Open Access Article
Open Access ArticleRuthenium-catalysed N-alkylation of anilines with primary carbohydrate alcohols via borrowing hydrogen strategy†
Kouki
Tsuge
a,
Shunnichi
Kubota
a,
Kana
Sakamoto
a,
Kenji
Kitayama
b and
Takahiro
Nishimura
 *a
*a
aDepartment of Chemistry, Graduate School of Science, Osaka Metropolitan University, Sumiyoshi, Osaka 558-8585, Japan. E-mail: tnishi@omu.ac.jp
bDaicel Corporation, Grand Front Osaka Tower-B, 3-1, Ofuka-cho, Kita-ku, Osaka 530-0011, Japan
First published on 17th May 2023
Abstract
Ruthenium-catalysed N-alkylation of anilines with sugar derivatives proceeded via the borrowing hydrogen strategy. Primary carbohydrate alcohols were successfully applied to N-alkylation of aniline derivatives to give the corresponding aminosugars in high yields.
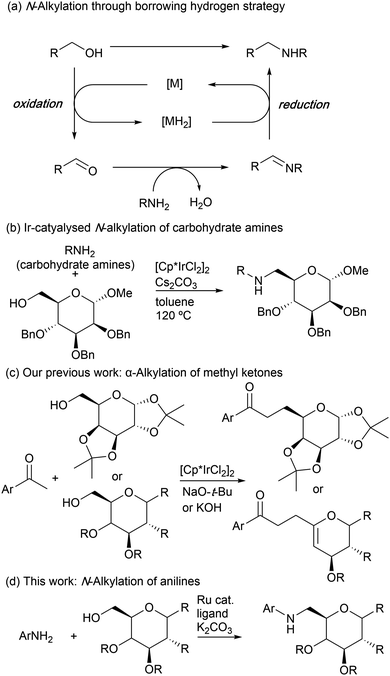
Transition-metal-catalysed N-alkylation of amines via the borrowing hydrogen strategy is a desirable process for the formation of carbon–nitrogen bonds, enabling alcohols to be employed directly as alkylating agents (Scheme 1a).1 A variety of catalytic systems based on the late-transition metals have been developed for the N-alkylation of amines by using simple alcohols.2–5 Biomass-derived alcohols, such as ethylene glycol, 1,3-propanediol, isohexides, and so on, have also been recognized as important reaction partners in the borrowing hydrogen strategy.6 However, carbohydrate alcohols have been scarcely used for the direct N-alkylation reaction. In this respect, in 2011, Cumpstey and Martín-Matute reported the first example of N-alkylation of alkylamines derived from sugars with primary carbohydrate alcohols catalysed by an Ir(III) complex, where amine-linked pseudodisaccharides are successfully synthesized in a single step through borrowing hydrogen strategy (Scheme 1b).7 In this context, we recently reported α-alkylation of methyl ketones with primary carbohydrate alcohols as alkylating agents (Scheme 1c).8 The reaction is efficiently catalysed by an Ir(III) complex in the presence of a strong base. During our studies on the catalytic functionalization of sugar derivatives,8,9 it was found that a ruthenium complex was effective in catalyzing the borrowing hydrogen reaction between anilines and sugars (Scheme 1d). Here we describe that a ruthenium/dppf type ligand complex efficiently catalyses N-alkylation of anilines with primary carbohydrate alcohols, providing a new method for the synthesis of aminosugar derivatives.10 Aminosugars possess potential properties that take part in a variety of biological functions, and therefore, the development of the synthesis of the aminosugars is important for the understanding of their functions.11
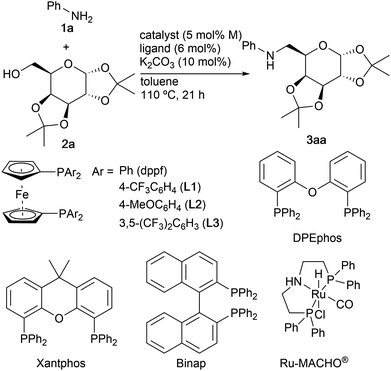
Our initial studies focused on the N-alkylation of aniline (1a) with 1,2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3,4-di-O-isopropylidene-α-D-galactopyranose (2a) in the presence of ruthenium complexes directed toward the catalytic synthesis of aminosugar 3aa (Table 1).12 Treatment of 1a (1.2 equiv.) with 2a (1.0 equiv.) in the presence of [RuCl2(p-cymene)]2 (5 mol% of Ru), dppf (6 mol%), and K2CO3 (10 mol%) in toluene, which is one of the reaction conditions reported by Williams and co-workers,2c at 110 °C for 21 h gave alkylated product 3aa in 31% yield (entry 1). The ligand and base were necessary to obtain 3aa (entries 2 and 3). The aryl groups on dppf ligands significantly influenced the reactivity. The use of ligand L1 substituted with p-(trifluoromethyl)phenyl groups improved the yield of 3aa up to 69% (entry 4). In contrast, methoxy-substituted L2 diminished the yield (entry 5), and ligand L3 inhibited the reaction, probably due to the bulkiness (entry 6). DPEphos, Xantphos, or Binap were not effective in catalyzing the present reaction (entries 7–9). The use of a slight excess (1.2 equiv.) of alcohol 2a toward aniline (1a) improved the yield up to 76% (entry 10). Na2CO3 and Cs2CO3 were less effective than K2CO3, thus giving 3aa in 45 and 53% yields, respectively (entries 11 and 12). The reaction in p-xylene at 140 °C gave 3aa in 87% yield (entry 13). The catalytic activity of [RuCl2(benzene)]2 was quite low (entry 14). Ru3(CO)122d and Ru-MACHO®2j did not work as catalysts (entries 15–17).
3,4-di-O-isopropylidene-α-D-galactopyranose (2a) in the presence of ruthenium complexes directed toward the catalytic synthesis of aminosugar 3aa (Table 1).12 Treatment of 1a (1.2 equiv.) with 2a (1.0 equiv.) in the presence of [RuCl2(p-cymene)]2 (5 mol% of Ru), dppf (6 mol%), and K2CO3 (10 mol%) in toluene, which is one of the reaction conditions reported by Williams and co-workers,2c at 110 °C for 21 h gave alkylated product 3aa in 31% yield (entry 1). The ligand and base were necessary to obtain 3aa (entries 2 and 3). The aryl groups on dppf ligands significantly influenced the reactivity. The use of ligand L1 substituted with p-(trifluoromethyl)phenyl groups improved the yield of 3aa up to 69% (entry 4). In contrast, methoxy-substituted L2 diminished the yield (entry 5), and ligand L3 inhibited the reaction, probably due to the bulkiness (entry 6). DPEphos, Xantphos, or Binap were not effective in catalyzing the present reaction (entries 7–9). The use of a slight excess (1.2 equiv.) of alcohol 2a toward aniline (1a) improved the yield up to 76% (entry 10). Na2CO3 and Cs2CO3 were less effective than K2CO3, thus giving 3aa in 45 and 53% yields, respectively (entries 11 and 12). The reaction in p-xylene at 140 °C gave 3aa in 87% yield (entry 13). The catalytic activity of [RuCl2(benzene)]2 was quite low (entry 14). Ru3(CO)122d and Ru-MACHO®2j did not work as catalysts (entries 15–17).
| Entry | Catalyst | Ligand | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.24 mmol), 2a (0.20 mmol), [RuCl2(p-cymene)]2 (0.0050 mmol, 5 mol% of Ru), and base (10 mol%) in toluene (0.30 mL) at 110 °C for 21 h. b Determined by 1H NMR. c Without K2CO3. d Performed with 1a (0.20 mmol) and 2a (0.24 mmol). e With Na2CO3 instead of K2CO3. f With Cs2CO3 instead of K2CO3. g At 140 °C in p-xylene. h Isolated yield. | |||
| 1 | [RuCl2(p-cymene)]2 | dppf | 31 |
| 2 | [RuCl2(p-cymene)]2 | — | 0 |
| 3c | [RuCl2(p-cymene)]2 | dppf | 0 |
| 4 | [RuCl2(p-cymene)]2 | L1 | 69 |
| 5 | [RuCl2(p-cymene)]2 | L2 | 6 |
| 6 | [RuCl2(p-cymene)]2 | L3 | 0 |
| 7 | [RuCl2(p-cymene)]2 | DPEphos | 15 |
| 8 | [RuCl2(p-cymene)]2 | Xantphos | 6 |
| 9 | [RuCl2(p-cymene)]2 | Binap | 0 |
| 10d | [RuCl2(p-cymene)]2 | L1 | 76 |
| 11de | [RuCl2(p-cymene)]2 | L1 | 45 |
| 12df | [RuCl2(p-cymene)]2 | L1 | 53 |
| 13dg | [RuCl2(p-cymene)]2 | L1 | 87 (82)h |
| 14 | [RuCl2(benzene)]2 | L1 | 7 |
| 15cd | Ru3(CO)12 | — | 0 |
| 16d | Ru3(CO)12 | — | 0 |
| 17d | Ru-MACHO® | — | 0 |
Scheme 2 summarizes the results obtained for the reaction of several primary carbohydrate alcohols. The reactions of O-methylated α-glucose 2b, β-glucose 2c, and β-galactose 2d with aniline (1a) gave the corresponding sugars 3ab–3ad in 42–53% yields. Alcohol 2e having benzyl ether moieties and 2f with a free hydroxy group reacted with 1a to give N-alkylated products 3ae and 3af in 25% and 35% yields, respectively. The reaction of C-glycoside 2g and deoxyglucose 2h also proceeded to give the corresponding aminosugars 3ag and 3ah.
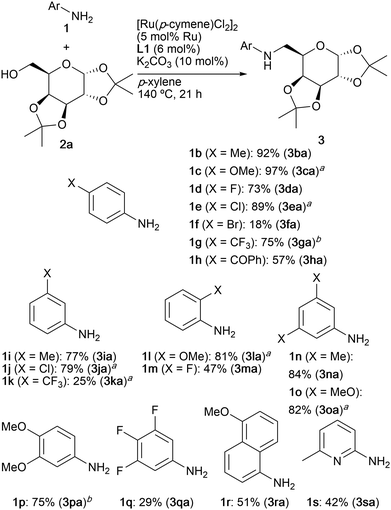
A variety of aniline derivatives 1 participated in the reaction with carbohydrate alcohol 2a as summarized in Scheme 3. N-Alkylation of anilines having electron-donating and -withdrawing substituents (2a–2m) at the o-, m-, and p-positions proceeded to give the corresponding aminosugars in 18–97% yields, where anilines substituted with electron-withdrawing groups displayed the low reactivity. In particular, the loss of the catalytic activity was observed in reaction of p-bromoaniline (1f). Dimethyl (1n) and dimethoxyanilines (1o and 1p) reacted with 2a to give aminosugars 3na, 3oa, and 3pa, respectively, in high yields. Modest yields were observed for 3,4,5-trifluoroaniline (1q), 5-methoxy-1-naphthylamine (1r), and 6-methyl-2-aminopyridine (1s). In sharp contrast, N-methylaniline or aliphatic amines such as n-butylamine and piperidine were not alkylated under the present reaction conditions.
In summary, we have developed ruthenium-catalysed N-alkylation of anilines with primary carbohydrate alcohols. A variety of aniline derivatives were applied to the reaction to give the corresponding aminosugars in high yields. Several O-protected sugar derivatives could be used as alkylating agents for N-alkylation of aniline derivatives.
Conflicts of interest
There are no conflicts to declare.Notes and references
- For selected recent reviews, see: (a) E. Podyacheva, O. I. Afanasyev, D. V. Vasilyev and D. Chusov, ACS Catal., 2022, 12, 7142 CrossRef CAS; (b) B. G. Reed-Berendt, D. E. Latham, M. B. Dambatta and L. C. Morrill, ACS Cent. Sci., 2021, 7, 570 CrossRef CAS PubMed; (c) T. Irrgang and R. Kempe, Chem. Rev., 2019, 119, 2524 CrossRef CAS PubMed; (d) B. G. Reed-Berendt, K. Polidano and L. C. Morrill, Org. Biomol. Chem., 2019, 17, 1595 RSC; (e) A. Corma, J. Navas and M. J. Sabater, Chem. Rev., 2018, 118, 1410 CrossRef CAS PubMed; (f) Q. Yang, Q. Wang and Z. Yu, Chem. Soc. Rev., 2015, 44, 2305 RSC; (g) S. Bähn, S. Imm, L. Neubert, M. Zhang, H. Neumann and M. Beller, ChemCatChem, 2011, 3, 1853 CrossRef; (h) G. Guillena, D. J. Ramón and M. Yus, Chem. Rev., 2010, 110, 1611 CrossRef CAS PubMed; (i) M. H. S. A. Hamid, P. A. Slatford and J. M. J. Williams, Adv. Synth. Catal., 2007, 349, 1555 CrossRef CAS.
- For selected examples using Ru catalysts, see: (a) Y. Watanabe, Y. Tsuji and Y. Ohsugi, Tetrahedron Lett., 1981, 22, 2667 CrossRef CAS; (b) M. H. S. A. Hamid and J. M. J. Williams, Chem. Commun., 2007, 725 RSC; (c) M. H. S. A. Hamid, C. L. Allen, G. W. Lamb, A. C. Maxwell, H. C. Maytum, A. J. A. Watson and J. M. J. Williams, J. Am. Chem. Soc., 2009, 131, 1766 CrossRef CAS PubMed; (d) D. Hollmann, A. Tillack, D. Michalik, R. Jackstell and M. Beller, Chem. – Asian J., 2007, 2, 403 CrossRef CAS PubMed; (e) R. N. Monrad and R. Madsen, Org. Biomol. Chem., 2011, 9, 610 RSC; (f) A. B. Enyong and B. Moasser, J. Org. Chem., 2014, 79, 7553 CrossRef CAS PubMed; (g) S. P. Shan, X. Xiaoke, B. Gnanaprakasam, T. T. Dang, B. Ramalingam, H. V. Huynh and A. M. Seayad, RSC Adv., 2015, 5, 4434 RSC; (h) M. Kaloğlu, Inorg. Chim. Acta, 2019, 498, 119163 CrossRef; (i) A. E. Putra, Y. Oe and T. Ohta, Eur. J. Org. Chem., 2013, 6146 CrossRef; (j) O. Ogata, H. Nara, M. Fujiwhara, K. Matsumura and Y. Kayaki, Org. Lett., 2018, 20, 3866 CrossRef CAS PubMed; (k) M. Maji, K. Chakrabarti, B. Paul, B. C. Roy and S. Kundu, Adv. Synth. Catal., 2018, 360, 722 CrossRef CAS; (l) X.-J. Yu, H.-Y. He, L. Yang, H.-Y. Fu, X.-L. Zheng, H. Chen and R.-X. Li, Catal. Commun., 2017, 95, 54 CrossRef CAS; (m) S. Agrawal, M. Lenormand and B. Martín-Matute, Org. Lett., 2012, 14, 1456 CrossRef CAS PubMed; (n) S. Demir, F. Coskun and I. Özdemir, J. Organomet. Chem., 2014, 755, 134 CrossRef CAS; (o) Ö. Ulu, N. Gürbüz and I. Özdemir, Tetrahedron, 2018, 74, 645 CrossRef; (p) S. N. R. Donthireddy, P. M. Illam and A. Rit, Inorg. Chem., 2020, 59, 1835 CrossRef CAS PubMed; (q) R. Savela, D. Vogt and R. Leino, Eur. J. Org. Chem., 2020, 3030 CrossRef CAS; (r) F.-L. Yang, Y.-H. Wang, Y.-F. Ni, X. Gao, B. Song, X. Zhu and X.-Q. Hao, Eur. J. Org. Chem., 2017, 3481 CrossRef CAS; (s) V. R. Jumde, L. Gonsalvi, A. Guerriero, M. Peruzzini and M. Taddei, Eur. J. Org. Chem., 2015, 1829 CrossRef CAS; (t) K. O. Marichev and J. M. Takacs, ACS Catal., 2016, 6, 2205 CrossRef CAS PubMed; (u) R. Ramachandran, G. Prakash, M. Nirmala, P. Viswanathamurthi and J. G. Malecki, J. Organomet. Chem., 2015, 791, 130 CrossRef CAS; (v) S. Gayathri, P. Viswanathamurthi, R. Bertani and P. Sgarbossa, ACS Omega, 2022, 7, 33107 CrossRef CAS PubMed; (w) F. V. Rossi, J. T. Starr, D. P. Uccello and J. A. Young, Org. Lett., 2020, 22, 5890 CrossRef CAS PubMed.
- For pioneering examples using Rh catalysts, see: (a) R. Grigg, T. R. B. Mitchell, S. Sutthivaiyakit and N. Tongpeny, J. Chem. Soc., Chem. Commun., 1981, 611 RSC; (b) N. Tanaka, M. Hatanaka and Y. Watanabe, Chem. Lett., 1992, 575 CrossRef CAS; (c) C. Liu, S. Liao, Q. Li, S. Feng, Q. Sun, X. Yu and Q. Xu, J. Org. Chem., 2011, 76, 5759 CrossRef CAS PubMed.
- For pioneering examples using Ir catalysts, see: (a) K. Fujita, Z. Li, N. Ozeki and R. Yamaguchi, Tetrahedron Lett., 2003, 44, 2687 CrossRef CAS; (b) K. Fujita, Y. Enoki and R. Yamaguchi, Tetrahedron, 2008, 64, 1943 CrossRef CAS; (c) R. Kawahara, K. Fujita and R. Yamaguchi, J. Am. Chem. Soc., 2010, 132, 15108 CrossRef CAS PubMed; (d) G. Cami-Kobeci, P. A. Slatford, M. K. Whittlesey and J. M. J. Williams, Bioorg. Med. Chem. Lett., 2005, 15, 535 CrossRef CAS PubMed; (e) O. Saidi, A. J. Blacker, M. M. Farah, S. P. Marsden and J. M. J. Williams, Chem. Commun., 2010, 46, 1541 RSC; (f) B. Blank, M. Madalska and R. Kempe, Adv. Synth. Catal., 2008, 350, 749 CrossRef CAS.
- For selected examples of catalytic N-alkylation by use of transition-metal catalysts other than Ru, Rh, and Ir, see: (a) R. Martínez, D. J. Ramón and M. Yus, Org. Biomol. Chem., 2009, 7, 2176 RSC; (b) A. Martínez-Asencio, D. J. Ramón and M. Yus, Tetrahedron Lett., 2010, 51, 325 CrossRef; (c) M. Peña-López, P. Piehl, S. Elangovan, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2016, 55, 14967 CrossRef PubMed; (d) S. Rösler, M. Ertl, T. Irrgang and R. Kempe, Angew. Chem., Int. Ed., 2015, 54, 15046 CrossRef PubMed; (e) M. Vellakkaran, K. Singh and D. Banerjee, ACS Catal., 2017, 7, 8152 CrossRef CAS.
- (a) N. K. Gupta, P. Reif, P. Palenicek and M. Rose, ACS Catal., 2022, 12, 10400 CrossRef CAS; (b) S. Hameury, H. Bensalem and K. de Oliveira Vigier, Catalysts, 2022, 12, 1306 CrossRef CAS.
- I. Cumpstey, S. Agrawal, K. E. Borbas and B. Martín-Matute, Chem. Commun., 2011, 47, 7827 RSC.
- K. Tsuge, S. Kubota, K. Sakamoto, K. Kitayama and T. Nishimura, Adv. Synth. Catal., 2023, 365, 971 CrossRef CAS.
- K. Sakamoto, M. Nagai, Y. Ebe, H. Yorimitsu and T. Nishimura, ACS Catal., 2019, 9, 1347 CrossRef CAS.
- For a review of the synthesis of aminosugar derivatives, see: R. Sangwan, A. Khanam and P. K. Mandal, Eur. J. Org. Chem., 2020, 5949 CrossRef CAS.
- L. Cipolla and F. Peri, Mini-Rev. Med. Chem., 2011, 11, 39 CrossRef CAS PubMed.
- For an example of the synthesis of 3aa by N-arylation of 6-amino-6-deoxy-1,2:3,4-di-O-isopropylidene-α-D-galactopyranose, see: K. B. Pal, M. Mahanti and U. J. Nilsson, Org. Lett., 2018, 20, 616 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc01931d |
| This journal is © The Royal Society of Chemistry 2023 |