Open Access Article
Open Access ArticleC1 functionalization of imidazo heterocycles via carbon dioxide fixation†
Michael
Fragkiadakis
a,
Paraskevi-Kleio
Anastasiou
a,
Ioannis
Volyrakis
a,
Apostolos
Pantousas
b,
Constantinos C.
Stoumpos
 b and
Constantinos G.
Neochoritis
b and
Constantinos G.
Neochoritis
 *a
*a
aDepartment of Chemistry, University of Crete, Voutes, 70013, Heraklion, Greece. E-mail: kneochor@uoc.gr
bDepartment of Materials Science & Technology, University of Crete, Voutes, 70013, Heraklion, Greece
First published on 13th November 2023
Abstract
Utilizing CO2 as a one-carbon building block in the preparation of high-value chemical entities is a cornerstone of modern organic synthesis. Herein, we exemplify this strategy through a mild, one-pot methodology that gives rapid access to N-heteroaryl substituted 6-, 8- and 9-membered carbamates via CO2 fixation.
The United Nations launched seventeen “Sustainable Development Goals (SDGs)” in 2015. The 7th SDG is dedicated to affordable, renewable, efficient and clean energy.1 In addition, the EU has set targets of at least 55% cuts in greenhouse gas emissions (from 1990 levels), a 32% share for renewable energy and 32.5% improvement in energy efficiency under the European Green Deal scheme.2 To achieve these goals attaining self-sufficiency in sustainable energy and raw materials, scaling up renewable carbon and circular carbon feedstocks are of note. A net-zero carbon future for synthetic methodology has to be the governing paradigm; thus, development of C1 chemistry is a key challenge.3
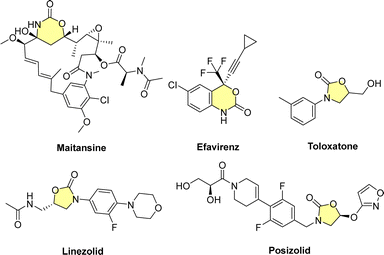
Employing carbon dioxide as a building block has gained a prominent place in C1 chemistry, especially in recent years; on one hand it is a waste that has to be minimized,4 on the other it is an inexpensive and abundant C1-building block.5–13 However, CO2 valorization still remains problematic due to its thermodynamic stability and chemical inertness; therefore, highly reactive or sensitive substrates in combination with harsh conditions are needed in many cases. Catalysed coupling of CO2 with epoxides and aziridines towards polycarbonates/polycarbamates14 and cyclic carbonates/carbamates15,16 and photocatalyzed electrophilic addition of CO2 to enones,17 represent some of the most recent methodologies using CO2 as a feedstock. Cyclic carbamates belong to a particular class of heterocycles where C1 chemistry based on CO2 fixation has been extensively employed in order to replace traditional methods that employed the highly toxic phosgene gas and CO.18,19 These entities, in particular, the N-aryl five- or six-membered cyclic carbamates (namely, oxazolidinones and oxazinanones – NAOs), have attracted considerable attention from synthetic chemists not only because they are extremely useful building blocks,20–22 but also due to the fact that they have been widely utilized as therapeutic agents; there are anti-cancer, antiviral and anti-inflammatory NAOs.23,24 Most importantly, NAOs have proved highly efficient as broad spectrum Gram-positive antibiotics (Fig. 1).25 In addition, due the high significance of medium-size N-heterocycles we still need more tools in our arsenal for their efficient and sustainable construction.26–29
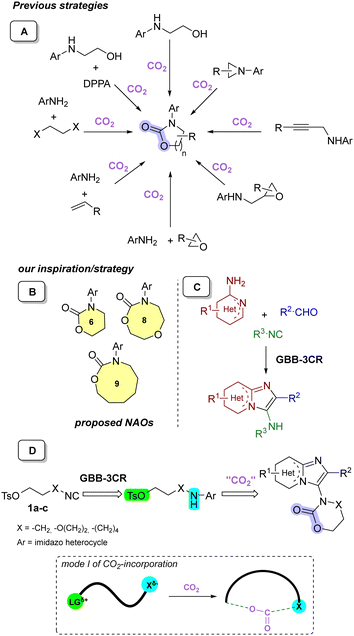
The direct fixation of CO2 in the synthesis of NAOs requires prefunctionalized, highly reactive substrates, i.e. propargyl amines30,31 or N-aziridines,32–34 amino alcohols35,36 and epoxy amines,37–39 even in solvent-free procedures.40 Three-component reactions involving CO2/haloalkane/anilines,41,42 CO2/epoxide/anilines43 and CO2/alkene/anilines44 have also been reported (Scheme 1A).45 The main issues with these synthetic processes, which can be divided into three modes of action,11 are the relatively limited substrate scope; the scarcity of examples bearing aryl groups, especially heteroaryls, the frequent employment of high pressure CO2 (>10 bar) and the use of metal catalysts/organocatalysts.46–48
In order to overcome these issues, we envisioned providing access to a variety of unprecedented N-aryl substituted oxazinanones via an efficient and straightforward synthetic pathway. Moreover, to the best of our knowledge some of our target scaffolds, such as the 9-membered N-aryl substituted oxazonanones and the 8-membered N-aryl substituted dioxazocanones, are currently unknown entities (Scheme 1B). The Groebke–Blackburn–Bienaymé three component reaction (GBB-3CR)49–51 was chosen for two main reasons beyond its versatility with three points of diversification and the ease with which it can be performed (Scheme 1C). First of all, it would give access to a variety of imidazo[1,2-a]-heterocycles, including, the privileged scaffold imidazo[1,2-a]pyridine, a well-known moiety in many marketed drugs, such as zolpidem, minodronic acid, etc.52 Secondly, we hypothesized that our recently developed isocyano alkyl tosylates 1a–c in a GBB-3CR53 can constitute an excellent mode I-substrate11 with a nucleophilic center (Xδ−, highlighted in cyan), which triggers the CO2 incorporation, combined with an electrophilic site (LGδ+, highlighted in green) (Scheme 1D).
Hence, after quite some experimentation (Table S1, ESI†), we found that treatment of the GBB-derivatives 2a–j (used without purification) with 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and under CO2 flow (1 atm) in DMF at room temperature led to the targeted carbamates 3a–j in only 1 hour with good to very good yields of the desired products (Scheme 2). The substrate scope is indeed broad; aliphatic and (hetero)aromatic aldehydes, various substituted 2-amino pyridines and thiazoles with different isocyano alkyl tosylates 1a,b all afforded the corresponding carbamates 3 (Schemes 2 and 3). We were also able to access the unprecedented 8-membered dioxazocanone 3j. Notably, substrates with groups such as cyclopropyl units and double bonds yielded the corresponding carbamates 3h and 3i, respectively, in high yields without any side reactions being observed (see mechanism, ESI,† Scheme S1). The reaction is scalable, as it was performed on a 5 mmol scale without any issues or a substantial difference in yield. The single crystal structures of 3a and 3g reveal a T-shape orientation of the imidazopyridine scaffold with the carbamate. Carbon dioxide fixation was also examined in the absence of any base, however, unreacted starting material was recovered. In addition, when the GBB-3CR adduct 2a was treated with either DBU or even with carbonates like NaHCO3 in the absence of carbon dioxide flow, deprotection of the tosyloxy group yielding compound 4 was seen. Probably, there is a –OTs exchange towards an intermediate carbonate with its subsequent basic hydrolysis to the corresponding alcohol.54,55
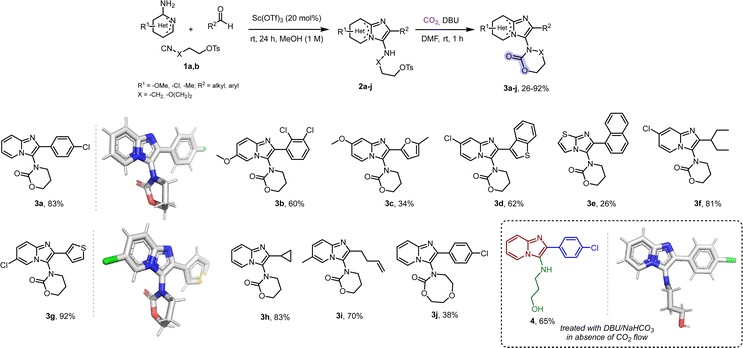 | ||
| Scheme 2 The synthesis of substituted 6- and 8-membered carbamates through a one-pot process. The yields referred to are overall yields after two steps. X-ray structures of 3a (CCDC 2294778†) and 3g (CCDC 2294775†) were obtained. Deprotection of the GBB adduct 2a gave the corresponding alcohol 4, shown with its single crystal structure (CCDC 2294777†). | ||
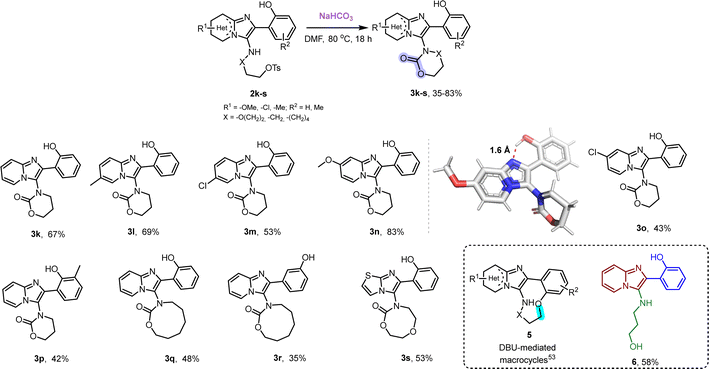 | ||
| Scheme 3 The synthesis of substituted 6-, 8- and 9-membered carbamates in the presence of substituted salicyl aldehydes in a one-pot process. The yields referred to are overall yields after two steps. X-ray structure of 3n (CCDC 2294776†) was obtained. | ||
Carbonate salts as C1 feedstocks instead of carbon dioxide have rarely been applied to the synthesis of oxazolidinones and especially to the case of oxazinanones.56–59 Very interestingly, when we employed a salicyl aldehyde as our oxo-component in the GBB-3CR, we noticed that just treatment with NaHCO3 in the absence of any metal-catalyst or metal-additive afforded the carbamates 3k–s in high yields (Scheme 3).
The reaction is also working at rt with slightly worst yields and conversion. ortho- and meta-substituted salicyl aldehydes yielded the corresponding products; dependent on the chain size of the isocyano alkyl tosylates (1a–c), different ring sizes of carbamates were formed including six-membered rings and the difficult to access eight- and nine-membered rings.60 The single crystal structure of 3n features not only the T-shape orientation, but also an intramolecular hydrogen bond N–H of 1.6 Å, rigidifying the scaffold. Using an alternative base, such as Et3N, which does not include a carbonate moiety, led to the deprotection of the tosyl group to yield the corresponding alcohol 6 (see mechanism, ESI,† Scheme S2). Treatment with DBU gave rise to medium-sized macrocycles.53 After treatment of the GBB-3CR adducts without the phenolic group, such as 2a–j, with NaHCO3, we observed deprotection of –OTs group and formation of 4 instead of re-fixing of CO2 (Scheme 2).
Taking advantage of the structural features of the GBB-3CR scaffolds, we were able to construct a library of 19 diverse N-heteroaryl substituted carbamates with different ring sizes via fixing CO2. The substrate scope is broad and the C1 feedstock proceeds either through base-catalyzed reaction under a CO2 flow (1 atm) or by employing a carbonate salt in the presence of salicyl aldehydes. The procedures occur rapidly in a one-pot fashion under mild reaction conditions without any metal-catalyst or metal-additive. Single crystal structures reveal the structural conformation of the unprecedented carbamate products.
C. G. N. conceptualized and directed the project. M. F., P. K. A and I. V. performed the syntheses and collected the analytical data. A. P and C. S. determined the single crystal X-ray structures. C. G. N. and M. F. contributed to the manuscript writing. The research project (to C. G. N) was supported by the Hellenic Foundation for Research and Innovation (H. F. R. I.) under the ‘‘2nd Call for H. F. R. I. Research Projects to support Post-Doctoral Researchers’’ (Project Number: 0911) and Empeirikion Idryma. We acknowledge the mass spectrometry facility of the University of Crete and we would like sincerely thank Dr Tamsyn Montagnon for her valuable remarks.
Conflicts of interest
There are no conflicts to declare.Notes and references
- United Nations Department of Economic and Social Affairs (UNDESA) and the Secretariat of the United Nations Framework Convention on Climate Change (UNFCCC), 2022.
- C. Fetting, The European Green Deal, 2020 Search PubMed.
- X.-F. Wu, P. J. Dyson and B. A. Arndtsen, J. Org. Chem., 2023, 88, 4891–4893 CrossRef CAS PubMed.
- W. Lamb and G. Grassi, Emissions Gap Report 2022, 2022 Search PubMed.
- S. Dabral and T. Schaub, Adv. Synth. Catal., 2019, 361, 223–246 CrossRef CAS.
- Q. Liu, L. Wu, R. Jackstell and M. Beller, Nat. Commun., 2015, 6, 5933 CrossRef PubMed.
- T. Sakakura, J.-C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365–2387 CrossRef CAS PubMed.
- M. Mikkelsen, M. Jørgensen and F. C. Krebs, Energy Environ. Sci., 2010, 3, 43–81 RSC.
- A. Pinaka and G. C. Vougioukalakis, Coord. Chem. Rev., 2015, 288, 69–97 CrossRef CAS.
- M. Aresta, A. Dibenedetto and A. Angelini, Chem. Rev., 2014, 114, 1709–1742 CrossRef CAS PubMed.
- S. Wang and C. Xi, Chem. Soc. Rev., 2019, 48, 382–404 RSC.
- S. Pradhan, S. Roy, B. Sahoo and I. Chatterjee, Chem. – Eur. J., 2021, 27, 2254–2269 CrossRef CAS PubMed.
- W. Hang, D. Li, S. Zou and C. Xi, J. Org. Chem., 2023, 88, 5007–5014 CrossRef CAS PubMed.
- T. Kessaratikoon, T. Theerathanagorn, D. Crespy and V. D’Elia, J. Org. Chem., 2023, 88, 4894–4924 CrossRef CAS PubMed.
- T. Fujihara and Y. Tsuji, Front. Chem., 2019, 7, 1–8 CrossRef PubMed.
- H. Koizumi, K. Takeuchi, K. Matsumoto, N. Fukaya, K. Sato, M. Uchida, S. Matsumoto, S. Hamura, J. Hirota, M. Nakashige and J.-C. Choi, J. Org. Chem., 2023, 88, 5015–5024 CrossRef CAS PubMed.
- S. Okumura, K. Torii and Y. Uozumi, Org. Lett., 2023, 25, 5226–5230 CrossRef CAS PubMed.
- L. Cotarca and H. Eckert, Phosgenations: A Handbook, 2005 Search PubMed.
- M. E. Dyen and D. Swern, Chem. Rev., 1967, 67, 197–246 CrossRef CAS PubMed.
- P. Adams and F. A. Baron, Chem. Rev., 1965, 65, 567–602 CrossRef CAS.
- S. G. Davies, A. C. Garner, P. M. Roberts, A. D. Smith, M. J. Sweet and J. E. Thomson, Org. Biomol. Chem., 2006, 4, 2753–2768 RSC.
- C. R. McElroy, F. Aricò, F. Benetollo and P. Tundo, Pure Appl. Chem., 2011, 84, 707–719 Search PubMed.
- P. Prasher, T. Mall and M. Sharma, Drug Dev. Res., 2023, 84, 397–405 CrossRef CAS PubMed.
- G. F. S. Fernandes, C. B. Scarim, S.-H. Kim, J. Wu and D. Castagnolo, RSC Med. Chem., 2023, 14, 823–847 RSC.
- T. A. Mukhtar and G. D. Wright, Chem. Rev., 2005, 105, 529–542 CrossRef CAS PubMed.
- F. Hu, X. Li, Z. Ding, L. Wang, C. Ge, L. Xu and S.-S. Li, ACS Catal., 2022, 12, 943–952 CrossRef CAS.
- G. Bai, F. Dong, L. Xu, Y. Liu, L. Wang and S.-S. Li, Org. Lett., 2019, 21, 6225–6230 CrossRef CAS PubMed.
- S.-S. Li, L. Zhou, L. Wang, H. Zhao, L. Yu and J. Xiao, Org. Lett., 2018, 20, 138–141 CrossRef CAS PubMed.
- X. Ma, X. Zhang, J. M. Awad, G. Xie, W. Qiu and W. Zhang, Green Chem., 2019, 21, 4489–4494 RSC.
- M. Yoshida, Y. Komatsuzaki and M. Ihara, Org. Lett., 2008, 10, 2083–2086 CrossRef CAS PubMed.
- J. Hu, J. Ma, Q. Zhu, Z. Zhang, C. Wu and B. Han, Angew. Chem., Int. Ed., 2015, 54, 5399–5403 CrossRef CAS PubMed.
- A. W. Miller and S. T. Nguyen, Org. Lett., 2004, 6, 2301–2304 CrossRef CAS PubMed.
- D. Adhikari, A. W. Miller, M.-H. Baik and S. T. Nguyen, Chem. Sci., 2015, 6, 1293–1300 RSC.
- P. Sonzini, C. Damiano, D. Intrieri, G. Manca and E. Gallo, Adv. Synth. Catal., 2020, 362, 2961–2969 CrossRef CAS.
- T. Niemi, I. Fernández, B. Steadman, J. K. Mannisto and T. Repo, Chem. Commun., 2018, 54, 3166–3169 RSC.
- J. Paz, C. Pérez-Balado, B. Iglesias and L. Muñoz, J. Org. Chem., 2010, 75, 3037–3046 CrossRef CAS PubMed.
- J. Rintjema, R. Epping, G. Fiorani, E. Martín, E. C. Escudero-Adán and A. W. Kleij, Angew. Chem., Int. Ed., 2016, 55, 3972–3976 CrossRef CAS PubMed.
- Y. Lee, J. Choi and H. Kim, Org. Lett., 2018, 20, 5036–5039 CrossRef CAS PubMed.
- Y. Toda, M. Shishido, T. Aoki, K. Sukegawa and H. Suga, Chem. Commun., 2021, 57, 6672–6675 RSC.
- S. Arshadi, A. Banaei, S. Ebrahimiasl, A. Monfared and E. Vessally, RSC Adv., 2019, 9, 19465–19482 RSC.
- T. Niemi, J. E. Perea-Buceta, I. Fernández, O.-M. Hiltunen, V. Salo, S. Rautiainen, M. T. Räisänen and T. Repo, Chem. – Eur. J., 2016, 22, 10355–10359 CrossRef CAS PubMed.
- C. Mei, Y. Zhao, Q. Chen, C. Cao, G. Pang and Y. Shi, ChemCatChem, 2018, 10, 3057–3068 CrossRef CAS.
- B. Wang, E. H. M. Elageed, D. Zhang, S. Yang, S. Wu, G. Zhang and G. Gao, ChemCatChem, 2014, 6, 278–283 CrossRef CAS.
- L. Feng, X. Li, C. Xu and S. M. Sadeghzadeh, Catal. Lett., 2020, 150, 1729–1740 CrossRef CAS.
- L. Wang, C. Qi, W. Xiong and H. Jiang, Chin. J. Catal., 2022, 43, 1598–1617 CrossRef CAS.
- J. Ye, L. Song, W. Zhou, T. Ju, Z. Yin, S. Yan, Z. Zhang, J. Li and D. Yu, Angew. Chem., Int. Ed., 2016, 55, 10022–10026 CrossRef CAS PubMed.
- Z. Zhang, J. Ye, D. Wu, Y. Zhou and D. Yu, Chem. – Asian J., 2018, 13, 2292–2306 CrossRef CAS PubMed.
- J.-H. Ye, T. Ju, H. Huang, L.-L. Liao and D.-G. Yu, Acc. Chem. Res., 2021, 54, 2518–2531 CrossRef CAS PubMed.
- K. Groebke, L. Weber and F. Mehlin, Synlett, 1998, 661–663 CrossRef CAS.
- C. Blackburn, B. Guan, P. Fleming, K. Shiosaki and S. Tsai, Tetrahedron Lett., 1998, 39, 3635–3638 CrossRef CAS.
- H. Bienaymé and K. Bouzid, Angew. Chem., Int. Ed., 1998, 37, 2234–2237 CrossRef.
- A. Boltjes and A. Dömling, Eur. J. Org. Chem., 2019, 7007–7049 CrossRef CAS PubMed.
- M. Fragkiadakis, P. Anastasiou, M. Zingiridis, M. E. Triantafyllou-Rundell, A. Reyes Romero, C. C. Stoumpos and C. G. Neochoritis, J. Org. Chem., 2023, 88, 12709–12715 CrossRef CAS PubMed.
- Y. R. Jorapur and D. Y. Chi, J. Org. Chem., 2005, 70, 10774–10777 CrossRef CAS PubMed.
- S. B. Singh, D. E. Kaelin, J. Wu, L. Miesel, C. M. Tan, P. T. Meinke, D. Olsen, A. Lagrutta, P. Bradley, J. Lu, S. Patel, K. W. Rickert, R. F. Smith, S. Soisson, C. Wei, H. Fukuda, R. Kishii, M. Takei and Y. Fukuda, ACS Med. Chem. Lett., 2014, 5, 609–614 CrossRef CAS PubMed.
- P. D. G. Greenwood and J. Waser, Eur. J. Org. Chem., 2019, 5183–5186 CrossRef CAS.
- H. Jin, Y. Yang, J. Jia, B. Yue, B. Lang and J. Weng, RSC Adv., 2014, 4, 26990–26992 RSC.
- Y. Osa, Y. Hikima, Y. Sato, K. Takino, Y. Ida, S. Hirono and H. Nagase, J. Org. Chem., 2005, 70, 5737–5740 CrossRef CAS PubMed.
- L. Fehr, L. Sewald, R. Huber and M. Kaiser, Eur. J. Org. Chem., 2023, e202300135 CrossRef CAS.
- T. Huber, R. E. Wildermuth and T. Magauer, Chem. – Eur. J., 2018, 24, 12107–12120 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General procedures, characterization data for products (NMR and MS) and crystallographic data. CCDC 2294775–2294778. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc04597h |
| This journal is © The Royal Society of Chemistry 2023 |