Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
From dots to tubes – the reversed scenario of bottom-up external-catalyst-free synthesis of N-doped carbon nanotubes†
Anna
Kolanowska
 *ab,
Dariusz
Łukowiec
*ab,
Dariusz
Łukowiec
 c,
Maciej
Krzywiecki
c,
Maciej
Krzywiecki
 d,
Joanna
Bok-Badura
d,
Joanna
Bok-Badura
 e and
Sławomir
Boncel
e and
Sławomir
Boncel
 *af
*af
aNanoCarbon Group, Faculty of Chemistry, Department of Organic Chemistry, Bioorganic Chemistry and Biotechnology, Silesian University of Technology, Krzywoustego 4, 44-100 Gliwice, Poland. E-mail: anna.kolanowska@polsl.pl; slawomir.boncel@polsl.pl
bBiotechnology Centre, Silesian University of Technology, Krzywoustego 8, 44-100 Gliwice, Poland
cMaterials Research Laboratory, Faculty of Mechanical Engineering, Silesian University of Technology, Konarskiego 18A, 44-100 Gliwice, Poland
dInstitute of Physics–CSE, Silesian University of Technology, Konarskiego 22B, 44-100 Gliwice, Poland
eFaculty of Chemistry, Department of Inorganic, Analytical Chemistry and Electrochemistry, Silesian University of Technology, Krzywoustego 6, 44-100 Gliwice, Poland
fCentre for Organic and Nanohybrid Electronics (CONE), Silesian University of Technology, Konarskiego 22B, 44-100 Gliwice, Poland
First published on 1st June 2023
Abstract
We present a novel external-catalyst-free route for the synthesis of N-doped carbon nanotubes (N-CNTs) from amino-acid-derived carbon dots (CDs) as sustainable resources. N-CNTs (∼4–26 at% of N) were comprehensively characterized by complementary techniques while the synthetic strategy emerges as an important alternative and, simultaneously, a simply-scalable approach.
Carbon nanotubes (CNTs), with their electronically tunable nitrogen-doped structural variants (N-CNTs), continue to appeal to both scientists and technologists worldwide.1 This attraction is driven by their ‘all-in-one’ superb mechanical, electrical, thermal, chemical and biological properties.2 (N-)CNTs are synthesized mainly via arc discharge, laser ablation, and chemical vapor deposition (CVD),3 while the latter method has emerged as the most scalable route yielding the purest, readily applicable (N-)CNTs.4 Although this method allows for the synthesis of (N-)CNTs from different carbon sources and in a variety of states of matter, such as gases,5 liquids6 or solids,7 the employment of catalytic amounts of metallic nanoparticles is typically reported as essential for the synthesis of (N-)CNTs.8
In 2004, the family of carbon nanoallotropes grew to include carbon dots (CDs) – fluorescent nanomaterials with a carbon core and oxygen/nitrogen functionalities on their surface.9,10 Those novel and per se unique 0D carbon nanomaterials serve as biocompatible fluorescent probes,11 drug delivery systems,12 sensors,13 light emitters,14 and photocatalysts.15 One of the top-down methods of CD synthesis is a controlled, fragmentary decomposition of CNTs,16 or graphite/graphene,17 whereas bottom-up synthetic methods are based on a progressive polyaromatization and graphitization of low-molecular-weight precursors.18 And although the synthesis of CNTs and N-CNTs has been studied for more than three decades, the catalyst-free methods were elaborated only for CNTs (with a single report on N-CNTs) (Table S1, ESI†).19–23 But more importantly, there are no reports on the synthesis of N-CNTs from CDs as a carbon source while, as mentioned above, only the reverse scenario was realized.
Here, we report the unique and innovative synthesis and characterization of multi-walled N-CNTs by external-catalyst-free decomposition of amino-acid-derived CDs. Similar to happenstances in the history of carbon nanomaterials, N-CNTs were accidentally discovered in the black deposits – left after thermogravimetric analysis (TGA) of solid amino-acid-derived CDs (displaying from green to blue fluorescence). Our results clearly demonstrate that CDs can be used as structurally tunable and scalable synthetic precursors of N-CNTs.
Firstly, CDs were synthesized from six structurally different natural L-amino acids according to the previously reported protocol (ESI).18 Next, CDs (10 mg) were placed in an alumina crucible, heated at the 20 °C min−1 rate to 800 °C, kept thereat for 3 min, and left to cool down to room temperature – with the total synthesis time of ∼1 h in a TGA furnace (TGA 8000, PerkinElmer) (Scheme 1). The whole process was run under a nitrogen flow (20 mL min−1). The highest yield (calculated from the TGA curves describing the synthesis procedure, i.e. the percentage ratio of the residual mass and the initial sample weight) of 35% was found for N-CNTs from the His-CDs.
 | ||
| Scheme 1 Scheme of TGA-based amino-acid-CD-N-CNT synthesis; insets show photographs of yellow CDs and a TGA pan with the black N-CNT product. | ||
In parallel, the yield of the Phe-CD-N-CNT product was 18% (Fig. S1, ESI†). The N-CNT yields were ambiguously related to the CD precursor size whereas the highest yields were achieved for CDs with a few-nanometre size. And so, the highest N-CNT yield was achieved for compact His-CDs with a diameter ∼1.5 nm; contrarily, for Cys- and Asp-CDs – of a diameter even smaller than 1 nm – the yields of N-CNTs were lower than 10%. This complexity indicates rather the surface and core physicochemistry as the key parameters affecting the yield (Fig. S1, ESI†).
The morphologies and microscopic structure of N-CNTs were characterized by scanning electron microscopy (SEM) (Phenom Pro Desktop SEM, Thermo Fischer Scientific) equipped with an energy-dispersive X-ray spectroscopy (EDX) detector, transmission electron microscopy (TEM) (S/TEM Titan 80–300, 300 kV, Field Electron and Ion Company), TGA (TGA 8000, PerkinElmer), Raman spectroscopy (inVia Confocal Raman microscope, Renishaw, excitation beam wavelength: 633 nm), and X-ray photoelectron spectroscopy (XPS) (PreVac EA15, PreVac). A Varian 710-ES spectrometer equipped with a V-groove nebulizer and a reduced-volume Sturman–Masters type spray chamber was used to determine possible inorganic N-CNT contaminants.
Following the ‘from dots to tubes’ transformation (Fig. 1), we firstly encounter a TEM image showing a dense agglomerate (due to a sample preparation for the TEM analysis, i.e. lyophilized CD samples were re-dispersed in ethanol using an ultrasonic bath) of ∼2 nm-wide Phe-CDs containing amorphous shells and semi-graphitic cores (Fig. 1a and Fig. S1, ESI†).18 TGA synthesis transformed the yellow-orange Phe-CDs into black Phe-CD-N-CNTs of a well-defined, aerogel-like morphology at the macroscale (density 6.7 g dm−3). As shown in the SEM image (Fig. 1b), Phe-CD-N-CNTs were found as flattened, needle-like N-CNT crystallites resembling leaves of a garden plant Yucca filamentosa (an inset in Fig. 1b). Furthermore, TEM imaging revealed that the crystallites were composed from the sets of several-micron-long, thick N-CNTs (outer and inner diameters of N-CNTs were found in the range of ∼70–90 nm and ∼5–10 nm, respectively), with occasionally copresent much thinner, intertwined tubes (again, similarly to threads in Yucca filamentosa leaves) (Fig. 1c and d). Only a smaller number (ca. 10%) of N-CNTs was found as practically coreless, semi-amorphous fibre-like 1D-objects. The interlayer spacing in the range of ∼0.4 nm resembled that of the only-carbon nanotubes of the multi-wall nature.24 Indeed, both perfectly crystalline sp2-carbon-rich (Fig. 1f) and corrugated, wrinkled though well-graphitized (Fig. 1g) domains could be found among the N-CNT walls. Generally, analogous trends were found for all other N-CNTs obtained from CDs synthesized from the other amino-acids (Fig. S2 and S3, ESI†).
TGA curves – acquired under a nitrogen and air atmosphere – for Phe-CDs and the resultant Phe-CD-derived N-CNTs are presented in Fig. 2. Importantly, no significant weight loss up to ∼800 °C in N2 (Fig. 2a) – with the corresponding thermal resistance up to 500 °C in air (Fig. 2b) – was observed only for N-CNTs.
Simultaneously, upon pyrolysis, the derivative thermogravimetry (DTG) analysis showed a single endothermic peak at 696 °C, and at the indicated below temperature for the other amino acids: 758 °C (Lys), 722 °C (His), 687 °C (Gly), and 650 °C (Asp), while uniquely three peaks – at 592, 743, and 801 °C – were found for Cys-CDQ-N-CNTs containing 3.5 ± 0.7 at% of sulphur (Fig. S4a and b, ESI†).
Pursuing further the chemical structure of the N-CNTs, we have recorded their Raman spectra (Fig. 2c and d). Indeed, the spectra of all the herein studied N-CNTs showed typical graphitic features: the D-band (a disorder mode) (1345–1369 cm−1) deriving from sp3-hybridized carbon atoms, the G-band (graphitic mode) (1582–1593 cm−1) arising from the E2g vibration of the graphitic (purely C-sp2) hybridization, and the second order features corresponding to the 2D-, D + G-, and 2G-combination modes (2780–2897 cm−1).
The relatively high ID/IG ratios, i.e. 0.99–1.14 (Table S2, ESI†), indicated that the N-CNTs had a comparatively large number of structural defects understood as CNT lattice deformations caused by the presence of both N-sp3- and N-sp2-atom-doping scenarios, with a portion of oxygen functionalities.25 Specifically, for the most crystalline Phe-CD-N-CNT sample (Fig. 2d), the purely C-sp2 domain displayed, statistically, crystallite sizes as large as ∼36 nm (based on the Tuinstra-Koenig relationship) (with ∼34 nm for the least crystalline Cys-CD-N-CNT) (Table S2, ESI†).26
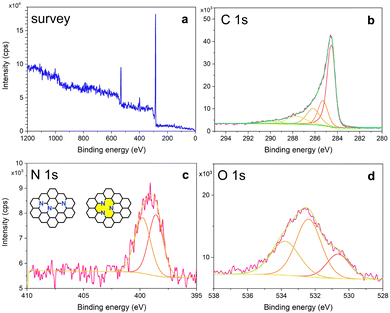
Fig. 3 shows XPS spectra of Phe-CD-N-CNTs, shown in a full, non-deconvoluted survey version (a), with the main peak of photoemission for C 1s (b). Definitely, the key peak for the C-sp2 atoms – such as in N-CNTs – could be found at the binding energy (BE) of ∼284.5 eV. The peak is broad and bears a long asymmetric tail toward the higher BE values. The C 1s core level was deconvoluted into a few subpeaks located around BE of ca. 284 (C–C), 285 (C–O), 286 (C–N), 288 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and 290 (COOH) eV. In turn, Fig. 3c shows the XPS spectrum in the N 1s BE region, as deconvoluted into the two peaks at 398.5 and 401 eV. Those peaks correspond to the near-equally intensive: (a) gap-generating pyridinic-, and (b) graphitic-like nitrogen atoms, respectively (an inset shows the corresponding structural formulae where the yellow inner space confirms the gap-generating pyridinic-like motifs).27 Hence, the nitrogen atoms in the N-CNT products occur not only as a part of the functional (as in terminating/tip/gap) groups, but they were successfully incorporated centrally into the N-CNT walls (peak a 398.5 eV after deconvolution of N 1s BE region).27–29 Indeed, XPS – according to Lazar et al.27 – was recalled as the most powerful spectroscopic method for distinguishing between different N-doping types in graphene as different forms of the nitrogen incorporation have markedly different BEs: the calculated XPS BE of the N 1s state for graphitic and pyridinic in N-CNTs are around 401.5 and 397.9 eV, respectively. Additionally, Fig. 3d shows XPS spectra obtained in the O 1s BE region with the least intense three peaks at 532, 533 and 534 eV. The peak at 533 eV could be assigned to the C–O–C bond, the one at 532 nm to the C
O), and 290 (COOH) eV. In turn, Fig. 3c shows the XPS spectrum in the N 1s BE region, as deconvoluted into the two peaks at 398.5 and 401 eV. Those peaks correspond to the near-equally intensive: (a) gap-generating pyridinic-, and (b) graphitic-like nitrogen atoms, respectively (an inset shows the corresponding structural formulae where the yellow inner space confirms the gap-generating pyridinic-like motifs).27 Hence, the nitrogen atoms in the N-CNT products occur not only as a part of the functional (as in terminating/tip/gap) groups, but they were successfully incorporated centrally into the N-CNT walls (peak a 398.5 eV after deconvolution of N 1s BE region).27–29 Indeed, XPS – according to Lazar et al.27 – was recalled as the most powerful spectroscopic method for distinguishing between different N-doping types in graphene as different forms of the nitrogen incorporation have markedly different BEs: the calculated XPS BE of the N 1s state for graphitic and pyridinic in N-CNTs are around 401.5 and 397.9 eV, respectively. Additionally, Fig. 3d shows XPS spectra obtained in the O 1s BE region with the least intense three peaks at 532, 533 and 534 eV. The peak at 533 eV could be assigned to the C–O–C bond, the one at 532 nm to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, while the other at 534 eV could be assigned to –C
O bonds, while the other at 534 eV could be assigned to –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(OH) and
O(OH) and ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C–OH bonds. Analogous relationships were found for N-CNTs obtained from the other amino-acids (Fig. S5–S7, ESI†). Additionally, in order to determine all the inorganic impurities in the exemplary Phe-CD-N-CNT sample, we have performed inductively coupled plasma atomic emission spectrometry (ICP-AES). As a result, seven metallic elements were found above the detection threshold with lead, iron, sodium, and aluminium, as the most significant contaminants present at as low as 10.4, 2.1, 1.8, 1.2 μg mg−1 N-CNTs, respectively (Table S3, ESI†). Therefore, a role of the Al2O3-pan surface and traces of the above metallic impurities in the possible catalytic activity cannot be fully ruled out. At the same time, the CDs themselves might also be considered as dual in nature, i.e. the CDs could serve both as the catalyst and the main carbon/nitrogen source, while their function is rather inseparable. We should also emphasize that we have performed N-CNT synthesis in a laboratory furnace using a quartz tube as the reactor.
C–OH bonds. Analogous relationships were found for N-CNTs obtained from the other amino-acids (Fig. S5–S7, ESI†). Additionally, in order to determine all the inorganic impurities in the exemplary Phe-CD-N-CNT sample, we have performed inductively coupled plasma atomic emission spectrometry (ICP-AES). As a result, seven metallic elements were found above the detection threshold with lead, iron, sodium, and aluminium, as the most significant contaminants present at as low as 10.4, 2.1, 1.8, 1.2 μg mg−1 N-CNTs, respectively (Table S3, ESI†). Therefore, a role of the Al2O3-pan surface and traces of the above metallic impurities in the possible catalytic activity cannot be fully ruled out. At the same time, the CDs themselves might also be considered as dual in nature, i.e. the CDs could serve both as the catalyst and the main carbon/nitrogen source, while their function is rather inseparable. We should also emphasize that we have performed N-CNT synthesis in a laboratory furnace using a quartz tube as the reactor.
Interestingly from the application point-of-view, we have manufactured different electroconductive nanotube coatings (ESI). Phe-CD-N-CNTs emerged as the most conductive 1D fillers in the series of Phe-CD-N-CNTs (surface resistivity of coatings ca. 120 Ω sq−1), in-house CVD synthesized ultralong MWCNTs (d = 60 nm, l = 0.8 mm, ca. 250 Ω sq−1), and commercial NC7000™ MWCNTs (d = 10 nm, l = 1.5 μm, ca. 100 kΩ sq−1) (Table S4 and Fig. S8, ESI†).
In conclusion, N-CNTs of tunable morphology and surface physicochemistry could be synthesised from amino-acid-derived CDs in a swift, external-catalyst-free procedure. The so obtained N-CNTs had a high ID/IG-ratio due to the presence of mainly nitrogen functional groups, thus enabling protocols for novel materials applicable as from-transparent-to-translucent electrodes, multifunctional coatings and self-standing films, or needle-like drug delivery systems, while the above list has now only a rather tentative character. We believe that this new synthetic approach, proceeding in the absence of intentionally introduced metallic nanoparticles and, in fact, excluding the pre-functionalization step, significantly extends the applicability of N-CNTs not only in the field of biomedical materials. For a better understanding of the process of synthesis, a detailed study of the growth mechanisms, and the scaled-up synthesis are currently under investigation.
This work was supported by the National Science Centre PRELUDIUM-18 grant (UMO-2019/35/N/ST5/02563) (A. K.). S. B. is very grateful for the financial support from the National Science Centre (Poland) grant No. 2020/39/B/ST5/02562 in the framework of the OPUS-20 program. S. B. also acknowledges the supporting actions from EU’s Horizon 2020 ERA-Chair project ExCEED, grant agreement No. 952008.
Conflicts of interest
There are no conflicts to declare.References
- I.-Y. Jeon, H.-J. Noh and J.-B. Baek, Chem. – Asian J., 2020, 15, 2282–2293 CrossRef CAS PubMed.
- R. Dubey, D. Dutta, A. Sarkar and P. Chattopadhyay, Nanoscale Adv., 2021, 2, 5722–5744 RSC.
- S. Rathinavel, K. Priyadharshini and D. Panda, Mater. Sci. Eng. B, 2021, 268, 115095 CrossRef CAS.
- J. H. Ahn, M. N. S. Koo, H. Chan, I. Kim, J. W. Hur, J. H. Lee and J. G. Ok, Micro Nano Syst. Lett., 2019, 7, 11 CrossRef.
- G. P. Gakis, S. Termine, A.-F. A. Trompeta, I. G. Aviziotis and C. A. Charitidis, Chem. Eng. J., 2022, 445, 136807 CrossRef CAS.
- O. O. Cilsal, M. C. Cakir and A. Uguz, Surf. Eng., 2022, 38, 472–481 CrossRef CAS.
- N. A. Fathy and S. El-Shafey, Int. J. Environ. Sci. Technol., 2023, 20, 293–306 CrossRef CAS.
- S. Y. Lim and M. M. Norani, Adv. Mater. Res., 2011, 364, 232–237 Search PubMed.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed.
- P. K. Yadav, S. Chandra, V. Kumar, D. Kumar and S. H. Hasan, Catalysts, 2023, 13, 422 CrossRef CAS.
- W. Zhang, H. Zhong, P. Zhao, A. Shen, H. Li and X. Liu, Food Control, 2022, 133, 108591 CrossRef.
- G. Calabrese, G. De Luca, G. Nocito, M. G. Rizzo, S. P. Lombardo, G. Chisari, S. Forte, E. L. Sciuto and S. Conoci, Int. J. Mol. Sci., 2021, 22, 11783 CrossRef CAS PubMed.
- M. Li, T. Chen, J. J. Gooding and J. Liu, ACS Sens., 2019, 4, 1732–1748 CrossRef CAS PubMed.
- P. He, Y. Shi, T. Meng, T. Yuan, Y. Li, X. Li, Y. Zhang, L. Fan and S. Yang, Nanoscale, 2020, 12, 4826–4832 RSC.
- Z. W. Heng, W. C. Chong, Y. L. Pang and C. H. Koo, J. Environ. Chem. Eng., 2021, 9, 105199 CrossRef CAS.
- J. Zhou, C. Booker, R. Li, X. Zhou, T.-K. Sham, X. Sun and Z. Ding, J. Am. Chem. Soc., 2007, 129, 744–745 CrossRef CAS PubMed.
- S. Kang, Y. K. Jeong, K. H. Jung, Y. Son, S.-C. Choi, G. S. An, H. Han and K. M. Kim, RSC Adv., 2019, 9, 38447–38453 RSC.
- A. Kolanowska, G. Dzido, M. Krzywiecki, M. M. Tomczyk, D. Lukowiec, S. Ruczka and S. Boncel, ACS Omega, 2022, 7, 41165–41176 CrossRef CAS PubMed.
- Y. Sun, R. Kitaura, J. Zhang, Y. Miyata and H. Shinohara, Carbon, 2014, 68, 80–86 CrossRef CAS.
- Z.-Y. Zeng and J.-H. Lin, RSC Adv., 2014, 4, 40251–40258 RSC.
- S. Huang, Q. Cai, J. Chen, Y. Qian and L. Zhang, J. Am. Chem. Soc., 2009, 131, 2094–2095 CrossRef CAS PubMed.
- B. Liu, W. Ren, L. Gao, S. Li, S. Pei, C. Liu, C. Jiang and H.-M. Cheng, J. Am. Chem. Soc., 2009, 131, 2082–2083 CrossRef CAS PubMed.
- X. Xu, S. Huang, Y. Hu, J. Lu and Z. Yang, Mater. Chem. Phys., 2012, 133, 95–102 CrossRef CAS.
- K. P. Maity and V. Prasad, Mater Res. Express, 2019, 6, 0850a2 CrossRef CAS.
- V. D. Blank, E. V. Polyakov, D. V. Batov, B. A. Kulnitskiy, U. Bangert, A. Gutiérrez-Sosa, A. J. Harvey and A. Seepujak, Diamond Relat. Mater., 2003, 12, 864–869 CrossRef CAS.
- L. G. Cançado, K. Takai, T. Enoki, M. Endo, Y. A. Kim, H. Mizusaki, A. Jorio, L. N. Coelho, R. Magalhães-Paniago and M. A. Pimenta, Appl. Phys. Lett., 2006, 88, 163106 CrossRef.
- P. Lazar, R. Mach and M. Otyepka, J. Phys. Chem. C, 2019, 123, 10695–10702 CrossRef CAS.
- M. Ayiania, M. Smith, A. J. R. Hensley, L. Scudiero, J.-S. McEwen and M. Garcia-Perez, Carbon, 2020, 162, 528–544 CrossRef CAS.
- J. D. Bagley, D. K. Kumar, K. A. See and N.-C. Yeh, RSC Adv., 2020, 10, 39562–39571 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc01785k |
| This journal is © The Royal Society of Chemistry 2023 |