Open Access Article
Open Access ArticleSimple and fast screening for structure-selective G-quadruplex ligands†
Yoshiki
Hashimoto
a,
Yoshiki
Imagawa
a,
Kaho
Nagano
a,
Ryuichi
Maeda
a,
Naho
Nagahama
a,
Takeru
Torii
a,
Natsuki
Kinoshita
a,
Nagisa
Takamiya
a,
Keiko
Kawauchi
a,
Hisae
Tatesishi-Karimata
b,
Naoki
Sugimoto
 ab and
Daisuke
Miyoshi
ab and
Daisuke
Miyoshi
 *a
*a
aFrontiers of Innovative Research in Science and Technology, Konan university, 7-1-20 Minatojima-minamimachi, Chuo-ku, Kobe, Hyogo 650-0047, Japan
bFrontier Institute for Biomolecular Engineering Research, Konan university, 7-1-20 Minatojima-minamimachi, Chuo-ku, Kobe, Hyogo 650-0047, Japan
First published on 5th April 2023
Abstract
Structural selectivity of G-quadruplex ligands is essential for cellular applications since there is an excess of nucleic acids forming duplex structures compared to G-quadruplex structures in living cells. In this study, we developed new structure-selective G-quadruplex ligands utilizing a simple and fast screening system. The affinity, selectivity, enzymatic inhibitory activity and cytotoxicity of the structure-selective G-quadruplex ligands were demonstrated along with a structural selectivity–cytotoxicity relationship of G-quadruplex ligands.
Guanine-rich sequences can form G-quadruplex (G4) structures, which encapsulate two to four planar layers of four guanine bases (G-quartets) via Hoogsteen hydrogen bonds.1,2 The human genome contains about 7 × 106 putative G4-forming G2–5N1–7G2–5N1–7G2–5N1–7G2–5 sequences3 which are frequently observed in human telomeres and promoters of genes, especially oncogenes.4 The most well-known and extensively studied G4-forming sequence in the human genome is the telomere, which is at the ends of chromosomes and is composed of d(GGGTTA)n. At the very 3′-end, the telomere has a characteristic single-stranded G-rich overhang which has a propensity to form G4 and inhibit telomerase activity.5–9 This finding has spurred huge interest in the development of G4-targeting small molecules (G4 ligands) to inhibit telomerase activity and to obstruct telomere elongation by binding and stabilizing the telomeric G4. The human genome comprises ca. 6 × 109 bases, most of which fold into canonical duplex structures. The telomere overhang regions involve 200 bases in each of the 46 chromosome ends, and approximately 1.8 × 104 bases in total. However, the number of duplex-forming bases is over 105 times that of the number of G4-forming telomeric bases. G4 ligands therefore require high structural selectivity towards the duplex for cellular applications but only a few structure-selective G4 ligands, such as pyridostatin and telomestatin, have been reported.10,11 Zhang et al. developed a key intracellular ligand displacement assay and demonstrated that small molecules, which bind to G4 in the presence of excess DNA duplex in a test tube experiment, can bind G4 in living cells.12 This G4 ligand screening assay which can be used in the presence of excess DNA duplex is a promising approach to identifying G4-selective ligands that remain functional in cells. However, although G4 screening methods have been developed,13–15 a simple, one-pot screening system applicable in the presence of excess DNA duplex has yet to be reported.
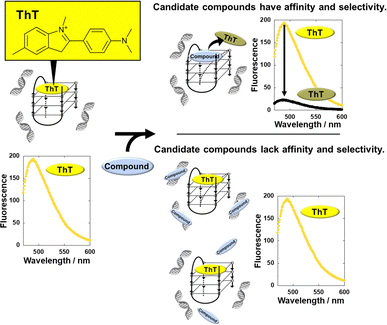
Here, we developed a thioflavin T (ThT)-displacement assay (TD assay). ThT was identified as a G4-selective fluorescence indicator,16 raising the possibility of screening for G4 ligands in the presence of excess DNA duplex to identify G4 ligands with high selectivity for G4 over duplex. In the proposed scheme (Fig. 1), ThT binds G4 and emits fluorescence even in the presence of excess DNA duplex. If the test molecule binds G4 but cannot bind duplex, ThT is displaced, leading to a decrease in fluorescence intensity. However, fluorescence intensity would not change if the test molecule has affinity for duplex or if it binds neither G4 nor duplex.
We confirmed that ThT binds to a G4-forming target oligonucleotide derived from human telomere DNA (22AG: 5′-AGGGTTAGGGTTAGGGTTAGGG-3′) in a structure-selective manner. 22AG was titrated into ThT in the absence or presence of excess calf thymus DNA duplex (CT-DNA) (2 mM nucleotide concentration). The fluorescence intensity of ThT at 485 nm (F485) drastically increased upon the addition of 22AG (Fig. S1A, ESI†) under both conditions. In addition, the binding stoichiometry of ThT and 22AG G4 was confirmend to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S2, ESI†). The dissociation constant, Kd-ThT, at 25 °C was calculated to be 7.0 μM and 13 μM in the absence and presence of 2 mM CT-DNA, respectively (Fig. S1B, see ESI† for details regarding evaluation of the Kd-ThT values of ThT). This result confirmed that ThT binds to 22AG G4 selectively and maintains its binding affinity even in the presence of excess DNA duplex. Note that it was reported that ThT boound to DNA duplex, although its fluoresence emission with DNA duplex was much weaker than that with DNA G4.17 The small difference between the Kd values may reflect the ThT binding with CT-DNA. From these results, ThT is a suitable fluorescence probe for the screening of G4 ligands in the presence of excess DNA duplex.
1 (Fig. S2, ESI†). The dissociation constant, Kd-ThT, at 25 °C was calculated to be 7.0 μM and 13 μM in the absence and presence of 2 mM CT-DNA, respectively (Fig. S1B, see ESI† for details regarding evaluation of the Kd-ThT values of ThT). This result confirmed that ThT binds to 22AG G4 selectively and maintains its binding affinity even in the presence of excess DNA duplex. Note that it was reported that ThT boound to DNA duplex, although its fluoresence emission with DNA duplex was much weaker than that with DNA G4.17 The small difference between the Kd values may reflect the ThT binding with CT-DNA. From these results, ThT is a suitable fluorescence probe for the screening of G4 ligands in the presence of excess DNA duplex.
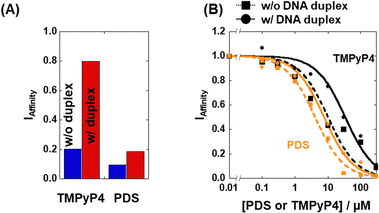
The affinity index (IAffinity) showing the degree of ThT displacement by a test compound was evaluated from the F485 value of ThT as follows: (F485 with the compound)/(F485 without the compound). The smaller the IAffinity value, the higher the affinity of the compound with 22AG, which forms a mixed G4 under these experimental conditions.18 We demonstrated the concept behind the TD assay by using the two G4 ligands TMPyP4 and pyridostatin (PDS) (Fig. 2A). TMPyP4,19 a non-selective G4 ligand, provided IAffinity values of 0.18 and 0.79 in the absence and presence of excess DNA duplex, respectively. In contrast, PDS, a typical G4-selective ligand, exhibited IAffinity values in the absence (0.09) and presence (0.12) of excess DNA duplex, showing that PDS maintains G4 binding affinity even in the presence of excess DNA duplex. Furthermore, we measured the F485 value of ThT with various concentrations of TMPyP4 and PDS and evaluated the observed Kd (Ki) values in the absence and presence of excess DNA duplex (please see ESI† for evaluation of the Ki values of the compounds). The Ki values of TMPyP4 were 0.95 ± 0.07 μM and 5.5 ± 0.3 μM in the absence and presence of excess DNA duplex, respectively (Fig. 2B), whereas the values for PDS were 0.43 ± 0.03 μM and 0.72 ± 0.08 μM, respectively. Although other factors, such as a direct binding of ThT with the added compound with ThT and a fluorescence quenching of ThT by the added compound, these Ki values confirm that the TD assay is suitable for screening structure-selective G4 ligands.13
We conducted the TD assay for an in-house library composed of 35 small molecules (Fig. S3, ESI†) which we considered to show structure-selective G4 binding. Fig. 3 shows the IAffinity values in the absence (blue) and in the presence of excess DNA duplex (red). The compounds were arranged in the order of increasing IAffinity value in the absence of excess DNA duplex. Many of the compounds with small IAffinity values in the absence of excess duplex showed larger values in the presence of excess duplex. For example, the IAffinity values of compound 1 (methyl violet 2B; MV) were 0.22 and 0.76, respectively, demonstrating that the binding of MV is reduced by excess DNA duplex. The five compounds that showed the lowest IAffinity values in the presence of excess DNA duplex are listed in Table S1 (ESI†), together with their Ki values. Furthermore, the selectivity index (ISelectivity), an indicator of selectivity of a compound, was evaluated as follows: ISelectivity = (Ki without excess DNA duplex)/(Ki with excess DNA duplex). A compound with an ISelectivity value close to 1 is more selective. The ISelectivity values of TMPyP4 and PDS were 0.17 and 0.59, respectively, consistent with previous findings and supporting the validity of the TD assay.
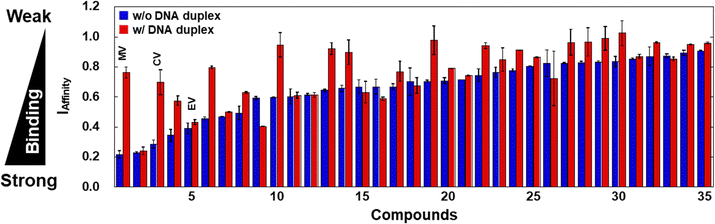 | ||
| Fig. 3 Affinity index (IAffinity) of 20 μM compounds in the absence and presence of excess DNA duplex. All measurements were carried out in KCl buffer at 25 °C. Error bars represent mean ± SD; n = 3. | ||
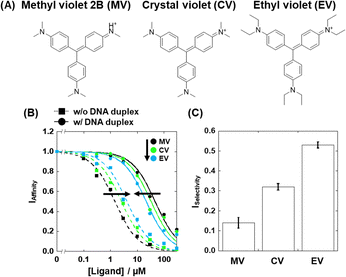
We identified compound 3 (crystal violet; CV, Fig. 4A) as a moderate G4-selective compound, although it was previously reported as a structure-selective G4 ligand.20 We also identified compound 1 (methyl violet; MV) and compound 5 (ethyl violet; EV), which possess the same structural scaffold as CV but have different lengths of terminal alkyl chains (Fig. 4A). The Ki values of these compounds are evaluated (Fig. 4B) and listed in Table S1 (ESI†). The binding affinities of these compounds in the absence of excess DNA duplex were MV > CV > EV, whereas in the presence of excess DNA duplex the binding affinity order was the opposite. Thus, the order of the ISelectivity values is EV > CV > MV (Table S1, ESI† and Fig. 4C), suggesting that the bulkiness of the terminal alkyl chain, which impedes non-selective electrostatic interaction of the positive functional groups with negatively charged DNA duplex, is an important factor for the structural selectivity of G4 ligands. In addition, structural and thermal studies of 22AG showed that a local structure change and thermal stabilization were induced by MV, CV, and EV (Fig. S4, ESI†), confirming the binding of the compounds to 22AG.
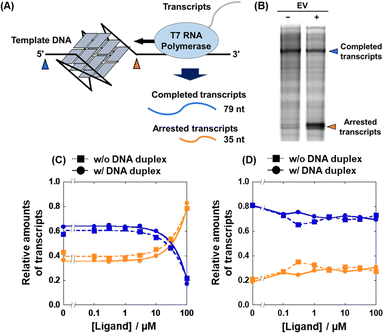
Based on the Ki values of the compounds screened, we further attempted to evaluate the inhibitory effects of EV on the transcription reaction. We used two transcription reaction templates: 22AG template containing the 22AG sequence (Table S2, ESI†) which folds into a mixed G4, and mut22AG template containing a mutated 22AG sequence which cannot fold into the G4 structure. T7 RNA polymerase stop assays with the two templates were performed in the absence and presence of excess DNA duplex (Fig. 5A). Inhibition of the polymerase reaction by G4 results in a short transcript (about 35 nucleotides long) and the amount of completed transcript (79 nucleotides long) is decreased. Fig. 5B shows a denaturing polyacrylamide gel of the transcripts at 0 μM and 100 μM EV in the absence of excess DNA duplex, demonstrating the inhibitory effect of EV on transcription. As shown in Fig. S5 (ESI†) and Fig. 5C, the amount of arrested (shown in orange) and completed (shown in blue) transcript increased and decreased, respectively, with increasing concentration of EV, when the G4 template was used in the absence of excess DNA duplex (squares with dotted line). In contrast, the transcription reaction with the mut22AG template was not inhibited regardless of the concentration of EV (squares with dotted line in Fig. 5D): the relative amounts of the transcripts (completed and arrested) were independent of the EV concentration. Therefore, transcriptional inhibition by EV is likely mediated by the G4 formed in the template. More importantly, this trend was maintained even in the presence of excess DNA duplex (circles with dotted lines in Fig. 5C and D). Although similar results were obtained for MV and CV, their inhibitory effects were weaker than that of EV (Fig. S6, ESI†). These results demonstrate that the binding of and transcriptional inhibition by EV are preserved even in the presence of excess DNA duplex and occur in a G4 structure-specific manner.
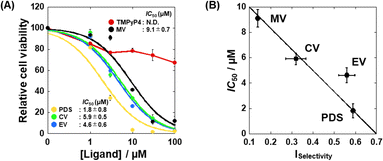
Based on the G4-specific binding of and enzyme inhibition by EV, we examined the effects of MV, CV, and EV, as well as TMPyP4 and PDS on the proliferation of HeLa cells. Fig. S7 (ESI†) shows images of HeLa cells 48 hours after the addition of 100 μM of each test compound. No significant decrease in cell viability was observed with TMPyP4, consistent with a previous study,21 whereas the other compounds decreased cell viability. Fig. 6A shows the viability of HeLa cells in the presence of various concentrations of TMPyP4, PDS, MV, EV, or EV. The IC50 value of the compounds were obtained from plots in Fig. 6A (see ESI† for the evaluation of the IC50 values). We then plotted the IC50 value versus the ISelectivity values to find a relationship between structural selectivity and anti-proliferative effect (Fig. 6B). Interestingly, there is a strong correlation between the anti-proliferative effect and the structural selectivity, showing that the structural selectivity of the G4 ligand is essential for the anti-proliferative effect on cancer cells. In addition, EV, a simple and relatively small compound, exhibits sufficient structural selectivity to reduce cancer cell viability.
Although the strong correlation between the selectivity and the anti-proliferative effect was observed, these componds may bind not only DNA G4 but also proteins. We then attempted to carry out the TD assay and the T7 polymerase assay of MV, CV, and EV in the presence of excess protein, HSA (human serum albmin). In the TD assay, it was found that the fluoresence decremet ThT was observed in the presence of HSA, although the degree of the decrement was smaller than that in the presence of exess DNA duplex (Fig. S8, ESI†). Moreover, the results of T7 RNA polymerase assay in the presence of 0, 5, 10, or 20 μM HSA showed that these compounds preserve the G4-specific inhibititory effect on T7 RNA polymerase (Fig. S9, ESI†). These results suggest that these compounds barely maintain the bindng affinity and selectivity in the presence of the protein, although further studies with other proteins are required. Thus, it is possible to consider that the between the anti-proliferative effect and the structural selectivity is reasonable.
In summary, we have established a new structure-selective G4 ligand screening system, the TD assay, which allowed the identification of novel structure-selective G4 ligands that showed a G4-specific transcriptional inhibitory effect and cell proliferative inhibition. These results clearly demonstrate that structural selectivity by G4 ligands is critical for cellular applications. Moreover, we quantitatively evaluated the effect of bulkiness of the positively charged functional group on the affinity and selectivity of the G4 ligands. Although the rational design of structure-selective G4 ligands remains difficult, a series of structure-selective G4 ligands identified using the TD assay will be useful for the rational design of structure-selective G4 ligands.
This work was supported by JSPS KAKENHI grant numbers 22H04975, 21H05107, 21H02062, 20K21259, and 17H06351, a Research Grant of the Asahi Glass Foundation, Japan, the Hirao Taro Foundation of Konan Gakuen for Academic Research, Japan, and JST SPRING, grant number JPMJSP2117.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Gellert, M. N. Lipsett and D. R. Davies, Proc. Natl. Acad. Sci. U. S. A., 1962, 48, 2013–2018 CrossRef CAS.
- D. Sen and W. Gilbert, Nature, 1988, 334, 364–366 CrossRef CAS.
- V. S. Chambers, G. Marsico, J. M. Boutell, M. Di Antonio, G. P. Smith and S. Balasubramanian, Nat. Biotechnol., 2015, 33, 877–881 CrossRef PubMed.
- S. Balasubramanian, L. H. Hurley and S. Neidle, Nat. Rev. Drug Discovery, 2011, 10, 261–275 CrossRef CAS PubMed.
- H. A. Pickett, J. D. Henson, A. Y. Au, A. A. Neumann and R. R. Reddel, Hum. Mol. Genet., 2011, 20, 4684–4692 CrossRef CAS PubMed.
- V. F. S. Kahl, J. A. M. Allen, C. B. Nelson, A. P. Sobinoff, M. Lee, T. Kilo, R. S. Vasireddy and H. A. Pickett, Front. Cell Dev. Biol., 2020, 8, 493 CrossRef PubMed.
- W. E. Wright, V. M. Tesmer, K. E. Huffman, S. D. Levene and J. W. Shay, Genes Dev., 1997, 11, 2801–2809 CrossRef CAS PubMed.
- A. De Cian, G. Cristofari, P. Reichenbach, E. De Lemos, D. Monchaud, M. P. Teulade-Fichou, K. Shin-Ya, L. Lacroix, J. Lingner and J. L. Mergny, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 17347–17352 CrossRef CAS PubMed.
- H. Yu, X. Gu, S. Nakano, D. Miyoshi and N. Sugimoto, J. Am. Chem. Soc., 2012, 134, 20060–20069 CrossRef CAS PubMed.
- R. Rodriguez, S. Müller, J. A. Yeoman, C. Trentesaux, J. F. Riou and S. Balasubramanian, J. Am. Chem. Soc., 2008, 130, 15758–15759 CrossRef CAS PubMed.
- M. Y. Kim, H. Vankayalapati, K. Shin-Ya, K. Wierzba and L. H. Hurley, J. Am. Chem. Soc., 2002, 124, 2098–2099 CrossRef CAS PubMed.
- S. Zhang, H. Sun, D. Yang, Y. Liu, X. Zhang, H. Chen, Q. Li, A. Guan and Y. Tang, Anal. Chim. Acta: X, 2019, 2, 100017 CAS.
- D. Monchaud, C. Allain and M. P. Teulade-Fichou, Bioorg. Med. Chem. Lett., 2006, 16, 4842–4845 CrossRef CAS PubMed.
- A. De Cian, L. Guittat, M. Kaiser, B. Saccà, S. Amrane, A. Bourdoncle, P. Alberti, M. P. Teulade-Fichou, L. Lacroix and J. L. Mergny, Methods, 2007, 42, 183–195 CrossRef CAS.
- C. Platella, D. Musumeci, A. Arciello, F. Doria, M. Freccero, A. Randazzo, J. Amato, B. Pagano and D. Montesarchio, Anal. Chim. Acta, 2018, 1030, 133–141 CrossRef CAS PubMed.
- V. Gabelica, R. Maeda, T. Fujimoto, H. Yaku, T. Murashima, N. Sugimoto and D. Miyoshi, Biochemistry, 2013, 52, 5620–5628 CrossRef CAS PubMed.
- P. Hanczyc, P. Rajchel-Mieldzioć, B. Feng and P. Fita, J. Phys. Chem. Lett., 2021, 12, 5436–5442 CrossRef CAS PubMed.
- A. T. Phan, V. Kuryavyi, K. N. Luu and D. J. Patel, Nucleic Acids Res., 2007, 35, 6517–6525 CrossRef CAS PubMed.
- R. T. S. Wheelhouse, D. Sun, H. Han, F. X. Han and L. H. Hurley, J. Med. Chem., 1998, 120, 3261–3262 CAS.
- D. M. Kong, Y. E. Ma, J. Wu and H. X. Shen, Chemistry, 2009, 15, 901–909 CrossRef CAS PubMed.
- H. Yaku, T. Murashima, D. Miyoshi and N. Sugimoto, J. Phys. Chem. B, 2014, 118, 2605–2614 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc00556a |
| This journal is © The Royal Society of Chemistry 2023 |