Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solvation entropy, enthalpy and free energy prediction using a multi-task deep learning functional in 1D-RISM†
Daniel J.
Fowles
and
David S.
Palmer
 *
*
Department of Pure and Applied Chemistry, University of Strathclyde, Thomas Graham Building, 295 Cathedral Street, Glasgow, Scotland G1 1XL, UK. E-mail: david.palmer@strath.ac.uk
First published on 14th February 2023
Abstract
Simultaneous calculation of entropies, enthalpies and free energies has been a long-standing challenge in computational chemistry, partly because of the difficulty in obtaining estimates of all three properties from a single consistent simulation methodology. This has been particularly true for methods from the Integral Equation Theory of Molecular Liquids such as the Reference Interaction Site Model which have traditionally given large errors in solvation thermodynamics. Recently, we presented pyRISM-CNN, a combination of the 1 Dimensional Reference Interaction Site Model (1D-RISM) solver, pyRISM, with a deep learning based free energy functional, as a method of predicting solvation free energy (SFE). With this approach, a 40-fold improvement in prediction accuracy was delivered for a multi-solvent, multi-temperature dataset when compared to the standard 1D-RISM theory [Fowles et al., Digital Discovery, 2023, 2, 177–188]. Here, we report three further developments to the pyRISM-CNN methodology. Firstly, solvation free energies have been introduced for organic molecular ions in methanol or water solvent systems at 298 K, with errors below 4 kcal mol−1 obtained without the need for corrections or additional descriptors. Secondly, the number of solvents in the training data has been expanded from carbon tetrachloride, water and chloroform to now also include methanol. For neutral solutes, prediction errors nearing or below 1 kcal mol−1 are obtained for each organic solvent system at 298 K and water solvent systems at 273–373 K. Lastly, pyRISM-CNN was successfully applied to the simultaneous prediction of solvation enthalpy, entropy and free energy through a multi-task learning approach, with errors of 1.04, 0.98 and 0.47 kcal mol−1, respectively, for water solvent systems at 298 K.
1 Introduction
State-of-the-art computational methodologies are capable of making accurate predictions of solvation free energy for aqueous systems. They are commonly used in the calculation of pKa,1–3 protein–ligand binding affinities4 and aqueous solubility.5,6 However, the development of computational approaches for determining solvation free energy often focus on aqueous systems, with a lack of progress for organic solvents or non-ambient temperatures. Further, although many methods exist for the prediction of solvation free energy, far fewer exist for the routine prediction of solvation entropy or enthalpy.Methods of simulating the solvated environment for a given system can generally be separated into one of two categories, implicit or explicit solvent models. The most common implicit models treat bulk solvent as a uniform polarisable medium defined by a dielectric constant, and have found extensive use through models such as the solvation model based on solute electron density (SMD)7 and the polarisable continuum model (PCM).8,9 However, implicit models rely on incomplete representations of important molecular level details such as short-ranged solute–solvent interactions. Typically, implicit models only predict for solvation free energy. Several such methods do exist for the routine prediction of other important thermophysical properties however, such as COSMOTherm.10 Fogolari et al. have discussed recent advances in the prediction of solvation thermodynamics,11 and Karplus et al. have proposed a method of estimating the configurational entropy difference between two states,12,13 both of which involve molecular dynamics (MD) simulations and implicit solvent. Explicit solvent models, such as those commonly used with MD, offer a viable alternative to implicit continuum based approaches,14 with which a variety of studies report methods for predicting solvation enthalpy or entropy. Lin et al. proposed a two-phase thermodynamic model for calculating the entropy of molecular fluids from the trajectory of MD simulations,15 and the inhomogeneous solvation theory (IST) has seen increased use for predicting solvation thermodynamics.16 However, the use of explicit solvent models and molecular dynamics simulations come at a far greater cost than their implicit counterparts, and often require time consuming and expensive simulations to model even a modest number of systems.
The reference interaction site model (RISM) is a third approach, capable of calculating solvation dependent thermodynamic parameters at a lower computational cost than explicit models, whilst modelling specific solute–solvent interactions. The RISM theory uses a simplified form of the high-dimensional molecular Ornstein–Zernike (MOZ) equations to model solvent density distribution around a solute molecule through a set of correlation functions, from which two distinct methods have been developed. The most commonly used of these is 3D-RISM, which approximates the MOZ equations by a set of three-dimensional integral equations. With the recent development of several semi-empirical17,18 and theoretical free energy functionals,19,20 3D-RISM has found frequent use as a method to predict SFE.21–25 Solvation enthalpies and entropies can also be obtained through 3D-RISM with the decomposition of solvation free energy into the entropic contribution using temperature derivatives.26 With this method, solvation enthalpies and entropies were reported to within 2.12 and 1.93 kcal mol−1 of experiment, respectively. However, as solvation entropies were extrapolated from calculated free energies, for which state-of-the-art semi-empirical or theoretical free energy functionals are necessary to obtain reasonable agreement to experiment, any errors found within free energy calculations can also be found in the associated solvation entropy and enthalpy. By contrast, the 1D-RISM theory, in which the MOZ equations are approximated as a set of one-dimensional integral equations, is rarely used for quantitative calculations of solvation thermodynamics because it is considered to be too inaccurate in its common form.
Within the RISM framework, solvation free energy predictions are made analytically using one of several available free energy functionals. In 1D-RISM many of these functionals fail to accurately predict the energetic parameters of the chemical system under investigation. These functionals, such as the Hyper-Netted Chain (1D-RISM/HNC) model,27 are too inaccurate for routine use and typically achieve absolute prediction errors above 20 kcal mol−1. Much effort has been put into improving the predictive capabilities of 1D-RISM based functionals for SFE calculations. Some of these improved models, such as the Gaussian Fluctuations (1D-RISM/GF) and Partial Wave models (1D-RISM/PW), can more accurately predict SFE than previous methods.28,29 Although reasonable qualitative agreement with experimental data has been reported, large predictive errors are still commonly observed for many chemical systems.
In previous work, we proposed a method of accurately predicting solvation free energy.30 This method, pyRISM-CNN, combined our in-house 1D-RISM solver, pyRISM,31 with a deep learning based free energy functional and was shown to accurately predict SFE for organic molecules in aqueous solvent at 273–373 K, as well as carbon tetrachloride or chloroform solvent systems at 298 K. Compared to the standard 1D-RISM theory, the pyRISM-CNN functional reduced the predictive error by up to 40-fold, obtaining a prediction accuracy below 1 kcal mol−1 of experiment across each tested solvent. Moving from 1D-RISM calculation to pyRISM-CNN prediction requires minimal additional computational expense as the solvation free energy density (SFED) functions that are used as input to the CNN model can be generated as part of the typical 1D-RISM workflow.
Here, we report three further developments to the pyRISM-CNN methodology. Solvation free energy data at 298 K has been introduced for methanol solvent systems, for a total of four solvents alongside carbon tetrachloride, chloroform and water. Organic molecular ions have also been introduced for water and methanol solvent systems, allowing pyRISM-CNN to predict SFE for both neutral and ionised solutes, either as a combined input or independently. Accurate predictions of SFE can be obtained for both neutral molecules and molecular ions without the need for additional descriptors or corrections. The pyRISM-CNN functional has also been successfully applied to the simultaneous and accurate prediction of solvation enthalpy, entropy and free energy through a multi-task learning approach. By expanding the range of chemical systems for which SFE predictions can be made, as well as enabling the accurate prediction of solvation enthalpy, entropy and free energy, pyRISM-CNN has expanded the potential application for the RISM theory.
2 Theory
2.1 1D-RISM
The details of the general RISM theory have been discussed in depth elsewhere,32 and so only the 1D-RISM theory will be explained here. 1D-RISM uses an approximated one-dimensional form of the molecular Ornstein–Zernike equation with spherically symmetric site–site correlation functions for the modelling of molecular solutions. Both solute and solvent molecules are modelled as sets of spherically symmetric sites, with one site per atom. There are three types of site–site correlation functions that are considered in RISM, each of which varies with site–site separation only: intramolecular correlation functions, total correlation functions and direct correlation functions. The intramolecular correlation functions describe the structure of a given molecule. For two sites within a molecule, s and s', the intramolecular correlation function is written as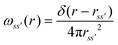 | (1) |
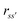 is the distance between sites and
is the distance between sites and 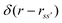 is the Dirac delta function.
is the Dirac delta function.
Intermolecular solute–solvent correlations are defined for each pair of solute and solvent sites by the total correlation functions hsα(r) and direct correlation functions csα(r). Here, s refers to a solute site and α to a solvent site. The total correlation functions are closely related to the radial distribution function (RDF) as
| hsα(r) = gsα(r) − 1 | (2) |
The total and direct correlation functions are related via a set of RISM equations
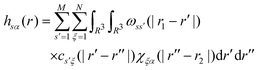 | (3) |
| χξα(r) = ωsolvξα(r) + ρhsolvξα(r) | (4) |
The solvent–solvent site hsolvξα(r) and ωsolvξα(r) are obtained from preliminary solvent–solvent 1D-RISM calculations and molecular structure. To complete the set of RISM equations, closure relations must be introduced
| hsα(r) = exp(−βusα(r) + γsα(r) + Bsα(r)) − 1 | (5) |
The exact bridge functions are typically unknown and so an approximation is needed to solve for the total correlation functions and direct correlation functions. A commonly used closure is the Kovalenko and Hirata (KH) closure33
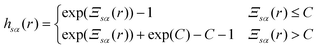 | (6) |
There are multiple expressions available within RISM for determining solvation free energy once the total and direct correlation functions have been solved. The functional is usually selected to be consistent with the closure used within the 1D-RISM calculations. The Gaussian fluctuations approximation (GF),34 KH35 and hypernetted chain (HNC)27 expressions are shown below.
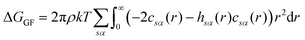 | (7) |
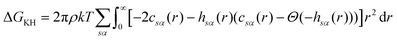 | (8) |
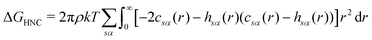 | (9) |
2.2 pyRISM
The pyRISM program31 includes a general method of obtaining variables which contain solvation and desolvation relevant descriptors from the standard 1D-RISM free energy functionals. Previously this method was applied within the RISM-MOL framework and has been described in detail elsewhere,36,37 so only a short summary of the process will be described here. Each of the free energy functionals described in eqn (7)–(9) can be condensed into a generalised form: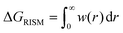 | (10) |
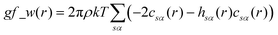 | (11) |
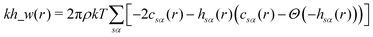 | (12) |
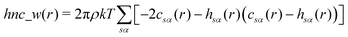 | (13) |
When the 1D-RISM equations are solved, the total and direct correlation functions are represented on a fine grid. The values of the SFED functions at selected grid points provide variables that are denoted as m_w_n, where m is the 1D-RISM free energy functional from which the variable is based and n is the grid point at which the variable is evaluated. Machine learning algorithms are then trained on these variables and the subsequent model can be used for solvation free energy prediction. pyRISM is made freely available as open-source software.
3 Methods
3.1 Dataset preparation
Experimental solvation free energies of small organic molecules were obtained from three different sources: the Minnesota Solvation Database (MSD),38 those developed by Chamberlin et al.39,40 and Zanith et al.41 The MSD contains experimental solvation free energies in water and several organic solvents at 298 K. Experimental data obtained from Chamberlin et al. includes hydration free energies in a 273–373 K temperature range, and data obtained from Zanith et al. includes solvation free energies for small organic molecules in methanol at 298 K.A multi-solvent, multi-temperature dataset of neutral and ionised compounds was prepared from the available experimental solvation free energies. Ionised compounds were obtained exclusively from the MSD and consisted of ionised organic solutes. A total of four solvents made up the neutral portion of the dataset: water, methanol, chloroform and carbon tetrachloride, with 659, 25, 109 and 79 solutes respectively. Of the 659 water solutes, 272 were taken from Chamberlin et al., and included hydration free energy data in a 273–373 K temperature range. By using free energies over a range of temperatures, a total of 3053 datapoints were available. The remaining 387 solutes were taken from the MSD at 298 K, for a total of 3440 datapoints. The ionised portion of the dataset consisted of anions and cations in water and methanol solvents. By solvent, 48 anions and 29 cations were present in methanol, and 56 anions and 47 cations in water.
Experimental solvation enthalpies, entropies and free energies of small organic molecules were taken from several different sources. This second dataset contained a full set of experimental solvation thermodynamic parameters for solute molecules in water at 298 K. Every molecule present in this second dataset can also be found in the solvation free energy dataset. Solvation enthalpies for solutes in water were obtained from the Acree dataset42 and Abraham et al.43 Solvation entropies were taken from Garza.44 Experimental solvation free energies from the MSD were assigned to each molecule. If an experimental solvation enthalpy, entropy and free energy were not all available for a given molecule then a pseudo-experimental value would be calculated from the other two available terms using ΔG = ΔH − TΔS. In total, solvation data was obtained for 139 solutes in water. Where necessary, experimental values were converted to the Ben Naim standard state.45,46Table 1 provides a breakdown of the available experimental data for each solvent. A spreadsheet detailing the available experimental data by source can be found as part of the ESI.†
| Dataset | Solvent | Temperature range | SFE functional | Datapoints |
|---|---|---|---|---|
| ΔGexp,neutralsolv | Carbon tetrachloride | 298 K | KH/HNC/GF | 79 |
| Chloroform | 298 K | 109 | ||
| Methanol | 298 K | 25 | ||
| Water | 273–373 K | 3440 | ||
| ΔGexp,ionisedsolv | Methanol | 298 K | KH/HNC/GF | 77 |
| Water | 298 K | 103 | ||
| ΔHexpsolv, TΔSexpsolv, ΔGexp,neutralsolv | Water | 298 K | KH/HNC/GF | 139 |
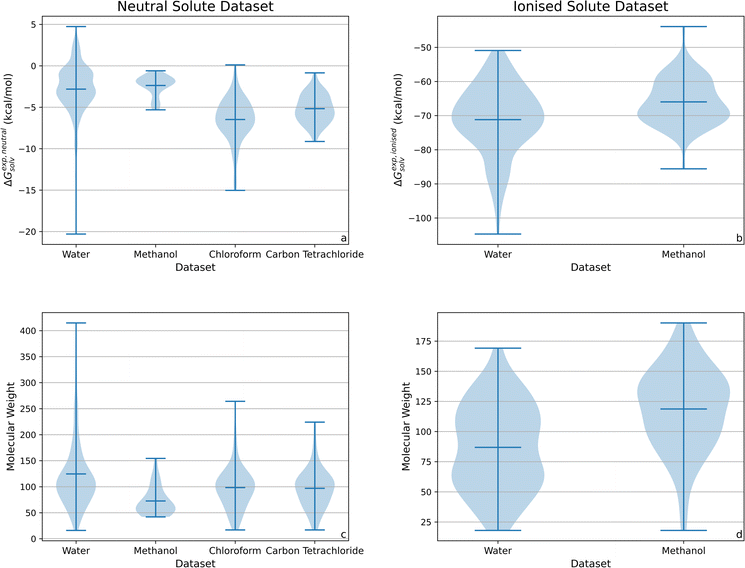
Fig. 1 provides violin plots which show the distribution of experimental solvation free energy and molecular weight by solvent across the neutral and ionised datasets. Additional violin plots of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and the number of rotatable bonds per solute are available in Section S1 of the ESI.† Tables containing the mean and standard deviation (SD) of each experimental property across the neutral and ionised datasets, as well as the mean and SD of experimental solvation enthalpy, entropy and free energy values, can also be found in Section S1 of the ESI.†
P and the number of rotatable bonds per solute are available in Section S1 of the ESI.† Tables containing the mean and standard deviation (SD) of each experimental property across the neutral and ionised datasets, as well as the mean and SD of experimental solvation enthalpy, entropy and free energy values, can also be found in Section S1 of the ESI.†
3.2 Solute structure generation
The InChi47 function within Open Babel48 was used to generate a unique InChi descriptor for each solute within the MSD, Chamberlin et al. and Zanith et al. datasets. Any duplicate molecules were then removed using the “unique” function within Open Babel. Dataset sizes shown in Table 1 refer to solutes present after duplicate molecules were removed.Solute coordinate files were taken from the MSD. The corresponding coordinate files were not available for 10 solute molecules taken from Zanith et al., and so were obtained from the PubChem chemical database49 as a 2D coordinate file. These files were converted to 3D structures and the lowest energy conformer found through a conformational search carried out using Open Babel. The conformational search involved a systematic rotor search of each solute with the GAFF forcefield.50
3.3 1D-RISM calculations
1D-RISM calculations were carried out with pyRISM using the KH closure within a system of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 384 grid points over 20.48 Å from the solute. Aqueous solvent calculations used the dielectrically consistent reference interaction site model (DRISM),51,52 while organic solvent calculations applied the extended reference interaction site model (XRISM).53 Organic solvent calculations were found to converge more consistently with XRISM than with DRISM. Solvation free energy calculations with the KH, HNC and GF free energy functionals were performed for both aqueous and organic solvent systems. For all calculations it was assumed that solute molecules were embedded within an infinitely dilute aqueous solution. A convergence tolerance of 10−12 was set for all calculations, with a minimum tolerance of 10−5 if the initial calculation failed to converge. The impact from lowering the minimum convergence tolerance to 10−5, as well as the choice of model for 1D-RISM calculations (DRISM or XRISM) on the quality of SFED generated has previously been found to be negligible.30
384 grid points over 20.48 Å from the solute. Aqueous solvent calculations used the dielectrically consistent reference interaction site model (DRISM),51,52 while organic solvent calculations applied the extended reference interaction site model (XRISM).53 Organic solvent calculations were found to converge more consistently with XRISM than with DRISM. Solvation free energy calculations with the KH, HNC and GF free energy functionals were performed for both aqueous and organic solvent systems. For all calculations it was assumed that solute molecules were embedded within an infinitely dilute aqueous solution. A convergence tolerance of 10−12 was set for all calculations, with a minimum tolerance of 10−5 if the initial calculation failed to converge. The impact from lowering the minimum convergence tolerance to 10−5, as well as the choice of model for 1D-RISM calculations (DRISM or XRISM) on the quality of SFED generated has previously been found to be negligible.30
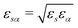 . GAFF50 parameters were assigned to solute molecules using the Antechamber and tLEaP programs within Amber18.
. GAFF50 parameters were assigned to solute molecules using the Antechamber and tLEaP programs within Amber18.
3.4 Obtaining RISM solvation free energy density functions
Solute specific SFED functions were obtained as a 1D-RISM calculation output using the pyRISM program. A SFED function was generated for each free energy functional used, totalling three sets per solute. As the grid used to represent the 1D-RISM total and direct correlation functions was very fine, leading to multiple correlated variables, a coarser grid-spacing was used to obtain a representation of the SFED that was suitable for machine learning. To minimise the inclusion of redundant data and to exclude data at long solute–solvent separations in the region where SFEDs approach zero, only every 40th grid point from r = 0 Å to r = 8 Å was considered. This approach produced 160 SFED descriptors per SFE functional for each solute 1D-RISM calculation. These SFED were then used as an input to machine learning models to predict experimental solvation free energy, enthalpy and entropy.3.5 Convolutional neural network models
Convolutional neural network (CNN) models were trained on all three SFED variations (KH, HNC and GF) for each dataset. Models were validated by nested cross-validation (CV), with hyper parameters tuned by an inner 5-fold CV loop. Final tuned model performance was evaluated by an outer 50-fold Monte Carlo cross-validation loop with a 70% train/30% test split. A stratified sampling approach was taken for multi-temperature and multi-solvent data to ensure datapoints were separated by molecule before splitting into training, validation and test sets. Each variable was centered by subtracting its mean value in the training data, and scaled by dividing by the standard deviation of its values in the training data.Single and multi-output convolutional neural networks were built using the ‘sequential’ and ‘functional’ model packages in Tensorflow59 respectively, and accessed using Keras60 with a Python implementation. Single output CNN were trained on SFED generated from the solvation free energy dataset, and multi-output CNN were trained on SFED generated from the solvation enthalpy, entropy and free energy dataset. The multi-task algorithm considered solvation enthalpy, entropy and free energy with an equal weighting. The CNN training datasets contained enthalpy, entropy and free energy values for each solute without any missing data. Final CNN architecture consisted of three blocks of Conv1D-MaxPooling1D-BatchNormalisation with a subsequent Flatten layer, and was based on the refined CNN architecture applied in our previous work.30 Single output models contained a single Dense output layer while multi-output models had three separate Dense output layers connected to the Flatten layer. Convolutional layers were created using the ‘Conv1D’ layer package in Keras with 32 output filters, a kernal size of 3 and stride length of 2. No padding was included and the rectified linear activation function (ReLu)61 was used. Each of the subsequent layers were also taken from Keras, with the max pool size within MaxPooling1D layers set to 2. Default parameters were used for BatchNormalisation and Flatten layers. The loss function and metric was set to ‘mse’ (mean squared error), with the ‘Adam’ optimiser.62 Each model could run for a maximum of 60 epochs with a patience of 20 epochs included through the Keras ‘EarlyStopping’ callback.
3.6 Statistical analysis
Solvation thermodynamics predictions were evaluated against experimental values of solvation enthalpy, entropy or free energy using the coefficient of determination (R2) and root mean squared deviation (RMSD).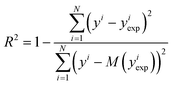 | (14) |
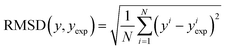 | (15) |
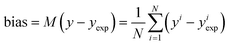 | (16) |
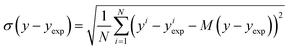 | (17) |
The bias provides the systematic error, while the standard deviation gives the random error that is not explained by the model. The bias and standard deviation are connected to the RMSD by:
| RMSD(y,yexp)2 = M(y − yexp)2 + σ(y − yexp)2 | (18) |
A model which reports an RMSD greater than the standard deviation of the experimental data provides less accurate predictions than the null model provided by the mean of the experimental data.
Statistical analyses were performed in a Python environment using the ‘sklearn.metrics’ module available in scikit-learn.63
4 Results and discussion
4.1 Solvation free energy – neutral & ionised datasets
Convolutional neural network models were trained on datasets of neutral or ionised solutes, separately. For both the neutral and ionised datasets, three variations were tested: KH, HNC and GF generated SFED, for a total of six models. Only models trained on GF based SFED will be discussed here, as it has been previously shown that the choice of free energy functional only marginally effects SFE prediction accuracy.30 Models associated with KH and HNC generated SFED can be found in Section S2 of the ESI.†Table 2 provides a breakdown of the test set based performance for both models trained on GF generated SFED.| ΔGexp,neutralsolv dataset | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neutral solvation free energy | By solvent | Full dataset | ||||||||
| Solvent | Temperature | Datapoints | R 2 | RMSD | Bias | SDEP | R 2 | RMSD | Bias | SDEP |
| Carbon Tetrachloride | 298 K | 79 | 0.63 | 1.00 | 0.69 | 0.68 | 0.93 | 0.99 | 0.28 | 0.93 |
| Chloroform | 298 K | 109 | 0.81 | 1.12 | 0.65 | 0.87 | ||||
| Methanol | 298 K | 25 | 0.42 | 0.73 | 0.16 | 0.64 | ||||
| Water | 273–373 K | 3440 | 0.95 | 0.96 | 0.11 | 0.93 | ||||
| ΔGexp,ionisedsolv dataset | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ionised solvation free energy | By solvent | Full dataset | ||||||||
| Solvent | Temperature | Datapoints | R 2 | RMSD | Bias | SDEP | R 2 | RMSD | Bias | SDEP |
| Methanol | 298 K | 77 | 0.61 | 3.60 | 0.51 | 3.06 | 0.74 | 3.96 | 0.35 | 3.62 |
| Water | 298 K | 103 | 0.73 | 4.10 | 0.21 | 3.71 | ||||
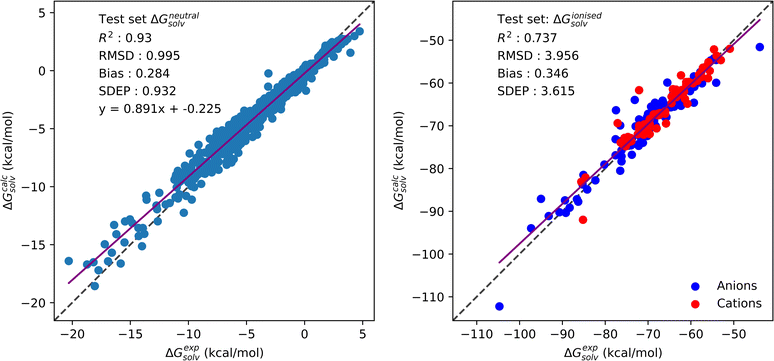
In our previous study of pyRISM-CNN, a CNN model trained on the GF generated multi-solvent, multi-temperature SFED dataset achieved an RMSD of 0.97 kcal mol−1 and R2 of 0.94. This dataset consisted of the carbon tetrachloride, chloroform and water based solute data presented in the neutral SFE section of Table 2 under ‘Solvent’, ‘Temperature’ and ‘Datapoints’. Here, we have expanded that benchmark dataset to include experimental solvation free energies for 25 solute molecules in methanol. As can be seen in Table 2, including this additional solvent does not impact upon the overall model performance, with an RMSD of 0.99 kcal mol−1 and R2 of 0.93. Individually, SFE predictions made for methanol based solutes are no less accurate than for other solvents, with an RMSD of 0.73 kcal mol−1 and R2 of 0.42. The relatively low R2 is likely due to the small number of datapoints available for methanol, which represent a smaller spread of experimental SFE values than are available for the other solvents. The performance of this CNN model, trained on the expanded neutral solute dataset, further shows the generalisability and capabilities of this approach.
Similarly to the neutral solute dataset, a CNN trained on SFED generated from ionised organic solutes is capable of making accurate solvation free energy predictions. From the ionised SFE section of Table 2, the CNN trained on ionised solute data can be seen to accurately predict SFE to within 3.96 kcal mol−1 of experiment. This accuracy is not limited to a single solvent as RMSD of 3.60 and 4.10 kcal mol−1 are achieved for predictions of solutes in methanol and water solvents, respectively. Fig. 2 provides the correlation plots of experimental SFE against predicted values for the neutral and ionised solute datasets. Ionised solute predictions are colour coded as anions and cations.
Solvation free energy predictions made with pyRISM-CNN are of comparable accuracy to the current state-of-the-art of the more computationally expensive 3D-RISM based methods. The semi empirical universal correction (UC) free energy functional paired with 3D-RISM has been shown to accurately predict hydration free energies for neutral and ionised solutes. Labute et al. calculated HFE values for a dataset of 504 neutral organic molecules, obtaining an RMSD of 1.18 kcal mol−1.17 A similar approach has been proposed by Casillas et al.,64 in which an RMSD of 1.44 kcal mol−1 was achieved for 642 molecules from the Freesolv database. Sumi et al. performed hydration free energy calculations on an earlier version of the Freesolv database using the reference-modified density functional formulation, with which an RMSD of 1.46 kcal mol−1 was reached.65 The authors also compared their approach against 3D-RISM/NgB and molecular DFT calculations performed on the same dataset, which managed RMSD of 1.29 and 1.80 kcal mol−1, respectively. Tielker et al. performed HFE calculations on neutral and ionised organic solutes obtained from the MSD using the embedded cluster RISM theory (EC-RISM).66 By including multiple solvent-specific empirical parameters, 3D-RISM calculated hydration free energies with an RMSD of 1.52, 4.48 and 2.91 kcal mol−1 were obtained for neutral, anionic and cationic solutes, respectively. Fewer studies have reported hydration free energy predictions for ionised datasets. Misin et al. reported HFE calculations for molecular ions involving 3D-RISM/PC+ and a correction for the Galvani potential, with an RMSD of 4.84 kcal mol−1.23 Johnson et al. treated 48 molecular ions in water with a variety of free energy functionals in 3D-RISM without a Galvani correction, achieving an RMSD of 6.51 kcal mol−1 with 3D-RISM/UC.26
4.2 Solvation free energy – combined dataset
Convolutional neural network models were trained on a combined dataset consisting of neutral and ionised solute SFED. In total, three separate models were tested with SFED generated from each free energy functional: KH, HNC and GF. Only models trained on GF based SFED will be discussed here. Models associated with KH and HNC generated SFED can be found in Section S3 of the ESI.†Compared to the individual neutral and ionised datasets, use of a combined dataset results in only a small reduction in SFE prediction accuracy across solvents and neutral/ionised solutes. A breakdown of solvation free energy predictions for the combined dataset can be found in Table 3. By solvent, neutral solute RMSD increases by 0.23, 0.22 and 0.51 kcal mol−1 for chloroform, methanol and water, respectively, when compared against CNN trained on the individual neutral dataset. For carbon tetrachloride, RMSD decreases by 0.02 kcal mol−1. Comparing ionised predictions, a CNN trained on the individual ionised dataset performed better than their combined dataset counterpart, with prediction errors increasing by 0.67 and 1.70 kcal mol−1 for methanol and water, respectively. A drop in SFE prediction accuracy is not unexpected for several reasons: experimental solvation free energies for ionised organic solutes are typically ten times greater than their neutral counterparts, and neutral solute data outnumbers ionised solute data by 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Fig. 3 shows the correlation plot of experimental SFE against predicted values for the combined neutral and ionised solute dataset.
1. Fig. 3 shows the correlation plot of experimental SFE against predicted values for the combined neutral and ionised solute dataset.
| ΔGexp,neutralsolv, ΔGexp,ionisedsolv dataset | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Solvent | Temperature | Datapoints | By solvent | Full dataset | ||||||
| R 2 | RMSD | Bias | SDEP | R 2 | RMSD | Bias | SDEP | |||
| Neutral solvation free energy | ||||||||||
| Carbon Tetrachloride | 298 K | 79 | 0.68 | 0.98 | 0.31 | 0.93 | 0.99 | 3.03 | 0.09 | 2.97 |
| Chloroform | 298 K | 109 | 0.74 | 1.35 | 0.29 | 1.32 | ||||
| Methanol | 298 K | 25 | 0.40 | 0.95 | −0.27 | 0.91 | ||||
| Water | 273–373 K | 3440 | 0.88 | 1.47 | −0.20 | 1.45 | ||||
| Ionised solvation free energy | ||||||||||
| Methanol | 298 K | 77 | 0.52 | 4.27 | 0.40 | 3.83 | ||||
| Water | 298 K | 103 | 0.59 | 5.80 | 0.48 | 5.55 | ||||
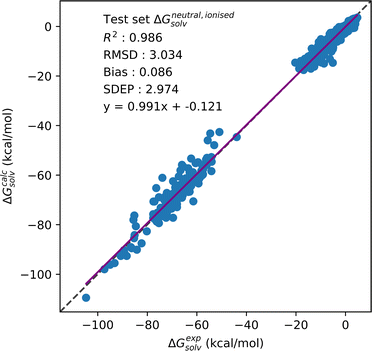 | ||
| Fig. 3 Correlation plot showing solvation free energy predictions made with the combined neutral and ionised dataset. SFED were generated using the GF functional. | ||
4.3 Solvation enthalpy, entropy & free energy
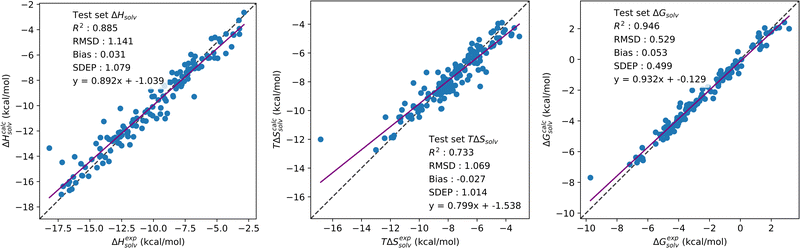
Multi-task CNN models were trained on the solvation thermodynamics dataset consisting of neutral organic solutes with experimental solvation enthalpy, entropy and free energy values. In total, three separate models were tested with SFED generated from each free energy functional: KH, HNC and GF. Single task CNN models were also trained on individual solvation enthalpy, entropy and free energy datasets across the KH, HNC and GF functionals, for a total of 9 single task models. Each single task model performed comparably to the multi-task CNN across each solvation property, with all of the statistics available in Section S5 of the ESI.† Correlation plots comparing pseudo-calculated values for solvation enthalpy, entropy and free energy parameters from single and multi-output models are available in Section S6 of the ESI.† Pseudo-calculated values are those determined using ΔG = ΔH − TΔS and the predicted values for the two corresponding parameters. From these plots, it can be noted that multi-output CNN better learn the correlation between each solvation parameter than single output models.Solvation enthalpy, entropy and free energy predictions from multi-output CNN trained on SFED generated from the KH, HNC or GF free energy functional can be found in Table 4. Across each free energy functional, solvation enthalpy, entropy and free energy predictions are made with accuracies nearing or below 1 kcal mol−1. By functional, RMSD of 1.06, 1.04 and 1.14 kcal mol−1 are obtained for solvation enthalpy predictions with KH, HNC and GF respectively. Similar values of 1.00, 0.98 and 1.07 kcal mol−1 are obtained for solvation entropy. Solvation free energy predictions remain the most accurate and closely resemble accuracies obtained with single task CNN, with RMSD of 0.53, 0.47 and 0.53 kcal mol−1. These results suggest that use of a multi-task algorithm allows for the accurate prediction of all three properties simultaneously, as can be seen by comparing Tables 2–4. Fig. 4 provides the correlation plots of experimental solvation enthalpy, entropy and free energy against predicted values for the multi-task CNN trained on GF generated SFED.
| Multi-output CNN – ΔHexpsolv, TΔSexpsolv, ΔGexp,neutralsolv dataset | |||||
|---|---|---|---|---|---|
| Functional | Parameter | R 2 | RMSD | Bias | SDEP |
| KH | Solvation enthalpy | 0.90 | 1.06 | 0.14 | 1.00 |
| Solvation entropy | 0.77 | 1.00 | 0.10 | 0.95 | |
| Solvation free energy | 0.94 | 0.53 | 0.07 | 0.50 | |
| HNC | Solvation enthalpy | 0.90 | 1.04 | 0.06 | 1.01 |
| Solvation entropy | 0.79 | 0.98 | 0.03 | 0.95 | |
| Solvation free energy | 0.95 | 0.47 | 0.04 | 0.45 | |
| GF | Solvation enthalpy | 0.89 | 1.14 | 0.03 | 1.08 |
| Solvation entropy | 0.73 | 1.07 | −0.03 | 1.01 | |
| Solvation free energy | 0.95 | 0.53 | 0.05 | 0.50 | |
Several methods have been reported for the prediction of solvation enthalpy and entropy, although few report predictions for both alongside solvation free energy. MD based free energy perturbation (FEP) calculations have been reported for a dataset of 239 neutral small molecules in water, achieving an average unsigned error (AUE) of 1.10 kcal mol−1.67 Comparisons can also be made against the SMD, which has been tested extensively against both aqueous and organic solvents at 298 K with which to calculate SFE for neutral and ionised solutes.7 By solvent, AUE of 0.52, 0.84 and 0.59 kcal mol−1 were reported for neutral solutes in carbon tetrachloride, chloroform and water respectively, and AUE of 2.47 and 4.40 kcal mol−1 were also reported for ionised solutes in methanol and water. Although not directly comparable, here with GF based pyRISM-CNN models, RMSD values of 1.00, 1.12 and 0.96 kcal mol−1 were made for neutral solutes in carbon tetrachloride, chloroform and water, and 3.60 and 4.10 kcal mol−1 for ionised solutes in methanol and water. Jaquis et al. reported solvation enthalpy predictions for ethanol solvent systems using a deep learning feedforward neural network and chemistry development kit (CDK) descriptors, with a test set RMSD of 1.58 kcal mol−1.68 Similarly, Chung et al. developed a deep learning neural network model for the prediction of solvation enthalpy and free energy, reporting an RMSD of 0.75 and 0.71 kcal mol−1 respectively.69 Irudayam et al. reported an MD based method of calculating the hydration entropy from individual entropic components, alongside solvation free energy.70 With this method, hydration free energies are calculated with a mean unsigned error of 2.5 kJ mol−1. The authors reported that solvation entropies, and enthalpies by extension using ΔG = ΔH − TΔS, are typically underestimated, and conclude that forcefield based methods are unable to account for the temperature dependence of solvation. Hydration free energies and hydration enthalpies/entropies were reported by Johnson et al. for a dataset of 1123 and subset of 74 molecules, respectively, calculated with the 3D-RISM theory and PC+ free energy functional.26 Across solvation free energy, enthalpy and entropy, RMSD of 1.43, 2.12 and 1.93 kcal mol−1 were obtained respectively. However, empirical corrections to the standard 3D-RISM theory were necessary to obtain values with reasonable agreement to experiment.
This multi-task approach can be readily extended to organic solvent systems alongside the aqueous systems tested here. There are, however, limited samples available with which to train machine learning models. Increasing the availability of high quality experimental data in the literature would help to resolve this problem.
5 Conclusions
Previously, we presented a new method for accurately predicting solvation free energy, pyRISM-CNN, by combining the 1D-RISM solver, pyRISM, with a deep learning based free energy functional. With this deep learning approach, solvation free energy predictions could be made to within 1 kcal mol−1 of experiment across several different solvents and at temperatures beyond 298 K. These accuracies marked a 40-fold improvement in prediction accuracy when compared to the standard 1D-RISM theory. Here, we have reported several developments to the pyRISM-CNN methodology: enabling the prediction of SFE for organic molecular ions, to within 4 kcal mol−1 of experiment; introduced methanol as a solvent with which to train the pyRISM-CNN functional to predict solvation free energy; and successfully expanded pyRISM-CNN to a multi-task algorithm with the accurate and simultaneous prediction of solvation enthalpy, entropy and free energy for water solvent systems at 298 K. With this multi-task learning approach, three thermodynamic parameters fundamental to solvation processes can be accurately obtained without the need for extensive sampling, and can be determined as part of a standard 1D-RISM calculation with minimal additional computational expenditure.Author contributions
Conceptualization: DJF, DSP. Methodology: DJF, DSP. Software: DJF. Validation/verification: DJF, DSP. Formal analysis: DJF. Investigation: DJF. Resources: DSP. Data curation: DJF. Writing – original draft: DJF. Writing – review & editing: DJF, DSP. Visualization: DJF. Supervision: DSP. Project administration: DSP. Funding acquisition: DSP.Data availability
The pyRISM v0.1.1 code for solving 1D RISM equations and computing solvation free energy density functions can be found as freely available and open-source software at https://zenodo.org/record/7107645. Future versions of this software will be released at https://github.com/2AUK/pyRISM. Software and datasets used to develop the pyRISM-CNN models have been uploaded as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
D. J. F. and D. S. P. thank the EPSRC for funding via Prosperity Partnership EP/S035990/1. The authors thank the ARCHIE-WeSt High-Performance Computing Centre (http://www.archie-west.ac.uk) for computational resources.References
- L. Xu and M. L. Coote, J. Phys. Chem. A, 2019, 123, 7430–7438 CrossRef CAS PubMed.
- M. S. Bodnarchuk, D. M. Heyes, D. Dini, S. Chahine and S. Edwards, J. Chem. Theory Comput., 2014, 10, 2537–2545 CrossRef CAS PubMed.
- F. R. Dutra, C. de Souza Silva and R. Custodio, J. Phys. Chem. A, 2021, 125, 65–73 CrossRef CAS PubMed.
- S. Genheden, T. Luchko, S. Gusarov, A. Kovalenko and U. Ryde, J. Phys. Chem. B, 2010, 114, 8505–8516 CrossRef CAS PubMed.
- D. S. Palmer, J. L. McDonagh, J. B. O. Mitchell, T. van Mourik and M. V. Fedorov, J. Chem. Theory Comput., 2012, 8, 3322–3337 CrossRef CAS PubMed.
- D. J. Fowles, D. S. Palmer, R. Guo, S. L. Price and J. B. O. Mitchell, J. Chem. Theory Comput., 2021, 17, 3700–3709 CrossRef CAS PubMed.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef CAS PubMed.
- J. Tomasi, B. Mennucci and E. Cances, J. Mol. Struct., 1999, 464, 211–226 CrossRef CAS.
- S.-T. Lin and C.-M. Hsieh, J. Chem. Phys., 2005, 125, 124103 CrossRef PubMed.
- J. E. Bara, J. D. Moon, K. R. Reclusado and J. W. Whitley, Ind. Eng. Chem. Res., 2013, 52, 5498–5506 CrossRef CAS.
- F. Fogolari, A. Corazza and G. Esposito, Front. Mol. Biosci., 2018, 5, 11 CrossRef PubMed.
- M. Karplus, T. Ichiye and B. M. Pettitt, Biophys. J., 1987, 52, 1083–1085 CrossRef CAS PubMed.
- V. Ovchinnikov, M. Cecchini and M. Karplus, J. Phys. Chem. B, 2013, 117, 750–762 CrossRef CAS PubMed.
- D. L. Mobley, C. I. Bayly, M. D. Cooper, M. R. Shirts and K. A. Dill, J. Chem. Theory Comput., 2009, 5, 350–358 CrossRef CAS PubMed.
- S.-T. Lin, P. K. Maiti and W. A. G. III, J. Phys. Chem. B, 2010, 114, 8191–8198 CrossRef CAS PubMed.
- F. Waibl, J. Kraml, M. L. Fernández-Quintero, J. R. Loefer and K. R. Liedl, J. Comput.-Aided Mol. Des., 2022, 36, 101–116 CrossRef CAS PubMed.
- J.-F. Truchon, B. M. Pettitt and P. Labute, J. Chem. Theory Comput., 2014, 10, 934–941 CrossRef CAS PubMed.
- D. S. Palmer, A. I. Frolov, E. L. Ratkova and M. V. Fedorov, J. Phys.: Condens. Matter, 2010, 22, 492101 CrossRef PubMed.
- V. Sergiievskyi, G. Jeanmairet, M. Levesque and D. Borgis, J. Chem. Phys., 2015, 143, 184116 CrossRef PubMed.
- M. Misin, M. V. Fedorov and D. S. Palmer, J. Chem. Phys., 2015, 142, 091105 CrossRef PubMed.
- S. Tanimoto, N. Yoshida, T. Yamaguchi, S. L. Ten-no and H. Nakano, J. Chem. Inf. Model., 2019, 59, 3770–3781 CrossRef CAS PubMed.
- D. Roy and A. Kovalenko, J. Phys. Chem. A, 2019, 123, 4087–4093 CrossRef CAS PubMed.
- M. Misin, M. V. Fedorov and D. S. Palmer, J. Phys. Chem. B, 2016, 120, 975–983 CrossRef CAS PubMed.
- M. Misin, P. A. Vainikka, M. V. Fedorov and D. S. Palmer, J. Phys. Chem., 2016, 145, 194501 CrossRef PubMed.
- M. Misin, M. V. Fedorov and D. S. Palmer, J. Phys. Chem. B, 2016, 120, 975–983 CrossRef CAS PubMed.
- J. Johnson, D. A. Case, T. Yamazaki, S. Gusarov, A. Kovalenko and T. Luchko, J. Phys.: Condens. Matter, 2016, 28, 344002 CrossRef CAS PubMed.
- S. J. Singer and D. Chandler, Mol. Phys., 1985, 55, 621–625 CrossRef CAS.
- K. Sato, H. Chuman and S. Ten-no, J. Phys. Chem. B, 2005, 109, 17290–17295 CrossRef CAS PubMed.
- S. Ten-no, J. Jung, H. Chuman and Y. Kawashima, Mol. Phys., 2010, 108, 327–336 CrossRef CAS.
- D. J. Fowles, R. G. McHardy, A. Ahmad and D. S. Palmer, Digital Discovery, 2023, 2, 177–188 RSC.
- A. Ahmad, 2AUK/pyRISM: v0.1.1, 2022 DOI:10.5281/zenodo.7107645.
- E. L. Ratkova, D. S. Palmer and M. V. Fedorov, Chem. Rev., 2015, 115, 6312–6356 CrossRef CAS PubMed.
- A. Kovalenko and F. Hirata, J. Phys. Chem. B, 1999, 103, 7942–7957 CrossRef CAS.
- S. Ten-no, J. Chem. Phys., 2001, 115, 3724–3731 CrossRef CAS.
- F. Hirata, Molecular Theory of Solvation, Springer Dordrecht, Dordrecht, The Netherlands, 1st edn, 2003 Search PubMed.
- V. P. Sergiievskyi, W. Hackbusch and M. V. Fedorov, J. Comput. Chem., 2011, 32, 1982–1992 CrossRef CAS PubMed.
- D. S. Palmer, M. Misin, M. V. Fedorov and A. Llinas, Mol. Pharmaceutics, 2015, 12, 3420–3432 CrossRef CAS PubMed.
- A. V. Marenich, C. P. Kelly, J. D. Thompson, G. D. Hawkins, C. C. Chambers, D. J. Giesen, P. Winget, C. J. Cramer and D. G. Truhlar, Minnesota Solvation Database – version 2012, University of Minnesota, Minneapolis, 2012 Search PubMed.
- A. C. Chamberlin, C. J. Cramer and D. G. Truhlar, J. Chem. Phys. B, 2006, 110, 5665–5675 CrossRef CAS PubMed.
- A. C. Chamberlin, C. J. Cramer and D. G. Truhlar, J. Chem. Phys. B, 2008, 112, 3024–3039 CrossRef CAS PubMed.
- C. C. Zanith and J. R. P. Jr., J. Comput.-Aided Mol. Des., 2015, 29, 217–224 CrossRef CAS PubMed.
- A. Lang, Acree Enthalpy of Solvation Dataset, 2015 DOI:10.6084/m9.figshare.1572326.v1, Figshare.
- C. Mintz, M. Clark, W. E. A. Jr. and M. H. Abraham, J. Chem. Inf. Model., 2007, 47, 115–121 CrossRef CAS PubMed.
- A. J. Garza, J. Chem. Thoery Comput., 2019, 15, 3204–3214 CrossRef CAS PubMed.
- A. Ben-Naim, J. Phys. Chem., 1978, 82, 792–803 CrossRef CAS.
- A. Ben-Naim and Y. Marcus, J. Chem. Phys., 1984, 81, 2016–2027 CrossRef CAS.
- S. R. Heller, A. McNaught, I. Pletnev, S. Stein and D. Tchekhovskoi, J. Cheminform, 2015, 7, 23 CrossRef PubMed.
- N. M. O'Boyle, M. Banck, C. A. James, C. Morley, T. Vandermeersch and G. R. Hutchison, J. Cheminf., 2011, 3, 33 Search PubMed.
- S. Kim, J. Chen, T. Cheng, A. Gindulyte, J. He, S. He, Q. Li, B. A. Shoemaker, P. A. Thiessen, B. Yu, L. Zaslavsky, J. Zhang and E. E. Bolton, Nucleic Acids Res., 2020, 49, D1388–D1395 CrossRef PubMed.
- J. Wang, R. M. Wolf, J. W. Caldwell, P. A. Kollman and D. A. Case, J. Comput. Chem., 2004, 25, 1157–1174 CrossRef CAS PubMed.
- J. Perkyns and B. M. Pettitt, J. Chem. Phys., 1992, 97, 7656–7666 CrossRef CAS.
- J. Perkyns and B. M. Pettitt, Chem. Phys. Lett., 1992, 190, 626–630 CrossRef CAS.
- P. H. Lee and G. Maggiora, J. Phys. Chem., 1993, 97, 10175–10185 CrossRef CAS.
- L. Lue and D. Blankschtein, J. Phys. Chem., 1992, 96, 8582–8594 CrossRef CAS.
- F. Hirata, P. J. Rossky and B. M. Pettitt, J. Chem. Phys., 1983, 78, 4133 CrossRef CAS.
- G. N. Chuev, M. V. Fedorov and J. Crain, Chem. Phys. Lett., 2007, 448, 198–202 CrossRef CAS.
- H. A. D. A. Case, K. Belfon, I. Ben-Shalom, J. Berryman, S. Brozell, D. Cerutti, T. Cheatham, G. C. III, V. Cruzeiro, T. Darden, R. Duke, G. Giambasu, M. Gilson, H. Gohlke, A. Goetz, R. Harris, S. Izadi, S. Izmailov, K. Kasavajhala, M. Kaymak, E. King, A. Kovalenko, T. Kurtzman, T. Lee, S. LeGrand, P. Li, C. Lin, J. Liu, T. Luchko, R. Luo, M. Machado, V. Man, M. Manathunga, K. Merz, Y. Miao, O. Mikhailovskii, G. Monard, H. Nguyen, K. O'Hearn, A. Onufriev, F. Pan, S. Pantano, R. Qi, A. Rahnamoun, D. Roe, A. Roitberg, C. Sagui, S. Schott-Verdugo, A. Shajan, J. Shen, C. Simmerling, N. Skrynnikov, J. Smith, J. Swails, R. Walker, J. Wang, J. Wang, H. Wei, R. Wolf, X. Wu, Y. Xiong, Y. Xue, D. York, S. Zhao and P. Kollman, Amber 2018, 2018, http://ambermd.org/ Search PubMed.
- M. P. Allen and D. J. Tildesley, Computer Simulation of Liquids, Clarendon Press, Oxford, 1989 Search PubMed.
- M. Abadi, A. Agarwal, P. Barham, E. Brevdo, Z. Chen, C. Citro, G. S. Corrado, A. Davis, J. Dean, M. Devin, S. Ghemawat, I. Goodfellow, A. Harp, G. Irving, M. Isard, Y. Jia, R. Jozefowicz, L. Kaiser, M. Kudlur, J. Levenberg, D. Mané, R. Monga, S. Moore, D. Murray, C. Olah, M. Schuster, J. Shlens, B. Steiner, I. Sutskever, K. Talwar, P. Tucker, V. Vanhoucke, V. Vasudevan, F. Viégas, O. Vinyals, P. Warden, M. Wattenberg, M. Wicke, Y. Yu and X. Zheng, TensorFlow: Large-Scale Machine Learning on Heterogeneous Systems, 2015, https://www.tensorflow.org/, Software available from tensorflow.org Search PubMed.
- F. Chollet et al. , Keras, 2015, https://github.com/fchollet/keras.
- A. F. Agarap, Deep learning using rectified linear units (relu), 2018 DOI:10.48550/arXiv.1803.08375, arXiv.
- D. Kingma and J. Ba, Adam: A Method for Stochastic Optimization, 2014 DOI:10.48550/arXiv.1412.6980, arXiv.
- F. Pedregosa, G. Varoquaux, A. Gramfort, V. Michel, B. Thirion, O. Grisel, M. Blondel, P. Prettenhofer, R. Weiss, V. Dubourg, J. Vanderplas, A. Passos, D. Cournapeau, M. Brucher, M. Perrot and E. Duchesnay, JMLR, 2011, 12, 2825–2830 Search PubMed.
- L. Casillas, V. M. Grigorian and T. Luchko, Molecules, 2023, 28, 925 CrossRef PubMed.
- T. Sumi, A. Mitsutake and Y. Maruyama, J. Comput. Chem., 2015, 36, 1359–1369 CrossRef CAS PubMed.
- N. Tielker, D. Tomazic, J. Heil, T. Kloss, S. Ehrhart, S. Gussregen, K. F. Schmidt and S. M. Kast, J. Comput.-Aided Mol. Des., 2016, 30, 1035–1044 CrossRef CAS PubMed.
- D. Shivakumar, J. Williams, Y. Wu, W. Damm, J. Shelley and W. Sherman, J. Chem. Theory Comput., 2010, 6, 1509–1519 CrossRef CAS PubMed.
- B. J. Jaquis, A. Li, N. D. Monnier, R. G. Sisk, W. E. A. Jr. and A. S. I. D. Lang, J. Solut. Chem., 2019, 48, 564–573 CrossRef CAS.
- Y. Chung, F. H. Vermeire, H. Wu, P. J. Walker, M. H. Abraham and W. H. Green, J. Chem. Inf. Model., 2022, 62, 433–446 CrossRef CAS PubMed.
- S. J. Irudayam, R. D. Plumb and R. H. Henchman, Faraday Discuss., 2009, 145, 467–485 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cp00199g |
| This journal is © the Owner Societies 2023 |