Open Access Article
Open Access ArticleMixed noble metal–oxo clusters: platinum(IV)–gold(III) oxoanion [PtIV2AuIII3O6((CH3)2AsO2)6]−†‡
Jiayao
Zhang
 a,
Saurav
Bhattacharya
a,
Saurav
Bhattacharya
 ab,
Anja B.
Müller
a,
Levente
Kiss
ab,
Anja B.
Müller
a,
Levente
Kiss
 c,
Cristian
Silvestru
c,
Cristian
Silvestru
 c,
Nikolai
Kuhnert
c,
Nikolai
Kuhnert
 a and
Ulrich
Kortz
a and
Ulrich
Kortz
 *a
*a
aSchool of Science, Constructor University (formerly Jacobs University), Campus Ring 1, 28759 Bremen, Germany. E-mail: ukortz@constructor.university; u.kortz@jacobs-university.de; Web: https://ukortz.user.jacobs-university.de/
bDepartment of Chemistry, BITS Pilani K. K. Birla Goa Campus, 403726 Goa, India
cDepartment of Chemistry, Supramolecular Organic and Organometallic Chemistry Centre (SOOMCC), Faculty of Chemistry and Chemical Engineering, Babes-Bolyai University, 400028 Cluj-Napoca, Romania
First published on 17th April 2023
Abstract
The first discrete mixed platinum(IV)–gold(III) oxoanion [PtIV2AuIII3O6((CH3)2AsO2)6]− (1) was synthesized by reaction of H2Pt(OH)6 with H[AuCl4] in a simple one-pot procedure in aqueous solution at pH 7 and comprises two equivalent PtIVO6(As(CH3)2)3 units which are linked by three square-planar AuIIIO4 units. Polyanion 1 could be isolated as a potassium or sodium salt in good yield, which were structurally characterized in the solid state by single-crystal XRD and TGA, and in solution by multinuclear (1H, 13C, 195Pt) NMR, indicating that polyanion 1 is stable in solution, which was confirmed by ESI-MS studies. The sodium salt of 1 undergoes a clean single-crystal-to-single-crystal (SCSC) structural transformation upon rehydration and dehydration.
Polynuclear coordination complexes form a large and diverse class of inorganic compounds, and anionic metal–oxo clusters known as polyoxometalates (POMs) form an important subclass.1 The enormous structural and compositional diversity and associated unique combination of different physicochemical properties of POMs have had an impact in many areas of chemistry, material science, medicine, and catalysis.2 Classical POMs comprise early d-block metal ions in high oxidation states (e.g. WVI, VV) as addenda and mainly edge- and corner-shared MO6 octahedra as structural motifs. The incorporation of noble metals such as Pt, Pd, and Au in lacunary POMs has attracted extensive attention, not only due to structural features, but the combination of the catalytic properties of the noble metals with the ability of the POM fragment to act as a thermally stable, soluble and redox-active host, relevant for catalytic intermediates in numerous industrially relevant processes and devices, including easily accessible “green” H2O2/O2-based oxidations.3
In 2004, the first noble metal oxoanion, [PtIII12O8(SO4)12]4−, was reported by Wickleder's group and this species is composed of six dumbbell-shaped metal–metal bonded PtIII2 dimers coordinated by oxo and sulfato bridges,4a and recently the first polythioplatinate(II), [PtII3S2(SO3)6]10−, was reported by our group.4b The first member of a polyoxopalladate(II), [PdII13As8O34(OH)6]8− (Pd13As8), was discovered by our group in 2008 and this anion comprises a cuboid-shaped assembly with one central and 12 external PdII ions in a square-planar coordination geometry, and eight arsenato capping groups.5 To date, more than 80 polyoxopalladates(II) with a large compositional and structural variety (e.g. cube, star, bowl, dumbbell, wheel, and open-shell archetypes) have been prepared by using various external capping groups RXO3n− (X = PV, AsV, SeIV, VV; R = O, Ph, lone pair) and many different central metal ion guests M (M = s, p, d or f element).3b Recently, we reported the first examples of neutral palladium(II)–oxo clusters (POCs), [Pd16O8(OH)8((CH3)2AsO2)8] (Pd16), [Pd16O8(OH)5Cl3((CH3)2AsO2)8] (Pd16Cl), [Pd24O12(OH)8((CH3)2AsO2)16] (Pd24), and [Pd40O24(OH)16(CH3)2AsO2)16] (Pd40), by using dimethylarsinate as capping group.6 Very recently, we could isolate the first two examples of cationic palladium(II)–oxo clusters (POCs) by incorporating f-metal ions, [PdII6O12M8{(CH3)2AsO2}16(H2O)8]4+ (M = CeIV, ThIV),7a as well as the mixed-valent palladium(IV/II)–oxoanion [PdIVO6PdII6((CH3)2AsO2)6]2−.7b In 2010, we reported the first discrete polyoxoaurate, [AuIII4As4O20]8− (Au4As4), which was prepared via aqueous condensation of in situ formed [Au(OH)4]− in the presence of AsO43− capping groups.8 Interestingly, in the solid state two Au4As4 units are linked by a belt of five sodium counter cations, resulting in the cuboid assembly {Na5(Au4As4)2}, which resembles the polyoxo-12-palladate family {MPd12X8}. Later a similar strategy was employed to prepare the selenium(IV) analogue [AuIII4Se4O20]4− (Au4Se4), with the arsenate caps being replaced by lone pair containing selenite groups.9 When reacting PdII and AuIII ions at the same time with the arsenate capping group, the first fully inorganic, mixed gold–palladium–oxoanion [NaAuIII4PdII8O8(AsO4)8]11− (Au4Pd8) could be obtained.10 Very recently, three more 12-palladate cubes with arsenate capping groups were reported, [ScO8Pd12(AsO4)8]13− and [MO8Pd12(AsO4)8]14− (M = CoII, CuII).11 It becomes apparent that the polyoxoplatinate and polyoxoaurate chemistry has been stagnant ever since the reports of the early members (vide supra). The research on Pt–Au containing compounds is largely limited to bimetallic nanoparticles, which exhibit excellent catalytic activity.12 However, to date no polyoxoanion containing both platinum(IV) and gold(III) centers has been reported.
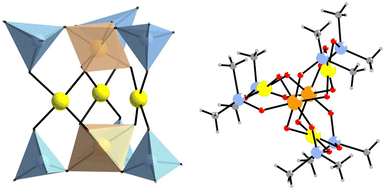
Herein, we report on the first example of a discrete mixed platinum(IV)–gold(III) oxoanion, [PtIV2AuIII3O6((CH3)2AsO2)6]− (1, see Fig. 1), which was synthesized in aqueous medium at 80 °C and isolated as a hydrated sodium salt, Na[PtIV2AuIII3O6((CH3)2AsO2)6]·NaCl·NaNO3·6H2O (Na-1) or a potassium salt, K[PtIV2AuIII3O6((CH3)2AsO2)6]·KCl·KAsO2(CH3)2·18H2O (K-1) in good yield.13 The polyanion 1 was prepared by reaction of H2[Pt(OH)6] with hydrogen tetrachloroaurate H[AuCl4] in a pH 7 sodium dimethylarsinate (also known as sodium cacodylate, Na-Cac) buffer, resulting in a rapid color change (the initial orange solution color becomes orange-red). It is well known that an acidification of a [Au(OH)4]− solution leads to the formation of insoluble Au(OH)3, and that chloride ions do not compete with OH− for AuIII in neutral or slightly alkaline solutions (pH 7.0 to 8.5).13 In our case, the in situ formed tetrahydroxogold(III) ion [Au(OH)4]− (or a closely related derivative) reacts smoothly with [Pt(OH)6]2− in the presence of cacodylate ions, which act as capping groups, thereby terminating the condensation process.
The polyanion 1 possesses a waterwheel structure with two PtIV ions linked by three square-planar coordinated AuIII ions and terminally coordinated by six cacodylate ligands, resulting in an assembly with C3h symmetry (Fig. 1). For K-1, the average AuIII–O bond lengths are 1.953(9) Å for the oxo ligands and 2.017(9) Å for the oxygen atoms of the cacodylate fragments. Bond valence sum (BVS) calculations showed no protonation for any bridging oxygen atoms (Table S5, ESI‡).14 The average AuIII–O bond lengths in K-1 (1.986 ± 0.010 Å) are comparable to those in other known gold(III)–oxo complexes, such as the square-planar Au2O2 core of [Au2{N2C10H7(CH2CMe3)-6}2(μ-O)2][PF6]2 (1.976(3) and 1.961(3) Å),15 the SrAu2(CH3COO)8) (1.979 ± 0.008) Å,16 or the polyoxoaurate Au4As4 (1.980 ± 0.023 Å),8 and quite a bit shorter than the Au–O distance in dimethylgold(III) hydroxide ((2.154 ± 0.148) Å).17 The Au···Au distance in K-1 (3.973 ± 0.070 Å) is significantly longer than in Au4As4 (3.246 ± 0.024 Å). The Pt–O bond lengths around the octahedrally coordinated Pt centers are quite regular, ranging from 1.960(9) to 2.063(9) Å. The same applies for the sodium salt Na-1. The three AuIII ions in 1 are located in the same plane and exhibit a slightly distorted square-planar coordination. The oxo ligands bridging to the platinum centers are situated on both sides of this {Au3} plane, while all O–As–O bridges connect a Pt and a Au atom (Fig. 1). In the solid-state lattice of Na-1, the polyanions are surrounded by a belt of six Na+ ions, three NO3− ions, and three Cl− ions, resulting in a supramolecular 2D layer with a hexagonal pattern (Fig. S2, ESI‡).
To complement our solid-state XRD results on 1 with solution studies, we performed 1H, 13C and 195Pt NMR measurements on Na-1 and K-1 redissolved in H2O/D2O. The 1H NMR spectrum of the reference Na-Cac in water exhibits sharp peaks at 4.7 and 1.4 ppm, respectively, corresponding to the protons of the cacodylate methyl groups and crystal water molecules. On the other hand, the 1H NMR spectrum of Na-1 exhibits peaks at 2.1 and 1.7 ppm that correspond to the two structurally and hence magnetically inequivalent cacodylate methyl groups (Fig. 2). The 1H NMR spectrum of K-1 exhibits three distinct peaks at 2.1, 1.7, and 1.6 ppm, respectively. The two downfield signals belong to 1, whereas the peak at 1.6 ppm corresponds to the methyl protons of free cocrystallized cacodylate ions. We also investigated the pH stability of polyanion 1 by NMR and the pH-dependent 1H NMR spectra show that polyanion 1 is stable at pH 2 to 10 (Fig. S4, ESI‡). The 13C NMR spectrum of Na-1 exhibits peaks at 22.8 and 17.1 ppm that correspond to the two structurally inequivalent cacodylate methyl groups (Fig. 2). The 13C NMR spectrum of Na-Cac exhibits a narrow peak at 17.3 ppm. The 13C NMR spectrum of K-1 exhibits three distinct sharp peaks (in analogy to its 1H NMR spectrum) at 22.8, 17.2, and 17.4 ppm corresponding to the methyl groups of the two structurally inequivalent cacodylate methyl groups of 1 and free cocrystallized cacodylate ions, respectively.
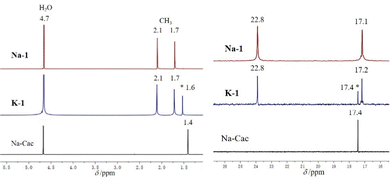 | ||
| Fig. 2 1H (left) and 13C (right) NMR spectra of Na-1 and K-1 dissolved in H2O/D2O compared to spectra of the sodium cacodylate reference. | ||
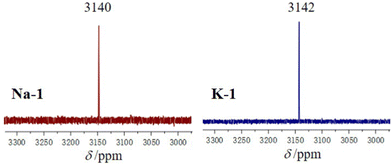
Next, we performed 195Pt NMR measurements on Na-1 and K-1 redissolved in water. This technique had been applied earlier for the platinum(IV)-containing decavanadate [H2PtIVV9O28]5−, exhibiting a clean signal at δ = 3832 ppm.18 We located the expected singlet for Na-1 and K-1 at 3140 and 3142 ppm, respectively (Fig. 3). The corresponding 195Pt NMR signal for the precursor H2[Pt(OH)6] appeared more downfield at 3294 ppm. We also performed time and temperature dependent 195Pt NMR measurements on fresh synthesis solutions of Na-1 (Fig. S5, ESI‡). After stirring at room temperature for 10 min and 40 min there are two peaks at 3316 and 3142 ppm, respectively, corresponding to the reagent H2Pt(OH)6 and polyanion 1. However, after heating at 40 °C for 30 min, the spectrum exhibits only a narrow peak at 3141 ppm, indicating that 1 is formed cleanly as the only product during the reaction procedure, suggesting a reaction yield of essentially 100%, which is extremely rare in POM chemistry. The combination of 1H, 13C, and 195Pt NMR is fully consistent with the solid-state structure of K-1 and Na-1 and hence provides unequivocal evidence for the presence of polyanion 1 also in solution.
Furthermore, the sodium and potassium salts of polyanion 1 were investigated by ESI-MS in the positive and negative ion modes. In the negative ion mode, signals centered around m/z 1898.55 were observed for both Na-1 and K-1. These could be clearly assigned to the singly-charged title polyanion [PtIV2AuIII3O6((CH3)2AsO2)6]− (1), see Fig. 4. In the positive ion mode spectrum of Na-1, a main group of signals was observed centered around m/z 983.75, corresponding to a doubly-charged species with an elemental composition of [Na3Pt2Au3O6(AsO2(CH3)2)6]2+ (Fig. S11, ESI‡). The isotope distribution was fully confirmed by comparison of the experimental spectrum to a simulated spectrum. Thus, the ESI-MS spectra of Na-1 and K-1 corroborate the solid-state structural analysis and show that polyanion 1 is structurally intact in solution, even under ESI conditions.
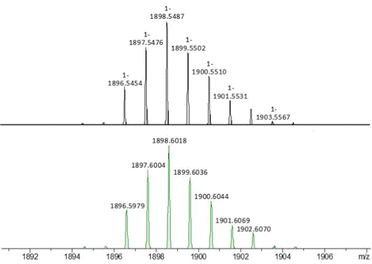 | ||
| Fig. 4 Simulated ESI-MS spectrum of Na-1 and K-1 in negative-ion mode (top) and experimental ESI-MS spectrum (bottom) of the singly-charged polyanion 1 (expanded view). | ||
We discovered that Na-1 undergoes a reversible single-crystal-to-single-crystal (SCSC) transformation in the solid state upon rehydration and hydration, where rehydration was achieved by keeping the sample in an atmosphere of water vapor at room temperature and dehydration was achieved by air drying for one day. The polyanion 1 crystallizes in the centrosymmetric space group P63/m when in the mother liquor (Na-1a), but after air drying for one day, it transforms to the non-centrosymmetric space group P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) (Na-1). The two crystal structures show the exact same polyanion as confirmed by single-crystal XRD, but the unit cell volume shrinks significantly from 2775.2(4) to 2130.9(12) Å3 upon air-drying for a day, and the packing arrangement is significantly different, because the layer sequence is AAA in Na-1 and ABA in Na-1a (Fig. S3, ESI‡). The structural transformation of Na-1a to Na-1 upon dehydration by air drying was also confirmed by powder-XRD (PXRD) measurements (Fig. 5 and Fig. S12, ESI‡), due to significantly different stacking of the 2-D layers. A pure phase of Na-1a is obtained in the crystals in the mother liquor, but after drying in air for one day, a transformation to the new phase Na-1 occurs. After 2 days, a completely pure phase of Na-1 is observed. The transformation from Na-1 to Na-1a is fully reversible during a rehydration and dehydration process. Rehydration of Na-1 in the presence of water vapor at room temperature results in Na-1a within half an hour. The rehydration behavior of Na-1 was also demonstrated by thermogravimetric analysis (TGA) and infrared spectroscopy (FT-IR) on Na-1 (Fig. S7 and S9, ESI‡). Single-crystal-to-single-crystal (SCSC) transformations constitute solid-state transitions induced by an external stimulus, such as light, heat, guest, or mechanochemical forces.19 While SCSC transformations in metal–organic frameworks (MOFs) and coordination polymers (CPs) are well-documented, examples for discrete complexes remain scarce. To our knowledge, only five other thermally-induced processes in POM structures have been described, involving high-, room- and low- temperature polymorphic transitions20 or structural variations by dehydration.21
(Na-1). The two crystal structures show the exact same polyanion as confirmed by single-crystal XRD, but the unit cell volume shrinks significantly from 2775.2(4) to 2130.9(12) Å3 upon air-drying for a day, and the packing arrangement is significantly different, because the layer sequence is AAA in Na-1 and ABA in Na-1a (Fig. S3, ESI‡). The structural transformation of Na-1a to Na-1 upon dehydration by air drying was also confirmed by powder-XRD (PXRD) measurements (Fig. 5 and Fig. S12, ESI‡), due to significantly different stacking of the 2-D layers. A pure phase of Na-1a is obtained in the crystals in the mother liquor, but after drying in air for one day, a transformation to the new phase Na-1 occurs. After 2 days, a completely pure phase of Na-1 is observed. The transformation from Na-1 to Na-1a is fully reversible during a rehydration and dehydration process. Rehydration of Na-1 in the presence of water vapor at room temperature results in Na-1a within half an hour. The rehydration behavior of Na-1 was also demonstrated by thermogravimetric analysis (TGA) and infrared spectroscopy (FT-IR) on Na-1 (Fig. S7 and S9, ESI‡). Single-crystal-to-single-crystal (SCSC) transformations constitute solid-state transitions induced by an external stimulus, such as light, heat, guest, or mechanochemical forces.19 While SCSC transformations in metal–organic frameworks (MOFs) and coordination polymers (CPs) are well-documented, examples for discrete complexes remain scarce. To our knowledge, only five other thermally-induced processes in POM structures have been described, involving high-, room- and low- temperature polymorphic transitions20 or structural variations by dehydration.21
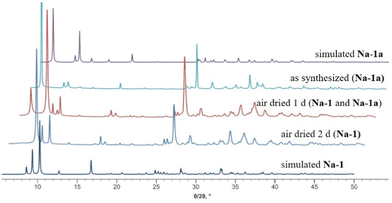 | ||
| Fig. 5 Experimental and simulated PXRD patterns of Na-1a and Na-1 during a dehydration process by air drying (simulated diffraction patterns derived from single-crystal data). | ||
We have synthesized and structurally characterized the first discrete mixed gold–platinum–oxoanion [PtIV2AuIII3O6((CH3)2AsO2)6]− (1) by using simple one-pot open-beaker techniques. The 195Pt NMR spectrum of redissolved solid 1 in water demonstrates solution stability, which provides much potential for further applications of 1 in catalysis and biomedicine. The reversible single-crystal-to-single-crystal transformation of Na-1a to Na-1 upon dehydration and rehydration is of particular interest, as concern solid state applications (sorption etc). The title polyanion 1 merges the areas of polyoxoplatinate and polyoxoaurate chemistry and pushes the area of polyoxo-noble-metalate chemistry to a higher synthetic and analytical level. Further studies in this area are ongoing.
U. K. thanks the German Research Council (DFG, KO 2288/26-1 and KO 2288/29-1) and Constructor University (formerly Jacobs University) for support. J. Z. sincerely acknowledges CSC (China Scholarship Council) for a doctoral fellowship.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) J. Berzelius, Poggendorff's Ann. Phys., 1826, 6, 369–380 CrossRef; (b) M. T. Pope, Y. Jeannin and M. Fournier, Heteropoly and Isopoly Oxometalates, Springer-Verlag, Berlin Heidelberg, 1983 CrossRef; (c) M. T. Pope, M. Sadakane and U. Kortz, Eur. J. Inorg. Chem., 2019, 340–342 CrossRef CAS.
- (a) M. T. Pope and A. Müller, Polyoxometalate Chemistry: From Topology via Self-Assembly to Applications, Kluwer Academic Publishers, Dordrecht, Netherlands, 2001 Search PubMed; (b) S. S. Wang and G. Y. Yang, Chem. Rev., 2015, 115, 4893–4962 CrossRef CAS PubMed; (c) M. R. Horn, A. Singh, S. Alomari, S. Goberna-Ferrón, R. Benages-Vilau, N. Chodankar, N. Motta, K. Ostrikov, J. MacLeod, P. Sonar, P. Gomez-Romero and D. Dubal, Energy Environ. Sci., 2021, 14, 1652–1700 RSC; (d) N. I. Gumerova and A. Rompel, Nat. Rev. Chem., 2018, 2, 0112 CrossRef CAS.
- (a) J. C. Goloboy and W. G. Klemperer, Angew. Chem., Int. Ed., 2009, 48, 3562–3564 CrossRef CAS PubMed; (b) P. Putaj and F. Lefebvre, Coord. Chem. Rev., 2011, 255, 1642–1685 CrossRef CAS; (c) I. A. Weinstock and A. Müller, Isr. J. Chem., 2011, 51, 176–178 CrossRef CAS; (d) N. V. Izarova, M. T. Pope and U. Kortz, Angew. Chem., Int. Ed., 2012, 51, 9492–9510 CrossRef CAS PubMed; (e) P. Yang and U. Kortz, Acc. Chem. Res., 2018, 51, 1599–1608 CrossRef CAS PubMed; (f) L. Rocchigiani and M. Bochmann, Chem. Rev., 2021, 121, 8364–8451 CrossRef CAS PubMed.
- (a) M. Pley and M. S. Wickleder, Angew. Chem., Int. Ed., 2004, 43, 4168–4170 CrossRef CAS PubMed; (b) A. Rajan, M. E. Mahmoud, F. Wang, S. Bhattacharya, A. S. Mougharbel, X. Ma, A. B. Müller, T. Nisar, D. H. Taffa, J. M. Poblet, N. Kuhnert, V. Wagner, M. Wark and U. Kortz, Inorg. Chem., 2022, 61, 11529–11538 CrossRef CAS PubMed.
- E. V. Chubarova, M. H. Dickman, B. Keita, L. Nadjo, F. Miserque, M. Mifsud, I. W. C. E. Arends and U. Kortz, Angew. Chem., Int. Ed., 2008, 47, 9542–9546 CrossRef CAS PubMed.
- (a) S. Bhattacharya, U. Basu, M. Haouas, P. Su, M. F. Espenship, F. Wang, A. Sole-Daura, D. H. Taffa, M. Wark, J. M. Poblet, J. Laskin, E. Cadot and U. Kortz, Angew. Chem., Int. Ed., 2021, 60, 3632–3639 CrossRef CAS PubMed; (b) S. Bhattacharya, X. Ma, A. S. Mougharbel, M. Haouas, P. Su, M. F. Espenship, D. H. Taffa, H. Jaensch, A. J. Bons, T. Stuerzer, M. Wark, J. Laskin, E. Cadot and U. Kortz, Inorg. Chem., 2021, 60, 17339–17347 CrossRef CAS PubMed.
- (a) S. Bhattacharya, A. Barba-Bon, T. A. Zewdie, A. B. Müller, T. Nisar, A. Chmielnicka, I. A. Rutkowska, C. J. Schürmann, V. Wagner, N. Kuhnert, P. J. Kulesza, W. M. Nau and U. Kortz, Angew. Chem., Int. Ed., 2022, 61, e202203114 CrossRef CAS PubMed; (b) X. Ma, S. Bhattacharya, T. Nisar, A. B. Müller, V. Wagner, N. Kuhnert and U. Kortz, Chem. Commun., 2023, 59, 904–907 RSC.
- N. V. Izarova, N. Vankova, T. Heine, R. Ngo Biboum, B. Keita, L. Nadjo and U. Kortz, Angew. Chem., Int. Ed., 2010, 49, 1886–1889 CrossRef CAS PubMed.
- Y. Xiang, N. V. Izarova, F. Schinle, O. Hampe, B. Keita and U. Kortz, Chem. Commun., 2012, 48, 9849–9851 RSC.
- N. V. Izarova, A. Kondinski, N. Vankova, T. Heine, P. Jäger, F. Schinle, O. Hampe and U. Kortz, Chem. – Eur. J., 2014, 20, 8556–8560 CrossRef CAS PubMed.
- P. Yang, M. Elcheikh Mahmoud, Y. Xiang, Z. Lin, X. Ma, J. H. Christian, J. K. Bindra, J. S. Kinyon, Y. Zhao, C. Chen, T. Nisar, V. Wagner, N. S. Dalal and U. Kortz, Inorg. Chem., 2022, 61, 18524–18535 CrossRef CAS PubMed.
- (a) S. Zhou, G. S. Jackson and B. Eichhorn, Adv. Funct. Mater., 2007, 17, 3099–3104 CrossRef CAS; (b) R. P. Doherty, J.-M. Krafft, C. Méthivier, S. Casale, H. Remita, C. Louis and C. Thomas, J. Catal., 2012, 287, 102–113 CrossRef CAS; (c) N. Biswakarma, D. Dowerah, S. D. Baruah, P. J. Sarma, N. K. Gour and R. C. Deka, Mol. Catal., 2021, 515, 111910 CrossRef CAS.
- (a) G. Jander and G. Krien, Z. Anorg. Allg. Chem., 1960, 304, 154–163 CrossRef CAS; (b) P. G. Jones, H. Rumpel, E. Schwarzmann and G. M. Sheldrick, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1979, 35, 1435–1437 CrossRef; (c) D. Vlassopoulos and S. A. Wood, Geochim. Cosmochim. Acta, 1990, 54, 3–12 CrossRef CAS.
- I. D. Brown and D. Altermatt, Acta Crystallogr., Sect. B, 1985, 41, 244–247 CrossRef.
- M. A. Cinellu, G. Minghetti, M. V. Pinna, S. Stoccoro, A. Zucca, M. Manassero and M. Sansoni, J. Chem. Soc., Dalton Trans., 1998, 1735–1741 RSC.
- P. G. Jones, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1984, 40, 804–805 CrossRef.
- (a) M. G. Miles, G. E. Glass and R. S. Tobias, J. Am. Chem. Soc., 1966, 88, 5738–5744 CrossRef CAS; (b) G. E. Glass, J. H. Konnert, M. G. Miles, D. Britton and R. S. Tobias, J. Am. Chem. Soc., 1968, 90, 1131–1138 CrossRef CAS; (c) S. J. Harris and R. S. Tobias, Inorg. Chem., 1969, 8, 2259–2264 CrossRef CAS.
- (a) U. Lee, H. C. Joo, K. M. Park, S. S. Mal, U. Kortz, B. Keita and L. Nadjo, Angew. Chem., Int. Ed., 2008, 47, 793–796 CrossRef CAS PubMed; (b) S. Dugar, N. V. Izarova, S. S. Mal, R. Fu, H.-C. Joo, U. Lee, N. S. Dalal, M. T. Pope, G. B. Jameson and U. Kortz, New J. Chem., 2016, 40, 923–927 RSC.
- (a) A. Chaudhary, A. Mohammad and S. M. Mobin, Cryst. Growth Des., 2017, 17, 2893–2910 CrossRef CAS; (b) F. Hu, X. Bi, X. Chen, Q. Pan and Y. Zhao, Chem. Lett., 2021, 50, 1015–1029 CrossRef CAS; (c) E. Fernandez-Bartolome, A. Martinez-Martinez, E. Resines-Urien, L. Piñeiro-Lopez and J. Sanchez Costa, Coord. Chem. Rev., 2022, 452, 214281 CrossRef CAS.
- (a) L. -Z. Zhang, W. Gu, Z. Dong, X. Liu and B. Li, CrystEngComm, 2008, 10, 1318–1320 RSC; (b) S. Reinoso, M. H. Dickman, A. Praetorius and U. Kortz, Acta Crystallogr., 2008, E64, m614–165 Search PubMed.
- (a) A. Iturrospe, L. S. Felices, S. Reinoso, B. Artetxe, L. Lezama and J. M. Gutierrez-Zorrilla, Cryst. Growth Des., 2014, 14, 2318–2328 CrossRef CAS; (b) D. Barats-Damatov, L. J. W. Shimon, Y. Feldman, T. Bendikov and R. Neumann, Inorg. Chem., 2015, 54, 628–634 CrossRef CAS PubMed; (c) L. Fernandez-Navarro, A. Iturrospe, S. Reinoso, B. Artetxe, E. Ruiz-Bilbao, L. S. Felices and J. M. Gutierrez-Zorrilla, Cryst. Growth Des., 2020, 20, 3499–3509 CrossRef CAS.
Footnotes |
| † Dedicated to Prof. Michael T. Pope on the occasion of his 90th Birthday. |
| ‡ Electronic supplementary information (ESI) available: Analytical techniques, XRD details, IR and 195Pt NMR spectra, TGA, selected BVS values, bond lengths and mass spectrum. CCDC 2221906, 2221907 and 2249169. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc00243h |
| This journal is © The Royal Society of Chemistry 2023 |