One-step fragmentation of a 2D MXene across the fine 1D MnO2 surface and its supercapacitance
Niraj
Kumar
 *a,
V.
Gajraj
b,
Sanjay
Upadhyay
*a,
V.
Gajraj
b,
Sanjay
Upadhyay
 *a,
Chetana
S.
a,
Sanjay
Sankaranarayanan
*a,
Chetana
S.
a,
Sanjay
Sankaranarayanan
 c,
Ismail
Hossain
d,
Naveen Chandra
Joshi
a,
Neeraj
Priyadarshi
e and
Arijit
Sen
c,
Ismail
Hossain
d,
Naveen Chandra
Joshi
a,
Neeraj
Priyadarshi
e and
Arijit
Sen
 *f
*f
aDivision of Research & Innovation, Uttaranchal University, Dehradun 248007, Uttarakhand, India. E-mail: nirajunisci2k@gmail.com; bannudsanjay@gmail.com
bSection of Chemistry for Technologies (ChemTech), Department of Industrial Engineering, University of Padova, Via Marzolo 9, I-35131 Padova (P.D.), Italy
cDepartment of Electronics and Communication Engineering, Koneru Lakshmaiah Education Foundation, Hyderabad, 500 075, Telangana, India
dSchool of Natural Sciences and Mathematics, Ural Federal University, Yekaterinburg, 620000, Russia
eDepartment of Electrical Engineering, JIS College of Engineering, Kolkata 741235, India
fDepartment of Physics & Nanotechnology, SRM Institute of Science & Technology, Katttankulathur-603203, India. E-mail: arijits@srmist.edu.in
First published on 17th November 2022
Abstract
Functional materials are being studied for their promising applications. Here, for the first time, a novel approach is highlighted to bring down the morphologies of MXene into small fragments with the aid of finer one-dimensional (1D)/nanorods of MnO2. This unique grown morphology was characterized by high-resolution transmission electron microscopy (HRTEM), scanning electron microscopy (SEM), X-ray diffraction spectroscopy (XRD) and X-ray photoelectron spectroscopy (XPS). The BET surface area showed an enhancement in surface area from 39 to 201 m2 g−1 on incorporating 1D MnO2 with MXene. Morphological tension as developed between MnO2 and the MXene surface helped in the considerable improvement of the supercapacitive behaviour of MnO2. An increase of 92.4% in the capacitive behaviour of MnO2 was observed with 818.5 F g−1 at 3 A g−1. Electrochemical device characterization was undertaken to achieve a promising energy density of 77.2 W h kg−1 at 1725 W kg−1. Favourable stability retention of 192.3% in a 3-electrode system and stable performance with 80% retention in a 2 electrode system were achieved after 5000 cycles of galvanostatic charge–discharge. The hydrothermal growth process of (1D) MnO2 is quite effective in bringing MXenes down to fragments, thereby enhancing their overall activity for showcasing one of the best supercapacitive behaviours.
1. Introduction
Novel functional materials have attracted considerable attention for their considerable changes in physical and chemical behaviours. These nanomaterials have bright prospects for developing efficient renewable energy systems. Future renewable energy systems will benefit greatly from energy storage devices as they can compensate for the intermittent nature of renewable energy.1–2 Owing to their high-power density, long cycle life, and fast charge/discharge characteristics and long cycle life, supercapacitors are considered to be an important class of energy storage devices.3–4 Despite this, their practical applications are limited due to their lower energy density values than batteries.5–6 Thus, to meet the high power and energy density requirements of next-generation energy storage devices, significant research efforts have been devoted to the design and development of high-energy-density supercapacitors.7–9 To achieve this, new electrode materials with higher energy densities must be developed without compromising cycling stability or power density. Among the various transition metal oxides investigated for supercapacitors, MnO2 has attracted great interest due to its low cost and toxicity, friendly interfacial properties with carbon materials, high theoretical super capacitance and natural abundance.10–12 Further studies have revealed that the usage of hybrid electrode materials with high specific capacitances could result in higher energy densities.4–5,13–15 Hybrid composite electrodes can be prepared by dispersing/inserting a supercapacitor material like a transition metal oxide/conducting polymer over carbon.16–19MXenes, a family of two-dimensional early transition metal carbides, are emerging as innovative electrode materials due to their superior properties, such as high in-plane electrical conductivity, large surface areas, and metallic conductivity on their hydrophilic surfaces.20–24 Gogotsi et al. have demonstrated that MXenes can act as an intercalation electrode for a wide variety of cations, such as Na+, Li+, K+, Mg2+, Al3+ and NH4+.25–27 By adapting post-etching annealing conditions or by coating MXene sheets with metal ions, the electrochemical energy storage properties of MXenes can be altered.28 Electrodes made of MXenes by conventional routes possess lower gravimetric specific capacitances than those made of graphene.29–30 This technical constraint can be overcome by surface coating the MXene with supercapacitive materials, which will significantly improve its electrochemical energy storage performance. To the best of our knowledge, MnO2/MXene hybrid materials have not been studied extensively for their electrochemical energy storage properties.
In the present work, we have tried to increase the supercapacitive behaviour of MnO2 by incorporating it with MXenes. We have done this by modifying our previously reported hydrothermal synthesis technique to grow fine 1D MnO2.31–40 Herein, we report for the first time that heavy clustered sheets of MXenes can be dispersed into small fragments by force generated during the growth process of fine 1D MnO2 to develop a superior functional material. The resulting hybrid material is one of the best electrochemical super capacitances due to the novel surface enhancement of 1D MnO2. Standard characterization techniques have been employed to study its morphology, structure and growth mechanism.
2. Experimental
2.1. Synthesis
First, 1 g of Ti3AlC2 MAX phase powder was mixed with a solution of 40 wt% hydrofluoric acid (HF) with continuous stirring under normal conditions. The stirring was continued for 5 days, followed by ultrasonication. The supernatant was removed, and the resulting precipitate was washed several times with ethanol and distilled water. The process was repeated and finally, Ti3C2 MXene powder was obtained after drying. Then, a known weight of KMnO4 (potassium permanganate) was mixed into 37 ml of distilled water under continuous magnetic stirring and 10 wt% of the as-prepared MXene powder was added to the solution. NaNO2 (sodium nitrite) was added to make the molar ratio between KMnO4 and NaNO2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Finally, under continuous stirring, 3 ml of a 0.3 M H2SO4 solution was slowly added dropwise and the solution was then treated hydrothermally at 170 °C for 12 h to obtain the MnO2/MXene nanocomposite after properly washing and drying the resulting precipitate. For a comparative analysis, the same procedure was repeated in the absence of MXene to obtain fine one-dimensional MnO2 structures.
3. Finally, under continuous stirring, 3 ml of a 0.3 M H2SO4 solution was slowly added dropwise and the solution was then treated hydrothermally at 170 °C for 12 h to obtain the MnO2/MXene nanocomposite after properly washing and drying the resulting precipitate. For a comparative analysis, the same procedure was repeated in the absence of MXene to obtain fine one-dimensional MnO2 structures.
2.2. Fabrication of the working electrode
In the preparation of the working electrode, super p carbon, polyvinylidene fluoride (PVDF) and the as-prepared material were taken at a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, respectively. N-Methyl-2-pyrrolidone (NMP) was added to make a slurry out of these materials after grinding. The slurry was easily coated over a 1 cm2 surface area of 0.05 mm thick stainless steel plate to be used as a working electrode after drying. The test was followed in 1 M KOH electrolyte with a mass of the active material of 1 mg. In contrast to a 3-electrode system, the electrode size was cut down to half of its length for a 2-electrode system.
8, respectively. N-Methyl-2-pyrrolidone (NMP) was added to make a slurry out of these materials after grinding. The slurry was easily coated over a 1 cm2 surface area of 0.05 mm thick stainless steel plate to be used as a working electrode after drying. The test was followed in 1 M KOH electrolyte with a mass of the active material of 1 mg. In contrast to a 3-electrode system, the electrode size was cut down to half of its length for a 2-electrode system.
3. Results and discussions
3.1. XRD
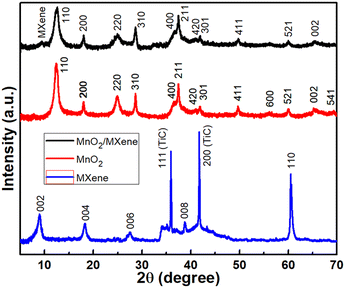
Fig. 1 shows the XRD patterns of all the prepared samples. The MXene sample exhibited peaks at 2θ values of about 9.0, 18.3, 27.5, 38.7, 41.6 and 60.4, attributed to the Ti3C2 phase with miller indices of (002), (004), (006), (008) and (110).41–45 In addition, peaks at 2θ values of 35.9 and 41.6 were attributed to TiC with miller indices of (111) and (200). XRD measurements of MnO2 exhibited peaks at 2θ values of 12.3, 17.9, 24.8, 28.5, 36.6, 37.5, 41.0, 41.82, 49.6, 56.1, 60.0, 65.2, 69.4 and 72.7 degrees. According to JCPDS 44-0141, these peaks are related to α-MnO2 with a tetragonal phase for miller indices (110), (200), (220), (310), (400), (211), (420), (301), (411), (600), (521), (002), (541) and (312), respectively. The composite sample, MnO2/MXene, exhibited all the above peaks plus an additional peak at 9.0 degrees corresponding to the (002) plane of MXene. No other impurity peaks in the XRD patterns were observed due to the high purity of the as-prepared samples. The presence of only a minute peak for MXene is related to its morphologically reduced shape and may be in strictly two dimensions, which results in unnoticeable lattices by passing X-rays. Therefore, the structural composition could be further verified by XPS analysis.3.2. XPS
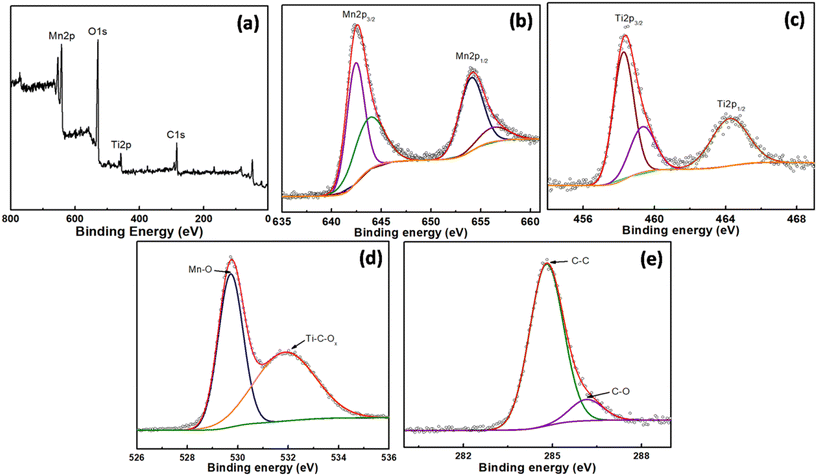
The chemical valence states and surface composition of MnO2/MXene were analyzed using XPS (Fig. 2). The obtained survey spectrum (Fig. 2a) indicated the presence of Mn, Ti, and C elements, which corroborate the EDS analysis. The Mn2p signal (Fig. 2b) consists of four peaks at 642.4, 643.9, 654.1, and 656.3 eV. Two of the peaks at 642.4 and 654.1 eV correspond to Mn (2p3/2 and 2p1/2, respectively) of the trivalent manganese ion (Mn3+), while the other two peaks at 643.9 and 656.3 eV correspond to the tetravalent manganese ion (Mn4+).46 The presence of Mn3+ species indicates the oxygen vacancies on the surface of the synthesized manganese dioxide. The Ti 2P signal (Fig. 2c) consists of two peaks at 458.3 and 459.3 corresponding to Ti–O (2p3/2), whilst the third peak at 464.1 eV represents O (2p1/2) bonds.46,47 The O1s peaks (Fig. 2d) observed at 529.7, and 531.9 eV arise from the Mn–O and Ti–C–OX, respectively and confer fruitful bonding with carbon.48 The C1s peaks (Fig. 2e) consist of two divisions at 284.8 and 286.1 eV, which represent C–C and C–O bonding, respectively.473.3. SEM & TEM
The SEM image shown in Fig. 3a corresponds to a fine one-dimensional (1D) structure of MnO2 with a diameter in the range of 10–80 nm. Energy Dispersive X-ray Spectroscopy (EDS) analysis of the MnO2 sample shown in the inset in Fig. 3a has characteristic peaks of only Mn and O elements. Two-dimensional MXene sheets can be visualized in SEM images shown in Fig. 3b. The MnO2/MXene sample images at different time intervals of the hydrothermal reaction are shown in Fig. 3(c and d). After approximately 6 h of the hydrothermal reaction (Fig. 3c), fine two-dimensional sheets of MXene were seen to be stacked together layer by layer, surrounded by minute particles considered to be the mixed reagents of KMnO4 and NaNO2. The reagents were distributed well over the MXene surface like a uniform coating (Fig. 3c). These reagents act as precursors for the growth of 1D MnO2 following redox kinetics. It was also predicted that these precursors were intercalated inside the thin layers of the MXene sheet. Herein, nitrite ions function as a perfect reducing agent for KMnO4 with the help of an adequate amount of acid content, as depicted below.| 2MnO4− + 3NO2− + 2H+ → 2MnO2 + 3NO3− + H2O | (a) |
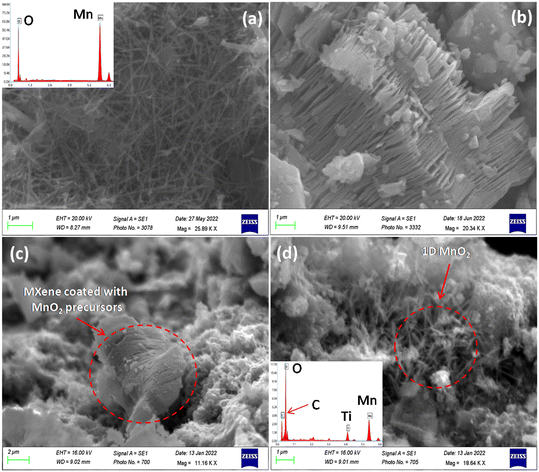 | ||
| Fig. 3 SEM images of (a) 1D MnO2 and its EDS as the inset, (b) MXene and MnO2/MXene after a hydrothermal reaction time of (c) 6, and (d) 12 h, and the EDS in the inset. | ||
In the TEM images shown in Fig. 4(a–c), fragmented MXene sheets, as highlighted, are seen to be settled around 1D structures. The precise attachment between MXene and MnO2 could be due to the weak van der Waals forces, which need to be studied in the near future. The HRTEM image shown in Fig. 4d provides the approximate interlayer spacing of MXene sheets as 0.95 nm.49,50 Also seen in the image is a d-spacing of 0.48 nm in the (200) plane for MnO2. This shows close interaction between MXene and MnO2.
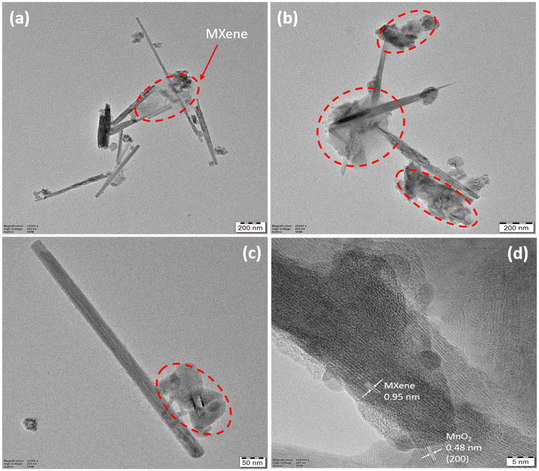 | ||
| Fig. 4 (a–c) TEM images of MnO2/MXene, (d) HRTEM image of MnO2/MXene with an interlayer spacing of 0.95 nm for MXene and d-spacing of 0.48 nm in the (200) plane for MnO2. | ||
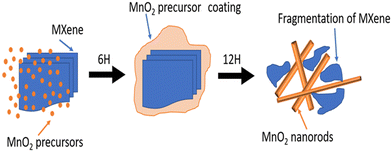
The phenomenon of fragmentation of MXene across 1D structures can be observed from the schematic shown in Fig. 5. As portrayed, after 6 h of hydrothermal reaction, a uniform coating of MnO2 precursors over MXene layered structures was formed. This coated material was then transformed into fine 1D structures after 12 h of hydrothermal reaction. Interestingly, the phenomenon of the growth of 1D structures deeply affects the layered structures of MXenes. As a fruitful effect, the layered structures of MXene are broken down into small fragments. Even after this breakdown, these fragmented parts of MXenes are seen to be morphologically attached to the 1D structures therein, giving rise to a novel blend of nanocomposites in the form of MnO2/MXene.
3.4. BET
Surface analysis by the Brunauer–Emmett–Teller (BET) method is highlighted with isotherms of nitrogen (N2) absorption–desorption and Barrett–Joyner–Halenda (BJH) pore size distribution (PSD) as an inset of Fig. 6(a and b) for MnO2 and MnO2/MXene, respectively. The 1D MnO2 structure exhibits a type H4 loop under a type IV isotherm, indicating a mesoporous structure.51 The specific surface area of 1D MnO2 was found to be 39 m2 g−1. The PSD histogram of MnO2 showed the greatest distribution around 5–10 nm giving rise to an average pore diameter of 7.4 nm. For the MnO2/MXene sample, the isotherm was observed to be a type H1 loop within a type IV isotherm, which again showcases the mesoporous structure. However, the specific surface area considerably increased to 201 m2 g−1, and the PSD histogram showed the greatest distribution around 4–9 nm, predicting an average pore diameter of 6.3 nm. This increase in specific surface area and reduction in average pore diameter can be attributed to the fragmented sheets of MXene being precisely attached to the fine 1D structures of MnO2.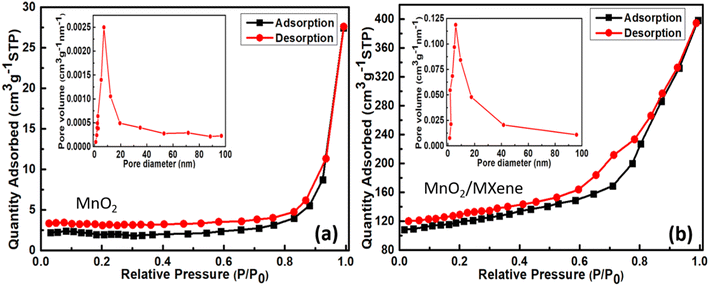 | ||
| Fig. 6 Isotherms of nitrogen absorption–desorption and BJH pore size distribution as an inset of the samples: (a) MnO2 and (b) MnO2/MXene. | ||
3.5. Supercapacitive behaviour
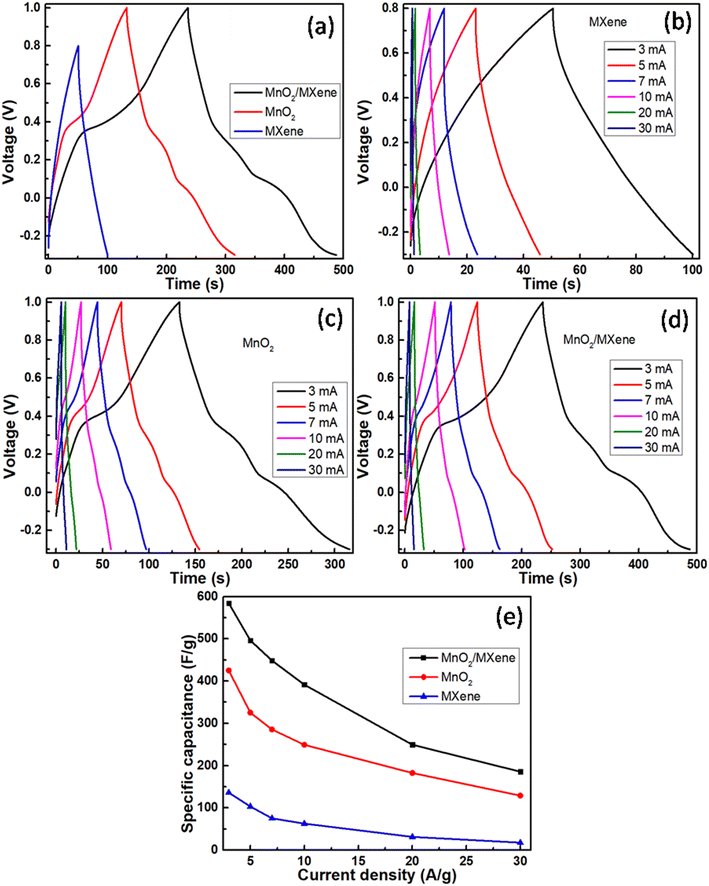
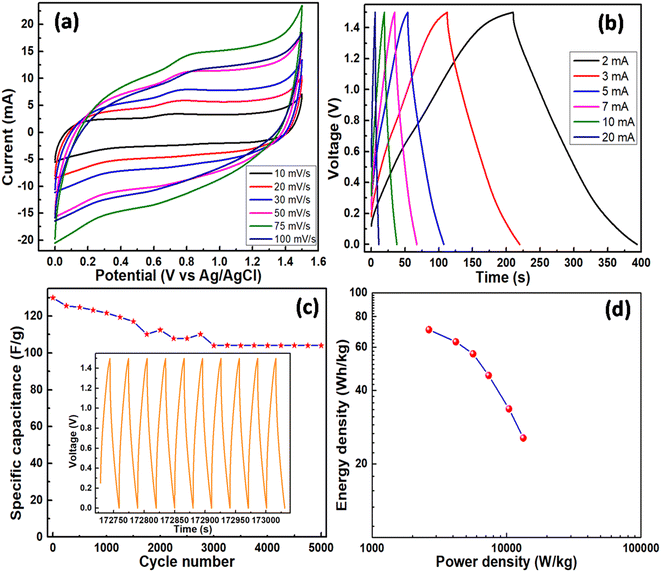
Cyclic voltammetry measurements in a potential window of −0.3 to 0.8 V for the MXene sample and −0.3 to 1.0 V for MnO2 and MnO2/MXene at 20 mV s−1 are shown in Fig. 7a. It was observed that the pristine MXene sample exhibited a considerably smaller area under the CV curve with undiscernible redox peaks. This indicates its poor electrochemical activity in contrast to MnO2 and MnO2/MXene samples. For MnO2 and MnO2/MXene samples, the larger curve area with two similar anodic oxidation peaks at around 0.15 and 0.65 V and two cathodic reduction peaks at around 0.0 and 0.2 V revealed the two-stage oxidation and reduction reaction of electrode surfaces. This suggests a promising charge flow in and out of the working electrodes, which is favourable for supercapacitive behaviour. In both the MnO2-based electrodes, the transition between Mn4+ and Mn3+ is reversible. Faradaic activity following the intercalation and de-intercalation of K+ ions as well as charges into the electrode surface can be interpreted as follows:
| MnO2 + K+ + e− ↔ MnO·OK | (b) |
| Ti3C2 + K+ + e− ↔ (Ti3C2)−K+ | (c) |
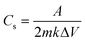 | (1) |
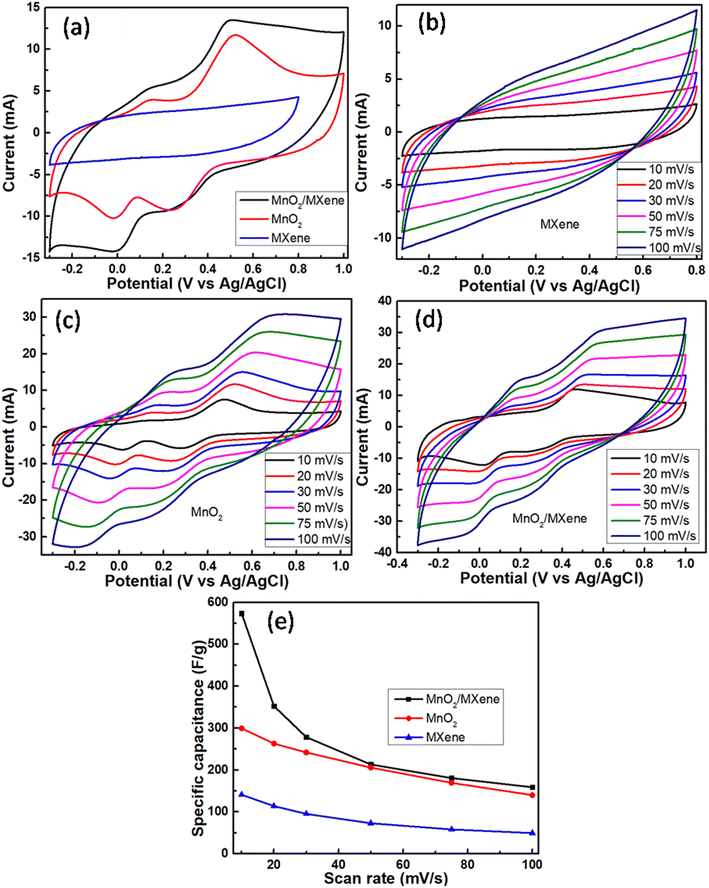 | ||
| Fig. 7 CV curves for (a) MXene, MnO2, and MnO2/MXene at 20 mV s−1, (b) MXene, (c) MnO2, (d) MnO2/MXene; (e) specific capacitances of MXene, MnO2 and MnO2/MXene at different scan rates. | ||
Galvanostatic charge–discharge measurements in a potential window of −0.3 to 0.8 V for the MXene sample and −0.3 to 1.0 V for MnO2 and MnO2/MXene at 3 A g−1 are shown in Fig. 8a. Fig. 8(b–d) show the measurements for MXene, MnO2 and MnO2/MXene, respectively, at current densities of 3, 5, 7, 10, 20 and 30 A g−1. The mathematical equation for calculating specific capacitance Cs in F g−1 using the GCD curve is as follows:
| Cs = IΔt/mΔV | (2) |
In line with the findings from CV waveforms, composite MnO2/MXene demonstrated superior capacitive behaviour as compared to MnO2 and MXene, respectively. Table 1 compares the new work to previously reported efforts.
| S. no. | Electrode | Specific capacitance (F g−1) | Current density (A g−1) | Ref. |
|---|---|---|---|---|
| 1 | MnO2/MXene | 340 | 1 | 47 |
| 2 | MnO2/Ti3C2Tx | 130.5 | 0.2 | 52 |
| 3 | MnO2/Ti3C2Tx | 452 | 1 | 53 |
| 4 | MnO2@MXene/CNT | 181.8 | 1 | 48 |
| 5 | MnO2/MXene | 611.5 | 1 | 54 |
| 6 | MnO2/MXene | 457 | 1 | 55 |
| 7 | MnO2 | 365 | 0.25 | 56 |
| 8 | MnO2/Fe3O4 | 243.7 | 0.1 | 57 |
| 9 | MnO2/Fe3O4 | 590 | 2.9 | 39 |
| 10 | MnO2 | 425.3 | 3 | This work |
| 11 | MnO2/MXene | 584.6 | 3 | This work |
The attained morphology may be used to explain why the developed material (MnO2/MXene) performs better capacitively than the previously reported works. When fine 1D materials are covered with MXene fragmented structures, the physical complexity of their surface is increased. It is clear from the TEM investigation that MXene is present in a variety of fragmented structures along with 1D morphologies. As a result of the interaction between 1D MnO2 and 2D MXene, the surface tension is supposed to be enhanced. The fragments of MXene are predicted to remain attached on the 1D MnO2 surface due to the weak van der Waals forces. The BET study, which shows a dramatic rise in the specific surface area of 1D MnO2 when mixed with MXene from 39 to 201 m2 g−1, provides insight into this interaction. However, this interaction does not support any chemical bonding between MnO2 and MXene, as XRD analysis showcased α-MnO2 with a tetragonal phase according to ICDD 44-0141. Therefore, this weak interaction could have resulted in a synergistic effect between the two interacting surfaces for a promising charge storage capability. In addition, as compared to pure MXene, the composite MXene was shown to have reduced morphologies. The reduction in morphology must be from the surface effects of 1D MnO2. This reveals that nanoscale materials try to interact closely with a substance having comparable dimensions for stability, thus giving rise to weak van der Waals forces. The surface tension is further increased by this change in form, creating an enhanced synergistic effect. Therefore, even though the pure MXene sample employed comparable crystallographic structures, the modification in morphology, as given, leads to greater capacitive behaviour. Surface tension that results from this significant morphological change may increase surface activity, creating more active spots for the convenient accommodation of charged particles. The material must react more quickly for both the non-faradaic and faradaic activities mentioned above due to the intricacy of the surface shape. However, the precise relationship between morphology and electrochemical kinetics may be examined thoroughly with an established synthesis and characterization approach, a topic that is debatable and requires more technological advancement.
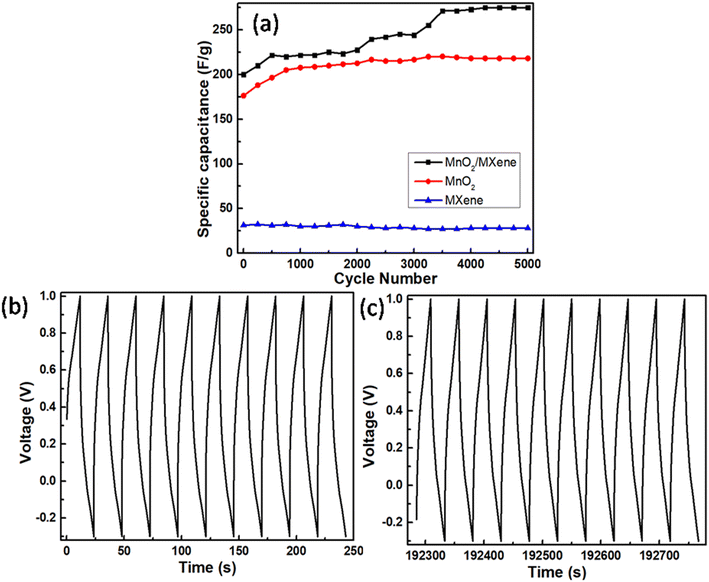
GCD cycles were performed for 5000 continuous cycles at 20 mA for all the samples to study their stability in capacity retention as presented in Fig. 9a. The MXene sample was quite stable with a good retention value of 89.54% after continuous running over 5000 GCD cycles at 20 mA. However, under similar conditions, MnO2 and MnO2/MXene electrodes showed variations. MnO2 and MnO2/MXene electrodes showed a gradual increase with prolonged cycling. These increases could account for the breaking of interfacial layers between electrode and electrolyte surfaces, which is a common phenomenon in a 3-electrode system. However, the MnO2 electrode was more stable and showed a steady response after 1000 cycles with a capacity retention of 123.6%, whilst the MnO2/MXene electrode took a longer cycling duration of 3500 cycles to get stabilized with a capacity retention of 137.3%. This stabilization could be accounted for by the creation of a continuous pathway for charged particles across the interfacial layers. In the case of the MnO2/MXene electrode, the longer cycling duration for stabilization directly reflects the breaking of a much large number of interfacial layers. The larger surface area of MnO2/MXene (201 m2 g−1 against 39 m2 g−1 of MnO2) sample observed through BET analysis and the complexity in morphology observed through TEM analysis suggest the existence of a large number of interfacial layers. For a steady reference, the first and last 10 GCD cycles of the MnO2/MXene sample are presented in Fig. 9(b and c), respectively.
Using the following equations and discharge duration as t in seconds, the energy density, E (Wh kg−1) and power density, P (W kg−1), were computed as 77.2, 67.3, 57.5, 48.6, 39.1, and 24.1 Wh kg−1, and 1725.0, 2587.5, 4404.2, 6035.7, 8635.3, and 17388.0 W kg−1, respectively (Fig. 10d, Ragone plot).
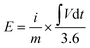 | (3) |
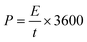 | (4) |
A comparison of energy density as achieved in the 2-electrode configuration with the available reports is presented in below Table 2:
| S. no. | Electrode | Energy density (Wh kg−1) | Power density (W kg−1) | Ref. |
|---|---|---|---|---|
| 1 | MnO2/CNT//Ti3C2Tx | 36 | 3200 | 58 |
| 2 | MnO2/Ti3C2Tx | 12.25 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
52 |
| 3 | MnO2/Ti3C2Tx | 14.3 | 790 | 53 |
| 4 | rGO//Ti3C2Tx | 23.7 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
59 |
| 5 | MnO2@Ti3C2Tx//activated carbon | 31.4 | 180 | 60 |
| 6 | MnO2/Ti3C2 | 8.3 | 221.33 | 61 |
| 7 | MnO2/Ti3C2Tx | 14.42 | 700 | 62 |
| 8 | Ti3C2Tx/MWCNT//rGO | 20 | 65 | 63 |
| 9 | Ti3C2/CuS//Ti3C2 | 15.4 | 750.2 | 64 |
| 10 | Ti3C2/nickel foam//Ti3C2 | 18.1 | 397.8 | 65 |
| 11 | MnO2/CNTs//activated carbon | 13.3 | 600 | 66 |
| 12 | MnO2/MXene | 77.2 | 1725 | This work |
The results predict that the as-prepared material could be one of the best in class for utilization as supercapacitor electrodes. It is noteworthy that the same elemental composition of MXene with MnO2 can give different results based on their morphologies. The fine morphological tuning of MXene sheets as fragments over 1D MnO2 has gained a considerable advantage for adding surface activity to MnO2, resulting in enormously high capacitive performance. The presented study is an addition to the exploration of fascinating material properties.67–74
4. Conclusions
We have successfully presented a simple one-step synthesis method for morphologically fragmenting the 2D sheets of MXene across the hydrothermally grown 1D MnO2 structure. TEM analysis presented weak bonds between fragmented parts of MXene and the 1D MnO2 surface. BET analysis further supported the promising interactions between MXene and MnO2 with an increment in surface area from 39 to 201 m2 g−1. As a consequence, promising supercapacitive behaviour was achieved to the extent of 818.5 F g−1 at 3 A g−1 in a three-electrode system. Also, the device performance was successfully tested in a two-electrode symmetric supercapacitor system with 77.2 Wh kg−1 at 1725 W kg−1. A stable electrode material was thus developed with a capacity retention of 80% as tested over 5000 GCD cycles in the two-electrode configurations. The improved surface activity could be attributed to the novel morphological structure of MnO2/MXene, following considerable enhancement in the overall charge distribution.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support provided by Uttaranchal University under the seed money project scheme for accomplishing the work reported in this research article. We further acknowledge the DST-FIST, Government of India (via Project No. SR/FST/PS-II/2021/190) along with SRM-SCIF and Nanotechnology Research Center (NRC) for providing some of the important characterization facilities at SRMIST. Also, the authors are thankful to the Ministry of Science and Higher Education of the Russian Federation (Ural Federal University Program of Development within the Priority-2030 Program) for funding.References
- Y. Deng, Y. Xie, K. Zoua and X. Ji, Review on recent advances in nitrogen-doped carbons: preparations and applications in supercapacitors, J. Mater. Chem. A, 2016, 4, 1144–1173 RSC.
- S. P. S. Badwal, S. S. Giddey, C. Munnings, A. I. Bhatt and A. F. Hollenkamp, Emerging electrochemical energy conversion and storage technologies, Front. Chem., 2014, 2, 79 Search PubMed.
- Z. Yu, L. Tetard, L. Zhai and J. Thomas, Supercapacitor electrode materials: nanostructures from 0 to 3 dimensions, Energy Environ. Sci., 2015, 8, 702–730 RSC.
- G. Yu, X. Xie, L. Pan, Z. Bao and Y. Cui, Hybrid nanostructured materials for high-performance electrochemical capacitors, Nano Energy, 2013, 2, 213–234 CrossRef CAS.
- G. Wang, L. Zhang and J. Zhang, A review of electrode materials for electrochemical supercapacitors, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- P. Simon and Y. Gogotsi, Materials for electrochemical capacitors, Nat. Mater., 2008, 7, 845–854 CrossRef CAS.
- J. Yan, Q. Wang, T. Wei and Z. Fan, Recent Advances in ddesign and fabrication of electrochemical supercapacitors with high energy densities, Adv. Energy Mater., 2014, 4, 1300816–1300849 CrossRef.
- F. Shi, L. Li, X. L. Wang, C. D. Gu and J. P. Tu, Metal oxide/hydroxide-based materials for supercapacitors, RSC Adv., 2014, 4, 41910–41921 RSC.
- R. Ramachandran, S. M. Chen and G. G. Kumar, An overview of electrochemical energy storage devices of various electrodes and morphological studies of supercapacitors, Int. J. Electrochem. Sci., 2015, 10, 10355–10388 CAS.
- J. C. Zhao, J. Wang and J. L. Xu, Synthesis and electrochemical characterization of mesoporous Mno2, J. Chem., 2015, 2015, 76803–76808 Search PubMed.
- J. Cao, X. Li, Y. Wang, F. C. Walsh, J. H. Ouyang, D. Jia and Y. Zhou, Materials and fabrication of electrode scaffolds for deposition of Mno2 and their true performance in supercapacitors, J. Power Sources, 2015, 293, 657–674 CrossRef CAS.
- W. Wei, X. Cui, W. Chen and D. G. Ivey, Manganese Oxide-Based Materials as Electrochemical Supercapacitor Electrodes, Chem. Soc. Rev., 2011, 40, 1697–1721 RSC.
- H. Choi and H. Yoon, Nanostructured electrode materials for electrochemical capacitor applications, Nanomaterials, 2015, 5, 906–936 CrossRef CAS PubMed.
- B. S. Lou, P. Veerakumar, S. M. Chen, V. Veeramani, R. Madhu and S. B. Liu, Ruthenium nanoparticles decorated Curl-Like porous carbons for high performance supercapacitors, Sci. Rep., 2016, 6, 19949–19960 CrossRef CAS.
- M. Meyyappan, Nanostructured materials for supercapacitors, J. Vac. Sci. Technol., A, 2013, 31, 050803–050817 CrossRef.
- Y. Sun and G. Shi, Graphene/Polymer composites for energy applications, J. Polym. Sci., Part B: Polym. Phys., 2013, 51, 231–253 CrossRef CAS.
- H. Ma, J. He, D. B. Xiong, J. Wu, Q. Li, V. Dravid and Y. Zhao, Nickel Cobalt hydroxide@reducedgraphene oxide hybrid nanolayers for high performance asymmetric supercapacitors with remarkable cycling stability, ACS Appl. Mater. Interfaces, 2016, 8, 1992–2000 CrossRef CAS PubMed.
- R. Thangappan, S. Kalaiselvam, A. Elayaperumal, R. Jayavel, M. Arivanandhan, R. Karthikeyan and Y. Hayakawa, Graphene decorated with MoS2nanosheets: A synergetic energy storage composite electrode for supercapacitorapplications, Dalton Trans., 2016, 45, 2637–2646 RSC.
- T. N. Quang, B. K. Kang, S. N. Tiruneh and D. H. Yoon, Design of advanced MnO/N-Gr 3d walls through polymer cross-linking for high-performance supercapacitor, Chem. – Eur. J., 2016, 22, 1652–1657 CrossRef PubMed.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, 25th Anniversary Article: Mxenes: A new family of two-dimensional materials, Adv. Mater., 2014, 26, 992–1005 CrossRef CAS PubMed.
- G. R. Bhimanapati, Z. Lin, V. Meunier, Y. Jung, J. Cha, S. Das, D. Xiao, Y. Son, M. S. Strano, V. R. Cooper, L. Liang, S. G. Louie, E. Ringe, W. Zhou, S. S. Kim, R. R. Naik, B. G. Sumpter, H. Terrones, F. Xia, Y. Wang, J. Zhu, D. Akinwande, N. Alem, J. A. Schuller, R. E. Schaak, M. Terrones and J. A. Robinson, Recent advances in two-dimensional materials beyond graphene, ACS Nano, 2015, 9, 11509–11539 CrossRef CAS PubMed.
- S. J. Kim, M. Naguib, M. Zhao, C. Zhang, H. T. Jung, M. W. Barsoum and Y. Gogotsi, High mass loading, Binder-Free Mxeneanodes for high areal Capacity Li-Ion Batteries, Electrochim. Acta, 2015, 163, 246–251 CrossRef CAS.
- Y. Xie, M. Naguib, V. N. Mochalin, M. W. Barsoum, Y. Gogotsi, X. Yu, K. W. Nam, X. Q. Yang, A. I. Kolesnikov and P. R. C. Kent, Role of Surface Structure on Li-Ion Energy Storage Capacity of TwoDimensional Transition-Metal Carbides, J. Am. Chem. Soc., 2014, 136, 6385–6394 CrossRef CAS PubMed.
- Y. Xie, Y. Dall'Agnese, M. Naguib, Y. Gogotsi, M. Barsoum, H. L. Zhuang and P. R. C. Kent, Prediction and Characterization of MxeneNanosheet Anodes for Non-Lithium-Ion Batteries, ACS Nano, 2014, 8, 9606–9615 CrossRef CAS.
- M. R. Lukatskaya, O. Mashtalir, C. E. Ren, Y. Dall'Agnese, P. Rozier, P. L. Taberna, M. Naguib, P. Simon, M. W. Barsoum and Y. Gogotsi, Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide, Science, 2013, 341, 1502–1505 CrossRef CAS.
- M. R. Lukatskaya, C. Ren, O. Mashtalir, Y. Dall'Agnese, M. Naguib, P. Simon, M. Barsoum and Y. Gogotsi, Capactive Performance of 2d titaniumcarbide based Mxenesowing to cation intercalation, Abstr. Pap. Am. Chem. Soc., 2014, 247 Search PubMed.
- Z. Ling, C. E. Ren, M. Q. Zhao, J. Yang, J. M. Giammarco, J. Qiu, M. W. Barsoum and Y. Gogotsi, Flexible and conductive Mxenefilms and nanocomposites with high capacitance, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 16676–16681 CrossRef CAS.
- R. B. Rakhi, B. Ahmed, M. N. Hedhili, D. H. Anjum and H. N. Alshareef, Effect of postetchannealing gas composition on the structural and electrochemical properties of Ti2ctx Mxeneelectrodes for supercapacitorapplications, Chem. Mater., 2015, 27, 5314–5323 CrossRef CAS.
- Y. Bai, R. B. Rakhi, W. Chen and H. N. Alshareef, Effect of PH-Induced chemical modification of hydrothermally reduced graphene oxide on supercapacitor performance, J. Power Sources, 2013, 233, 313–319 CrossRef CAS.
- P. Lian, X. Zhu, S. Liang, Z. Li, W. Yang and H. Wang, Large reversible capacity of high quality graphene sheets as an anode material for Lithium-Ion batteries, Electrochim. Acta, 2010, 55, 3909–3914 CrossRef CAS.
- N. Kumar, J. R. Rodriguez, V. G. Pol and A. Sen, Facile synthesis of 2D graphene oxide sheet enveloping ultrafine 1D LiMn2O4 as interconnected framework to enhance cathodic property for Li-ion battery, Appl. Surf. Sci., 2019, 463, 132–140 CrossRef CAS.
- N. Kumar, K. G. Prasad, A. Sen and T. Maiyalagan, Enhanced pseudocapacitance from finely ordered pristine α-MnO2 nanorods at favourably high current density using redox additive, Appl. Surf. Sci., 2018, 449, 492–499 CrossRef CAS.
- N. Kumar, K. G. Prasad, T. Maiyalagan and A. Sen, Precise control of morphology of ultrafine LiMn2O4 nanorods as a supercapacitor electrode via a two-step hydrothermal method, CrystEngComm, 2018, 20, 5707–5717 RSC.
- N. Kumar, S. Bhaumik, A. Sen, A. P. Shukla and S. D. Pathak, One-pot synthesis and first-principles elasticity analysis of polymorphic MnO2 nanorods for tribological assessment as friction modifiers, RSC Adv., 2017, 7, 34138–34148 RSC.
- N. Kumar, A. Sen, K. Rajendran, R. Rameshbabu, J. Ragupathi, H. A. Therese and T. Maiyalagan, Morphology and phase tuning of α- and β-MnO2nanocacti evolved at varying modes of acid count for their well-coordinated energy storage and visible-light-driven photocatalytic behaviour, RSC Adv., 2017, 7, 25041–25053 RSC.
- N. Kumar, P. Dineshkumar, R. Rameshbabu and A. Sen, Facile size-controllable synthesis of single crystalline β-MnO2 nanorods under varying acidic strengths, RSC Adv., 2016, 6, 7448–7454 RSC.
- N. Kumar, P. Dineshkumar, R. Rameshbabu and A. Sen, Morphological analysis of ultra-fine α-MnO2 nanowires under different reaction conditions, Mater. Lett., 2015, 158, 309–312 CrossRef CAS.
- N. Kumar, V. Gajraj, R. Rameshbabu, R. V. Mangalaraja, N. C. Joshi and N. Priyadarshi, Redox additive electrolyte assisted promising pseudocapacitance from strictly 1D and 2D blended structures of MnO2/rGO, Mater. Charact., 2022, 189, 111991 CrossRef CAS.
- N. Kumar, V. N. Thakur, V. Gajraj, M. Karthikeyan, A. Sen, N. Joshi and N. Priyadarshi, Morphological reduction of Fe3O4 by single step hydrothermal synthesis using 1D MnO2 as a template and its supercapacitive behaviour, CrystEngComm, 2022, 24, 4611–4621 RSC.
- N. Kumar, J. R. Rodriguez, V. G. Pol and A. Sen, Synergistically advancing Li storage property of hydrothermally grown 1D pristine MnO2 over a mesh-like interconnected framework of 2D graphene oxide, J. Solid State Electrochem., 2019, 23, 1443–1454 CrossRef CAS.
- M. Xue, Z. Wang, F. Yuan, X. Zhang, W. Wei, H. Tang and C. Li, Preparation of TiO2/Ti3C2Tx hybrid nanocomposites and their tribological properties as base oil lubricant additives, RSC Adv., 2017, 7, 4312–4319 RSC.
- M. Naguib, M. Kurtoglu and V. Presser, Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- Y. Liu, X. Zhang and S. Dong, Synthesis and tribological property of Ti3C2Tx nanosheets, J. Mater. Sci., 2016, 52, 1–10 CrossRef.
- J. Come, J. M. Black and M. R. Lukatskaya, Controlling the actuation properties ofMXene paper electrodes upon cation intercalation, Nano Energy, 2015, 17, 27–35 CrossRef CAS.
- S. J. Kim, M. Naguib and M. Zhao, High mass loading, binder-free MXene anodes for high areal capacity Li-ion batteries, Electrochim. Acta, 2015, 163, 246–251 CrossRef CAS.
- H. Zhou, Y. Lu, F. Wu, L. Fang, H. J. Luo, Y. X. Zhang and M. Zhou, MnO2nanorods/MXene/CC composite electrode for flexible supercapacitors with enhanced electrochemical performance, J. Alloys Compd., 2019, 802, 259–268 CrossRef CAS.
- S. Chen, Y. Xiang, W. Wu and C. Peng, A novel MnO2/MXene composite prepared by electrostatic self-assembly and its use as an electrode for enhanced supercapacitive performance, Inorg. Chem. Front., 2019, 6, 199–208 RSC.
- Q. Liu, J. Yang, X. Luo, Y. Miao, Y. Zhang, W. Xu, L. Yang, Y. Liang, W. Weng and M. Zhu, Fabrication of a fibrous MnO2@MXene/CNT electrode for high-performance flexible supercapacitor, Ceram. Int., 2020, 46, 11874–11881 CrossRef CAS.
- F. Zhang, Y. Zhou, Y. Zhang, D. Li and Z. Huang, Facile synthesis of sulfur@titanium carbide Mxene as high performance cathode for lithium-sulfur batteries, Nanophotonics, 2020, 9, 2025–2032 CrossRef CAS.
- M. Cao, F. Wang, L. Wang, W. Wu, W. Lv and J. Zhu, Room temperature oxidation of Ti3C2MXene for supercapacitorelectrodes, J. Electrochem. Soc., 2017, 164, A3933–A3942 CrossRef CAS.
- M. Kruk and M. Jaroniec, Gas adsorption characterizationof ordered organic-inorganic nanocomposite materials, Chem. Mater., 2001, 13, 3169–3183 CrossRef CAS.
- H. Jiang, Z. Wang, Q. Yang, M. Hanif, Z. Wang, L. Dong and M. Dong, A novel MnO2/Ti3C2TxMXene nanocomposite as high performance electrode materials for flexiblesupercapacitors, Electrochim. Acta, 2018, 290, 695–703 CrossRef CAS.
- X. Zhang, B. Shao, A. Guo, Z. Sun, J. Zhao, F. Cui and X. Yang, MnO2 nanoshells/Ti3C2TxMXene hybrid film as supercapacitor electrode, Appl. Surf. Sci., 2021, 560, 150040 CrossRef CAS.
- M. Mahmood, A. Rasheed, I. Ayman, T. Rasheed, S. Munir, S. Ajmal, P. O. Agboola, M. F. Warsi and M. Shahid, Synthesis of Ultrathin MnO2nanowire-intercalated 2D-MXenes for high-performance hybrid supercapacitors, Energy Fuels, 2021, 35, 3469–3478 CrossRef CAS.
- H. Peçenek, S. Yetiman, F. Dokan, M. S. Onses, E. Yılmaz and E. Sahmetlioglu, Effects of carbon nanomaterials and MXene addition on the performance of nitrogen doped MnO2 based supercapacitors, Ceram. Int., 2022, 48, 7253–7260 CrossRef.
- M. Huang, Y. Zhang, F. Li, L. Zhang, R. S. Ruoff, Z. Wen and Q. Liu, Self-assembly of mesoporous nanotubes assembled from interwoven Ultrathin Birnessite-type MnO2nanosheets for asymmetric supercapacitors, Sci. Rep., 2014, 4, 3878 CrossRef PubMed.
- J. Ding, J. Yang, S. Ji, S. Huo and H. Wang, Ionics (Kiel), Core-shell structured Fe3O4@MnO2 nanospheres to achieve high cycling stability as electrode for supercapacitors, Ionics, 2019, 25, 665–673 CrossRef CAS.
- J. Wu, Q. Li, C. E. Shuck, K. Maleski, H. N. Alshareef, J. Zhou and Y. G. L. Huang, An aqueous 2.1 V pseudocapacitor with MXene and V-MnO2 electrodes, Nano Res., 2022, 15, 535–541 CrossRef.
- S. P. Wang, X. L. Zhao, X. J. Yan, Z. W. Xiao, C. C. Liu, Y. J. Zhang and X. W. Yang, Regulating fast anionic redox for high-voltage aqueous hydrogen-ion-based energy storage, Angew. Chem., Int. Ed., 2019, 58, 205–210 CrossRef CAS PubMed.
- X. Li, J. Zhu, B. Zhang, Y. Jiao, J. Huang and F. Wang, Manganese dioxide nanosheets decorated on MXene (Ti3C2Tx) with enhanced performance for asymmetric supercapacitors, Ceram. Int., 2021, 47, 12211–12220 CrossRef CAS.
- W. Liu, Z. Wang, Y. Su, Q. Li, Z. Zhao and F. Geng, Molecularly stacking manganese dioxide/titanium carbide sheets to produce highly flexible and conductive film electrodes with improved pseudocapacitive performances, Adv. Energy Mater., 2017, 7, 1602834 CrossRef.
- R. B. Rakhi, B. Ahmed, D. Anjum and H. N. Alshareef, Direct chemical synthesis of MnO2nanowhiskers on transition-metal carbide surfaces for supercapacitor applications, ACS Appl. Mater. Interfaces, 2016, 8, 18806–18814 CrossRef CAS PubMed.
- A. M. Navarro-Su'arez, K. L. van Aken, T. Mathis, T. Makaryan, J. Yan, J. Carretero-Gonz'alez, T. Rojo and Y. Gogotsi, Development of asymmetric supercapacitors with titanium carbide-reduced graphene oxide couples as electrodes, Electrochim. Acta, 2018, 259, 752–761 CrossRef.
- Z. Pan, F. Cao, X. Hu and X. Ji, A facile method for synthesizing CuS decorated Ti3C2MXene with enhanced performance for asymmetric supercapacitors, J. Mater. Chem. A, 2019, 7, 8984–8992 RSC.
- J. Guo, Y. Zhao, A. Liu and T. Ma, Electrostatic self-assembly of 2D delaminated MXene (Ti3C2) onto Ni foam with superior electrochemical performance forsupercapacitor, Electrochim. Acta, 2019, 305, 164–174 CrossRef CAS.
- L. Li, Z. A. Hu, N. An, Y. Y. Yang, Z. M. Li and H. Y. Wu, Facile synthesis of MnO2/CNTs composite for supercapacitor electrodes with long cycle stability, J. Phys. Chem. C, 2014, 118, 22865–22872 CrossRef CAS.
- S. Sankaranarayanan, P. Kandasamy, R. Raju and B. Krishnan, Fabrication of gallium nitride and nitrogen doped single layer graphenehybrid heterostructures for high performance photodetectors, Sci. Rep., 2020, 10, 14507–14519 CrossRef CAS PubMed.
- S. Sanjay, K. Prabakaran and K. Baskar, Epitaxy of gallium nitride pyramids on few layergraphene for metal-semiconductor-metal based photodetectors, Mater. Chem. Phys., 2020, 240, 122189–122194 CrossRef CAS.
- S. Sankaranarayanan, P. Kandasamy and B. Krishnan, Catalytic Growth of Gallium Nitride Nanowires on Wet Chemically Etched Substrates by Chemical Vapor Deposition, ACS Omega, 2019, 4, 14772–14779 CrossRef CAS PubMed.
- S. Sanjay and K. Baskar, Fabrication of Schottky barrier diodes on clump of gallium nitridenanowires grown by chemical vapour deposition, Appl. Surf. Sci., 2018, 456, 526–531 CrossRef CAS.
- S. Sanjay, K. Prabakaran, S. Singh and K. Baskar, Growth of gold-palladium alloy catalyzed gallium nitride nanowires by chemical vapour deposition, Mater. Lett., 2018, 217, 100–103 CrossRef CAS.
- S. Sanjay, K. Prabakaran, S. Singh and K. Baskar, Catalyst-free deposition of few layer graphene on c-plane sapphire substrates by drop casting technique, J. Mater. Sci.: Mater. Electron., 2018, 29, 4413–4421 CrossRef CAS.
- S. Sankaranarayanan, P. Kandasamy, R. Raju, S. Gengan and B. Krishnan, Controlled growth of gallium nitride nanowires on silicon and their utility in high performance Ultraviolet-A photodetector, Sens. Actuators, A, 2021, 332, 113189–113200 CrossRef CAS.
- S. Sankaranarayanan, P. Kandasamy, R. Raju, S. Gengan and B. Krishnan, Enhancement of visible light photodetector performance for ultrafast switching using flower shaped gallium nitride nanostructures, Scr. Mater., 2021, 194, 113711–113716 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |