Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Access to isoindole-derived BODIPYs by an aminopalladation cascade†
Heinrich F.
von Köller‡
a,
Finn J.
Geffers‡
b,
Pedram
Kalvani
a,
Adrian
Foraita
b,
Patrick-Eric J.
Loß
b,
Burkhard
Butschke
 c,
Peter G.
Jones
d and
Daniel B.
Werz
c,
Peter G.
Jones
d and
Daniel B.
Werz
 *a
*a
aAlbert-Ludwigs-Universität Freiburg, Institute of Organic Chemistry, Albertstraße 21, 79104 Freiburg, Germany. E-mail: daniel.werz@chemie.uni-freiburg.de
bTechnische Universität Braunschweig, Institute of Organic Chemistry, Hagenring 30, 38106 Braunschweig, Germany
cAlbert-Ludwigs-Universität Freiburg, Institute of Inorganic and Analytical Chemistry, Albertstraße 21, 79104 Freiburg, Germany
dTechnische Universität Braunschweig, Institute of Inorganic and Analytical Chemistry, Hagenring 30, 38106 Braunschweig, Germany
First published on 13th November 2023
Abstract
Here, we present a new route to dyes of the BODIPY family. We first built up a N-Boc-protected dipyrromethene scaffold via an aminopalladation cascade. Subsequentially, the pyrrole moiety was deprotected and the BF2 unit inserted. Depending on the terminating reaction, BODIPYs with either aryl or alkynyl moieties were accessible.
Since their discovery in the 1960s by Treibs and Kreuzer, BODIPYs have become established in a wide range of different research areas.1–7 They have been recognized as valuable fluorescent tags,8,9 found application in bioimaging5,10–13 and been investigated as part of photodynamic therapy.14 Furthermore, they have been used as efficient photosensitizers,15,16 photocatalysts17,18 and photocages.19–24 BODIPYs are also considered as potential candidates for circularly polarized luminescence.25 Classically, dyes of the BODIPY family are built up by formation of a dipyrromethene through condensation and oxidation chemistry followed by the insertion of the chelating BF2 unit via BF3·OEt2. A broad pool of post-functionalization methods allows further decoration of the core structure, especially at the α- and β-positions, and therefore fine-tuning of the photophysical properties.26 However, the residue in the meso-position of BODIPYs is mainly restricted to alkyl and aryl groups because of the nature of the condensation precursors (often aldehydes and acyl chlorides). To allow modification at this position, a functional group suitable for cross-coupling reactions has to be pre-installed and then addressed by palladium-catalyzed cross-coupling chemistry.27–29 To complement classical textbook chemistry for assembling nitrogen-containing heterocycles, palladium-catalyzed reactions have also been developed that allow new modes of retrosynthesis and synthesis planning. This was achieved either in an aza-Wacker fashion with a PdII salt activating an alkene and allowing an amino group to attack,30,31 by an internal Buchwald–Hartwig type coupling32–35 or by oxidative addition of Pd0 into an N–O bond and subsequent Heck reaction.36 The latter strategy was first employed by Narasaka to synthesize pyrrole derivatives from O-pentafluorobenzoyloximes.37 Thereafter, this aminopalladation strategy was applied to the synthesis of pyridine, isoquinoline, indole and imidazole derivatives.38–42 Further mechanistic studies by Bower and co-workers revealed the electron-deficient P(3,5-(CF3)2C6H3)3 ligand to be generally more efficient than PPh3, whereas with electron-rich ligands such as P(tBu)3 a single electron pathway is favored, leading to the iminyl radical product instead of the aza-Heck product.43 In further work Bower presented the successful synthesis of perhydroindoles,44 dihydropyrroles,45 pyrrolidines and piperidines.46 Interception of the alkyl-Pd(II) intermediates by organometallic nucleophiles or alcohols was also demonstrated.47 Besides the use of oxime esters, oxidative addition into the N–O bond of N-(pentafluorobenzoyloxy)-amides followed by a Heck reaction also afforded access to various N-heterocycles.48,49 Our group employed N-(pentafluorobenzoyloxy)-amides to extend this concept to an aza-Heck reaction with internal alkynes, thereby obtaining tetrasubstituted enamines.50
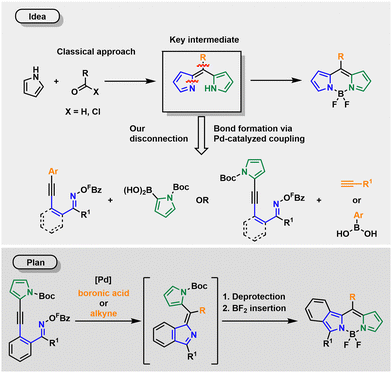
Inspired by the scope of this alkyne aminopalladation/Heck cascade, we envisioned a synthesis of dipyrromethenes 2, key intermediates in every BODIPY synthesis, via an aminopalladation cascade. Treating precursor 1 with Pd0 should result in an oxidative addition of Pd into the N–O bond of the oxime ester, followed by an intramolecular aza-Heck reaction leading to the formation of an isoindole moiety. In the presence of a base and a boronic acid or a terminal alkyne, Suzuki- or Sonogashira cross-couplings, respectively, should terminate this cascade. The required pyrrole moiety, if not already present in the precursor, might be introduced via a boronic acid. In both cases protection of the N–H is mandatory. Deprotection of the aminopalladation product followed by the treatment with BF3·OEt2 under basic conditions would then lead to the BODIPY scaffold (Scheme 1). To avoid β-hydride elimination after oxidative addition, R1 of the oxime ester 1 must not be a hydrogen atom.44
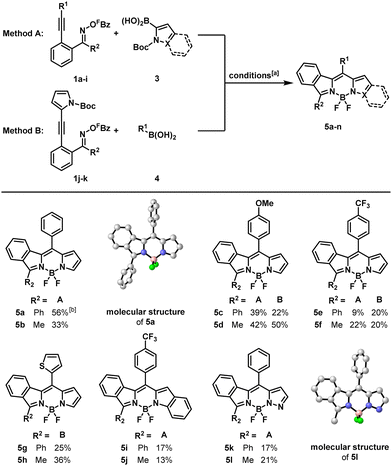
We started our investigations with precursor 1a and N-Boc-protected pyrrole boronic acid and subjected them to Kitamura's conditions51 to obtain the expected product in 56% yield. While DMF as a solvent halved the yield, 1,4-dioxane and toluene nearly shut down the reaction. Whereas triethylamine reduced the yield only slightly to 43%, inorganic bases (CsF, Cs2CO3) significantly decreased the yields. Additional ligands (JohnPhos, SPhos, dppe) had no beneficial effects on the yields, and the bidentate ligand dppe stopped the reaction completely. Even the ligand P(3,5-(CF3)2C6H3)3 only gave the product in 32% yield. Nevertheless, when the equivalents of the boronic acid were increased from 1.5 to 3.0, the reaction time could be reduced to 1 h (Table S2, see ESI†).
After the successful formation of the dipyrromethene, we treated it with oxalyl chloride in methanol to remove the Boc-protecting group.52 After aqueous work-up the crude product was reacted with BF3·OEt2 under basic conditions (Et3N) to insert the central BF2-unit, providing BODIPY 5a with a yield of 56% over three steps.
Since a mixture of (E)- and (Z)-isomers of intermediate 2a was formed, slightly raised temperatures of 50 °C were employed during the BF2 insertion step to allow isomerization. This synthesis was also performed on larger scale (1 mmol) to obtain BODIPY 5a in a slightly decreased yield of 46% over three steps.
With this optimized reaction sequence in hand (for Method A), we explored the scope of the reaction. First, we varied the aryl group (R1) that became the meso-substituent of the emerging BODIPY. When precursor 1c, bearing a p-methoxyphenyl group at this position, was subjected to our conditions, BODIPY 5c was obtained in 39%. A p-trifluoromethylphenyl group at the same position led to BODIPY 5e with a yield of only 9%. Next, we started from the acetophenone-derived precursors (R2 = Me). Employing the same residues for R1, BODIPYs 5b, 5d and 5f were formed with yields of 33%, 42% and 22%, respectively. Thereafter, we employed precursor 1g, already substituted with a pyrrole at the triple bond, and reacted it under the same conditions with various aryl boronic acids that now deliver the meso-residue (Method B). p-Methoxy and p-trifluoromethyl phenyl boronic acids gave BODIPYs 5c and 5e in 22% and 20% yield, respectively. Thienyl, indolinyl and pyrazolyl boronic acids gave BODIPYs 5g, 5i and 5k in 25%, 17% and 17% over 3 steps. The same boronic acids were also reacted with the acetophenone-derived precursor 1h. The resulting BODIPYs 5d, 5f, 5h, 5j and 5l were formed in improved yields of 50%, 20%, 36%, 13% and 21% (Scheme 2). It should be pointed out that, to the best of our knowledge, 5k and 5l are the first reported BODIPYs containing a pyrazole ring as part of their core structure. The structures of BODIPYs 5a, 5b and 5l were also elucidated by X-ray diffraction analyses (see also ESI†).55
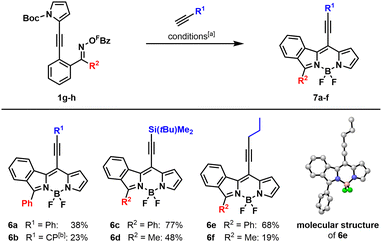
As a modification of the Pd-catalyzed cascade, we explored a Sonogashira reaction as the terminating step. Optimization studies of the reaction conditions revealed Pd(P(3,5-(CF3)2C6H3)3)3 to be the best catalyst together with triethylamine as base in THF. Both electron-richer (PPh3, PCy3) and also electron-poorer (P(C6F5)3) ligands proved to decrease the yield. Furthermore, it was shown that the presence of a copper co-catalyst is not beneficial to the reaction. Product formations also suffered from other organic and inorganic bases (Cs2CO3, Et2NH, DIPEA). When other solvents (1,4-dioxane, MeCN, DMF) were tried, a significant drop in yield was observed (Table S3, see ESI†).
After having established the conditions for the aminopalladation-Sonogashira cascade, we found that deprotection attempts with oxalyl chloride led to decomposition of the substrate instead of cleavage of the Boc group. Therefore, we applied TFA/DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at r.t. to obtain the deprotected dipyrromethene smoothly,53 into which then the BF2 unit was inserted.
1) at r.t. to obtain the deprotected dipyrromethene smoothly,53 into which then the BF2 unit was inserted.
Next, we explored the scope of this cascade reaction. Generally, the benzophenone-derived precursor 1g (R2 = Ph) gave the corresponding BODIPY in better yields than the acetophenone-derived precursor 1h (R2 = Me). Using TBS-protected acetylene and pent-1-yne as alkynes gave BODIPYs 6c and 6e in 77% and 68% yield for the benzophenone derivatives and BODIPYs 6d and 6f in 48% and 19% yield for the acetophenone derivatives. However, when phenyl acetylene and cyclopropyl acetylene were used as coupling partners for precursor 1g (R2 = Ph), BODIPYs 6a and 6b were obtained only in mediocre yields of 38% and 23%, respectively (Scheme 3). Subjecting phenyl acetylene and cyclopropyl acetylene to the reaction conditions with precursor 1h (R2 = Me) led to the corresponding BODIPYs in poor yields and with stability problems that resulted in major purification issues. Attempts to use ethyl propiolate as an electron-poor alkyne under the optimized reaction conditions failed completely. The structure of BODIPY 6e was also confirmed by X-ray diffraction analysis.55
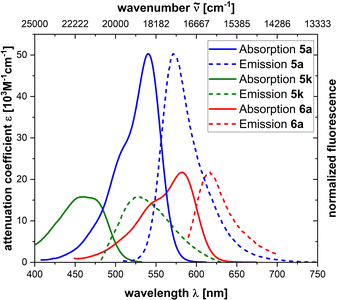
The photophysical properties of a selection of our prepared BODIPYs are summarized in Table S1 (see ESI†) and Fig. 1. BODIPYs 5a–5l absorbed and emitted light between 517 nm and 600 nm. Those bearing a phenyl group at the α-position showed a bathochromic shift compared to those having a methyl group at the same position. Compared to the effect of this substituent, the impact of the substituent at the meso-aryl group is low. In spite of high attenuation coefficients (∼40 × 103 M−1 cm−1), only poor fluorescence was observed (quantum yields for 5a, 5b, 5e, and 5f between 1% and 4%). These values can be explained by an almost free rotation of the meso-aryl group, which allows non-radiative decay processes of the excited state.54
BODIPYs 6a–6f showed absorption and emission maxima in the range 544–617 nm. Again, α-phenyl substituted BODIPYs were bathochromically shifted compared to the α-methyl substituted BODIPYs. Stokes shifts lay between 961 cm−1 and 1119 cm−1. In contrast to meso-aryl substituted BODIPYs, BODIPYs 6e and 6f exhibited a distinct fluorescence with quantum yields of 59% and 84%, nicely demonstrating the effect of the rigid alkynyl moiety.
A marked blue shift was observed for pyrazole-derived BODIPYs 5k and 5l with 459 and 434 nm, respectively, as absorption maxima and 530 and 507 nm as emission maxima. Furthermore, their Stokes shifts significantly increased to 2919 cm−1 and 3318 cm−1, respectively. At the same time the attenuation also dropped to 15.8 and 16.2 × 103 M−1 cm−1, respectively. Additionally, quantum yields decreased below 1%, although we were still able to measure fluorescence spectra.
To conclude, we presented a novel approach to dipyrromethenes, key intermediates in the BODIPY synthesis, which employed an aminopalladation cascade starting from oxime esters. The oxidative addition of Pd into the N–O bond was followed by an intramolecular aza-Heck-reaction with an alkyne and was terminated by either a Suzuki or a Sonogashira cross-coupling reaction. The emerging N-Boc-protected diyprromethenes were deprotected and treated with BF3·OEt2 under basic conditions to obtain unsymmetrical isoindole-derived BODIPYs. A broad range of boronic acids can be used for this transformation. For the Sonogashira-terminated cascade reaction, terminal alkynes were used. Investigation of the photophysical properties revealed that a pyrazole-derived BODIPY analogue shows a significant blue-shift compared to commonly used BODIPYs.
This research was supported by the Studienstiftung des deutschen Volkes (PhD Fellowship to H. F. v. K.), DAAD (PhD Fellowship to P. K.) and financial support from the Deutsche Forschungsgemeinschaft (DFG) – EXC-2193/1 (livMatS Cluster of Excellence).
Conflicts of interest
The authors declare no conflict of interest.Notes and references
- A. Treibs and F.-H. Kreuzer, Justus Liebigs Ann. Chem., 1968, 718, 208–223 CrossRef CAS.
- G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS PubMed.
- A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS PubMed.
- R. Ziessel, G. Ulrich and A. Harriman, New J. Chem., 2007, 31, 496–501 RSC.
- L. J. Patalag, L. P. Ho, P. G. Jones and D. B. Werz, J. Am. Chem. Soc., 2017, 139, 15104–15113 CrossRef CAS PubMed.
- A. Patra, L. J. Patalag, P. G. Jones and D. B. Werz, Angew. Chem., Int. Ed., 2021, 60, 747–752 CrossRef CAS PubMed.
- L. J. Patalag, J. Hoche, M. Holzapfel, A. Schmiedel, R. Mitric, C. Lambert and D. B. Werz, J. Am. Chem. Soc., 2021, 143, 7414–7425 CrossRef CAS PubMed.
- L. Mendive-Tapia, L. Miret-Casals, N. D. Barth, J. Wang, A. de Bray, M. Beltramo, V. Robert, C. Ampe, D. J. Hodson, A. Madder and M. Vendrell, Angew. Chem., Int. Ed., 2023, 62, e202302688 CrossRef CAS PubMed.
- A. Barattucci, C. M. A. Gangemi, A. Santoro, S. Campagna, F. Puntoriero and P. Bonaccorsi, Org. Biomol. Chem., 2022, 20, 2742–2763 RSC.
- T. Wang, Z. Jiang and Z. Liu, Org. Lett., 2023, 25, 1638–1642 CrossRef CAS PubMed.
- C. S. Wijesooriya, J. A. Peterson, P. Shrestha, E. J. Gehrmann, A. H. Winter and E. A. Smith, Angew. Chem., Int. Ed., 2018, 57, 12685–12689 CrossRef CAS PubMed.
- T. Kowada, H. Maeda and K. Kikuchi, Chem. Soc. Rev., 2015, 44, 4953–4972 RSC.
- L. J. Patalag, S. Ahadi, O. Lashchuk, P. G. Jones, S. Ebbinghaus and D. B. Werz, Angew. Chem., Int. Ed., 2021, 60, 8766–8771 CrossRef CAS PubMed.
- L. Schneider, M. Kalt, S. Koch, S. Sithamparanathan, V. Villiger, J. Mattiat, F. Kradolfer, E. Slyshkina, S. Luber, M. Bonmarin, C. Maake and B. Spingler, J. Am. Chem. Soc., 2023, 145, 4534–4544 CrossRef CAS PubMed.
- T. Yogo, Y. Urano, Y. Ishitsuka, F. Maniwa and T. Nagano, J. Am. Chem. Soc., 2005, 127, 12162–12163 CrossRef CAS PubMed.
- R. Prieto-Montero, R. Sola-Llano, R. Montero, A. Longarte, T. Arbeloa, I. López-Arbeloa, V. Martínez-Martínez and S. Lacombe, Phys. Chem. Chem. Phys., 2019, 21, 20403–20414 RSC.
- M. A. Filatov, Org. Biomol. Chem., 2019, 18, 10–27 RSC.
- P. de Bonfils, L. Péault, P. Nun and V. Coeffard, Eur. J. Org. Chem., 2021, 1809–1824 CrossRef CAS.
- R. Weber, K. Chok, S. Junek, C. Glaubitz and A. Heckel, Chem. – Eur. J., 2023, 29, e202300149 CrossRef CAS PubMed.
- P. Shrestha, A. Mukhopadhyay, K. C. Dissanayake and A. H. Winter, J. Org. Chem., 2022, 87, 14334–14341 CrossRef CAS PubMed.
- J. A. Peterson, C. Wijesooriya, E. J. Gehrmann, K. M. Mahoney, P. P. Goswami, T. R. Albright, A. Syed, A. S. Dutton, E. A. Smith and A. H. Winter, J. Am. Chem. Soc., 2018, 140, 7343–7346 CrossRef CAS PubMed.
- D. Kand, P. Liu, M. X. Navarro, L. J. Fischer, L. Rousso-Noori, D. Friedmann-Morvinski, A. H. Winter, E. W. Miller and R. Weinstain, J. Am. Chem. Soc., 2020, 142, 4970–4974 CrossRef CAS PubMed.
- A. Poryvai, M. Galkin, V. Shvadchak and T. Slanina, Angew. Chem., Int. Ed., 2022, 61, e202205855 CrossRef CAS PubMed.
- P. Shrestha, D. Kand, R. Weinstain and A. H. Winter, J. Am. Chem. Soc., 2023, 145 Search PubMed , asap.
- T. Mori, Circularly Polarized Luminescence of Isolated Small Organic Molecules, Springer Singapore, Singapore, 2020 Search PubMed.
- R. G. Clarke and M. J. Hall, Adv. Heterocycl. Chem., 2019, 128, 181–261 CrossRef CAS.
- T. V. Goud, A. Tutar and J.-F. Biellmann, Tetrahedron, 2006, 62, 5084–5091 CrossRef CAS.
- E. Lager, J. Liu, A. Aguilar-Aguilar, B. Z. Tang and E. Peña-Cabrera, J. Org. Chem., 2009, 74, 2053–2058 CrossRef CAS PubMed.
- V. Leen, P. Yuan, L. Wang, N. Boens and W. Dehaen, Org. Lett., 2012, 14, 6150–6153 CrossRef CAS PubMed.
- B. Åkermark, J. E. Bäckvall, L. S. Hegedus, K. Zetterberg, K. Siirala-Hansén and K. Sjöberg, J. Organomet. Chem., 1974, 72, 127–138 CrossRef.
- L. S. Hegedus, G. F. Allen, J. J. Bozell and E. L. Waterman, J. Am. Chem. Soc., 1978, 100, 5800–5807 CrossRef CAS.
- A. S. Guram, R. A. Rennels and S. L. Buchwald, Angew. Chem., Int. Ed. Engl., 1995, 34, 1348–1350 CrossRef CAS.
- J. P. Wolfe, R. A. Rennels and S. L. Buchwald, Tetrahedron, 1996, 52, 7525–7546 CrossRef CAS.
- J. P. Wolfe, S. Wagaw, J.-F. Marcoux and S. L. Buchwald, Acc. Chem. Res., 1998, 31, 805–818 CrossRef CAS.
- A. J. Peat and S. L. Buchwald, J. Am. Chem. Soc., 1996, 118, 1028–1030 CrossRef CAS.
- A. Minatti and K. Muñiz, Chem. Soc. Rev., 2007, 36, 1142–1152 RSC.
- H. Tsutsui and K. Narasaka, Chem. Lett., 1999, 45–46 CrossRef CAS.
- H. Tsutsui and K. Narasaka, Chem. Lett., 2001, 526–527 CrossRef CAS.
- M. Kitamura, S. Chiba, O. Saku and K. Narasaka, Chem. Lett., 2002, 606–607 CrossRef CAS.
- M. Kitamura and K. Narasaka, Chem. Rec., 2002, 2, 268–277 CrossRef CAS PubMed.
- S. Chiba, M. Kitamura, O. Saku and K. Narasaka, Bull. Chem. Soc. Jpn., 2004, 77, 785–796 CrossRef CAS.
- S. Zaman, K. Mitsuru and A. D. Abell, Org. Lett., 2005, 7, 609–611 CrossRef CAS PubMed.
- N. J. Race, A. Faulkner, M. H. Shaw and J. F. Bower, Chem. Sci., 2016, 7, 1508–1513 RSC.
- A. Faulkner and J. F. Bower, Angew. Chem., Int. Ed., 2012, 51, 1675–1679 CrossRef CAS PubMed.
- N. J. Race and J. F. Bower, Org. Lett., 2013, 15, 4616–4619 CrossRef CAS PubMed.
- I. R. Hazelden, R. C. Carmona, T. Langer, P. G. Pringle and J. F. Bower, Angew. Chem., Int. Ed., 2018, 57, 5124–5128 CrossRef CAS PubMed.
- A. Faulkner, J. S. Scott and J. F. Bower, J. Am. Chem. Soc., 2015, 137, 7224–7230 CrossRef CAS PubMed.
- I. R. Hazelden, X. Ma, T. Langer and J. F. Bower, Angew. Chem., Int. Ed., 2016, 55, 11198–11202 CrossRef CAS PubMed.
- C. Jing, B. T. Jones, R. J. Adams and J. F. Bower, J. Am. Chem. Soc., 2022, 144, 16749–16754 CrossRef CAS PubMed.
- F. J. Geffers, F. R. Kurth, P. G. Jones and D. B. Werz, Chem. – Eur. J., 2021, 27, 14846–14850 CrossRef CAS PubMed.
- M. Kitamura, Y. Moriyasu and T. Okauchi, Synlett, 2011, 643–646 CrossRef CAS.
- N. George, S. Ofori, S. Parkin and S. G. Awuah, RSC Adv., 2020, 10, 24017–24026 RSC.
- D. M. Shendage, R. Fröhlich and G. Haufe, Org. Lett., 2004, 6, 3675–3678 CrossRef CAS PubMed.
- A. B. Zaitsev, R. Méallet-Renault, E. Y. Schmidt, A. I. Mikhaleva, S. Badré, C. Dumas, A. M. Vasil'tsov, N. V. Zorina and R. B. Pansu, Tetrahedron, 2005, 61, 2683–2688 CrossRef CAS.
- Deposition Numbers 2298671–2298673, and 2284407 contain the supplementary crystallographic data for this paper†.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2284407 and 2298671–2298673. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc04913b |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif)