Open Access Article
Open Access ArticleLocal reactivity of metal–insulator–semiconductor photoanodes imaged by photoinduced electrochemiluminescence microscopy†
Julie
Descamps
a,
Yiran
Zhao
 b,
Julie
Le-Pouliquen
c,
Bertrand
Goudeau
a,
Patrick
Garrigue
a,
Karine
Tavernier
c,
Yoan
Léger
c,
Gabriel
Loget
b,
Julie
Le-Pouliquen
c,
Bertrand
Goudeau
a,
Patrick
Garrigue
a,
Karine
Tavernier
c,
Yoan
Léger
c,
Gabriel
Loget
 *bd and
Neso
Sojic
*bd and
Neso
Sojic
 *a
*a
aUniversity of Bordeaux, Bordeaux INP, ISM, UMR CNRS 5255, Pessac 33607, France. E-mail: sojic@u-bordeaux.fr
bUniv Rennes, CNRS, ISCR (Institut des Sciences Chimiques de Rennes)-UMR6226, Rennes F-35000, France. E-mail: gabriel.loget@cnrs.fr
cUniv Rennes, INSA Rennes, CNRS, Institut FOTON-UMR 6082, F-35000, Rennes, France
dInstitute of Energy and Climate Research, Fundamental Electrochemistry (IEK-9), Forschungszentrum Jülich GmbH, Jülich, 52425, Germany
First published on 19th September 2023
Abstract
Localized photoinduced electrochemiluminescence (PECL) is studied on photoanodes composed of Ir microbands deposited on n-Si/SiOx. We demonstrate that PECL microscopy precisely imaged the hole-driven heterogeneous photoelectrochemical reactivity. The method is promising for elucidating the local activity of photoelectrodes that are employed in solar energy conversion.
Photoelectrochemistry of illuminated semiconductors is a field of research that deals with the charge transfer of photogenerated charge carriers (electrons and holes) at the solid–liquid interface.1,2 It has promising applications in the field of energy conversion as the basis for artificial photosynthesis, in which solar energy is converted into H2,3,4 C1, or C25–7 products using water and/or CO2 as reactants. In these systems, semiconductor photoelectrodes, often coated with protection and/or catalytic layers are immersed in a liquid electrolyte.8–10 Upon light absorption, photogenerated charge carriers diffusing within the semiconductor, are collected at the catalyst and trigger redox reactions in the liquid phase. Photoelectrochemical characterization is mainly done by recording the current density (j) as a function of another parameter, for instance, the potential (E)/j (i.e., voltammetry) or time (t)/j (i.e., chronoamperometry) measurements, in which the j value corresponds to the faradaic charge transfer rate at the photoelectrode surface. Although these electroanalytical methods are straightforward for performance assessment, the j value only corresponds to the overall charge transfer rate, averaged over all the electrode surface, and does not allow for spatially-resolved determination of photoelectrochemical activity. However, probing the local activity is essential for elucidating the operation of photoelectrodes that are made by combining several materials, usually a semiconductor absorber, a protection layer, and a catalytic coating7 (in the form of thin films11,12 or particles10,13–16 dispersed over the surface). Due to the complexity of these interfaces, the photoelectrochemical reaction is not expected to occur at the same rate over the whole surface. Visualizing local photoelectrochemical activity remains a challenge, which has been investigated by several groups17 using different methods such as laser scanning techniques,18 scanning electrochemical microscope variants,19–24 or a combination of both.25
Electrochemiluminescence (ECL) is a light-emitting process initiated at an electrode surface.26 The light originates from a luminophore molecule that is brought to its excited state by a highly exergonic electron-transfer reaction and relaxes to its ground state by emitting a photon.27,28 This phenomenon is widely used for medical diagnosis applications and immunoassays,29–36 with the ECL model system comprising tris(bipyridine)ruthenium(II) complex ([Ru(bpy)3]2+) and tri-n-propylamine (TPrA) as the luminophore and the co-reactant, respectively. Since ECL offers an optical readout, it has evolved progressively into a microscopy technique.37–42 Single entities such as cells, bacteria, and organelles have been imaged by ECL with remarkable resolution.39,43–49 Notably, ECL has also been employed for visualizing local electron transfer and catalytic activity of single nano-objects and particles.50–55 Photoinduced ECL (PECL) combines semiconductor photoelectrochemistry and ECL.56 In this process, a light input (λexc) is electrochemically converted and generates a light output (λPECL) through an ECL reaction. Depending on the choice of the electrode semiconductor material and the ECL system implied, PECL can allow either a downconversion (λPECL < λexc)57 or an upconversion (λPECL < λexc) emission (Table S1, ESI†).56,58–63 So far, PECL microscopy has been reported in two studies, and used to probe upconversion PECL at Au nanoparticles localized on Si nanopillars64 or TiO2.62 In these reports, PECL was triggered through the oxidation of luminol via photogenerated holes.
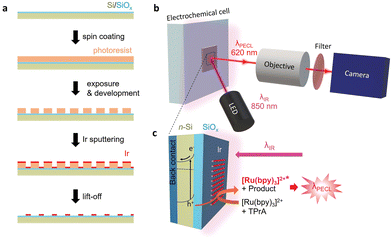
Herein, we report the detailed PECL microscopy study of inhomogeneous metal–insulator–semiconductor (MIS) photoanodes using the model [Ru(bpy)3]2+/TPrA system (Fig. 1). Such MIS-based architectures are often employed for manufacturing efficient and stable Si-based water-splitting photoanodes.65–68 In particular, MIS photoanodes based on Si and Ir have been previously employed for photoelectrochemical water splitting66,68–70 and it is known that n-Si/SiOx/Ir photoanodes, in their homogeneous (i.e., non-patterned) form exhibit exceptional PECL stability (Table S1, ESI†).63 Here, inhomogeneous n-Si/SiOx/Ir photoanodes are prepared by sputtering Ir microbands with a defined pattern. The operation of upconversion PECL and observation of the produced emission patterns allows resolving the solid/liquid photo-electrogenerated hole transfer at the local scale.
The MIS photoanodes were fabricated by chemical oxidation, photolithography and magnetron sputtering (Fig. S1, ESI†). First, Si/SiOx was prepared by oxidizing a moderately doped photoactive n-Si surface to create a ≈1.5 nm-thick tunnel SiOx layer, as described previously.67,71 Prior to the PECL experiments, a 2 nm-thick Ir pattern (Fig. S2, ESI†) was deposited on Si/SiOx using a lift-off process, described in Fig. 1a (see details in ESI†). Four types of Ir patterns with various width/pitch (50 μm/100 μm, 25 μm/50 μm, 10 μm/50 μm, and 5 μm/50 μm) were designed and produced. It is worth mentioning that the Si/SiOx and the Si/SiOx/Ir junctions were characterized by X-ray photoelectron spectroscopy and atomic force microscopy in a previous paper.63 For the PECL microscopy experiments, the photoanode was excited by side-illumination with a near-IR LED (λexc = 850 nm) to photogenerate charge carriers, as shown in Fig. 1b and c. The localized PECL emission was imaged with a microscope equipped with an IR filter (Fig. S3, ESI†).
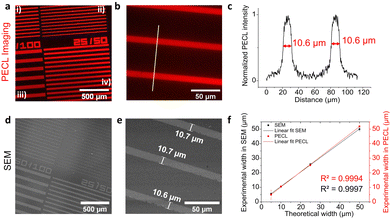
As shown in Fig. 2, microscopy images of PECL could be reliably recorded on n-Si/SiOx/Ir under near-IR illumination at 0.8 V (all potential are here referred vs. Ag/AgCl) in the electrolytic solution containing both [Ru(bpy)3]2+ and TPrA. Fig. 2a shows that the red PECL light is homogeneously emitted over the Ir microbands, for all the patterns. At higher magnification (Fig. 2b), the PECL sharply delimits the contour of the Ir surface area allowing an excellent definition of the patterns, as further demonstrated by the intensity profile in Fig. 2c (vide infra). The bands made of Ir thin film are easily viewed by PECL because Ir acts as a collector for photogenerated charges and presents good charge transfer properties, leading to the efficient oxidation of both [Ru(bpy)3]2+ and TPrA producing light locally.63
In contrast, Si surface is passivated in the aqueous solution at the anodic potentials required for ECL generation. In other words, PECL is preferentially emitted at the Ir patterns (Fig. 2a and b). The 2 nm-thick Ir pattern was not observable with the naked eye or under a wide-field microscope, and therefore, scanning electron microscopy (SEM) was required to characterize the thin film patterns. Fig. 2d and e presents the SEM pictures of the Ir microbands that allow for a precise measurements of their dimensions. Then, we compared their dimensions measured by SEM and by PECL. For PECL microscopy, we extracted the PECL intensity profiles along a line perpendicular to the long band axis. More precisely, their full-width at half-maximum (FWHM) values were compared with the width measured by SEM for all the patterns: 10 μm/50 μm in Fig. 2c and, 50 μm/100 μm, 25 μm/50 μm, and 5 μm/50 μm in Fig. S4c, f and i, ESI†). In Fig. 2f, the PECL and SEM width measurements were plotted as a function of the theoretical width. The linear fits of both measurements show a slope close to 1 (R2 = 0.9997 and R2 = 0.9994 for the SEM and the PECL, respectively). This reveals the accuracy of the Ir pattern manufacturing with the lift-off process on one hand, and, on the other hand, that the PECL is restricted to the conductive patterns. We selected a high concentration of TPrA (i.e. 0.1 M) for the PECL imaging experiments because (i) it generates a strong PECL signal and (ii) it confines the PECL-emitting layer at the electrode surface. Indeed, in the present experimental conditions, the size of the PECL reaction layer is typically ≈200 nm so much smaller than the dimensions of the bands.72 Control experiments, presented in Fig. S5 (ESI†), were performed under illumination at open-circuit conditions to ensure that the observed pattern was not caused by reflection of the IR light. Furthermore, Fig. S5c (ESI†) shows that polarization of the electrode at 0.8 V in the dark did not lead to PECL emission, confirming the necessity of photocarrier generation in the PECL process.
We further investigated the photoelectrochemistry of the n-Si/SiOx/Ir electrode. A cyclic voltammogram (CV) was recorded in the same electrolyte (5 mM [Ru(bpy)3]2+, 0.1 M TPrA, 0.1 M PBS at pH = 7.4) under IR illumination at λexc = 850 nm with a power density (PLED) of 6.6 mW cm−2 (red curve) and in the dark (black curve, Fig. 3a). The PECL images were captured with the microscope at a speed of 2 frames per second (fps). In Fig. 3b, the corresponding average PECL intensity was plotted against the applied potential for the 10 μm/50 μm microbands. This points out that the local PECL emission was only initiated from +0.57 V. These measurements can be compared with that of an analogous but non-photoactive p++-Si/SiOx/Ir MIS anode prepared on a highly-doped, degenerate p++-Si surface and studied in the dark. Indeed, ECL microscopy on these anodes revealed that they also promote local ECL at the Ir/electrolyte interface, however, at higher potentials than that required for their illuminated photoactive counterpart (n-Si/SiOx/Ir). This shift of ECL onset potential can be clearly observed in Fig. S6a (ESI†) where the CV obtained for p++-Si/SiOx/Ir and n-Si/SiOx/Ir under different illumination conditions are plotted together. This shift of onset potential (here ≈0.5 V) is characteristic of a conventional photoanode behavior and caused by the generation of a photovoltage, this was already observed (to a lower extent) on non-patterned n-Si/SiOx/Ir photoanodes.63,73
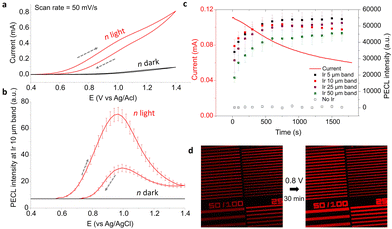 | ||
| Fig. 3 (a) Voltammetric measurement of n-Si/SiOx patterned with Ir microbands in the dark (black curve) and under LED illumination (red curve). The dashed grey arrows show the sweep direction. (b) Corresponding average PECL intensity of the 10 μm/50 μm Ir microbands under IR illumination (red curve) and in the dark (black curve). (c) Evolution of the current (line) and the PECL of each Ir band (squares) as a function of time at Eapp = 0.8 V under LED light. (d) PECL imaging before (left) and after (right) a potentiostatic measurement. Same solution as in Fig. 2. | ||
We then studied the evolution of PECL on the illuminated n-Si/SiOx/Ir over time by potentiostatic measurement at 0.8 V for 30 min while capturing microscopy pictures. The PECL intensity averages were measured on the pictures for the 50 μm/100 μm, 25 μm/50 μm, 10 μm/50 μm, and 5 μm/50 μm microbands. On Fig. 3c, it can be observed that the photocurrent decreases by −45% after 30 min, but, meanwhile, the PECL intensities increased and reached a plateau after about 10 min. The pictures at the beginning and at the end of the potentiostatic measurement, (recorded with the same conditions of the CCD camera with texpo = 0.5 s) complete this observation with a PECL that appears more intense at the end of the experiment (Fig. 3d). This behavior could be explained by the anodic passivation of the Si/SiOx surface that inhibits the flow of residual current onto the Ir-free area and the surface oxidation of Ir that could influence the ECL process. However, the pattern remains identical after 30 min, showing no obvious damage of the patterns. In addition to the PECL on n-Si/SiOx/Ir, the ECL intensity on the p++-Si/SiOx/Ir electrode was also recorded (in the dark at 1.2 V, Fig. S6b and S7, ESI†), confirming our observations.
To conclude, we have performed a PECL microscopy study of MIS n-Si/SiOx/Ir microbands. This junction has been considered so far as the most stable interface for manufacturing PECL photoanodes (Table S1, ESI†)63 and has implications in the field of photoelectrochemical solar energy conversion.66,68–70 Inhomogeneous photoactive surfaces comprising ultrathin Ir microbands were prepared on Si/SiOx and studied under an optical microscope by PECL under IR irradiation (λexc = 850 nm). The emission patterns (λPECL = 635 nm) were intense, stable, and presented geometrical features identical to that of the Ir microbands. These results clearly revealed, in the form of a snapshot, that photoelectrochemical charge transfer occurs at the surface Ir microbands and is inhibited at the SiOx surface. We anticipate that PECL microscopy can be used as a novel approach for understanding the complexity of inhomogeneous solid/liquid junctions and could help the further development in photoelectrochemical solar energy conversion.
The manuscript was written through contributions of all authors. This work was funded by ANR (LiCORN, ANR-20-CE29-0006).
Conflicts of interest
There are no conflicts to declare.References
- H. Gerischer, in Solar Energy Conversion, ed. B. O. Seraphin, Springer, Heidelberg, Berlin, 1979, pp. 115–172 Search PubMed.
- M. X. Tan, P. E. Laibinis, S. T. Nguyen, J. M. Kesselman, C. E. Stanton and N. S. Lewis, in Progress in Inorganic Chemistry, ed. K. D. Karlin, John Wiley & Sons, Inc., Hoboken, USA, 2007 Search PubMed.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446 CrossRef CAS PubMed.
- D. G. Nocera, Acc. Chem. Res., 2012, 45, 767–776 CrossRef CAS PubMed.
- T. Morikawa, S. Sato, K. Sekizawa, T. M. Suzuki and T. Arai, Acc. Chem. Res., 2022, 55, 933–943 CrossRef CAS PubMed.
- P. B. Pati, R. Wang, E. Boutin, S. Diring, S. Jobic, N. Barreau, F. Odobel and M. Robert, Nat. Commun., 2020, 11, 3499 CrossRef CAS PubMed.
- I. Roh, S. Yu, C.-K. Lin, S. Louisia, S. Cestellos-Blanco and P. Yang, J. Am. Chem. Soc., 2022, 144, 8002–8006 CrossRef CAS PubMed.
- D. Bae, B. Seger, P. C. K. Vesborg, O. Hansen and I. Chorkendorff, Chem. Soc. Rev., 2017, 46, 1933–1954 RSC.
- G. Loget, Curr. Opin. Colloid Interface Sci., 2019, 39, 40–50 CrossRef CAS.
- B. Fabre and G. Loget, Acc. Mater. Res., 2023, 4, 133–142 CrossRef CAS.
- L. Pan, J. H. Kim, M. T. Mayer, M.-K. Son, A. Ummadisingu, J. S. Lee, A. Hagfeldt, J. Luo and M. Grätzel, Nat. Catal., 2018, 1, 412 CrossRef CAS.
- I. A. Digdaya, G. W. P. Adhyaksa, B. J. Trześniewski, E. C. Garnett and W. A. Smith, Nat. Commun., 2017, 8, 15968 CrossRef CAS PubMed.
- F. A. L. Laskowski, S. Z. Oener, M. R. Nellist, A. M. Gordon, D. C. Bain, J. L. Fehrs and S. W. Boettcher, Nat. Mater., 2020, 19, 69 CrossRef CAS PubMed.
- G. Loget, B. Fabre, S. Fryars, C. Mériadec and S. Ababou-Girard, ACS Energy Lett., 2017, 2, 569–573 CrossRef CAS.
- K. Oh, V. Dorcet, B. Fabre and G. Loget, Adv. Energy Mater., 2020, 10, 1902963 CrossRef CAS.
- S. Lee, L. Ji, A. C. De Palma and E. T. Yu, Nat. Commun., 2021, 12, 3982 CrossRef CAS PubMed.
- D. V. Esposito, J. B. Baxter, J. John, N. S. Lewis, T. P. Moffat, T. Ogitsu, G. D. O’Neil, T. A. Pham, A. A. Talin, J. M. Velazquez and B. C. Wood, Energy Environ. Sci., 2015, 8, 2863–2885 RSC.
- G. Loget, S. So, R. Hahn and P. Schmuki, J. Mater. Chem. A, 2014, 2, 17740–17745 RSC.
- J. Lee, H. Ye, S. Pan and A. J. Bard, Anal. Chem., 2008, 80, 7445–7450 CrossRef CAS PubMed.
- B. D. B. Aaronson, J. C. Byers, A. W. Colburn, K. McKelvey and P. R. Unwin, Anal. Chem., 2015, 87, 4129–4133 CrossRef CAS PubMed.
- V. Badets, G. Loget, P. Garrigue, N. Sojic and D. Zigah, Electrochim. Acta, 2016, 222, 84–91 CrossRef CAS.
- F. Gelb, Y.-C. Chueh, N. Sojic, V. Keller, D. Zigah and T. Cottineau, Sustain. Energy Fuels, 2020, 4, 1099–1104 RSC.
- R. Gutkowski, C. Khare, F. Conzuelo, Y. U. Kayran, A. Ludwig and W. Schuhmann, Energy Environ. Sci., 2017, 10, 1213–1221 RSC.
- X. Zhou, Z. T. Gossage, B. H. Simpson, J. Hui, Z. J. Barton and J. Rodríguez-López, ACS Nano, 2016, 10, 9346–9352 CrossRef CAS PubMed.
- D. V. Esposito, I. Levin, T. P. Moffat and A. A. Talin, Nat. Mater., 2013, 12, 562–568 CrossRef CAS PubMed.
- Z. Liu, W. Qi and G. Xu, Chem. Soc. Rev., 2015, 44, 3117–3142 RSC.
- W. Miao and J.-P. Choi, in Electrogenerated chemiluminescence ed. A. J. Bard, Marcel Dekker, New York, 2004, pp. 213–271 Search PubMed.
- Analytical Electrogenerated Chemiluminescence, ed. N. Sojic, RSC Publishing, Cambridge, 2020 Search PubMed.
- F. Du, Y. Chen, C. Meng, B. Lou, W. Zhang and G. Xu, Curr. Opin. Electrochem., 2021, 28, 100725 CrossRef CAS.
- A. Zanut, A. Fiorani, S. Canola, T. Saito, N. Ziebart, S. Rapino, S. Rebeccani, A. Barbon, T. Irie, H.-P. Josel, F. Negri, M. Marcaccio, M. Windfuhr, K. Imai, G. Valenti and F. Paolucci, Nat. Commun., 2020, 11, 2668 CrossRef CAS PubMed.
- M. Guo, D. Du, J. Wang, Y. Ma, D. Yang, M. A. Haghighatbin, J. Shu, W. Nie, R. Zhang, Z. Bian, L. Wang, Z. J. Smith and H. Cui, Chem. Biomed. Imaging, 2023, 1, 179–185 CrossRef CAS.
- Y. Wang, J. Ding, P. Zhou, J. Liu, Z. Qiao, K. Yu, J. Jiang and B. Su, Angew. Chem., Int. Ed., 2023, 62, e202216525 CrossRef CAS PubMed.
- E. Faatz, A. Finke, H.-P. Josel, G. Prencipe, S. Quint and M. Windfuhr, Analytical Electrogenerated Chemiluminescence, RSC, Cambridge, 2020, pp. 443–470 Search PubMed.
- X. Yang, J. Hang, W. Qu, Y. Wang, L. Wang, P. Zhou, H. Ding, B. Su, J. Lei, W. Guo and Z. Dai, J. Am. Chem. Soc., 2023, 145, 16026–16036 CrossRef CAS PubMed.
- F. Deiss, C. N. LaFratta, M. Symer, T. M. Blicharz, N. Sojic and D. R. Walt, J. Am. Chem. Soc., 2009, 131, 6088–6089 CrossRef CAS PubMed.
- H. Qi and C. Zhang, Anal. Chem., 2020, 92, 524–534 CrossRef CAS PubMed.
- A. Zanut, A. Fiorani, S. Rebeccani, S. Kesarkar and G. Valenti, Anal. Bioanal. Chem., 2019, 411, 4375–4382 CrossRef CAS PubMed.
- J. Zhang, S. Arbault, N. Sojic and D. Jiang, Ann. Rev. Anal. Chem., 2019, 12, 275–295 CrossRef CAS PubMed.
- J. Dong, Y. Lu, Y. Xu, F. Chen, J. Yang, Y. Chen and J. Feng, Nature, 2021, 596, 244–249 CrossRef CAS PubMed.
- X. Gou, Z. Xing, C. Ma and J.-J. Zhu, Chem. Biomed. Imaging, 2023, 1, 414–433 CrossRef CAS.
- S. Rebeccani, A. Zanut, C. I. Santo, G. Valenti and F. Paolucci, Anal. Chem., 2022, 94, 336–348 CrossRef CAS PubMed.
- L. Ding, P. Zhou, Y. Yan and B. Su, Chem. Biomed. Imaging, 2023 DOI:10.1021/cbmi.3c00066.
- Y. Ma, C. Colin, J. Descamps, S. Arbault and N. Sojic, Angew. Chem., Int. Ed., 2021, 60, 18742–18749 CrossRef CAS PubMed.
- H. Gao, W. Han, H. Qi, Q. Gao and C. Zhang, Anal. Chem., 2020, 92, 8278–8284 CrossRef CAS PubMed.
- W. Zhao, H.-Y. Chen and J.-J. Xu, Chem. Sci., 2021, 12, 5720–5736 RSC.
- H. Ding, P. Zhou, W. Fu, L. Ding, W. Guo and B. Su, Angew. Chem., Int. Ed., 2021, 60, 11769–11773 CrossRef CAS PubMed.
- Y. Zhou, J. Dong, P. Zhao, J. Zhang, M. Zheng and J. Feng, J. Am. Chem. Soc., 2023, 145, 8947–8953 CrossRef CAS PubMed.
- Y. Wang, W. Guo, Q. Yang and B. Su, J. Am. Chem. Soc., 2020, 142, 1222–1226 CrossRef CAS PubMed.
- J. Descamps, C. Colin, G. Tessier, S. Arbault and N. Sojic, Angew. Chem., Int. Ed., 2023, 135, e202218574 CrossRef.
- J. Dong, Y. Xu, Z. Zhang and J. Feng, Angew. Chem., Int. Ed., 2022, 61, e202200187 CrossRef CAS PubMed.
- M.-M. Chen, C.-H. Xu, W. Zhao, H.-Y. Chen and J.-J. Xu, J. Am. Chem. Soc., 2021, 143, 18511–18518 CrossRef CAS PubMed.
- M.-J. Zhu, J.-B. Pan, Z.-Q. Wu, X.-Y. Gao, W. Zhao, X.-H. Xia, J.-J. Xu and H.-Y. Chen, Angew. Chem., Int. Ed., 2018, 130, 4074–4078 CrossRef.
- Y. Lu, X. Huang, S. Wang, B. Li and B. Liu, ACS Nano, 2023, 17, 3809–3817 CrossRef CAS PubMed.
- K. Wu, R. Chen, Z. Zhou, X. Chen, Y. Lv, J. Ma, Y. Shen, S. Liu and Y. Zhang, Angew. Chem., Int. Ed., 2023, 135, e202217078 CrossRef.
- X. Hu, S. Yu, C. Wang, X. Zhang, J. Pan and H. Ju, Anal. Chem., 2023, 95, 4496–4502 CrossRef CAS PubMed.
- Y. Zhao, L. Bouffier, G. Xu, G. Loget and N. Sojic, Chem. Sci., 2022, 13, 2528–2550 RSC.
- J. Yu, H. Saada, R. Abdallah, G. Loget and N. Sojic, Angew. Chem., Int. Ed., 2020, 132, 15269–15272 CrossRef.
- Y. Zhao, J. Descamps, Y. Léger, L. Santinacci, S. Zanna, N. Sojic and G. Loget, Electrochim. Acta, 2023, 444, 142013 CrossRef CAS.
- D. Laser and A. J. Bard, Chem. Phys. Lett., 1975, 34, 6 CrossRef.
- Y. B. Vogel, N. Darwish and S. Ciampi, Cell Rep. Phys. Sci., 2020, 1, 100107 CrossRef.
- Y. Zhao, J. Yu, G. Xu, N. Sojic and G. Loget, J. Am. Chem. Soc., 2019, 141, 13013–13016 CrossRef CAS PubMed.
- J.-W. Xue, C.-H. Xu, W. Zhao, H.-Y. Chen and J.-J. Xu, Nano Lett., 2023, 23, 4572–4578 CrossRef CAS PubMed.
- Y. Zhao, J. Descamps, S. Ababou-Girard, J.-F. Bergamini, L. Santinacci, Y. Léger, N. Sojic and G. Loget, Angew. Chem., Int. Ed., 2022, 61, e20220186 Search PubMed.
- Y. Zhao, J. Descamps, N. H. Al Bast, M. Duque, J. Esteve, B. Sepulveda, G. Loget and N. Sojic, J. Am. Chem. Soc., 2023, 145, 17420–17426 CrossRef CAS PubMed.
- S. Hu, M. R. Shaner, J. A. Beardslee, M. Lichterman, B. S. Brunschwig and N. S. Lewis, Science, 2014, 344, 1005–1009 CrossRef CAS PubMed.
- A. G. Scheuermann, J. P. Lawrence, K. W. Kemp, T. Ito, A. Walsh, C. E. D. Chidsey, P. K. Hurley and P. C. McIntyre, Nat. Mater., 2016, 15, 99–105 CrossRef CAS PubMed.
- G. Loget, C. Mériadec, V. Dorcet, B. Fabre, A. Vacher, S. Fryars and S. Ababou-Girard, Nat. Commun., 2019, 10, 3522 CrossRef CAS PubMed.
- S. E. Jun, Y.-H. Kim, J. Kim, W. S. Cheon, S. Choi, J. Yang, H. Park, H. Lee, S. H. Park, K. C. Kwon, J. Moon, S.-H. Kim and H. W. Jang, Nat. Commun., 2023, 14, 609 CrossRef CAS PubMed.
- O. L. Hendricks, R. Tang-Kong, A. S. Babadi, P. C. McIntyre and C. E. D. Chidsey, Chem. Mater., 2019, 31, 90–100 CrossRef CAS.
- M. Ben-Naim, D. W. Palm, A. L. Strickler, A. C. Nielander, J. Sanchez, L. A. King, D. C. Higgins and T. F. Jaramillo, ACS Appl. Mater. Interfaces, 2020, 12, 5901–5908 CrossRef CAS PubMed.
- J. Dabboussi, R. Abdallah, L. Santinacci, S. Zanna, A. Vacher, V. Dorcet, S. Fryars, D. Floner and G. Loget, J. Mater. Chem. A, 2022, 10, 19769–19776 RSC.
- A. Chovin, P. Garrigue, P. Vinatier and N. Sojic, Anal. Chem., 2004, 76, 357–364 CrossRef CAS PubMed.
- Y. Zhao, J. Descamps, B. Le Corre, Y. Léger, A. Kuhn, N. Sojic and G. Loget, J. Phys. Chem. Lett., 2022, 13, 5538–5544 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc03702a |
| This journal is © The Royal Society of Chemistry 2023 |