Open Access Article
Open Access ArticleA narrow-band deep-blue MRTADF-type organic afterglow emitter†
Guangming
Wang‡
,
Shuhui
Ding‡
,
Jiuyang
Li‡
,
Zi
Ye
,
Wen
Xia
,
Xuefeng
Chen
and
Kaka
Zhang
 *
*
Key Laboratory of Synthetic and Self-Assembly Chemistry for Organic Functional Molecules, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, People's Republic of China. E-mail: zhangkaka@sioc.ac.cn
First published on 19th September 2023
Abstract
We report a multi-resonant thermally activated delayed fluorescent (MRTADF) afterglow emitter with unprecedented long emission lifetime > 100 ms, full-width at half-maximum < 40 nm, and deep-blue emission color of CIEy at 0.048. Such emitters remain rarely achieved and would show potential applications in multiplexed bioimaging and high-density information encryption.
Room-temperature phosphorescence (RTP) and organic afterglow materials feature long-lived excited states1–10 and display applications in high-contrast bioimaging (negligible background fluorescence in time-gated imaging mode),11–13 oxygen sensing and mapping (based on organic triplets’ sensitivity towards molecular oxygen),14–16 and advanced anticounterfeiting and information encryption (on the basis of afterglow patterns/colours and their changes after ceasing excitation source).17–20 It has been reported that multiplexed bioimaging requires the use of narrow-band luminescent probes to minimize spectral crosstalk.21–23 In the aspect of optical anticounterfeiting and information encryption, the realization of high-density information storage relies on narrow-band emitters to address the issue of “spectral crowding”.24–26 However, despite the tremendous development of the field of organic afterglow materials in the past decade,1–20 most of the reported afterglow emitters exhibit broad emission bands with full-width at half-maximum (FWHM) larger than 50 nm and even around 100 nm. Such emission spectral broadening is mainly caused by two factors: (1) vibronic coupling between excited states and ground states; (2) structural relaxation of excited states.
The multiple resonance effect has been reported to enable the localization of the HOMO and LUMO on different atoms, reduce the vibronic coupling and consequently narrow the emission spectra.27–32 Recent studies have shown that multi-resonant thermally activated delayed fluorescent (MRTADF) emitters serve as promising candidates for developing high-quality and energy-saving OLED displays. It is noteworthy that such MRTADF emitters of narrow-band emission possess reverse intersystem crossing rate (kRISC) of 103 to 106 s−1.27–32 It is well known that large kRISC is required to avoid efficiency roll off of OLED displays at relatively high luminance. However, large kRISC of 103 to 106 s−1 means short delayed fluorescence lifetimes of 10−6 to 10−3 s, which is detrimental to the fabrication of afterglow materials with emission lifetimes longer than 100 ms. Recent studies on TADF-type organic afterglow by us and others reveal that the requirement of kRISC in afterglow material design is the opposite to that in OLED display fabrication; moderate kRISC of 10−1 to 101 s−1 is favored for the design of TADF-type afterglow materials.33–38 Probably because of this, despite the fact that many narrow-band MRTADF emitters for OLEDs have been reported, MRTADF-type organic afterglow materials with emission lifetimes ≥ 100 ms and narrow-band emission remain rarely explored.
The key to achieving narrow-band MRTADF-type organic afterglow is to use a MRTADF emitter featuring moderate kRISC of 10−1 to 101 s−1, which is a prerequisite for the realization of long emission lifetimes ![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) 100 ms. Given that most organic systems in the absence of heavy atom effects and benzophenone functional groups possess phosphorescence rates (kP) of 10−2–102 s−1, such moderate kRISC is sufficient to open the TADF pathway for converting organic triplets into singlets. Another key point is the employment of suitable crystalline or glassy matrices to reduce organic triplets’ nonradiative decay rate (knr) and oxygen quenching rate (kq) to small values < kRISC.39–43
100 ms. Given that most organic systems in the absence of heavy atom effects and benzophenone functional groups possess phosphorescence rates (kP) of 10−2–102 s−1, such moderate kRISC is sufficient to open the TADF pathway for converting organic triplets into singlets. Another key point is the employment of suitable crystalline or glassy matrices to reduce organic triplets’ nonradiative decay rate (knr) and oxygen quenching rate (kq) to small values < kRISC.39–43
Here we report the emergence of MRTADF-type organic afterglow via doping 10-phenylacridone (compound 1) into a phenyl benzoate (PhB) matrix (Fig. 1a). The resultant afterglow materials exhibit narrow-band emission with FWHM of 38 nm, deep-blue emission color of CIEy at 0.048, and long emission lifetime of 0.2 s. By incorporating 1 into the emulsion polymerization system, narrow-band deep-blue aqueous afterglow dispersion can be obtained, displaying promising applications.
Compound 1 (purchased from Leyan Company, Shanghai) was purified by recrystallization in spectroscopic grade dichloromethane/n-hexane. Its high purity was confirmed by HPLC (Fig. S1, ESI†). The solution of 1 in dichloromethane exhibits a UV-vis absorption maximum at 394 nm (ε = 9.99 × 103 M−1 cm−1) and deep-blue emission (under 365 nm UV lamp) with λem at 406 nm (FWHM, 35 nm; τF, 5.33 ns) and photoluminescence quantum yield (PLQY) of 52% (Fig. 1b and Fig. S2, ESI†). The absorption and fluorescence spectra obey the mirror image rule (Fig. 1b), with small Stokes shift of 750 cm−1 estimated from spectral maxima.
Single-component 1 samples don’t show room-temperature afterglow either in solution or the solid state (Fig. S3, ESI†). Recent studies by us and others have demonstrated that, in two-component afterglow systems, suitable organic matrices assist luminescent dopants’ intersystem crossing (ISC) and protect dopants’ triplet excited states from nonradiative decay and oxygen quenching.33–36 PhB crystalline matrices, which also don’t show room-temperature afterglow (Fig. S4, ESI†), have a melting point of around 70 °C and thus can protect dopants’ triplets by the rigid environment at room temperature.40,42 PhB has a high T1 level (approximately 3.4 eV estimated by TD-DFT, Text S1, ESI†), which can resist excited state energy transfer from dopant to matrix and subsequent afterglow quenching.39,44 Upon doping 0.001 wt% 1 into PhB, the resultant 1-PhB-0.001% solid sample exhibits deep-blue afterglow under ambient conditions (Fig. 1c). Room-temperature delayed emission spectra (1 ms delay, excited at 365 nm) show narrow emission band at 406 nm with CIE coordinates (0.158, 0.048) and small FWHM of 38 nm (Fig. 1c and Fig. S5 and Text S2, ESI†). The PLQY of the 1-PhB-0.001% sample has been measured to be 19.4%. The emission lifetime can be fit into double-exponential decay with τ1 = 31 ms (7.6%) and τ2 = 199 ms (92.4%) (Fig. 1d), where the τ2 part is responsible for the deep-blue afterglow. The 1-PhB-0.01% sample has similar photophysical properties (Fig. S6, ESI†) to the 1-PhB-0.001% sample. Other matrices such as benzoic acid, boric acid, and 4-methoxybenzophenone were tested, but the resultant 1-matrix samples showed weak room-temperature afterglow and the afterglow color is cyan rather than deep-blue (Fig. S7, ESI†).
It is noteworthy that the steady-state emission spectra of the 1-PhB-0.001% sample almost coincide with the delayed emission spectra (excited at 365 nm, Fig. 1c). In the literature, there are only several instances exhibiting the coincidence of steady-state and delayed emission spectra. (1) Excited state energy transfer from the afterglow donor to the fluorescence acceptor can give rise to spectral coincidence,45 which only occurs when the afterglow donor is sufficiently excited. PhB matrices show no absorption at 365 nm (Fig. S4, ESI†), so the energy transfer mechanism is not the case here. (2) Intermolecular charge transfer and retarded charge recombination in donor–acceptor systems have been reported to show organic long persistent luminescence (OLPL) with identical steady-state and delayed emission spectra.46 The PhB matrix has a low-lying HOMO (−6.43 eV, Fig. S8, ESI†) and high-lying LUMO (−2.01 eV) compared to 1 (−5.53 eV and −2.61 eV, Fig. S9, ESI†), so the OLPL mechanism should be absent here. (3) Organic systems with strong ISC, such as the benzophenone system, would exhibit identical steady-state and delayed emission spectra.42,47 This is not the case here, as revealed by variable temperature delayed emission spectra (vide infra). (4) The impurity mechanism may give spectral coincidence between the steady-state and delayed emission spectra.48 Here 1 has high purity as confirmed by HPLC (Fig. S1, ESI†). 1 and PhB matrices don’t show room-temperature afterglow (Fig. S3 and S4, ESI†). Besides, the excitation spectra of the 1-PhB-0.001% sample exhibit similar maxima to 1's UV-vis absorption spectra (Fig. S10, ESI†). The UV-vis spectra show an electronic absorption property of 1, while excitation spectra reveal the absorption property of the luminescent component in the system. The match between the UV-vis and excitation spectra suggests that the luminescent property originates from 1 rather than impurities. Moreover, the mirror image of the UV-vis and emission spectra in the 1 system also support that the luminescent property here is exclusively from 1 dispersed in PhB matrices.
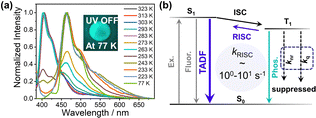
To further investigate the afterglow mechanism, variable temperature delayed emission spectra (1 ms delay) have been collected (Fig. 2a). At 77 K, the 1-PhB-0.001% sample shows bluish green afterglow (Fig. 2a and Fig. S11, ESI†), and the delayed emission band at 464 nm can be assigned as phosphorescence decay of 1's triplets; the T1 level was obtained from the phosphorescence maximum to be 2.67 eV. The phosphorescence decay rate (kP) was estimated from the phosphorescence lifetime (837 ms, Fig. S12, ESI†) to be 1.2 s−1. By increasing the temperature, the delayed fluorescence band at 406 nm (S1 level, 3.05 eV) increases and then dominates the whole delayed emission spectra at 293 K and higher temperature (Fig. 2a). The delayed fluorescence is exclusively attributed to thermally activated delayed fluorescence (TADF), since triplet–triplet annihilation (TTA that can also give rise to delayed fluorescence) should be absent at such low doping concentration of 0.001 wt%.49 Based on temperature-dependent emission decay, the Arrhenius plot of the kRISC gives an experimental ΔEST of 0.39 eV (Fig. S13, ESI†), which agrees with the experimental results obtained from fluorescence and phosphorescence maxima (0.38 eV). Power-dependent delayed emission spectra at room temperature (Fig. S14, ESI†) show a quasi-linear relationship between delayed fluorescence intensity and excitation power further confirming the TADF-type afterglow mechanism. The 1-PhB-0.001% sample under ambient conditions exhibits τ1 = 31 ms (7.6%) and τ2 = 199 ms (92.4%), as shown in Fig. 1d. The τ1 part, which is largely overestimated,50 originates from prompt fluorescence and the accurate value has been obtained by a picosecond pulse laser to be 3.36 ns (Fig. S15, ESI†). The τ2 part originates from TADF afterglow, from which kRISC was roughly estimated to be 100–101 s−1. The rigid crystalline PhB matrices, as well as intermolecular interaction between 1 and PhB, efficiently protect 1's triplets from quenching. With the suppressed knr and kq, the moderate kRISC can explain the emergence of TADF afterglow with 0.2 s lifetime under ambient conditions (Fig. 2b).
Conventional TADF emitters with ΔEST of around 0.2 eV or lower for OLEDs usually possess kRISC of 103 to 106 s−1. It has been reported that the increase of ΔEST by 0.1 eV would result in the decrease of kRISC by approximately 100-fold.51 Here, the moderate ΔEST value (0.38 eV) agrees with the observation of TADF-type afterglow with moderate kRISC of 100–101 s−1 under ambient conditions. To gain a further insight, we conduct a theoretical investigation (Tables S1–S8, ESI†). 1 in dichloromethane was simulated utilizing the polarizable continuum model (PCM). The geometry structures and frequency analyses of the ground and excited states of 1 have been obtained by DFT and TD-DFT methods, employing the TPSSh functional and the 6-31+G(d,p) basis set.52 For ISC, the electronic energies and spin–orbit coupling matrix elements (SOCMEs) have been calculated by the ORCA 5.0.3 program with spin–orbit mean-field methods at the STEOM-DLPNO-CCSD/def2-TZVP theoretical level based on the optimized geometries. Using the simplified formula proposed by Kaji and coworkers,52,53 the fluorescence decay rate of the S1 state (kF) is calculated to be 3.05 × 108 s−1 (Table S5, ESI†), which is consistent with the experimental result (0.97 × 108 s−1) in dichloromethane. The S1-to-T1 ISC rate is calculated by Marcus theory to be 1.19 × 107 s−1, which allows the population of 1's triplets. According to our previous study,33–36 PhB matrices can also assist the population of dopants’ triplets via dipole–dipole interactions (PhB's ground state dipole moment, 2.02 debye; 1's S1 state dipole moment, 17.36 debye; Fig. S16, ESI†). kRISC at 300 K is calculated by Marcus theory to be 6.62 s−1, which also agrees with experimental observations. Given that the experimental kP is 1.2 s−1, such kRISC is enough to enable TADF-type afterglow with emission lifetime > 100 ms under ambient conditions where the nonradiative decay and oxygen quenching of 1's triplets are suppressed in the rigid PhB matrices.
The narrow-band emission of the 1-PhB-0.001% sample can be attributed to the multiple resonance effect of the nitrogen atom and carbonyl group on compound 1 (Fig. 1a); such arrangement reduces vibronic coupling between the excited states and ground states (Fig. S9, ESI†).27–32 Besides, the relatively rigid structure of compound 1 restricts structural relaxation of the excited states, which can also narrow the emission band (Text S2, ESI†). Narrow-band MRTADF-type organic afterglow materials with emission lifetime > 100 ms have been rarely reported in the literature, despite the fact that many narrow-band MRTADF emitters (with emission lifetime of 10−6 to 10−3 s) have been developed for OLED devices.
To explore the function of the present MRTADF afterglow system, we prepare aqueous afterglow dispersions in view of their promising biomedical applications.12–16 Most of the reported methods for aqueous afterglow material fabrication are based on mechanical processing of solid-state afterglow materials, which would disrupt the rigid environment for the protection of organic triplets and cause a drastic loss of afterglow performance. Here, we introduce 1 into the emulsion polymerization system to prepare an aqueous afterglow dispersion (experimental details in the ESI†).54,55 The resultant 1-PMMA emulsion inherits the narrow-band deep-blue MRTADF-type organic afterglow property of 1, exhibiting a delayed emission band at 409 nm with FWHM of 40 nm at room temperature (Fig. 3a). The emission lifetime of the 1-PMMA emulsion can be fit into double-exponential decay with τ1 = 78 ms (19.5%) and τ2 = 400 ms (80.5%) (Fig. 3b); glassy PMMA latex protect 1's triplets. Dynamic light-scattering (DLS) measurement shows that the average hydrodynamic diameter of the 1-PMMA emulsion is 78 nm with a polydispersity index of 0.058 (Fig. 3c). Given the small Stokes shift of compound 1, we use 405 nm visible light to excite the 1-PMMA emulsion. The afterglow photograph shown in Fig. 3d and delayed emission spectra (excited at 405 nm) demonstrate the visible-light-excitable property of the 1-PMMA emulsion with a deep-blue afterglow band at 409 nm and FWHM of 40 nm (Fig. 3d). Such MRTADF afterglow emulsion in an aqueous medium would display potential bioimaging applications.
In summary, we achieve narrow-band deep-blue MRTADF-type organic afterglow by doping 1 into small organic crystalline matrices or glassy PMMA emulsion latex. These MRTADF afterglow materials possess emission lifetimes longer than 100 ms, which is among the highest values in the reported MRTADF emitters. In the field of organic afterglow materials, narrow-band MRTADF afterglow materials have been rarely reported, which would show potential applications in multiplexed bioimaging and high-density information encryption.
We thank the National Natural Science Foundation of China (22175194), Hundred Talents Program from Shanghai Institute of Organic Chemistry (Y121078), and Pioneer Hundred Talents Program of Chinese Academy of Sciences (E320021).
Conflicts of interest
There are no conflicts to declare.Notes and references
- V. W.-W. Yam, V. K.-M. Au and S. Y.-L. Leung, Chem. Rev., 2015, 115, 7589–7728 CrossRef CAS PubMed.
- W. Zhao, Z. He and B. Z. Tang, Nat. Rev. Mater., 2020, 5, 869–885 CrossRef CAS.
- N. Gan, H. Shi, Z. An and W. Huang, Adv. Funct. Mater., 2018, 28, 1802657 CrossRef.
- X. Ma, J. Wang and H. Tian, Acc. Chem. Res., 2019, 52, 738–748 CrossRef CAS PubMed.
- S. Hirata, Adv. Opt. Mater., 2017, 5, 1700116 CrossRef.
- Kenry, C. Chen and B. Liu, Nat. Commun., 2019, 10, 2111 CrossRef CAS PubMed.
- Q. Li and Z. Li, Acc. Chem. Res., 2020, 53, 962–973 CrossRef CAS PubMed.
- J. Guo, C. Yang and Y. Zhao, Acc. Chem. Res., 2022, 55, 1160–1170 CrossRef CAS PubMed.
- H. Nie, Z. Wei, X.-L. Ni and Y. Liu, Chem. Rev., 2022, 122, 9032–9077 CrossRef CAS PubMed.
- A. Forni, E. Lucenti, C. Botta and E. Cariati, J. Mater. Chem. C, 2018, 6, 4603–4626 RSC.
- X. Zhen, Y. Tao, Z. An, P. Chen, C. Xu, R. Chen, W. Huang and K. Pu, Adv. Mater., 2017, 29, 1606665 CrossRef PubMed.
- X.-F. Wang, H. Xiao, P.-Z. Chen, Q.-Z. Yang, B. Chen, C.-H. Tung, Y.-Z. Chen and L.-Z. Wu, J. Am. Chem. Soc., 2019, 141, 5045–5050 CrossRef CAS PubMed.
- Y. Wang, H. Gao, J. Yang, M. Fang, D. Ding, B. Z. Tang and Z. Li, Adv. Mater., 2021, 33, 2007811 CrossRef CAS PubMed.
- G. Zhang, G. M. Palmer, M. W. Dewhirst and C. L. Fraser, Nat. Mater., 2009, 8, 747–751 CrossRef CAS PubMed.
- Y. Yu, M. S. Kwon, J. Jung, Y. Zeng, M. Kim, K. Chung, J. Gierschner, J. H. Youk, S. M. Borisov and J. Kim, Angew. Chem., Int. Ed., 2017, 56, 16207–16211 CrossRef CAS PubMed.
- Y. Zhou, W. Qin, C. Du, H. Gao, F. Zhu and G. Liang, Angew. Chem., Int. Ed., 2019, 58, 12102–12106 CrossRef CAS PubMed.
- Z. An, C. Zheng, Y. Tao, R. Chen, H. Shi, T. Chen, Z. Wang, H. Li, R. Deng, X. Liu and W. Huang, Nat. Mater., 2015, 14, 685–690 CrossRef CAS PubMed.
- X. Yan, H. Peng, Y. Xiang, J. Wang, L. Yu, Y. Tao, H. Li, W. Huang and R. Chen, Small, 2022, 18, 2104073 CrossRef CAS PubMed.
- X. Yu, H. Zhang and J. Yu, Aggregate, 2021, 2, 20–34 CrossRef CAS.
- Z. He, H. Gao, S. Zhang, S. Zheng, Y. Wang, Z. Zhao, D. Ding, B. Yang, Y. Zhang and W. Z. Yuan, Adv. Mater., 2019, 31, 1807222 CrossRef PubMed.
- J. Han, A. Xia, Y. Huang, L. Ni, W. Chen, Z. Jin, S. Yang and F. Jin, ACS Synth. Biol., 2019, 8, 2536–2546 CrossRef CAS PubMed.
- M. Li, S. Tian, F. Meng, M. Yin, Q. Yue, S. Wang, W. Bu and L. Luo, Nano Lett., 2022, 22, 4544–4551 CrossRef CAS PubMed.
- I. L. Medintz, H. T. Uyeda, E. R. Goldman and H. Mattoussi, Nat. Mater., 2005, 4, 435–446 CrossRef CAS PubMed.
- Y. Lei, W. Dai, J. Guan, S. Guo, F. Ren, Y. Zhou, J. Shi, B. Tong, Z. Cai, J. Zheng and Y. Dong, Angew. Chem., Int. Ed., 2020, 59, 16054–16060 CrossRef CAS PubMed.
- L. Lei, Y. Wang, W. Xu, R. Ye, Y. Hua, D. Deng, L. Chen, P. N. Prasad and S. Xu, Nat. Commun., 2022, 13, 5739 CrossRef CAS PubMed.
- Y. Su, Y. Zhang, Z. Wang, W. Gao, P. Jia, D. Zhang, C. Yang, Y. Li and Y. Zhao, Angew. Chem., Int. Ed., 2020, 59, 9967–9971 CrossRef CAS PubMed.
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Adv. Mater., 2016, 28, 2777–2781 CrossRef CAS PubMed.
- Y. Kondo, K. Yoshiura, S. Kitera, H. Nishi, S. Oda, H. Gotoh, Y. Sasada, M. Yanai and T. Hatakeyama, Nat. Photonics, 2019, 13, 678–682 CrossRef CAS.
- J. M. Ha, S. H. Hur, A. Pathak, J.-E. Jeong and H. Y. Woo, NPG Asia Mater., 2021, 13, 53 CrossRef CAS.
- Y. Zhang, D. Zhang, J. Wei, Z. Liu, Y. Lu and L. Duan, Angew. Chem., Int. Ed., 2019, 58, 16912–16917 CrossRef CAS PubMed.
- T. Wang, X. Yin, X. Cao and C. Yang, Angew. Chem., Int. Ed., 2023, 62, e202301988 CrossRef CAS PubMed.
- X. Wu, B.-K. Su, D.-G. Chen, D. Liu, C.-C. Wu, Z.-X. Huang, T.-C. Lin, C.-H. Wu, M. Zhu, E. Y. Li, W.-Y. Hung, W. Zhu and P.-T. Chou, Nat. Photonics, 2021, 15, 780–786 CrossRef CAS.
- X. Wang, Y. Sun, G. Wang, J. Li, X. Li and K. Zhang, Angew. Chem., Int. Ed., 2021, 60, 17138–17147 CrossRef CAS PubMed.
- Y. Pan, J. Li, X. Wang, Y. Sun, J. Li, B. Wang and K. Zhang, Adv. Funct. Mater., 2022, 32, 2110207 CrossRef CAS.
- G. Wang, J. Li, X. Li, X. Wang, Y. Sun, J. Liu and K. Zhang, Chem. Eng. J., 2022, 431, 134197 CrossRef CAS.
- G. Wang, X. Chen, J. Liu, S. Ding and K. Zhang, Sci. China: Chem., 2023, 66, 1120–1131 CrossRef CAS.
- Y. Liang, C. Xu, H. Zhang, S. Wu, J. Li, Y. Yang, Z. Mao, S. Luo, C. Liu, G. Shi, F. Sun, Z. Chi and B. Xu, Angew. Chem., Int. Ed., 2023, 62, e202217616 CrossRef CAS PubMed.
- J. Wang, Y. Yang, K. Li, L. Zhang and Z. Li, Angew. Chem., Int. Ed., 2023, 62, e202304020 CrossRef CAS PubMed.
- S. Hirata, K. Totani, J. Zhang, T. Yamashita, H. Kaji, S. R. Marder, T. Watanabe and C. Adachi, Adv. Funct. Mater., 2013, 23, 3386–3397 CrossRef CAS.
- J. Li, X. Wang, Y. Pan, Y. Sun, G. Wang and K. Zhang, Chem. Commun., 2021, 57, 8794–8797 RSC.
- X. Chen, G. Wang, X. Chen, X. Deng and K. Zhang, J. Mater. Chem. C, 2022, 10, 11634–11641 RSC.
- Y. Su, M. Wu, G. Wang, J. Li, X. Chen, X. Li, G. Wang and K. Zhang, Chem. Commun., 2023, 59, 1525–1528 RSC.
- S. Guo, W. Dai, X. Chen, Y. Lei, J. Shi, B. Tong, Z. Cai and Y. Dong, ACS Mater. Lett., 2021, 3, 379–397 CrossRef CAS.
- N. Notsuka, R. Kabe, K. Goushi and C. Adachi, Adv. Funct. Mater., 2017, 27, 1703902 CrossRef.
- S. Kuila and S. J. George, Angew. Chem., Int. Ed., 2020, 59, 9393–9397 CrossRef CAS PubMed.
- R. Kabe and C. Adachi, Nature, 2017, 550, 384–387 CrossRef CAS PubMed.
- W. Z. Yuan, X. Y. Shen, H. Zhao, J. W. Y. Lam, L. Tang, P. Lu, C. Wang, Y. Liu, Z. Wang, Q. Zheng, J. Z. Sun, Y. Ma and B. Z. Tang, J. Phys. Chem. C, 2010, 114, 6090–6099 CrossRef CAS.
- C. Chen, Z. Chi, K. C. Chong, A. S. Batsanov, Z. Yang, Z. Mao, Z. Yang and B. Liu, Nat. Mater., 2021, 20, 175–180 CrossRef CAS PubMed.
- Z. Lin, R. Kabe, K. Wang and C. Adachi, Nat. Commun., 2020, 11, 191 CrossRef CAS PubMed.
- H. Noda, H. Nakanotani and C. Adachi, Chem. Lett., 2019, 48, 126–129 CrossRef CAS.
- P. K. Samanta, D. Kim, V. Coropceanu and J.-L. Brédas, J. Am. Chem. Soc., 2017, 139, 4042–4051 CrossRef CAS PubMed.
- K. Shizu and H. Kaji, Commun. Chem., 2022, 5, 53 CrossRef CAS PubMed.
- K. Shizu and H. Kaji, J. Phys. Chem. A, 2021, 125, 9000–9010 CrossRef CAS PubMed.
- J. Liu, Y. Sun, G. Wang, X. Chen, J. Li, X. Wang, Y. Zou, B. Wang and K. Zhang, Adv. Opt. Mater., 2022, 10, 2201502 CrossRef CAS.
- X. Zhai, Y. Zeng, X. Deng, Q. Lou, A. Cao, L. Ji, Q. Yan, B. Wang and K. Zhang, Chem. Commun., 2023, 59, 10500–10503 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, and spectroscopic data. See DOI: https://doi.org/10.1039/d3cc04012g |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2023 |