Open Access Article
Open Access ArticleA photoresponsive gold catalyst based on azobenzene-functionalized NHC ligands†
Jianghua
Liu
,
Eduard O.
Bobylev
,
Bas
de Bruin
 and
Joost N. H.
Reek
and
Joost N. H.
Reek
 *
*
Homogeneous, Supramolecular and Bio-Inspired Catalysis (HomKat), Van ‘t Hoff Institute for Molecular Sciences (HIMS), University of Amsterdam (UvA), Science Park 904, Amsterdam 1098XH, The Netherlands. E-mail: j.n.h.reek@uva.nl
First published on 13th June 2023
Abstract
An azobenzene-bearing N-heterocyclic carbene-based gold catalyst is reported of which the reactivity in a cyclization reaction depends on the isomeric state of the azobenzene. The configurations of the catalyst can be reversibly switched by light and are stable during the reaction, effectively leading to a switchable catalyst system.
Development of artificial switchable catalysts allowing control over the rate and selectivity of reactions by external stimuli has emerged as an important research area.1 The interest has been fueled by the potential in regulating organic transformations in new ways. In addition, stimuli-responsive catalysis in living systems may find application in smart medicine,2 and as such, transition metal catalysis in living systems has become an emerging frontier in recent years.3 Switchable catalysts can provide spatial and temporal control in catalysis which may be relevant for diverse applications in synthetic chemistry as well as in biology. Various external stimuli can be used, including light,4 heat,5 ultrasound,6 and even magnetic fields.7 Among the external stimuli, light8 is attractive because it is a clean, non-invasive stimulus, and it can remotely control reactions in time and space. Photoswitchable organometallic catalysts containing photochromic ligands (i.e. spiropyran, azobenzene, diarylethenes, biindanes, etc.) and transition metal centers (i.e. Rh, Ru, Au, Cu, Pd and etc.) have been reported, and applied in organic solvents.9
Azobenzene (Azo) is the most widely studied photo-responsive group.10 It can be reversibly switched between trans and cis configurations under UV light and visible light, and also heating can be used for isomerization from cis to trans. As photoisomerization changes the configurations of azobenzene, it may provide useful handle for regulating reactions by light-controlled steric effects.11 Gold complexes are increasingly popular as catalyst as they display unique reactivity12 and allow a variety of cyclization reactions under mild conditions.13 In addition, gold complexes typically display low toxicity14 and have reactivity that is complementary to reactions found in nature,15 and therefore they have been used under biorelevant conditions.16 We have reported previously switchable gold catalysts by encapsulation of the complex, which effectively shifted the equilibrium from dinuclear to mononuclear.17 However, photoswitchable gold complexes used as catalysts for cyclization reactions under mild conditions have not been reported so far.
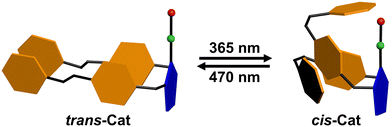
Here, we report an N-heterocyclic carbene (NHC) ligand18 with two azobenzene functional groups as photoswitchable units, and the coordination to gold to form a complex (Azo-NHC-Au) that can be used in catalysis. The azobenzene units can be switched with light and this in turn controls the activity of the gold complex in a cyclization reaction. The trans and cis configurations of the Azo-NHC-Au catalysts can be interconverted by irradiation with UV and visible light (Scheme 1). The trans Azo-NHC-Au complex displays rates that are more than two times higher compared to that of the cis-isomer.
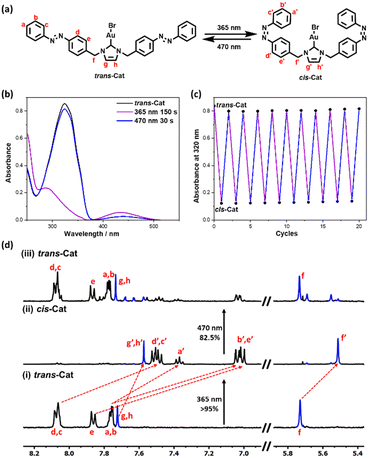
The photoswitchable bis-azobenzene N-heterocyclic carbene ligand and the gold complex (Azo-NHC-Au) thereof were synthesized19 using standard procedures and fully characterized by 1H NMR, 13C NMR and FD-Mass (See Supplementary, Scheme S1 and Fig. S1–S6, ESI†). The ligand (Azo-NHC) and gold complex (Azo-NHC-Au) are isolated as the thermodynamically stable trans-L isomer and trans-Cat, respectively, as confirmed by the absorption band at 320 nm in UV-vis (Fig. 1b). The photoisomerization of the catalyst was investigated by 1H NMR and UV-vis spectroscopy. Trans-Cat has a maximum absorption at 320 nm, characteristic of the trans azobenzene unit (Fig. 1b and Fig. S9, ESI†). Upon irradiation of a THF:H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution of the trans-Cat with 365 nm light, this 320 nm absorption band decreased, while a new band at 440 nm, typical for the cis-azobenzene (cis-Azo), arises (Fig. S9b, ESI†), indicating that trans to cis- isomerization efficiently occurred. The photostationary state (PSS) is achieved after 150 s 365 nm irradiation and contains 95% cis-Azo and 5% trans-Azo as calculated by Abs(PSS)/Abs(trans-Azo) ratio (Fig. S10, ESI†).20 Exposing the solution after this period of time to 470 nm light leads to a decrease of the 440 nm band (associated to cis-Azo) and an increase of the 320 nm absorbtion (the band of the trans-Azo) again. This experiment shows that the trans-Cat can be reformed and the PSS state was now reached after irradiation for 30 s at 470 nm (Fig. S9c, ESI†). Importantly, the reversible isomerization of the catalyst could be repeated at least ten times without any signs of degradation (Fig. 1c and Fig. S9d, ESI†). Similar photoisomerization experiments on the Azo-NHC ligand showed that it has similar photochromic behavior as observed for the catalyst (Fig. S7 and S8, ESI†).
1 solution of the trans-Cat with 365 nm light, this 320 nm absorption band decreased, while a new band at 440 nm, typical for the cis-azobenzene (cis-Azo), arises (Fig. S9b, ESI†), indicating that trans to cis- isomerization efficiently occurred. The photostationary state (PSS) is achieved after 150 s 365 nm irradiation and contains 95% cis-Azo and 5% trans-Azo as calculated by Abs(PSS)/Abs(trans-Azo) ratio (Fig. S10, ESI†).20 Exposing the solution after this period of time to 470 nm light leads to a decrease of the 440 nm band (associated to cis-Azo) and an increase of the 320 nm absorbtion (the band of the trans-Azo) again. This experiment shows that the trans-Cat can be reformed and the PSS state was now reached after irradiation for 30 s at 470 nm (Fig. S9c, ESI†). Importantly, the reversible isomerization of the catalyst could be repeated at least ten times without any signs of degradation (Fig. 1c and Fig. S9d, ESI†). Similar photoisomerization experiments on the Azo-NHC ligand showed that it has similar photochromic behavior as observed for the catalyst (Fig. S7 and S8, ESI†).
Next, the isomerization conversion efficiency of the Azo-NHC-Au complex was quantitatively studied by 1H NMR (Fig. 1d). Under 1H NMR conditions, the PSS state was reached after 300 s exposure to 365 nm light. The peaks of trans-Cat almost completely disappeared while a new set of signals attributed to the cis-Cat appeared. Based on the integrals of the peaks attributed to f (or g, h) with respect to f′ (or g′, h′), the conversion efficiency from trans-Azo to cis-Azo was calculated to be more than 95%. After irradiating the solution by blue light for 30 s, the conversion of cis-Azo to trans-Azo was determined as 82.5%. The isomerization efficiency calculated by 1H NMR is similar to those based on the UV-vis experiments.
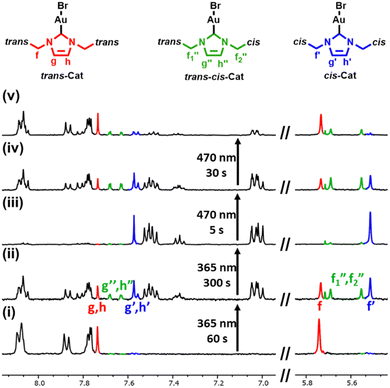
Next, we monitored the isomerization process in time by 1H NMR spectroscopy, to see if the photoisomerization of trans-Cat to cis-Cat proceeds via an isomer in which only one of the azobenzene units is switched (trans-cis-Cat) (Fig. 2). After irradiation of the solution containing the trans-Cat isomer for 60 s with 365 nm light, the signals attributed to both the trans-Cat and the cis-Cat are clearly visible (i.e., protons on imidazolium ring (Hg and Hh) and CH2 (Hf)) for trans-Cat and (Hg′, Hh′ and Hf′) for cis-Cat). Next to this a new set of signals is clearly visible (Hg′′, Hh′′, Hf1′′ and Hf2′′, in green in Fig. 2), which is attributed to the intermediate trans-cis-Cat. The first PSS is reached after irradiation with 365 nm for 300 s, and NMR shows that the ratio of these three configurations is 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 91 based on the integrals of f, f1′′, f2′′ f′ (trans-Cat:trans-cis-Cat:cis-Cat). Irradiation of this solution containing dominantly cis-Cat with 470 nm light for 5 s, shows that also this pathway proceeds via the trans-cis-Cat intermediate. After irradiation with 470 nm light for 30 s, another PSS state was obtained with the isomers present in the ratio 65
91 based on the integrals of f, f1′′, f2′′ f′ (trans-Cat:trans-cis-Cat:cis-Cat). Irradiation of this solution containing dominantly cis-Cat with 470 nm light for 5 s, shows that also this pathway proceeds via the trans-cis-Cat intermediate. After irradiation with 470 nm light for 30 s, another PSS state was obtained with the isomers present in the ratio 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (trans-Cat:trans-cis-Cat:cis-Cat).
4 (trans-Cat:trans-cis-Cat:cis-Cat).
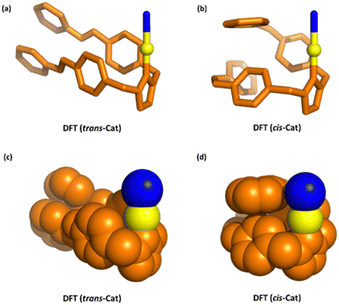
Next, the thermal relaxation of cis-Cat was studied by monitoring the solution, which is kept in the dark, every 1 h for 24 h by UV-vis and 1H NMR (Fig. S11, ESI†). Surprisingly, only 5% cis-Cat was thermally relaxed to trans-Cat after 24 h at dark, which is unusually slow compared to normal azobenzene. The typical thermal half-life of the Z-isomers of azobenzene at room temperature in acetonitrile is 4.7 h.21 Previously it has established that thermal stability of the Z-isomers can be improved by electronic effects, for example, the ortho fluoroazobenzenes are configurational more stable than the parent azobenzene.22 DFT calculations, show π–π stacking of benzene rings between two azobenzene units in the cis-Cat structure (Fig. 4), which might explain the thermostability of the ligands in the cis state (vide infra). This high thermal stability is crucial for the application in catalysis as it allows to use the cis-Cat sufficiently long for allowing significant substrate conversion.
To evaluate the catalytic performance of the trans-Cat and cis-Cat, the intramolecular cyclization of acetylenic acid was explored (Fig. 3a). We used conditions that do not require silver(I) salts to remove the halide from the gold catalyst,13,16,23 as silver is sensitive to the light that we use for photoisomerization. To that end water was used as co-solvent as it is known to activate gold complexes by bromide replacement. As such, the catalytic reactions were performed in THF-d8:D2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at room temperature and conversions were monitored every hour by 1H NMR for 24 h. The yield and rate constant k of the catalytic reactions as function of time are displayed in Fig. 3a and b and Fig. S12–S14 (ESI†). The trans-Cat shows good catalytic activity (k = 0.081 h−1) and provided 87% yield after 16 h. Under the same conditions using the cis-Cat, which was obtained by 365 nm light irradiating of the trans-Cat solution for 10 min, the reaction rate was much slower (k = 0.035 h−1) with 52% yield after 16 h, showing that the catalyst activity can be switched by light. The concentration of trans-Cat and cis-Cat during the reactions were also monitored based on the integrals of proton f. The unchanged concentrations during these experiments demonstrate the excellent geometric stability of both trans-Cat and cis-Cat under the reaction conditions.
1 at room temperature and conversions were monitored every hour by 1H NMR for 24 h. The yield and rate constant k of the catalytic reactions as function of time are displayed in Fig. 3a and b and Fig. S12–S14 (ESI†). The trans-Cat shows good catalytic activity (k = 0.081 h−1) and provided 87% yield after 16 h. Under the same conditions using the cis-Cat, which was obtained by 365 nm light irradiating of the trans-Cat solution for 10 min, the reaction rate was much slower (k = 0.035 h−1) with 52% yield after 16 h, showing that the catalyst activity can be switched by light. The concentration of trans-Cat and cis-Cat during the reactions were also monitored based on the integrals of proton f. The unchanged concentrations during these experiments demonstrate the excellent geometric stability of both trans-Cat and cis-Cat under the reaction conditions.
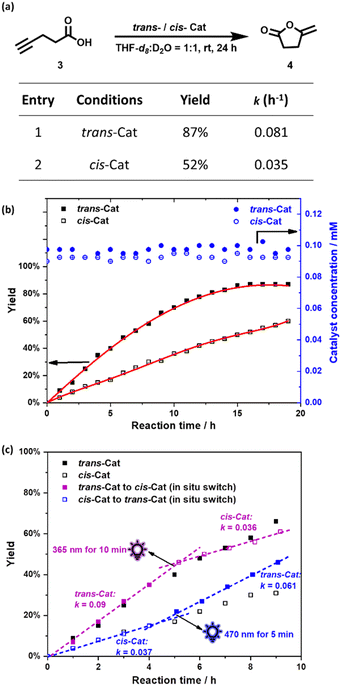 | ||
Fig. 3 (a) Cyclization reaction of acetylenic acid 3 to give enol lactone 4, with the yield and rate constant k displayed in the table; (b) The yield as function of time determined by 1H NMR spectroscopy in red and the concentration of the catalyst during the reaction (THF-d8:D2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 298 K); (c) In situ photoswitchable catalysis: (purple) switch from trans-Cat to cis-Cat, after 4 h (by 10 min irradiation 365 nm). (blue) switch from cis-Cat to trans-Cat, after 4 h (by 5 min irradiation with 470 nm). Conditions: Reagent concentrations: [3] = 1 mM, [trans- or cis-Cat] = 0.1 mM. The yield was determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard (error <5%). For full experimental details see the ESI.†) 1 at 298 K); (c) In situ photoswitchable catalysis: (purple) switch from trans-Cat to cis-Cat, after 4 h (by 10 min irradiation 365 nm). (blue) switch from cis-Cat to trans-Cat, after 4 h (by 5 min irradiation with 470 nm). Conditions: Reagent concentrations: [3] = 1 mM, [trans- or cis-Cat] = 0.1 mM. The yield was determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard (error <5%). For full experimental details see the ESI.†) | ||
The different reactivity displayed by trans-Cat and cis-Cat may be caused by the difference in steric hindrance of the ligand. Density functional theory (DFT) calculations (BP86, def2-SVP, D3 dispersion corrections, see ESI† for details) were carried out to investigate the effect of the configurations of the ligand. The input structures of the trans-Cat and cis-Cat complexes were pre-optimized with GFN2-xTB, and subsequently a conformational search was performed using the crest procedure, and the DFT-optimized the structures of trans-Cat and cis-Cat are displayed in Fig. 4. The Balls and stick model of trans-Cat clearly shows that the two azobenzene arms are parallel oriented to allow face-to-face stacking of the aromatic rings. The azobenzene in cis-Cat structure are arranged such that they form a face to edge stacking arrangement (Fig. 4a and b). The different orientation of the rings in the trans-Cat and cis-Cat creates a different steric environment around the gold center in the area where the substrate should coordinate. Clearly, the space-filling model, topographic steric map and %buried volume (%VBur) (Fig. S21, ESI†) of trans-Cat visualizes the larger available area for coordination in the trans-Cat compared to cis-Cat, which may explain the difference in reaction rate for the cyclization (Fig. 4c and d).
Finally, we explored if the reaction activity could be regulated by photoswitching the catalysts in situ. Firstly, the reaction started in the presence of the trans-Cat for 4 h, showing a similar yield (35%) and rate constant (k = 0.09) previously obtained for trans-Cat (k = 0.081). Then the reaction mixture was irradiated by 365 nm light for 10 min to obtain cis-Cat to slow down the reaction rate (k = 0.036) (Fig. 3c purple and Fig. S15 and S16, ESI†). In the next experiment, the reaction started with the cis-Cat for 4 h (k = 0.037). Then trans-Cat was obtained by 470 nm irradiating 5 min to accelerate the reaction rate to k = 0.061 (Fig. 3c blue and Fig. S17 and S18, ESI†). No products was found if the substrate solution without catalyst was irradiated with UV or blue light (Fig. S19 and S20, ESI†). These results show that reaction rate can be reversibly controlled by different light conditions.
In conclusion, we report an azobenzene-bearing N-heterocyclic carbene that can be reversibly switched from the trans to the cis configurations using light, also when coordinated to gold. The trans-Cat exhibited almost two times the catalytic activity compared to the cis-Cat. The configurations of catalysts can be reversibly switched between trans- and cis- under reaction conditions, and maintain stable during the reaction process. As gold-catalyzed cyclization reactions are of great significance for various applications including smart drugs and other biological processes, we think this photoswitchable catalyst may have potential to control catalytic reactions spatially and temporally in biological systems. As N-heterocyclic carbene ligands are used for many different catalytic processes we foresee that this ligand may be more broadly applicable.
We gratefully acknowledge the University of Amsterdam and China Scholarship Council (CSC; No. 201906200131) for financial support.
Conflicts of interest
There are no conflicts to declare.Notes and references
-
(a) V. Blanco, D. A. Leigh and V. Marcos, Chem. Soc. Rev., 2015, 44, 5341–5370 RSC
; (b) M. Vlatković, B. S. L. Collins and B. L. Feringa, Chem. – Eur. J., 2016, 22, 17080–17111 CrossRef PubMed
; (c) J. Choudhury, Tetrahedron Lett., 2018, 59, 487–495 CrossRef CAS
; (d) U. Lüning, Angew. Chem., Int. Ed., 2012, 51, 8163–8165 CrossRef PubMed
; (e) Y. Tang, Y. He and Q. Fan, Chin. J. Org. Chem., 2020, 40, 3672–3685 CrossRef CAS
.
- D. Jiang, D. Ni, Z. T. Rosenkrans, P. Huang, X. Yan and W. Cai, Chem. Soc. Rev., 2019, 48, 3683–3704 RSC
.
-
(a) P. K. Sasmal, C. N. Streu and E. Meggers, Chem. Commun., 2013, 49, 1581–1587 RSC
; (b) Y. Bai, J. Chen and S. C. Zimmerman, Chem. Soc. Rev., 2018, 47, 1811–1821 RSC
; (c) M. Martínez-Calvo and J. L. Mascareñas, Coord. Chem. Rev., 2018, 359, 57–79 CrossRef
; (d) M. O. N. van de L’Isle, M. C. Ortega-Liebana and A. Unciti-Broceta, Curr. Opin. Chem. Biol., 2021, 61, 32–42 CrossRef PubMed
; (e) A. H. Ngo, S. Bose and L. H. Do, Chem. – Eur. J., 2018, 24, 10584–10594 CrossRef CAS PubMed
; (f) D. P. Nguyen, H. T. H. Nguyen and L. H. Do, ACS Catal., 2021, 11, 5148–5165 CrossRef CAS PubMed
; (g) Y. Liu and Y. Bai, ACS Appl. Bio Mater., 2020, 3, 4717–4746 CrossRef CAS PubMed
.
- S. Neri, S. G. Martin, C. Pezzato and L. J. Prins, J. Am. Chem. Soc., 2017, 139, 1794–1997 CrossRef CAS PubMed
.
-
(a) H. Ye, K. Yang, J. Tao, Y. Liu, Q. Zhang, S. Habibi, Z. Nie and X. Xia, ACS Nano, 2017, 11, 2052–2059 CrossRef CAS PubMed
; (b) D. E. Bergbreiter, V. M. Mariagnanam and L. Zhang, Adv. Mater., 1995, 7, 69 CrossRef CAS
.
- W. P. Li, C. H. Su, Y. C. Chang, Y. J. Lin and C. S. Yeh, ACS Nano, 2016, 10, 2017–2027 CrossRef CAS PubMed
.
- J. Lee, S. Dubbu, N. Kumari, A. Kumar, J. Lim, S. Kim and I. S. Lee, Nano Lett., 2020, 20, 6981–6988 CrossRef CAS PubMed
.
- Y. Tao, H. F. Chan, B. Shi, M. Li and K. W. Leong, Adv. Funct. Mater., 2020, 30, 2005029 CrossRef CAS PubMed
.
-
(a) Z. Freixa, Catal. Sci. Technol., 2020, 10, 3122–3139 RSC
; (b) M. Kondo, K. Nakamura, C. G. Krishnan, H. Sasai and S. Takizawa, Chem. Rec., 2023, e202300040 CrossRef PubMed
; (c) P. J. Gilissen, X. Chen, J. D. Graaf, P. Tinnemans, B. L. Feringa, J. A. Elemans and R. J. Nolte, Chem. – Eur. J., 2023, e202203539 CrossRef CAS PubMed
.
- F. A. Jerca, V. V. Jerca and R. Hoogenboom, Nat. Rev. Chem., 2022, 6, 51–69 CrossRef PubMed
.
-
(a) C. Z.-J. Ren, P. Solís Muñana, J. Dupont, S. S. Zhou and J. L.-Y. Chen, Angew. Chem., 2019, 131, 15398–15402 CrossRef
; (b) A. Kunfi, I. Jablonkai, T. Gazdag, P. J. Mayer, P. P. Kalapos, K. Németh, T. Holczbauer and G. London, RSC Adv., 2021, 11, 23419–23429 RSC
; (c) S. Park, S. Byun, H. Ryu, H. Hahm, J. Lee and S. Hong, ACS Catal., 2021, 11, 13860–13865 CrossRef CAS
; (d) T. Arif, C. Cazorla, N. Bogliotti, N. Saleh, F. Blanchard, V. Gandon, R. Métivier, J. Xie, A. Voituriez and A. Marinetti, Catal. Sci. Technol., 2018, 8, 710–715 RSC
.
-
(a) M. Mato, A. Franchino, C. G. Morales and A. M. Echavarren, Chem. Rev., 2021, 121(14), 8613–8684 CrossRef CAS PubMed
; (b) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180–3211 CrossRef CAS PubMed
; (c) B. Huang, M. Hu and F. D. Toste, Trends Chem., 2020, 2(8), 707–720 CrossRef CAS PubMed
; (d) D. J. Gorin, B. D. Sherry and F. D. Toste, Chem. Rev., 2008, 108, 3351–3378 CrossRef CAS PubMed
; (e) S. Witzel, A. S. K. Hashmi and J. Xie, Chem. Rev., 2021, 121, 8868–8925 CrossRef CAS PubMed
.
-
(a) K. Belger and N. Krause, Eur. J. Org. Chem., 2015, 220–225 CrossRef CAS
; (b) M. J. Rodríguez-Álvarez, C. Vidal, S. Schumacher, J. Borge and J. García-Álvarez, Chem. – Eur. J., 2017, 23, 3425–3431 CrossRef PubMed
.
- Y. Wang, M. Liu, R. Cao, W. Zhang, M. Yin, X. Xiao, Q. Liu and N. Huang, J. Med. Chem., 2013, 56, 1455–1466 CrossRef CAS PubMed
.
- A. S. K. Hashmi and M. Rudolph, Chem. Soc. Rev., 2008, 37, 1766–1775 RSC
.
-
(a) T. C. Chang, K. Vong, T. Yamamoto and K. Tanaka, Angew. Chem., Int. Ed., 2021, 60, 12446–12454 CrossRef CAS PubMed
; (b) C. Vidal, M. Tomás-Gamasa, P. Destito, F. López and J. L. Mascareñas, Nat. Commun., 2018, 9, 1–9 CrossRef CAS PubMed
.
-
(a) A. C. H. Jans, A. Gómez-Suárez, S. P. Nolan and J. N. H. Reek, Chem. – Eur. J., 2016, 22, 14836–14839 CrossRef CAS PubMed
; (b) A. C. H. Jans, X. Caumes and J. N. H. Reek, ChemCatChem, 2019, 11, 287–297 CrossRef CAS PubMed
.
- S. D. Gonzaez, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612–3676 CrossRef PubMed
.
- F. Nahra, N. V. Tzouras, A. Collado and S. P. Nolan, Nat. Protoc., 2021, 16, 1476–1493 CrossRef CAS PubMed
.
- C. Y. Huang, A. Bonasera, L. Hristov, Y. Garmshausen, B. M. Schmidt, D. Jacquemin and S. Hecht, J. Am. Chem. Soc., 2017, 139, 15205–15211 CrossRef CAS PubMed
.
- X. M. Liu, X. Y. Jin, Z. X. Zhang, J. Wang and F. Q. Bai, RSC Adv., 2018, 8, 11580–11588 RSC
.
-
(a) D. Bléger, J. Schwarz, A. M. Brouwer and S. Hecht, J. Am. Chem. Soc., 2012, 134, 20597–20600 CrossRef PubMed
; (b) C. Knie, M. Utecht, F. Zhao, H. Kulla, S. Kovalenko, A. M. Brouwer, P. Saalfrank, S. Hecht and D. Bléger, Chem. – Eur. J., 2014, 20, 16492–16501 CrossRef CAS PubMed
.
- A. Franchino, M. M. Magraner and A. M. Echavarren, Bull. Chem. Soc. Jpn., 2021, 94, 1099–1117 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc01726e |
| This journal is © The Royal Society of Chemistry 2023 |