A novel Li+-doped CsCu2I3 single crystal for dual gamma–neutron detection†
Dongdong
Liu
a,
Qinhua
Wei
 *a,
Yufeng
Tong
a,
Peng
Xiang
a,
Peiqing
Cai
*a,
Yufeng
Tong
a,
Peng
Xiang
a,
Peiqing
Cai
 b,
Gao
Tang
a,
Hongsheng
Shi
c and
Laishun
Qin
*a
b,
Gao
Tang
a,
Hongsheng
Shi
c and
Laishun
Qin
*a
aCollege of Materials and Chemistry, China Jiliang University, Hangzhou 310018, China
bCollege of Optical and Electronic Technology, China Jiliang University, Hangzhou 310018, China
cXinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Urumqi 830011, China
First published on 9th December 2022
Abstract
Halide perovskites are among the most attractive research hotspots in optoelectronic applications due to their excellent luminescence properties. In this paper, Li+ was successfully incorporated into a one-dimensional perovskite CsCu2I3 host and constructed as a novel neutron/gamma detection scintillator via the Bridgman method. XRD and ICP-MS were carried out, and the results were discussed. The Li-doped CsCu2I3 crystal exhibits a main emission peak at 575 nm originating from strongly localized 1D exciton emission and a high photoluminescence quantum efficiency of 18.7%. The CsCu2I3:Li crystal has a light yield of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 photons per MeV and an energy resolution of 11.5% under 137Cs irradiation. The figure-of-merit (FoM) of the CsCu2I3:Li crystal has been estimated (about 0.8) under 252Cf radiation source. The crystal has a potential application for dual gamma–neutron detection.
900 photons per MeV and an energy resolution of 11.5% under 137Cs irradiation. The figure-of-merit (FoM) of the CsCu2I3:Li crystal has been estimated (about 0.8) under 252Cf radiation source. The crystal has a potential application for dual gamma–neutron detection.
Efficient neutron detectors have been used widely in many fields, such as homeland security, crystallography and medicine.1 Because of the increasing application of neutron detection, scintillators that can simultaneously detect or distinguish neutrons and gamma rays have attracted wide attention. A number of inorganic scintillating crystals containing 6Li have been discovered and developed for neutron detection, including LiI:Eu (ref. 2) and elpasolite-structured crystals like Cs2LiYCl6:Ce (CLYC:Ce),3 Cs2LiLaBr6:Ce (CLLB:Ce),4 and LiCaAlF6:Eu.5 However, these crystals have some disadvantages, such as extremely high hygroscopicity and incongruent melting.
In recent years, because of the advantages of confinement exciton emission, a large Stokes shift, good air stability and high photoluminescence quantum yield (PLQY), perovskite-structured metal halide materials have attracted more and more attention for radiation detectors.6–8 At present, many promising Cu-based low-dimensional perovskite scintillators with excellent scintillation properties have been discovered, including Cs3Cu2I5 (ref. 9) and CsCu2I3.10 They can be used as general scintillators to detect a wide energy range from soft X-ray to hard gamma radiation. Recently, Tl+ doped Cs3Cu2I5 single crystal was developed and has a high light output of 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons per MeV under 137Cs source. Meanwhile, it presents an extremely low X-ray induced afterglow of 0.03% at 10 ms and an excellent energy resolution of 3.4% at 662 keV.11 In our previous work, a 6Li+ doped Cs3Cu2I5 crystal was also prepared and presented excellent neutron and gamma discrimination (FoM > 2.0).12 However, the Cs3Cu2I5 crystal still undergoes incongruent melting. Fortunately, the CsCu2I3 crystal exhibits congruent melting and has a lower melting point (371 °C) and good air stability. It has the great advantage of the preparation of large-size single crystals. Moreover, as previously reported,13 the pure CsCu2I3 single crystal has a high light yield (16
000 photons per MeV under 137Cs source. Meanwhile, it presents an extremely low X-ray induced afterglow of 0.03% at 10 ms and an excellent energy resolution of 3.4% at 662 keV.11 In our previous work, a 6Li+ doped Cs3Cu2I5 crystal was also prepared and presented excellent neutron and gamma discrimination (FoM > 2.0).12 However, the Cs3Cu2I5 crystal still undergoes incongruent melting. Fortunately, the CsCu2I3 crystal exhibits congruent melting and has a lower melting point (371 °C) and good air stability. It has the great advantage of the preparation of large-size single crystals. Moreover, as previously reported,13 the pure CsCu2I3 single crystal has a high light yield (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons per MeV) and a good energy resolution of 7.8% under 137Cs source at 662 keV. Similarly, the CsCu2I3 crystal has potential application in the neutron detection field by doping with Li+. In this paper, an environmentally friendly scintillator with good air-stability for dual gamma-ray and neutron detection is developed by enriched 6Li+ doping. The single crystal of 6Li-doped CsCu2I3 has a scintillation yield of 10
000 photons per MeV) and a good energy resolution of 7.8% under 137Cs source at 662 keV. Similarly, the CsCu2I3 crystal has potential application in the neutron detection field by doping with Li+. In this paper, an environmentally friendly scintillator with good air-stability for dual gamma-ray and neutron detection is developed by enriched 6Li+ doping. The single crystal of 6Li-doped CsCu2I3 has a scintillation yield of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 photons per MeV under 137Cs source at 662 keV. The FoM value is estimated to be about 0.8 under 252Cf source.
900 photons per MeV under 137Cs source at 662 keV. The FoM value is estimated to be about 0.8 under 252Cf source.
Anhydrous high-purity CuI (99.999%, ALDRICH), CsI and 6LiI (99.99%, 6Li enrichment above 95%, APL Engineered Materials, Inc.) were used as starting materials. High-quality Li+-doped CsCu2I3 single crystals with a size of Φ 12 mm × 30 mm were grown successfully via a self-seeding vertical Bridgman technique with a capillary tube, as shown in Fig. S1a (ESI†). The temperature field for CsCu2I3 growth is shown in Fig. S1b.† The experimental results show that the CsCu2I3 crystal is easy to crack during the cooling and machining processes, as shown in Fig. S1c.† In order to avoid structural defects and crystal cracks, a suitable temperature gradient (20 °C cm−1), slow growth rate (<1 mm h−1), and cooling rate (<5 °C h−1) are crucial for obtaining a high-quality CsCu2I3 crystal. The incorporation of Li ions was confirmed by XRD and inductively coupled plasma mass spectrometry (ICP-MS). Furthermore, the PLQY, X-ray excited emission luminescence (XEL) and decay time were also measured. Finally, the application potential of CsCu2I3:Li crystals in the field of dual gamma–neutron detection is explored. The detail of the growth process and characterization methods can be seen in the ESI.†
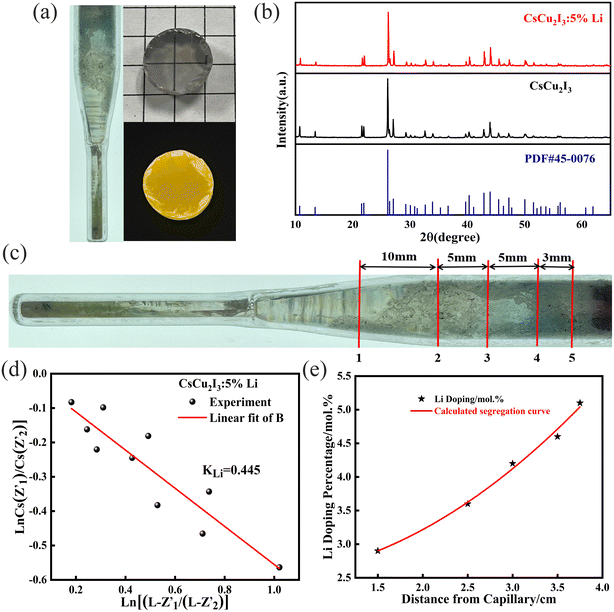
The transparent and free of impurities crystal sample with a size of Φ 12 mm × 3 mm was machined, as shown in Fig. 1a. Bright yellow light can be observed under ultraviolet light. The powder XRD patterns of the as-grown CsCu2I3 and CsCu2I3:6Li are shown in Fig. 1b. The patterns were matched well with that of the PDF card #45-0076,14 and no other phase can be identified. Therefore, the Li+ doped CsCu2I3 crystal still belongs to the orthogonal space group Cmcm. Two spatially oriented [CuI4]3− tetrahedral units are alternately connected to form a 1D [Cu2I6]4− tetrahedral unit chain separated by Cs, thus forming the 1D electronic structure of CsCu2I3,15 as exhibited in Fig. S2a.† This unique localized structure can result in the formation of self-trapping excitons. From Fig. S2b,† it can be found that the position of diffraction peaks shifts to a higher angle after Li+ doping. The radius of Li+ (0.59 Å) is closer to Cu+ (0.60 Å), while it is far less than the radius of Cs+ (1.74 Å). Therefore, the crystal lattice shrinks when Cu+ is replaced by Li+. It is rational that Li+ tends to substitute the site of Cu+.
In order to further prove the successful incorporation of Li ions and calculate the segregation coefficient of Li+ in the CsCu2I3 crystal, the actual Li concentration was measured by an ICP-MS method. First, five different positions were determined along the direction of the CsCu2I3:5mol%Li crystal growth axis for a small amount of sampling, as shown in Fig. 1c. The Li+ doping concentration was confirmed and increased from 2.92 mol% to 5.13 mol% along the direction of the crystal growth, as listed in Table S1.† Because of the slow growth rate of the CsCu2I3 crystal, the growth process can be regarded as quasi-static. Therefore, the segregation coefficient KLi of the CsCu2I3:5mol%Li crystal can be calculated using the following formula:16
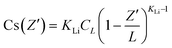 | (1) |
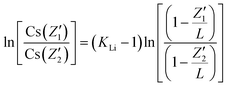 | (2) |
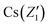 ) and (Z2,
) and (Z2, 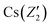 ), respectively. A segregation coefficient KLi of 0.45 was obtained by linear fitting in the CsCu2I3 crystal, as shown in Fig. 1d. The calculated curve is in good agreement with the actual doping concentration, as shown in Fig. 1e. The segregation coefficient is low (<1), indicating that a large number of Li ions will be enriched in the tail of the CsCu2I3 crystal.
), respectively. A segregation coefficient KLi of 0.45 was obtained by linear fitting in the CsCu2I3 crystal, as shown in Fig. 1d. The calculated curve is in good agreement with the actual doping concentration, as shown in Fig. 1e. The segregation coefficient is low (<1), indicating that a large number of Li ions will be enriched in the tail of the CsCu2I3 crystal.
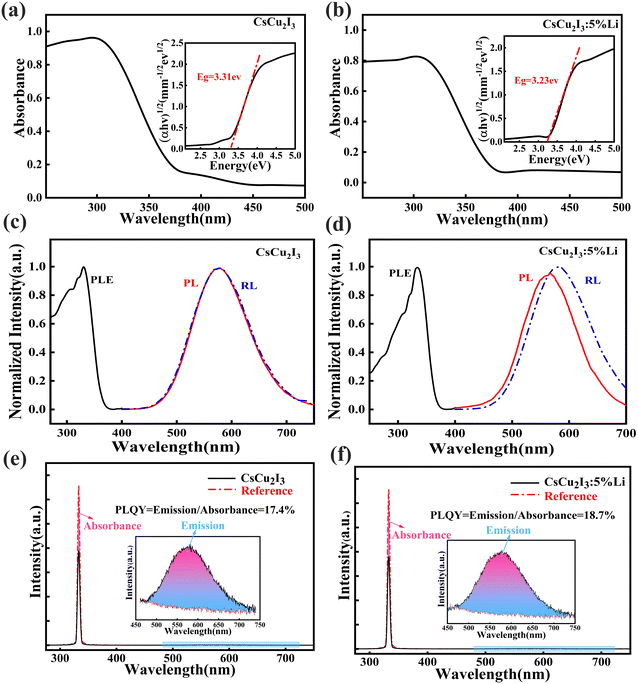
The optical absorption spectra of pure CsCu2I3 and CsCu2I3:5 mol%Li crystals are shown in Fig. 2(a and b), and the inset is the fitted optical band gap. Based on the onset of the light absorption edge, the optical band gaps of pure CsCu2I3 and 6Li+-doped CsCu2I3 crystals are calculated to be 3.31 eV and 3.23 eV, respectively. The band gap was narrowed after Li+ doping. In order to discuss the effect of Li+ doping on the luminescence properties, the PL, PLE, and RL spectra of pure and Li+-doped CsCu2I3 were measured, as shown in Fig. 2(c and d). The Li+-doped CsCu2I3 crystal has a broad excitation and emission bands peaking at 334 and 575 nm, respectively, which are consistent with those of the pure CsCu2I3 crystal. The Li+-doped CsCu2I3 crystal still has a large Stokes shift of 1.54 eV and no self-absorption. The RL spectra under X-ray excitation are similar to the PL spectra with a slight redshift. The red shift maybe related with the ionization luminescence mechanism, which is more efficient in capturing charge carriers and can be more observed in RL spectra, as discussed in ref. 17. Obviously, the strategy of Li+ doping does not introduce a new luminescent center. The PL decay curves of pure CsCu2I3, as well as CsCu2I3:5mol%Li, were measured at room temperature (λex = 334 nm; λem = 575 nm), as shown in Fig. S3.† The decay curves of pure CsCu2I3 and CsCu2I3:5mol%Li crystal can be well fitted using two-components. The decay time of the CsCu2I3 crystal is 82 ns (56%) and 1332 ns (44%). Compared to the pure CsCu2I3 crystal, the proportion of the fast component increased slightly from 56% to 63%, while the decay time of the slow component decreased from 1332 ns to 1204 ns after Li+ doping. The fast decay component is attributed to trap-assisted recombination of the photo-excited electrons and holes at the CsCu2I3 surface, while the slow decay component is related with the recombination inside CsCu2I3, as reported in a previous study.14 Obviously, Li+-doping was beneficial to improving the fast decay component. The PLQY of the pure and Li+-doped CsCu2I3 crystal was measured at room temperature. A blank quartz plate was also measured in an integrating sphere under excitation as a reference, using an absolute photoluminescence measurement system with an integrating sphere, as shown in Fig. 2(e and f). It is found that the PLQY improved from 17.4% to 18.7% when Li+ was introduced. This indicates that the PLQY has a slight improvement after Li+ doping.
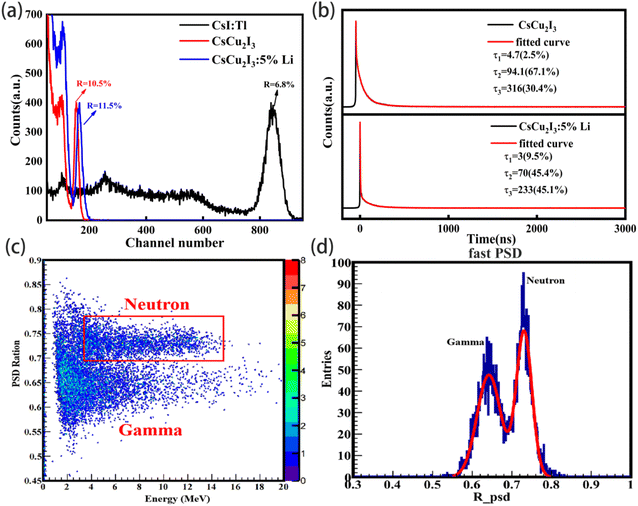
The energy resolution, light yield and decay time of the pure and CsCu2I3:5 mol%Li crystals were measured under 137Cs gamma-ray source at 662 keV with a Hamamatsu R6231-100 PMT. To estimate the absolute light yield of the crystal samples, the standard sample of the CsI:Tl crystal was also chosen and measured as a reference. The pulse height spectra are plotted in Fig. 3a. Under 137Cs excitation source, the absolute light yield of the CsI:Tl standard sample is about 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons per MeV. The absolute light yield of the pure CsCu2I3 and CsCu2I3:5 mol%Li crystal samples was estimated to be 10
000 photons per MeV. The absolute light yield of the pure CsCu2I3 and CsCu2I3:5 mol%Li crystal samples was estimated to be 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 248 photons per MeV and 10
248 photons per MeV and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 photons per MeV, respectively. The light yield is improved by Li+ doping, which is consistent with the results of PLYQ. But, the energy resolution of the CsCu2I3 crystal is deteriorated from 10.5% to 11.5% after Li+ doping at 662 keV, which is worse than that of the CsI:Tl crystal (E.R. = 6.8%). The deterioration of the energy resolution is related to the transparency of the crystal and its degree of coloring. The scintillation decay profiles of both crystals were measured at room temperature under gamma ray. All the decay curves can be fitted well using a three-exponential function, as shown in Fig. 3b. The decay time of the CsCu2I3 crystal is 4.7 ns (2.5%), 94.1 ns (67.1%) and 316 ns (30.4%), which should be ascribed to the self-trapped exciton recombination. The fast component was shortened from 94.1 ns to 70 ns, while the slow component was reduced to 233 ns after Li+ doping. It is possible that the charge carrier mobility will be improved by Li+ doping, as observed in ref. 18. However, the mechanism is still unclear. More experiments need to be done.
900 photons per MeV, respectively. The light yield is improved by Li+ doping, which is consistent with the results of PLYQ. But, the energy resolution of the CsCu2I3 crystal is deteriorated from 10.5% to 11.5% after Li+ doping at 662 keV, which is worse than that of the CsI:Tl crystal (E.R. = 6.8%). The deterioration of the energy resolution is related to the transparency of the crystal and its degree of coloring. The scintillation decay profiles of both crystals were measured at room temperature under gamma ray. All the decay curves can be fitted well using a three-exponential function, as shown in Fig. 3b. The decay time of the CsCu2I3 crystal is 4.7 ns (2.5%), 94.1 ns (67.1%) and 316 ns (30.4%), which should be ascribed to the self-trapped exciton recombination. The fast component was shortened from 94.1 ns to 70 ns, while the slow component was reduced to 233 ns after Li+ doping. It is possible that the charge carrier mobility will be improved by Li+ doping, as observed in ref. 18. However, the mechanism is still unclear. More experiments need to be done.
Finally, the performance of the Li+-doped CsCu2I3 crystal was examined further for dual gamma ray and neutron detection application. A two-dimensional PSD scatter plot was generated for the neutron and gamma-ray events under a 252Cf radioactive source, as shown in Fig. 3c. It can be recognized that the gamma signal and the neutron signal have a relatively clear separation. Fig. 3d shows one-dimensional projection of the neutron and gamma-ray events to the Y axis. Gaussian fitting of the neutron and gamma ray peaks is performed to calculate the number of merits (FoM), which is used to evaluate the discrimination performance of the neutrons and gamma rays, which can be defined by the following equation.19
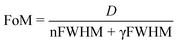 | (3) |
In summary, Li+-doped CsCu2I3 bulk single crystals were prepared by a Bridgman method for the first time. The Li+ effective segregation coefficient (keff) of the CsCu2I3:Li crystal was calculated to be about 0.45 using the deformed equilibrium segregation equation. The PLQY value increased slightly from 17.4% to 18.7% after Li+ doping. The absolute light yield of Li+-doped CsCu2I3 is about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 photons per MeV under 137Cs radiation, and Li+ doping also leads to a faster decay time under gamma-ray. Due to the successful combination of the 6Li isotope, the CsCu2I3 crystal material can be used in the field of dual gamma–neutron detection. The crystal quality and scintillation performance need to be improved in the future.
900 photons per MeV under 137Cs radiation, and Li+ doping also leads to a faster decay time under gamma-ray. Due to the successful combination of the 6Li isotope, the CsCu2I3 crystal material can be used in the field of dual gamma–neutron detection. The crystal quality and scintillation performance need to be improved in the future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support provided by the “Pioneer” and “Leading Goose” R&D Program of Zhejiang (Grant No. 2022C01046), the National Natural Science Foundation of China (NSFC) (No. 11975220, 51972291), the Natural Science Foundation of Zhejiang (No. LGG22E020001), and the Fundamental Research Funds for the Provincial Universities of Zhejiang (No. 2021YW14).References
- K. Yang, P. R. Menge and V. Ouspenski, IEEE Trans. Nucl. Sci., 2017, 64, 2406–2413 CAS.
- A. Syntfeld, M. Moszynski, R. Arlt, M. Balcerzyk, M. Kapusta, M. Majorov, R. Marcinkowski, P. Schotanus, M. Swoboda and D. Wolski, IEEE Trans. Nucl. Sci., 2005, 52, 3151–3156 CAS.
- W. M. Higgins, J. Glodo, U. Shirwadkar, A. Churilov, E. V. Loef, R. Hawrami, G. Ciampi, C. Hines and K. S. Shah, J. Cryst. Growth, 2010, 312, 1216–1220 CrossRef CAS.
- J. Lin, Q. H. Wei, D. Zhang, G. Tang, L. S. Qin and H. S. Shi, Cryst. Res. Technol., 2019, 54, 1900047 CrossRef CAS.
- T. Yanagida, N. Kawaguchi, Y. Fujimoto, K. Fukuda, Y. Yokota, A. Yamazaki, K. Watanabe, J. Pejchal, A. Uritani, T. Iguchi and A. Yoshikawa, Opt. Mater., 2011, 33, 1243–1247 CrossRef CAS.
- R. C. Lin, Q. Zhu, Q. L. Guo, Y. M. Zhu, W. Zheng and F. Huang, J. Phys. Chem. C, 2020, 124, 20469–20476 CrossRef CAS.
- F. Maddalena, L. Tjahjana, A. Xie, A. Arramel, S. Zeng, H. Wang, P. Coquet, W. Drozdowski, C. Dujardin, C. Dang and M. D. Birowosuto, Crystals, 2019, 9, 88 CrossRef.
- B. Yang, L. X. Yin, G. D. Niu, J. H. Yuan, K. H. Xue, Z. F. Tan, X. S. Miao, M. Niu, X. Y. Du, H. S. Song, E. Lifshitz and J. Tang, Adv. Mater., 2019, 31, 1904711 CrossRef CAS PubMed.
- X. M. Li, J. X. Chen, D. D. Yang, X. Chen, D. L. Geng, L. F. Jiang, Y. Wu, C. F. Meng and H. B. Zeng, Nat. Commun., 2021, 12, 1–9 CrossRef PubMed.
- R. C. Lin, Q. L. Guo, Q. Zhu, Y. M. Zhu, W. Zheng and F. Huang, Adv. Mater., 2019, 31, 1905079 CrossRef CAS PubMed.
- S. L. Cheng, M. Nikl, A. Beitlerova, R. Kucerkova, X. Y. Du, G. D. Niu, Y. C. Jia, J. Tang, G. H. Ren and Y. T. Wu, Adv. Opt. Mater., 2021, 9, 2100460 CrossRef CAS.
- P. Xiang, Q. H. Wei, C. E. Wang, P. Q. Cai, Y. F. Tong, G. Tang, F. Yang, H. S. Shi, Z. G. Liu and L. S. Qin, J. Mater. Chem. C, 2022, 10, 15400–15407 RSC.
- S. L. Cheng, A. Beitlerova, R. Kucerkova, E. Mihokova, M. Nikl, Z. Y. Zhou, G. H. Ren and Y. T. Wu, ACS Appl. Mater. Interfaces, 2021, 13, 12198–12202 CrossRef CAS PubMed.
- X. M. Mo, T. Li, F. C. Huang, Z. X. Li, Y. L. Zhou, T. Lin, Y. Ouyang, X. M. Tao and C. F. Pan, Nano Energy, 2021, 81, 105570 CrossRef CAS.
- Y. Hui, S. Y. Chen, R. C. Lin, W. Zheng and F. Huang, Mater. Chem. Front., 2021, 5, 7088–7107 RSC.
- X. G. Zhang, Z. Kang, Z. C. Cai, H. W. Zhai, Z. Yin, W. Q. Jie and T. Wang, J. Cryst. Growth, 2021, 573, 126308 CrossRef CAS.
- Q. Wang, C. E. Wang, Z. H. Wang, X. L. Sun, M. Nikl, X. P. Ouyang and Y. T. Wu, J. Phys. Chem. Lett., 2022, 13, 9066–9071 CrossRef CAS PubMed.
- F. Maddalena, A. Xie, A. Arramel, M. E. Witkowski, M. Makowski, B. Mahler, W. Drozdowski, T. Mariyappan, S. V. Springham, P. Coquet, C. Dujardin, M. D. Birowosuto and C. Dang, J. Mater. Chem. C, 2021, 9, 2504–2512 RSC.
- K. N. Li, X. P. Zhang, Q. Gui, P. Jin and G. Tian, Nucl. Sci. Tech., 2018, 29, 1–6 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ce01263d |
| This journal is © The Royal Society of Chemistry 2023 |