 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lighting up spin systems: enhancing characteristic 1H signal patterns of fluorinated molecules†
Marshall J.
Smith
 a,
Jack E.
Bramham
a,
Jack E.
Bramham
 a,
Mathias
Nilsson
a,
Mathias
Nilsson
 a,
Gareth A.
Morris
a,
Gareth A.
Morris
 a,
Laura
Castañar
a,
Laura
Castañar
 *ab and
Alexander P.
Golovanov
*ab and
Alexander P.
Golovanov
 *a
*a
aDepartment of Chemistry, The University of Manchester, Oxford Road, Manchester, M13 9PL, UK. E-mail: a.golovanov@manchester.ac.uk
bDepartment of Organic Chemistry, Faculty of Chemical Science, Complutense University of Madrid, Ciudad Universitaria s/n, Madrid 28040, Spain. E-mail: lcastana@ucm.es
First published on 1st September 2023
Abstract
Fluorine is becoming increasingly prevalent in medicinal chemistry, both in drug molecules and in molecular probes. The presence of fluorine allows convenient monitoring of such molecules in complex environments by NMR spectroscopy. However, sensitivity is a persistent limitation of NMR, especially when molecules are present at low concentrations. Here, sensitivity issues with 1H NMR are mitigated by sharing 19F photochemically-induced dynamic nuclear polarisation with 1H nuclei. Unlike direct 1H enhancement, this method enhances 1H signals without significantly distorting multiplet intensities, and has the potential to enable the use of suitable molecules as low-concentration probes.
Fluorinated molecules account for over 20% of pharmaceuticals currently available, and are commonly found in compound libraries used for drug screening.1,2 Fluorinated species are also increasingly used as probes for studying biological processes, due to the high sensitivity of 19F chemical shifts to the local environment, the absence of endogenous background signals, and 100% natural abundance.3–7 However, structural information obtained by direct observation of sparse fluorine atoms is limited.
1H NMR can provide much more information, by reporting multiple signals from the same molecule, and can be used to characterise the behaviour of a probe molecule in a complex system.8,9 Unfortunately, 1H spectra often suffer from signal overlap, especially in mixtures of protonated molecules, due to the limited chemical shift range and prominent signal multiplicity, making disentangling the spectra of individual species difficult. One strategy for mitigating the limitations of both nuclei is fluorine-edited selective TOCSY acquisition (FESTA), which exploits the exceptional chemical shift dispersion of 19F spectra to acquire 1H spectra containing only 1H signals that are within the same spin system as a selected 19F nucleus.10 FESTA has been previously demonstrated to be particularly powerful in mixture analysis, allowing characteristic 1H subspectra of fluorinated components to be obtained.10–12 Unfortunately, the beneficial ability to observe the characteristic 1H fingerprint patterns of particular fluorinated molecules comes with a sensitivity penalty, which may be critical if molecules are present at low concentrations. Here we introduce a method to address this.
Strategies to improve the sensitivity of NMR include using very high magnetic fields, cryoprobes,13 parahydrogen-induced polarisation (PHIP),14,15 and dynamic nuclear polarisation (DNP)16–18 techniques. Unfortunately, these methods generally come at substantial cost and require additional complex hardware, such as gyrotrons and dissolution apparatus or parahydrogen generators. A potentially more convenient and relatively low-cost alternative to these approaches is photochemically-induced dynamic nuclear polarization (photo-CIDNP)19–21 which, upon simple sample illumination, even using cheap LEDs, can lead to large signal enhancements in target molecules with low ionisation potentials, such as aromatic molecules. The relative enhancements obtained are larger for lower magnetic fields, so photo-CIDNP lends itself well to less expensive hardware, where the increase in sensitivity is at its most welcome.22
Illumination (historically with lasers, more recently with inexpensive LEDs)23–25 of a photosensitiser, such as fluorescein or flavin, present in the sample leads to a non-Boltzmann distribution of nuclear spins in a target molecule via a radical pair mechanism.26 A hyperpolarised signal arises because there is an overpopulation of nuclear spin states which experience faster intersystem crossing and less efficient paramagnetic relaxation.27 However, hyperpolarisation only occurs for nuclei that have a significant hyperfine coupling to an unpaired electron. Other signals from the molecule are not enhanced and, disappointingly, signal intensities may even be reduced, as the polarisation produced by photo-CIDNP can be absorptive or emissive.28 The net result is to distort the characteristic signal pattern of a probe molecule, potentially making the spectrum unrecognisable. These signal distortions and losses hinder the application of direct 1H photo-CIDNP as a signal enhancement approach for molecular detection and monitoring in mixtures. It has been noted, however, that there are significant benefits to using heavier heteroatoms such as 19F, 13C or 15N, which may result in greater hyperpolarization.29–33 Given the ability to transfer magnetization from heteronuclei to protons, sharing such hyperpolarisation enables more sensitive heteronuclear correlation experiments, or can highlight through-space interactions between heteronuclei and neighbouring protons.17,31,34
In this study, we demonstrate how the benefits of FESTA10 can be combined with those of photo-CIDNP to provide significant 1H signal enhancements across a spin system that includes fluorine. This enables observation of the characteristic 1H signals of a molecule with enhanced sensitivity. Using 6-fluoroindole (6FI), a common reagent in the preparation of fluorinated amino acids and antifungal and antibacterial agents,35–37 as a model we show that the direct photo-CIDNP effect is here much greater for 19F than for 1H (Fig. 1d and c). Transferring the large 19F hyperpolarisation to 1H using FESTA demonstrates that multiple 1H signals in the same spin system as fluorine can be greatly enhanced, with much less signal distortion than in direct 1H photo-CIDNP (Fig. 1g and c). This approach also would allow subspectra for specific fluorinated molecules in complex mixtures to be obtained, using a frequency-selective shaped 19F pulse for the initial excitation. The recently proposed NMRtorch approach38 was used to illuminate a sample containing 1 mM 6FI and a flavin mononucleotide as a photosensitizer prior to data acquisition. Matching control spectra were recorded without illumination.
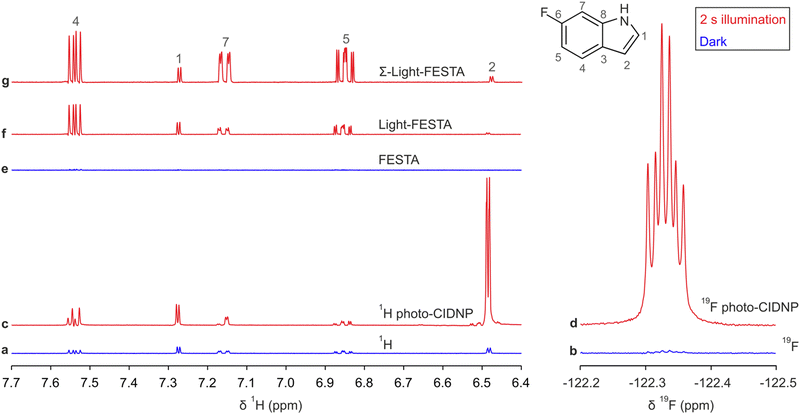 | ||
Fig. 1 (a) 500 MHz 1H and (b) 470 MHz 19F NMR spectra of 1 mM 6FI with 0.2 mM riboflavin 5′-monophosphate sodium salt in D2O. (c) 1H and (d) 19F NMR spectra with 2 s illumination using a single LED with nominal 460![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm peak emission. Example 1H FESTA spectra obtained (e) in the dark and (f) with 2 s illumination using the pulse sequence of Fig. 2 selectively exciting H7 (7.18 ppm). (g) Summation of three 1H FESTA spectra where H4, H5 and H7 were selected (further information can be found in the ESI†). Spectra (d), (f) and (g) were emissive, and are shown inverted with respect to the dark spectra to facilitate comparison. All spectra are scaled consistently for a given nucleus. nm peak emission. Example 1H FESTA spectra obtained (e) in the dark and (f) with 2 s illumination using the pulse sequence of Fig. 2 selectively exciting H7 (7.18 ppm). (g) Summation of three 1H FESTA spectra where H4, H5 and H7 were selected (further information can be found in the ESI†). Spectra (d), (f) and (g) were emissive, and are shown inverted with respect to the dark spectra to facilitate comparison. All spectra are scaled consistently for a given nucleus. | ||
As 6FI is only present at 1 mM concentration, 1H and FESTA NMR spectra recorded in the dark (Fig. 1a and e) suffer from limited signal-to-noise ratio. The 1H NMR spectrum acquired with illumination (i.e., direct 1H photo-CIDNP) showed a 32-fold absorptive signal enhancement of the integral of H2 (Fig. 1c). However, other 1H signals in the molecule showed only modest signal enhancements (<5-fold) and, importantly, the multiplet patterns of H4, H5 and H7 were all significantly distorted (Fig. S2, ESI†), complicating the recognition of this spectrum as belonging to 6FI. 19F photo-CIDNP (Fig. 1d), on the other hand, resulted in 83-fold emissive signal enhancement compared to the corresponding dark reference spectrum (Fig. 1b).
As only certain 6FI 1H signals are enhanced by direct 1H photo-CIDNP, methods of sharing the benefits of hyperpolarisation amongst multiple 1H signals were explored. Initially, 1H selective TOCSY39,40 (as previously proposed with photo-CIDNP by Goez et al.)41 was trialled to share the hyperpolarisation of H2 around the coupled 1H network. However, the signal enhancement was only efficiently shared between H2 and H1, and gave only ∼12-fold enhancement of each with the optimum mixing time (Fig. S3, ESI†). The distant aromatic protons of 6FI (H4, H5 and H7) showed no significant enhancement (Fig. S3, ESI†), because the small inter-ring couplings JHH (Table S2, ESI†) severely limit the effectiveness of TOCSY transfer.
To obtain more uniform enhancement across multiple 1H nuclei, the use of a new approach, Light-FESTA (Fig. 2), was investigated. This exploits the strong 19F photo-CIDNP enhancement and the ability of FESTA to transfer that enhancement across a 1H spin system with minimal multiplet distortion.10 In Light-FESTA the selection of different 19F-1H coupling partners, i.e., transferring F6 magnetisation to H4, H5 or H7, leads to preferential enhancement of different signals (Table 1). However, summing the spectra from these three separate Light-FESTA experiments into a single Σ-Light-FESTA spectrum (Fig. 1g) leads to a more even distribution of signal intensities for H4, H5 and H7. This makes the spectrum easily recognisable as that of 6FI and allows straightforward signal comparison with the reference1H NMR spectrum (Fig. 1a). This contrasts with the direct 1H photo-CIDNP spectrum (Fig. 1c), which is barely recognisable, with the signal intensities for H4, H5 and H7 being lower and their multiplet structures severely distorted.
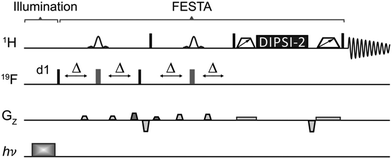 | ||
| Fig. 2 Light-FESTA pulse sequence. The narrow black and wide grey rectangles represent hard 90° and 180° radiofrequency pulses, respectively. The shaped pulses represent selective 180° radiofrequency pulse applied to a chosen 1H resonance coupled to 19F. The trapezoids on either side of the isotropic mixing element (DIPSI-2) represent zero-quantum coherence suppression elements.40 Field gradient pulses are employed to enforce the coherence transfer pathway. Illumination (hν) is applied during the recovery delay, d1. If more than one fluorine resonance is present, the first 19F pulse should be frequency-selective. Further details are provided in the ESI.† | ||
| H4 | H1 | H7 | H5 | H2 | |
|---|---|---|---|---|---|
| Direct 1H photo-CIDNP | 4.0 | 3.3 | 2.1 | 1.4 | 32.4 |
| Light-FESTA H5 (6.86 ppm) | 5.0 | 0.3 | 7.2 | 16.6 | 0.8 |
| Light-FESTA H7 (7.16 ppm) | 14.9 | 2.9 | 4.1 | 7.2 | 0.5 |
| Light-FESTA H4 (7.54 ppm) | 6.1 | 0.4 | 10.9 | 3.7 | 0.7 |
| Σ-Light-FESTA | 8.7 | 1.2 | 7.4 | 9.2 | 0.7 |
Light-FESTA provides a significant signal enhancement (Fig. 1f) compared to standard FESTA (Fig. 1e), with minimal multiplet distortion. The background noise level was unaffected by illumination with NMRtorch.38 The average sensitivity enhancement (defined as enhancement in the individual signal-to-noise ratios) was 34-fold for the H4–H5–H7 spin system (Table S3, ESI†), translating into a potential 1000-fold experimental time saving. Overall, Σ-Light-FESTA gives rise to >7 times more intense signals for the H4–H5–H7 spin system compared with a standard 1H NMR spectrum recorded without illumination (Table 1) and is up to 49 times more sensitive than a standard FESTA experiment (Table S3, ESI†). Extra benefits include the ability to select a molecule of interest in complex mixtures, by applying selective 19F and 1H pulses at defined frequencies. As the transfer of magnetisation to other 1H nuclei within a spin system is dependent on the isotropic mixing time (Fig. S4, ESI†), running several experiments with judicious choices of mixing times and summing the resulting spectra can lead to more even distribution of hyperpolarisation.
Finally, although 1D selective TOCSY did not yield satisfactory results for 6FI, in cases where there is an extensive scalar coupling network with appreciable JHH values, a selective TOCSY experiment may be advantageous. Another interesting possibility, currently being explored, is to design a pulse sequence that pools the 1H and the 19F hyperpolarisation, sharing both among their neighbours.
In summary, we have shown that 19F and direct 1H photo-CIDNP enhancements can be shared amongst multiple 1H nuclei within the same spin system, using FESTA or selective TOCSY, with the former being much more effective. Transferring the greater hyperpolarisation of 19F to 1H led to the enhancement of more signals, a more even distribution of signal intensities, and, in contrast to direct 1H photo-CIDNP, preservation of normal multiplet structure. This makes the molecular spectral fingerprint easily recognisable. Light-FESTA not only eliminated the sensitivity penalty of the parent FESTA experiment, with a 34-fold enhancement, but actually increased the sensitivity of proton detection sevenfold compared to a standard 1H NMR spectrum without illumination. Light-FESTA should therefore allow the analysis of molecules at low concentrations while retaining the selectivity advantages of FESTA. This approach will be particularly powerful when investigating fluorinated molecules known to show a photo-CIDNP effect. Such molecules could be used as probes to investigate complex system behaviour, or as constituents of compound libraries for drug screening.42,43 Sample illumination here used the LED-based NMRtorch, a recent general tool for photo-NMR.38 The approach presented in this paper can readily be extended to other spectral editing techniques, for example, to observe enhanced through-space interactions, determine molecular proximity, or quantify ligand–receptor interactions, as required in drug screening.34,44 Although demonstrated using 19F nuclei, Light-FESTA could be extended to achieve selective photo-CIDNP enhancement of 1H using other heteronuclei such as 13C or 15N.31,33
The NMRtorch hardware was manufactured by the last author (APG). All authors contributed to the design of the experiments and pulse sequences, the analysis of the results, and the writing of the manuscript.
The authors gratefully acknowledge the Engineering and Physical Sciences Research Council (grant numbers EP/R018790/1, EP/V04835X/1, and EP/R513131/1 Project Reference 2297284) and the University of Manchester (Dame Kathleen Ollerenshaw Fellowship to L. C.) and the Comunidad de Madrid (grant number 2022-T1/BMD-24030 to L. C.) for supporting this work. We acknowledge the use of the Manchester Biomolecular NMR Facility and are grateful to Matthew Cliff for help with NMR equipment. For the purpose of open access, the authors have applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising. All NMR experimental data, pulse sequence codes for Bruker spectrometers, and processing macro are freely available at https://doi.org/10.48420/23056013.
Conflicts of interest
APG is the named author in patent application PCT/GB2021/051254, International Publication Number WO 2021/250372 A1. All other authors declare no competing interests.References
- M. Inoue, Y. Sumii and N. Shibata, ACS Omega, 2020, 5, 10633–10640 CrossRef CAS PubMed
.
- I. Hyohdoh, N. Furuichi, T. Aoki, Y. Itezono, H. Shirai, S. Ozawa, F. Watanabe, M. Matsushita, M. Sakaitani, P.-S. Ho, K. Takanashi, N. Harada, Y. Tomii, K. Yoshinari, K. Ori, M. Tabo, Y. Aoki, N. Shimma and H. Iikura, ACS Med. Chem. Lett., 2013, 4, 1059–1063 CrossRef CAS PubMed
.
- H. Chen, S. Viel, F. Ziarelli and L. Peng, Chem. Soc. Rev., 2013, 42, 7971 RSC
.
- J.-X. Yu, R. R. Hallac, S. Chiguru and R. P. Mason, Prog. Nucl. Magn. Reson. Spectrosc., 2013, 70, 25–49 CrossRef CAS PubMed
.
- J. M. Edwards, J. P. Derrick, C. F. Van Der Walle and A. P. Golovanov, Mol. Pharmaceutics, 2018, 15, 2785–2796 CrossRef CAS PubMed
.
- H.-L. Bao and Y. Xu, Chem. Commun., 2020, 56, 6547–6550 RSC
.
- A. M. Gronenborn, Structure, 2022, 30, 6–14 CrossRef CAS PubMed
.
- Z. Xu, C. Liu, S. Zhao, S. Chen and Y. Zhao, Chem. Rev., 2019, 119, 195–230 CrossRef CAS PubMed
.
- Q. Liu, Q.-T. He, X. Lyu, F. Yang, Z.-L. Zhu, P. Xiao, Z. Yang, F. Zhang, Z.-Y. Yang, X.-Y. Wang, P. Sun, Q.-W. Wang, C.-X. Qu, Z. Gong, J.-Y. Lin, Z. Xu, S.-L. Song, S.-M. Huang, S.-C. Guo, M.-J. Han, K.-K. Zhu, X. Chen, A. W. Kahsai, K.-H. Xiao, W. Kong, F.-H. Li, K. Ruan, Z.-J. Li, X. Yu, X.-G. Niu, C.-W. Jin, J. Wang and J.-P. Sun, Nat. Commun., 2020, 11, 4857 CrossRef CAS PubMed
.
- L. Castañar, P. Moutzouri, T. M. Barbosa, C. F. Tormena, R. Rittner, A. R. Phillips, S. R. Coombes, M. Nilsson and G. A. Morris, Anal. Chem., 2018, 90, 5445–5450 CrossRef PubMed
.
- T. M. Barbosa, L. Castañar, P. Moutzouri, M. Nilsson, G. A. Morris, R. Rittner and C. F. Tormena, Anal. Chem., 2020, 92, 2224–2228 CrossRef CAS PubMed
.
- G. Dal Poggetto, J. V. Soares and C. F. Tormena, Anal. Chem., 2020, 92, 14047–14053 CrossRef CAS PubMed
.
- H. Kovacs, D. Moskau and M. Spraul, Prog. Nucl. Magn. Reson. Spectrosc., 2005, 46, 131–155 CrossRef CAS
.
- S. B. Duckett and R. E. Mewis, Acc. Chem. Res., 2012, 45, 1247–1257 CrossRef CAS PubMed
.
- W. Iali, P. J. Rayner and S. B. Duckett, Sci. Adv., 2018, 4, eaao6250 CrossRef PubMed
.
- A. Abragam and M. Goldman, Rep. Prog. Phys., 1978, 41, 395–467 CrossRef CAS
.
- J. H. Lee, Y. Okuno and S. Cavagnero, J. Magn. Reson., 2014, 241, 18–31 CrossRef CAS PubMed
.
- B. Plainchont, P. Berruyer, J.-N. Dumez, S. Jannin and P. Giraudeau, Anal. Chem., 2018, 90, 3639–3650 CrossRef CAS PubMed
.
- R. Kaptein, K. Dijkstra, F. Müller, C. G. Van Schagen and A. J. W. G. Visser, J. Magn. Reson. (1969-1992), 1978, 31, 171–176 CrossRef CAS
.
- M. Mompeán, R. M. Sánchez-Donoso, A. De La Hoz, V. Saggiomo, A. H. Velders and M. V. Gomez, Nat. Commun., 2018, 9, 108 CrossRef PubMed
.
- Y. Okuno, M. F. Mecha, H. Yang, L. Zhu, C. G. Fry and S. Cavagnero, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 11602–11611 CrossRef CAS PubMed
.
- J. Bernarding, C. Bruns, I. Prediger and M. Plaumann, Appl. Magn. Reson., 2022, 53, 1375–1398 CrossRef CAS
.
- P. Nitschke, N. Lokesh and R. M. Gschwind, Prog. Nucl. Magn. Reson. Spectrosc., 2019, 114–115, 86–134 CrossRef CAS PubMed
.
- J. Bernarding, F. Euchner, C. Bruns, R. Ringleb, D. Müller, T. Trantzschel, J. Bargon, U. Bommerich and M. Plaumann, Chem. Phys. Chem., 2018, 19, 2453–2456 CrossRef CAS PubMed
.
- H. Yang, H. Hofstetter and S. Cavagnero, J. Chem. Phys., 2019, 151, 245102 CrossRef PubMed
.
- G. L. Closs, J. Am. Chem. Soc., 1969, 91, 4552–4554 CrossRef CAS
.
- J. Hore and R. W. Broadhurst, Prog. Nucl. Magn. Reson. Spectrosc., 1993, 25, 345–402 CrossRef
.
- R. Kaptein, J. Chem. Soc. D, 1971, 732–733 RSC
.
- C. E. Lyon, J. A. Jones, C. Redfield, C. M. Dobson and P. J. Hore, J. Am. Chem. Soc., 1999, 121, 6505–6506 CrossRef CAS
.
- F. Khan, I. Kuprov, T. D. Craggs, P. J. Hore and S. E. Jackson, J. Am. Chem. Soc., 2006, 128, 10729–10737 CrossRef CAS PubMed
.
- J. H. Lee, A. Sekhar and S. Cavagnero, J. Am. Chem. Soc., 2011, 133, 8062–8065 CrossRef CAS PubMed
.
- Y. Okuno and S. Cavagnero, J. Magn. Reson., 2018, 286, 172–187 CrossRef CAS PubMed
.
- A. Sekhar and S. Cavagnero, J. Phys. Chem. B, 2009, 113, 8310–8318 CrossRef CAS PubMed
.
- I. Kuprov and P. J. Hore, J. Magn. Reson., 2004, 168, 1–7 CrossRef CAS PubMed
.
- R. Curtis-Marof, D. Doko, M. L. Rowe, K. L. Richards, R. A. Williamson and M. J. Howard, Org. Biomol. Chem., 2014, 12, 3808–3812 RSC
.
- O. N. Burchak, E. L. Pihive, L. Maigre, X. Guinchard, P. Bouhours, C. Jolivalt, D. Schneider, M. Maurin, C. Giglione, T. Meinnel, J.-M. Paris and J.-N. Denis, Biorg. Med. Chem., 2011, 19, 3204–3215 CrossRef CAS PubMed
.
- Y.-M. Na, Bull. Korean Chem. Soc., 2010, 31, 3467–3470 CrossRef CAS
.
- J. E. Bramham and A. P. Golovanov, Commun. Chem., 2022, 5, 90 CrossRef CAS PubMed
.
- H. Kessler, H. Oschkinat, C. Griesinger and W. Bermel, J. Magn. Reson. (1969-1992), 1986, 70, 106–133 CrossRef CAS
.
- M. J. Thrippleton and J. Keeler, Angew. Chem., Int. Ed., 2003, 42, 3938–3941 CrossRef CAS PubMed
.
- M. Goez, K. H. Mok and P. J. Hore, J. Magn. Reson., 2005, 177, 236–246 CrossRef CAS PubMed
.
- F. Torres, A. Renn and R. Riek, Magn. Reson., 2021, 2, 321–329 CrossRef CAS
.
- F. Torres, M. Bütikofer, G. R. Stadler, A. Renn, H. Kadavath, R. Bobrovs, K. Jaudzems and R. Riek, J. Am. Chem. Soc., 2023, 145, 12066–12080 CrossRef CAS PubMed
.
- P. Lameiras, S. Patis, J. Jakhlal, S. Castex, P. Clivio and J.-M. Nuzillard, Chem. – Eur. J., 2017, 23, 4923–4928 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Containing further experimental data, guidance on acquiring experiments and pulse sequence code for Bruker spectrometers. See DOI: https://doi.org/10.1039/d3cc03557c |
| This journal is © The Royal Society of Chemistry 2023 |
