very minor for CH3O2. For both radicals, the fraction having reacted with Cl-atoms (green symbols), is small, up to 5% for CH3O2 in the worst case of high initial radical concentration.
 | ||
| Fig. 3 Modeling results for conditions from first raw of Fig. 2 (highest excess of C2H5O2). Left graph CH3O2, right graph C2H5O2. Upper graphs are results for lowest Cl-concentration (blue symbols in Fig. 2), lower graph are results for highest Cl-concentration (green symbols in Fig. 2). Open black circles are CH3O2/C2H5O2 concentration, blue symbols represent CH3O2/C2H5O2 concentration having reacted through cross reaction with HO2, black symbol represent CH3O2/C2H5O2 concentration having reacted through self-reaction, green symbols represent CH3O2/C2H5O2 concentration having reacted with Cl-atoms, red symbols represent CH3O2/C2H5O2 concentration having reacted through cross reaction with C2H5O2/CH3O2. | ||
Determination of branching ratio
Simultaneously measured HO2 profiles allow in principle the estimation of the branching ratio for the radical and molecular path of the cross reaction. The right graph of Fig. 4 shows the HO2 profiles obtained for the series with the highest C2H5O2/CH3O2 ratio. The initial fast rise of HO2 has two origins: it is partially due to the reaction of Cl-atoms with the peroxy radicals (R6) and partially due to the small fraction of C2H5 radicals that form HO2 in reaction with O2(R11b) rather than the C2H5O2 radical. The first process is taken into account in the chemical model by adding a simplified reaction schema (see Table 3), the second process has been implemented to best represent the initial HO2 concentration and represents less than 1% of the initial C2H5 concentration. This observation is in excellent agreement with earlier works.24,52,65,66 These two processes are finished within a few hundred μs, and the branching ratio of the cross reaction then influences the HO2 concentration at longer reaction time. This is conceivable, because the HO2 concentration at longer reaction times represents the steady-state concentration between production from peroxy self-and cross reactions and the consumption through cross reaction of HO2 with the peroxy radicals. Best results are obtained with a branching ratio towards the radical channel of ϕ1a = 0.40, i.e. very similar to the branching fraction of the two self-reactions, ϕ12a = 0.32 and ϕ13a = 0.37 for C2H5O2 and CH3O2, respectively. To demonstrate the influence of the cross reaction on the HO2 profiles, the full black lines in the right graph represent for the highest radical concentration the simulation with the best rate constant and a branching ratio varied by ±0.2. It can be seen that such variation of the branching ratio makes the model clearly deviating from the experimental results and therefore we estimate the uncertainty of the branching fraction from the comparison between model and experiment to be better than ±0.2. | ||
| Fig. 4 Left graph: CH3O2 profiles for highest C2H5O2 excess: full lines represent best simulation with rate constants from Table 3 (k1 = 3.8 × 10−13 cm3 s−1), dashed lines represent a variation of k1 of ±1.5 × 10−13 cm3 s−1. Right graph: HO2 profiles for the same experiment. Full coloured line represents best model with a radical yield of 0.4, dashed lines in the right graph show the model with k1 varied as shown in left graph, but the branching ratio varied to best reproduce experiment (see text). The black lines show a variation of ±0.2 for the branching ratio for the highest radical concentration. | ||
However, a major problem for estimating the branching ratio in these experiments is, that even for the self-reactions of the simplest peroxy radicals CH3O2 and C2H5O2 there are still large uncertainty in rate constant and branching ratio. For CH3O2 the IUPAC recommendation54 since many years was k13 = 3.5 × 10−14 cm3 s−1 with a branching ratio of 0.37 for the radical channel. In a very recent work, Onel et al.12 have re-determined the rate constant and found only k13 = (2.0 ± 0.9) × 10−14 cm3 s−1, nearly 2 times slower, but they confirmed the radical yield as recommended by IUPAC. They convincingly argue that earlier experiments suffered from interferences of the fast reaction of Cl-atoms with CH3O2 and this would have increased the apparent rate constant. The rate constant for the self-reaction of C2H5O2 radicals was also recommended by IUPAC for many years at k12 = 7.6 × 10−14 cm3 s−1 with a radical yield of 0.63, based on the measurement of stable end products. Recently, Noell et al.14 and Shamas et al.24 obtained through direct radical measurements a much lower yield for the radical path and a subsequently higher rate constant (0.32 radical yield leading to k12 = (1.0 ± 0.2) × 10−13 cm3 s−1). A possible explanation for this disagreement could be a non-negligible yield of dimer-formation, ROOR, in the self-reaction of peroxy radicals. The decomposition of such dimer on reactor walls could lead to formation of aldehydes and thus appear as additional radical formation when measuring stable end products. The dimeric product has very recently been detected in the self-reaction of C2H5O225 using advanced vacuum ultraviolet (VUV) photoionization mass spectrometry with a yield of 10 ± 5%. The dimer has also been directly detected by CIMS with a yield of 23% in the self-reaction of HOC2H4O2 radicals and has been proven to decompose easily on quartz or metal surfaces.67 But even though the cross reaction (R1) is the major HO2 production path in the current experiments and the two self-reactions are only minor contributors, the above described uncertainties increase of course directly the uncertainty of the deduced yield in this work. Also, the HO2 signal quality is poor in these experiments due to the absorption of high CH4 and C2H6 concentrations, therefore we estimate the final uncertainty of the radical yield to be ϕ1a = 0.40 ± 0.20. It should be noted that the uncertainty in the branching ratio has negligible influence on the determination of the rate constant: a change in radical yield for (R1) from 0.2 to 0.6 is barely visible in the modelled absorption-time profiles at both wavelengths.
To demonstrate the sensitivity of the observed absorption-time profiles to the rate constant of the cross reaction, the left graph of Fig. 4 shows the CH3O2 profiles of the same experiments, i.e. high excess of C2H5O2. The full lines show again the model from Table 3, while the dashed lines represent a variation of k1 = (3.8 ± 1.5) × 10−13 cm3 s−1. Such variation brings the simulated profiles outside the experimental data. In these simulations, the branching ratio ϕ1a has been adapted to best reproduce the HO2 profiles (dashed coloured lines on the right graph): for the upper and lower limit of k1, ϕ1a was changed to 0.31 (for k1 = 4.8 × 10−13 cm3 s−1) and 0.54 (for k1 = 2.8 × 10−13 cm3 s−1) to best reproduce HO2. However, this variation has no influence on the CH3O2 profiles as can be seen in Fig. 3 the cross reaction with HO2 is only a minor path for CH3O2 and therefore a change in the branching ratio has a negligible effect on the CH3O2 profile. From these simulations we estimate the uncertainty of the rate constant of the cross reaction to be k1 = (3.8 ± 1.0) × 10−13 cm3 s−1.
The simulation corresponding to the lower limit of the rate constant (upper curves in Fig. 4) is close to the only published value for the cross reaction rate constant45 (k1 = 2.0 × 10−13 cm3 s−1), and is can be seen that the observed absorption time profiles are poorly reproduced by such a model. In the work of Villenave et al.45 no details are given on how the rate constant was obtained by solely measuring UV absorption profiles, and therefore no speculation about possible reasons for the disagreement can be proposed.
The geometric mean value rule is an empirical approach that allows for the estimation of cross-reaction rate coefficients from the self-recombination rate constants of the reacting partners68
 | ||
| Fig. 5 Series of lowest C2H5O2/CH3O2 ratio (lower raw in Fig. 3) with simulations using a rate constant for the cross reaction such as predicted by the geometric mean rule, if using data from Table 3 (full lines) and when using the recently determined rate constant for the CH3O2 self-reaction12 (dashed lines). | ||
It can be seen that this rate constant does not allow to reproduce the observed absorption-time profiles, as the decays at both wavelengths are clearly too slow. However, it has not been demonstrated that in the case of cross-reactions of peroxy radicals the geometric mean rule is a good approximation, in particular because there are no reliable determinations of the rate constants for self- and cross-reactions of peroxy radicals to validate the approach. Therefore, from the current experiments one cannot infer about the rate constant of the CH3O2 self-reaction. But it is clear that recent research using more selective detection methods for peroxy radicals, compared to UV absorption, have challenged long-standing results on even the simplest peroxy radicals, and more research is necessary to better understand their reactivity under low NOx conditions.
Conclusion
The rate constant for the cross reaction of the two most simple and abundant peroxy radicals, CH3O2 and C2H5O2, has been determined by following their concentration-time profiles in their respective Ö![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) electronic transition. A good selectivity has been obtained by working under excess of C2H5O2 and by monitoring CH3O2 radical at 7488.13 cm−1 and C2H5O2 radicals at 7602.25 cm−1. A rate constant for the cross reaction of k1 = (3.8 ± 1.0) × 10−13 cm3 s−1 and a yield for the radical channel of ϕ1a = 0.40 ± 0.20 have been obtained. The present rate constant is nearly two times faster than the only earlier value, but in excellent agreement with an estimation based on the mean geometric rule. This work shows again, that the chemistry of peroxy radicals under low NO conditions is still not well understood and more work is needed to improve the knowledge.
electronic transition. A good selectivity has been obtained by working under excess of C2H5O2 and by monitoring CH3O2 radical at 7488.13 cm−1 and C2H5O2 radicals at 7602.25 cm−1. A rate constant for the cross reaction of k1 = (3.8 ± 1.0) × 10−13 cm3 s−1 and a yield for the radical channel of ϕ1a = 0.40 ± 0.20 have been obtained. The present rate constant is nearly two times faster than the only earlier value, but in excellent agreement with an estimation based on the mean geometric rule. This work shows again, that the chemistry of peroxy radicals under low NO conditions is still not well understood and more work is needed to improve the knowledge.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is a contribution to the LabEx CaPPA project funded by the French National Research Agency under contract ANR-11-LABX-0005-01 and to the CPER research project ECRIN funded by the French Ministère de l'Enseignement Supérieur et de la Recherche. The authors thank the Regional Council “Hauts-de-France” and the “European Regional Development Fund” for their financial support to these projects. The authors thank Mohamed Assali and Mirna Shamas for assistance with initial experiments. C. Z. and C. L. thanks the Chinese Scholarship Council for financial support (no. 202006340125 (C. Z.) and no. 201908140178 (C. L.)). C. F. thanks the CAS for funding through PIFI no. 2018VMA0055.References
- J. J. Orlando and G. S. Tyndall, Chem. Soc. Rev., 2012, 41, 6294–6317 RSC.
- G. S. Tyndall, R. A. Cox, C. Granier, R. Lesclaux, G. K. Moortgat, M. J. Pilling, A. R. Ravishankara and T. J. Wallington, J. Geophys. Res., 2001, 106, 12157–12182 CrossRef CAS.
- C. Fittschen, Chem. Phys. Lett., 2019, 725, 102–108 CrossRef CAS.
- B. Veyret, J. C. Rayez and R. Lesclaux, J. Phys. Chem., 1982, 86, 3424–3430 CrossRef CAS.
- R. A. Cox and G. S. Tyndall, J. Chem. Soc., Faraday Trans., 1980, 76, 153–163 RSC.
- S. P. Sander and R. T. Watson, J. Phys. Chem., 1981, 85, 2960–2964 CrossRef CAS.
- K. McAdam, B. Veyret and R. Lesclaux, Chem. Phys. Lett., 1987, 133, 39–44 CrossRef CAS.
- M. J. Kurylo and T. J. Wallington, Chem. Phys. Lett., 1987, 138, 543–547 CrossRef CAS.
- M. E. Jenkin, R. A. Cox, G. D. Hayman and L. J. Whyte, J. Chem. Soc., Faraday Trans., 1988, 84, 913–930 RSC.
- F. G. Simon, W. Schneider and G. K. Moortgat, Int. J. Chem. Kinet., 1990, 22, 791–812 CrossRef CAS.
- P. D. Lightfoot, R. Lesclaux and B. Veyret, J. Phys. Chem., 1990, 94, 700–707 CrossRef CAS.
- L. Onel, A. Brennan, F. F. Østerstrom, E. Cooke, L. Whalley, P. W. Seakins and D. E. Heard, J. Phys. Chem. A, 2022, 126, 7639–7649 CrossRef CAS PubMed.
- F. C. Cattell, J. Cavanagh, R. A. Cox and M. E. Jenkin, J. Chem. Soc., Faraday trans. II, 1986, 82, 1999–2018 RSC.
- A. C. Noell, L. S. Alconcel, D. J. Robichaud, M. Okumura and S. P. Sander, J. Phys. Chem. A, 2010, 114, 6983–6995 CrossRef CAS PubMed.
- H. Adachi, N. Basco and D. G. L. James, Int. J. Chem. Kinet., 1979, 11, 1211–1229 CrossRef CAS.
- C. Anastasi, D. J. Waddington and A. Woolley, J. Chem. Soc., Faraday trans. I, 1983, 79, 505–516 RSC.
- J. Munk, P. Pagsberg, E. Ratajczak and A. Sillesen, J. Phys. Chem., 1986, 90, 2752–2757 CrossRef CAS.
- T. J. Wallington, P. Dagaut and M. J. Kurylo, J. Photochem. Photobiol., A, 1988, 42, 173–185 CrossRef CAS.
- D. Bauer, J. N. Crowley and G. K. Moortgat, J. Photochem. Photobiol., A, 1992, 65, 329–344 CrossRef CAS.
- F. F. Fenter, V. Catoire, R. Lesclaux and P. D. Lightfoot, J. Phys. Chem., 1993, 97, 3530–3538 CrossRef CAS.
- D. B. Atkinson and J. W. Hudgens, J. Phys. Chem. A, 1997, 101, 3901–3909 CrossRef CAS.
- H. Niki, P. D. Maker, C. M. Savage and L. P. Breitenbach, J. Phys. Chem., 1982, 86, 3825–3829 CrossRef CAS.
- T. J. Wallington, C. A. Gierczak, J. C. Ball and S. M. Japar, Int. J. Chem. Kinet., 1989, 21, 1077–1089 CrossRef CAS.
- M. Shamas, M. Assali, C. Zhang, X. Tang, W. Zhang, L. Pillier, C. Schoemaecker and C. Fittschen, ACS Earth Space Chem., 2022, 6, 181–188 CrossRef CAS.
- H. Yue, C. Zhang, X. Lin, Z. Wen, W. Zhang, S. Mostafa, P.-L. Luo, Z. Zhang, P. Hemberger, C. Fittschen and X. Tang, Int. J. Mol. Sci., 2023, 24, 3731 CrossRef CAS PubMed.
- Z. Wen, H. Yue, Y. Zhang, X. Lin, Z. Ma, W. Zhang, Z. Wang, C. Zhang, C. Fittschen and X. Tang, Chem. Phys. Lett., 2022, 806, 140034 CrossRef CAS.
- P. Dagaut, T. J. Wallington and M. J. Kurylo, J. Phys. Chem., 1988, 92, 3833–3836 CrossRef CAS.
- G. K. Moortgat, R. A. Cox, G. Schuster, J. P. Burrows and G. S. Tyndall, J. Chem. Soc., Faraday Trans., 1989, 85, 809–829 RSC.
- P. D. Lightfoot, B. Veyret and R. Lesclaux, J. Phys. Chem., 1990, 94, 708–714 CrossRef CAS.
- A. A. Boyd, P.-M. Flaud, N. Daugey and R. Lesclaux, J. Phys. Chem. A, 2003, 107, 818–821 CrossRef CAS.
- M. T. Raventós-Duran, M. McGillen, C. J. Percival, P. D. Hamer and D. E. Shallcross, Int. J. Chem. Kinet., 2007, 39, 571–579 CrossRef.
- P. D. Lightfoot, P. Roussel, F. Caralp and R. Lesclaux, J. Chem. Soc., Faraday Trans., 1991, 87, 3213–3220 RSC.
- P. Dagaut, T. J. Wallington and M. J. Kurylo, J. Phys. Chem., 1988, 92, 3836–3839 CrossRef CAS.
- M. M. Maricq and J. J. Szente, J. Phys. Chem., 1994, 98, 2078–2082 CrossRef CAS.
- M. T. Raventós-Duran, C. J. Percival, M. R. McGillen, P. D. Hamer and D. E. Shallcross, Phys. Chem. Chem. Phys., 2007, 9, 4338–4348 RSC.
- A. Bossolasco, E. P. Faragó, C. Schoemaecker and C. Fittschen, Chem. Phys. Lett., 2014, 593, 7–13 CrossRef CAS.
- C. Fittschen, L. K. Whalley and D. E. Heard, Environ. Sci. Technol., 2014, 118, 7700–7701 CrossRef PubMed.
- C. Yan, S. Kocevska and L. N. Krasnoperov, J. Phys. Chem. A, 2016, 120, 6111–6121 CrossRef CAS PubMed.
- E. Assaf, B. Song, A. Tomas, C. Schoemaecker and C. Fittschen, J. Phys. Chem. A, 2016, 120, 8923–8932 CrossRef CAS PubMed.
- C. Fittschen, M. Al Ajami, S. Batut, V. Ferracci, S. Archer-Nicholls, A. T. Archibald and C. Schoemaecker, Atmos. Chem. Phys., 2019, 19, 349–362 CrossRef CAS.
- R. L. Caravan, M. A. H. Khan, J. Zádor, L. Sheps, I. O. Antonov, B. Rotavera, K. Ramasesha, K. Au, M.-W. Chen, D. Rösch, D. L. Osborn, C. Fittschen, C. Schoemaecker, M. Duncianu, A. Grira, S. Dusanter, A. Tomas, C. J. Percival, D. E. Shallcross and C. A. Taatjes, Nat. Commun., 2018, 9, 4343 CrossRef PubMed.
- E. P. Faragó, C. Schoemaecker, B. Viskolcz and C. Fittschen, Chem. Phys. Lett., 2015, 619, 196–200 CrossRef.
- E. Assaf, C. Schoemaecker, L. Vereecken and C. Fittschen, Int. J. Chem. Kinet., 2018, 50, 670–680 CrossRef CAS.
- E. Assaf, S. Tanaka, Y. Kajii, C. Schoemaecker and C. Fittschen, Chem. Phys. Lett., 2017, 684, 245–249 CrossRef CAS.
- E. Villenave and R. Lesclaux, J. Phys. Chem., 1996, 100, 14372–14382 CrossRef CAS.
- J. Thiebaud and C. Fittschen, Appl. Phys. B, 2006, 85, 383–389 CrossRef CAS.
- A. E. Parker, C. Jain, C. Schoemaecker, P. Szriftgiser, O. Votava and C. Fittschen, Appl. Phys. B., 2011, 103, 725–733 CrossRef CAS.
- O. Votava, M. Mašát, A. E. Parker, C. Jain and C. Fittschen, Rev. Sci. Instrum., 2012, 83, 043110 CrossRef PubMed.
- E. Assaf, O. Asvany, O. Votava, S. Batut, C. Schoemaecker and C. Fittschen, J. Quant. Spectrosc. Radiat. Transfer, 2017, 201, 161–170 CrossRef CAS.
- E. N. Sharp, P. Rupper and T. A. Miller, Phys. Chem. Chem. Phys., 2008, 10, 3955–3981 RSC.
- E. P. Faragó, B. Viskolcz, C. Schoemaecker and C. Fittschen, J. Phys. Chem. A, 2013, 117, 12802–12811 CrossRef PubMed.
- C. Zhang, M. Shamas, M. Assali, X. Tang, W. Zhang, L. Pillier, C. Schoemaecker and C. Fittschen, Photonics, 2021, 8, 296 CrossRef CAS.
- L. S. Rothman, I. E. Gordon, Y. Babikov, A. Barbe, D. Chris Benner, P. F. Bernath, M. Birk, L. Bizzocchi, V. Boudon, L. R. Brown, A. Campargue, K. Chance, E. A. Cohen, L. H. Coudert, V. M. Devi, B. J. Drouin, A. Fayt, J. M. Flaud, R. R. Gamache, J. J. Harrison, J. M. Hartmann, C. Hill, J. T. Hodges, D. Jacquemart, A. Jolly, J. Lamouroux, R. J. Le Roy, G. Li, D. A. Long, O. M. Lyulin, C. J. Mackie, S. T. Massie, S. Mikhailenko, H. S. P. Müller, O. V. Naumenko, A. V. Nikitin, J. Orphal, V. Perevalov, A. Perrin, E. R. Polovtseva, C. Richard, M. A. H. Smith, E. Starikova, K. Sung, S. Tashkun, J. Tennyson, G. C. Toon, V. G. Tyuterev and G. Wagner, J. Quant. Spectrosc. Radiat. Transfer, 2013, 130, 4–50 CrossRef CAS.
- R. Atkinson, D. L. Baulch, R. A. Cox, J. N. Crowley, R. F. Hampson, R. G. Hynes, M. E. Jenkin, M. J. Rossi and J. Troe, Atmos. Chem. Phys., 2006, 6, 3625–4055 CrossRef CAS.
- R. X. Fernandes, K. Luther and J. Troe, J. Phys. Chem. A, 2006, 110, 4442–4449 CrossRef CAS PubMed.
- R. X. Fernandes, K. Luther, G. Marowsky, M. P. Rissanen, R. Timonen and J. Troe, J. Phys. Chem. A, 2015, 119, 7263–7269 CrossRef CAS PubMed.
- C. Fittschen, A. Frenzel, K. Imrik and P. Devolder, Int. J. Chem. Kinet., 1999, 31, 860–866 CrossRef CAS.
- E. Assaf, C. Schoemaecker, L. Vereecken and C. Fittschen, Phys. Chem. Chem. Phys., 2018, 20, 8707 RSC.
- R. Atkinson, D. L. Baulch, R. A. Cox, J. N. Crowley, R. F. Hampson, R. G. Hynes, M. E. Jenkin, M. J. Rossi and J. Troe, Atmos. Chem. Phys., 2004, 4, 1461–1738 CrossRef CAS.
- V. Daele and G. Poulet, J. de Chimie Physique, 1996, 93, 1081–1099 CrossRef CAS.
- R. Atkinson, D. L. Baulch, R. A. Cox, J. N. Crowley, R. F. Hampson, R. G. Hynes, M. E. Jenkin, M. J. Rossi, J. Troe and T. J. Wallington, Atmos. Chem. Phys., 2008, 8, 4141–4496 CrossRef CAS.
- R. Atkinson, D. L. Baulch, R. A. Cox, J. N. Crowley, R. F. Hampson, R. G. Hynes, M. E. Jenkin, M. J. Rossi and J. Troe, Atmos. Chem. Phys. Discuss, 2007, 7, 981–1191 CrossRef CAS.
- E. Delbos, C. Fittschen, H. Hippler, N. Krasteva, M. Olzmann and B. Viskolcz, J. Phys. Chem. A, 2006, 110, 3238–3245 CrossRef CAS PubMed.
- M. M. Maricq, J. J. Szente, E. W. Kaiser and J. Shi, J. Phys. Chem., 1994, 98, 2083–2089 CrossRef CAS.
- E. P. Clifford, J. T. Farrell, J. D. DeSain and C. A. Taatjes, J. Phys. Chem. A, 2000, 104, 11549–11560 CrossRef CAS.
- J. D. DeSain, S. J. Klippenstein, J. A. Miller and C. A. Taatjes, J. Phys. Chem. A, 2003, 107, 4415–4427 CrossRef CAS.
- S. E. Murphy, J. D. Crounse, K. H. Møller, S. P. Rezgui, N. J. Hafeman, J. Park, H. G. Kjaergaard, B. M. Stoltz and P. O. Wennberg, Environ. Sci.: Atmos., 2023, 3, 882–893 CAS.
- A. W. Jasper, S. J. Klippenstein and L. B. Harding, J. Phys. Chem. A, 2007, 111, 8699–8707 CrossRef CAS PubMed.
- S. J. Klippenstein, Y. Georgievskii and L. B. Harding, Phys. Chem. Chem. Phys., 2006, 8, 1133–1147 RSC.
- M. Assali, J. Rakovsky, O. Votava and C. Fittschen, Int. J. Chem. Kinet., 2020, 52, 197–206 CrossRef CAS.
| This journal is © the Owner Societies 2023 |

