 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fluorescence-based chemical tools for monitoring ultrasound-induced hydroxyl radical production in aqueous solution and in cells†
Cherie CY.
Wong
a,
Lu-Lu
Sun
b,
Meng-Jiao
Liu
c,
Eleanor
Stride
 d,
Jason L.
Raymond
d,
Jason L.
Raymond
 a,
Hai-Hao
Han
a,
Hai-Hao
Han
 *bc,
James
Kwan
*bc,
James
Kwan
 *a and
Adam C.
Sedgwick
*a and
Adam C.
Sedgwick
 *e
*e
aDepartment of Engineering Science, Parks Road, Oxford, OX1 3PJ, UK. E-mail: james.kwan@balliol.ox.ac.uk
bShandong Laboratory of Yantai Drug Discovery, Bohai Rim Advanced Research Institute for Drug Discovery, Yantai, Shandong 264117, P. R. China. E-mail: hanhaihao@simm.ac.cn
cMolecular Imaging Center, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203, P. R. China
dInstitute of Biomedical Engineering, Department of Engineering Sciences, Old Road Campus Research Building, University of Oxford, Headington, Oxford, OX3 7DQ, UK
eDepartment of Chemistry, Chemistry Research Laboratory, University of Oxford, Mansfield Road, OX1 3TA, UK. E-mail: adam.sedgwick@chem.ox.ac.uk
First published on 21st March 2023
Abstract
We report the synthesis of hydroxyl-radical (˙OH) responsive fluorescent probes that utilise the 3,5-dihydroxybenzyl (DHB) functionality. 4-Methylumbeliferone-DHB (Umb-DHB) and resorufin-DHB (Res-DHB) in the presence of ˙OH radicals resulted in significant increases in their respective fluorescent emission intensities at 460 nm and 585 nm. The incubation of Res-DHB in HeLa cells followed by therapeutic ultrasound (1 MHz) resulted in a significant increase in fluorescence emission intensity thus permitting the ability to monitor ultrasound-induced ˙OH production in live cells.
The use of external stimuli to activate small molecules has shown tremendous potential in biological, biomedical and chemical research.1–3 Recent efforts within the chemical community have utilized the production of ˙OH radicals derived from radiolysis to activate and/or release small molecules for both imaging and therapeutic applications.4,5 Although a mainstay of cancer therapy, ionizing radiation suffers from several limitations, including radiation-associated toxicity and dose-limiting treatments.6 An emerging alternative is the use of therapeutic ultrasound, which can be used for both localised ablation7 and delivery/activation of anti-cancer agents.8,9 The ability of ultrasound to propagate through soft tissues to much greater depths than light similarly offers advantages compared with photodynamic therapy and photocaging approaches.10–12 In a recent report by Peng and co-workers, ultrasound-induced cavitation effectively generated ˙OH radicals by sonolysis13 to activate methylene blue-based urea conjugates for both imaging and therapeutic applications.14 Inspired by this strategy, we rationalised the recently reported ˙OH radical responsive unit, 3,5-dihydroxylbenzyl (DHB)4 may afford a general “sonocaging” unit that is selectively removed by ˙OH radicals generated from ultrasound irradiation. It is proposed ˙OH radical-mediated aromatic hydroxylation results in self-immolation releasing the active molecule (Scheme 1).
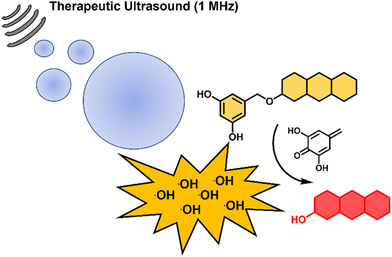 | ||
| Scheme 1 Schematic of acoustic cavitation resulting in the ˙OH-mediated deprotection of the 3,5-dihydroxybenzyl unit and release of a highly fluorescent molecule. Grey lines depict sound waves. | ||
Owing to the simplicity and non-invasive nature of fluorescence probes,15–20 in this study, we focused on the synthesis of DHB-functionalised 4-methylumbeliferone and resorufin fluorescent probes as shown in Fig. 1 (Umb-DHB and Res-DHB). We rationalised ˙OH radicals generated by ultrasound would selectively remove the DHB unit and lead to changes in their corresponding fluorescent emissions therefore allowing us to monitor the ultrasound-induced ˙OH production in solution and in mammalian cells.21,22
First, to attach the DHB unit onto each fluorophore, we synthesised a DHB-based alkylating reagent (see ESI† for full synthetic procedures and Schemes S1–S3). In brief, literature reported protocols were followed to isolate bis-silyl protected 3,5-dihydroxybenzyl alcohol 2.4 The synthetic transformation of 2 to a suitable alkylating agent proved difficult. After extensive method screening, a tosyl-based alkylating agent 3 was successfully synthesized (see ESI† – Scheme S1). Umb-DHB was obtained in 16% yield via refluxing 4-methylumbeliferone, 3, K2CO3 in THF followed by treating the crude mixture with TBAF (see ESI† – Scheme S2). Whereas Res-DHB was isolated in 27% yield by stirring resorufin, 3 and K2CO3 in DMF for 14 hours followed by the deprotection of the silyl ethers by treating the crude mixture with K2CO3 (5 eq.) and DMF (see ESI† – Scheme S3).23
Next, we tested the responsiveness of Umb-DHB and Res-DHB towards ˙OH radicals generated from the Fenton reaction. These measurements were needed to confirm the ˙OH radical responsiveness of the DHB functionality.4 It is important to note that the Fenton reaction favours acidic pH.24 However, most phenolic-based fluorophores are pH-sensitive. We therefore had to identify Fenton reaction conditions that reflected physiologically relevant pH values. Titrating Fe (II)–EDTA to solutions of either Umb-DHB (5 μM) or Res-DHB (5 μM) in PBS buffer, pH = 7.40 and hydrogen peroxide (H2O2, 10 mM) showed an Fe (II)-dependent (0–200 μM, 60 min incubation) increase in fluorescence emission intensity at 460 nm and 585 nm, respectively (Fig. 2a and b and Fig. S1 and S2, ESI†). A colour change from orange to pink was observed for Res-DHB, indicative of the release of resorufin (see ESI† – Fig. S3). Before evaluating the response of either probe to ultrasound irradiation, we tested their reactivity to other oxidants and biologically relevant species. Acoustic inertial cavitation produces several reactive molecules,25 including the reactive oxygen species (ROS): H2O2 and peroxynitrite (ONOO−).26 Surprisingly, the addition of ONOO− (100 μM) led to an instant increase in the fluorescence emission intensities of both Umb-DHB and Res-DHB (Fig. 1c and d). We hypothesise this ONOO−-mediated response is due to the rapid decomposition of peroxynitrous acid (ONOOH) to form ˙OH radicals.27,28 ONOO− generating the largest signal is rationalised by differences in the rate of ˙OH radical production from each source. The Fenton reaction is slow (>60 min) and suboptimal at pH = 7.4.24,29 This observation is supported by a similar response being seen using the traditional ˙OH radical fluorescent assay, terephthalic acid30 (TA) (see ESI† – Fig. S4). The potential formation of ONOO− during sonication provides another means of deprotection for the DHB functionality.26
With the responsiveness of Umb-DHB (5 μM) and Res-DHB (5 μM) towards ˙OH radicals identified; we turned our attention to monitoring changes in their respective fluorescence emissions when exposed to ultrasound (1 MHz) produced from a bespoke sonoreactor (see ESI† – methods). The bespoke sonoreactor was designed to create a region of high intensity ultrasound and hence acoustic cavitation in a water filled vessel. As seen in Fig. 3a and b, an increase in fluorescence emission was seen with increasing sonication time (0–6 min) at 1.2 W cm−2 time averaged power per unit area. The release of each fluorophore was confirmed by LC-MS analysis (see ESI† – Fig. S5–S8) and the TA assay confirmed the ultrasound-induced production of ˙OH radicals (see ESI† – Fig. S9).30 It is important to note that the ultrasound parameters need further optimisation in order to fully convert each probe to the corresponding fluorophores.
Resorufin was chosen in this study due to its extensive use in fluorescence probe design for imaging key chemical biomarkers in vitro and in vivo.31,32 We hypothesized that Res-DHB may have the potential to image ultrasound-induced production of ˙OH radicals in live cells. However, to demonstrate the potential of this strategy in biological settings, we had to first evaluate the fluorescence response of Res-DHB to ultrasound generated from a clinically used commercial instrument – WED-100 (1 MHz). The fluorescence response of Res-DHB correlated with intensity (0, 0.5, 1.5, 2 W cm−2 for 5 min) and increasing duration of ultrasound irradiation (0, 5, 10, 15, 20, 25, 30 min at 1.5 W cm−2) (see ESI† – Fig. S10). The release of resorufin was confirmed by mass spectrometry (see ESI† – Fig. S11). In addition, we synthesised other bis-functionalised benzyl resorufin derivatives (R1–R9) including 3,5-dimethoxylbenzyl33-functionalised resorufin in an effort to identify additional ˙OH radical responsive units. To our surprise and despite literature precedent with ionising radiation,4,33 only Res-DHB afforded noticeable changes in fluorescence emission intensity when sonicated for 5 min and 10 min (see ESI† – Fig. S12). This suggests that ionizing radiation may deprotect these types of protecting groups by additional mechanisms.
With the promise shown by Res-DHB using WED-100, we next turned to evaluating the responsiveness of Res-DHB to ultrasound-induced ˙OH radical production in live cells. CCK-8 assay was first performed to evaluate the cell viability of HeLa cells when incubated with different concentrations of Res-DHB. Both Res-DHB and the parent fluorophore resorufin were found non-toxic at the relevant fluorescence imaging concentrations (0–10 μM) and imaging timeframe (>2 h) (see ESI† – Fig. S13). No toxicity was seen for the duration of the ultrasound irradiation (5 min, 1.5 W cm−2) – see ESI† – Fig. S14. We next incubated Res-DHB (10 μM) for 120 min followed by sonication (1.5 W cm−2) for 5 minutes. As seen in Fig. 4, this treatment resulted in a significant increase (3-fold) in fluorescence emission compared to the HeLa cells not exposed to ultrasound irradiation. This increase in fluorescence emission was dependent on exposure time (0–10 min) and power (0–2 W cm−2) (see ESI† – Fig. S15 and S16). In addition, pork tissue (2 cm) placed between the cells followed by ultrasound treatment (1.5 W cm−2, 5 min) showed that distance did not impact the ultrasound induced fluorescence response (see ESI† – Fig. S17). However, due to the ROS-dependent nature of this strategy and the complexity of cellular environments, we subsequently tested the fluorescence response in the presence of the antioxidant GSH.34 As seen in Fig. 4, GSH had a clear inhibitory effect on the ultrasound-induced fluorescence response (Fig. 4). This data suggests that this ultrasound-induced activation could be limited to cell types due to GSH concentrations ranging vastly in cancers.35
In summary, we have synthesised two hydroxyl-radical (˙OH) responsive fluorescent probes (Umb-DHB and Res-DHB) that utilise the DHB functionality. Fluorescence changes were observed in solution and in cells when irradiated with therapeutic ultrasound frequency. However, GSH concentration was found to impact the ultrasound-induced fluorescence response in HeLa cells. These observations highlight the potential of ultrasound activation of small molecules, however further work is needed to identify the correct ultrasound parameters and cell types for this strategy to be appropriate. Further work is ongoing in our laboratories.
H.-H. H. thanks the National Natural Science Foundation of China (No. 22107029). A. C. S. would like to thank the Glasstone Research fellowship (University of Oxford) and Jesus College, Oxford for support as Junior Research Fellow. C. CY. W. thanks the Department of Engineering Science (University of Oxford) and Balliol College (University of Oxford) for their support through the DTP scholarship and the Dervouguilla Scholarship, respectively.
Conflicts of interest
There are no conflicts to declare.References
- L. Josa-Cullere and A. Llebaria, ChemPhotoChem, 2021, 5, 298–316 CrossRef CAS.
- K. S. Troelsen, E. D. D. Calder, A. Skwarska, D. Sneddon, E. M. Hammond and S. J. Conway, ChemMedChem, 2021, 16, 3691–3700 CrossRef CAS PubMed.
- M. S. Baker, H. Kim, M. G. Olah, G. G. Lewis and S. T. Phillips, Green Chem., 2015, 17, 4541–4545 RSC.
- Q. F. Fu, H. Y. Li, D. B. Duan, C. L. Wang, S. Y. Shen, H. M. Ma and Z. B. Liu, Angew. Chem., Int. Ed., 2020, 59, 21546–21552 CrossRef CAS PubMed.
- J. Geng, Y. C. A. Zhang, Q. Gao, K. Neumann, H. Dong, H. Porter, M. Potter, H. Ren, D. Argyle and M. Bradley, Nat. Chem., 2021, 13, 805–810 CrossRef CAS PubMed.
- K. Wang and J. E. Tepper, Ca-Cancer J. Clin., 2021, 71, 437–454 CrossRef PubMed.
- C. A. Speed, Rheumatology, 2001, 40, 1331–1336 CrossRef CAS PubMed.
- L. J. Delaney, S. Isguven, J. R. Eisenbrey, N. J. Hickok and F. Forsberg, Mater. Adv., 2022, 3, 3023–3040 RSC.
- T. Kondo, in Sonochemistry and the Acoustic Bubble, ed. F. Grieser, P.-K. Choi, N. Enomoto, H. Harada, K. Okitsu and K. Yasui, Elsevier, Amsterdam, 2015, pp. 207–230 DOI:10.1016/B978-0-12-801530-8.00009-8.
- G. Gunaydin, M. E. Gedik and S. Ayan, Front. Chem., 2021, 9 DOI:10.3389/fchem.2021.686303.
- R. Weinstain, T. Slanina, D. Kand and P. Klán, Chem. Rev., 2020, 120, 13135–13272 CrossRef CAS PubMed.
- E. Beguin, S. Shrivastava, N. V. Dezhkunov, A. P. McHale, J. F. Callan and E. Stride, ACS Appl. Mater. Interfaces, 2019, 11, 19913–19919 CrossRef CAS PubMed.
- M. Y. Choi, Molecules, 2019, 24, 3341, DOI:10.3390/molecules24183341.
- Y. Tong, M. Li, H. Huang, S. Long, W. Sun, J. Du, J. Fan, L. Wang, B. Liu and X. Peng, J. Am. Chem. Soc., 2022, 144, 16799–16807 CrossRef CAS PubMed.
- H. R. Bolland, E. M. Hammond and A. C. Sedgwick, Chem. Commun., 2022, 58, 10699–10702 RSC.
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC.
- X.-L. Hu, A. C. Sedgwick, D. N. Mangel, Y. Shang, A. Steinbrueck, K.-C. Yan, L. Zhu, D. W. Snelson, S. Sen, C. V. Chau, G. Juarez, V. M. Lynch, X.-P. He and J. L. Sessler, J. Am. Chem. Soc., 2022, 144, 7382–7390 CrossRef CAS PubMed.
- H.-H. Han, H. Tian, Y. Zang, A. C. Sedgwick, J. Li, J. L. Sessler, X.-P. He and T. D. James, Chem. Soc. Rev., 2021, 50, 9391–9429 RSC.
- W.-T. Dou, H.-H. Han, A. C. Sedgwick, G.-B. Zhu, Y. Zang, X.-R. Yang, J. Yoon, T. D. James, J. Li and X.-P. He, Sci. Bull., 2022, 67, 853–878 CrossRef CAS PubMed.
- H.-H. Han, H.-M. Wang, P. Jangili, M. Li, L. Wu, Y. Zang, A. C. Sedgwick, J. Li, X.-P. He, T. D. James and J. S. Kim, Chem. Soc. Rev., 2023, 52, 879–920 RSC.
- L. C. Moores, D. Kaur, M. D. Smith and J. S. Poole, J. Org. Chem., 2019, 84, 3260–3269 CrossRef CAS PubMed.
- A. R. Waggoner, Y. Abdulrahman, A. J. Iverson, E. P. Gibson, M. A. Buckles and J. S. Poole, J. Phys. Org. Chem., 2021, 34, e4278 CAS.
- Z.-Y. Jiang and Y.-G. Wang, Tetrahedron Lett., 2003, 44, 3859–3861 CrossRef CAS.
- C. Y. Chang, Y. H. Hsieh, K. Y. Cheng, L. L. Hsieh, T. C. Cheng and K. S. Yao, Water Sci. Technol., 2008, 58, 873–879 CrossRef CAS PubMed.
- K. Peng, F. G. F. Qin, R. Jiang, W. Qu and Q. Wang, Ultrason. Sonochem., 2022, 88, 106067 CrossRef CAS PubMed.
- G. Mark, H. P. Schuchmann and C. von Sonntag, J. Am. Chem. Soc., 2000, 122, 3781–3782 CrossRef CAS.
- J. W. Coddington, J. K. Hurst and S. V. Lymar, J. Am. Chem. Soc., 1999, 121, 2438–2443 CrossRef CAS.
- J. S. Beckman, T. W. Beckman, J. Chen, P. A. Marshall and B. A. Freeman, Proc. Natl. Acad. Sci. U. S. A., 1990, 87, 1620–1624 CrossRef CAS PubMed.
- S. Goldstein and G. Merényi, Methods Enzymol., 2008, 436, 49–61 CAS.
- J. C. Barreto, G. S. Smith, N. H. Strobel, P. A. McQuillin and T. A. Miller, Life Sci., 1995, 56, Pl89–Pl96 CrossRef CAS PubMed.
- J. W. Yoon, S. Kim, Y. Yoon and M. H. Lee, Dyes Pigm., 2019, 171, 107779 CrossRef CAS.
- L. Tian, H. Feng, Z. Dai and R. Zhang, J. Mater. Chem. B, 2021, 9, 53–79 RSC.
- J. M. Quintana, D. Arboleda, H. Hu, E. Scott, G. Luthria, S. Pai, S. Parangi, R. Weissleder and M. A. Miller, Bioconjugate Chem., 2022, 33, 1474–1484 CrossRef CAS PubMed.
- H. J. Forman, H. Zhang and A. Rinna, Mol. Aspects Med., 2009, 30, 1–12 CrossRef CAS PubMed.
- M. P. Gamcsik, M. S. Kasibhatla, S. D. Teeter and O. M. Colvin, Biomarkers, 2012, 17, 671–691 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc00364g |
| This journal is © The Royal Society of Chemistry 2023 |




