Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of a rhodium(III) dinitrogen complex using a calix[4]arene-based diphosphine ligand†
Jack
Emerson-King
 a,
Sudip
Pan
a,
Sudip
Pan
 b,
Matthew R.
Gyton
b,
Matthew R.
Gyton
 a,
Ralf
Tonner-Zech
a,
Ralf
Tonner-Zech
 b and
Adrian B.
Chaplin
b and
Adrian B.
Chaplin
 *a
*a
aDepartment of Chemistry, University of Warwick, Gibbet Hill Road, Coventry, CV4 7AL, UK. E-mail: a.b.chaplin@warwick.ac.uk
bWilhelm-Ostwald-Institut für Physikalische und Theoretische Chemie, Universität Leipzig, Linnéstraße 2, Leipzig, D-04103, Germany
First published on 23rd January 2023
Abstract
The synthesis and characterisation of the rhodium(III) dinitrogen complex [Rh(2,2′-biphenyl)(CxP2)(N2)]+ are described, where CxP2 is a trans-spanning calix[4]arene-based diphosphine and the dinitrogen ligand is projected into the cavity of the macrocycle.
Activation of dinitrogen by coordination to a transition metal is a process of immense biological and technological importance, helping to weaken the otherwise formidably strong nitrogen–nitrogen triple bond (De = 946 kJ mol−1) through M→N2 π-back donation.1,2 Molecular dinitrogen complexes have been reported for most of the transition elements. Mononuclear d6 systems have been the most heavily investigated, however, no well-defined rhodium(III) examples have previously been described.3 This paucity presumably reflects an incompatibility between the weak σ-donating, poor π-accepting character of dinitrogen and the relatively high oxidation state of the second-row transition metal.
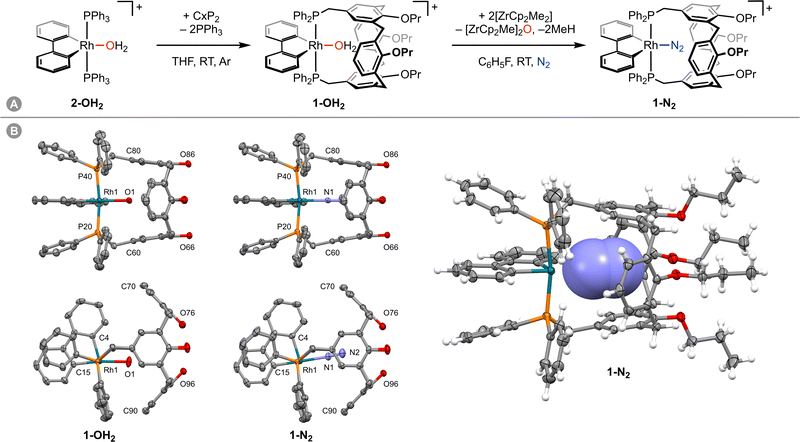
Inspired by the use of donor-functionalised cavitands as ligands in the literature and as part of our work exploring the chemistry of low-coordinate group 9 complexes supported by the high trans influence ancillary ligand 2,2′-biphenyl (biph),4,5 we became intrigued by the prospect of using a cavitand-based ligand to isolate a labile rhodium(III) dinitrogen complex.6 To this end, synthesis of [Rh(biph)(CxP2)(N2)][Al(ORF)4] (1-N2, RF = C(CF3)3; Fig. 1A) was targeted, reasoning that the previously reported diphosphine ligand CxP2 would position the {Rh(biph)}+ fragment across the upper rim of the constituent calix[4]arene scaffold and in doing so favour coordination of the small diatomic over solvent molecules. Only polynuclear and dinuclear derivatives of CxP2 have been reported previously.7 We herein describe the synthesis and characterisation of 1-N2 through dehydration of the corresponding rhodium(III) aqua complex 1-OH2, which can be obtained in 44% isolated yield by ligand substitution of trans-[Rh(biph)(PPh3)2(OH2)][Al(ORF)4] (2-OH2) with CxP2 in THF at room temperature (Fig. 1A).
In CD2Cl2 solution, isolated 1-OH2 is characterised by time-averaged C2v symmetry and a doublet 31P resonance at δ 13.2 (1JRhP = 120 Hz) at 298 K. Coordination of water within the calix[4]arene cavity is evidenced by a singlet 2H resonance at δ 0.84, which was washed out upon shaking with D2O and is significantly shielded relative to free water (δ 1.53) and 2-OH2 (δ 2.44). Crystals of 1-OH2 suitable for analysis by single crystal X-ray diffraction were obtained from CH2Cl2-hexane and demonstrate that the metal adopts a square pyramidal coordination geometry with the CxP2 ligand bound with near ideal trans geometry (P20–Rh1–P40 = 171.90(2)°) in the solid state (Fig. 1B). The coordinated water ligand is projected into the calix[4]arene cavity with a Rh1–O1 bond distance of 2.2046(14) Å and approximately linear C15–Rh1–O1 angle of 172.17(8)°. The formally vacant coordination site of the metal centre is sterically occluded by two phenyl groups of the CxP2 ligand, with carbon contacts >3.4 Å suggesting that any stabilisation by agostic bonding is minimal.8 In any case, these phenyl groups complete the encapsulation of the aqua ligand, which is contained within an almost uninterrupted van der Waals surface defined by the components of 1.
Treatment of 1-OH2 with an excess of the potent drying agent [ZrCp2Me2]9 in CD2Cl2 under an atmosphere of dinitrogen resulted in smooth conversion into a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dynamic equilibrium mixture of new rhodium(III) CxP2 complexes we assign as 1-N2 (δ31P 16.1, 1JRhP = 117 Hz) and 1-DCM (δ31P 4.4, 1JRhP = 117 Hz) within 24 h at room temperature. These assignments were substantiated by freeze-pump-thaw degassing the solution to remove dinitrogen and a control reaction carried out under an atmosphere of argon, both of which resulted in exclusive formation of 1-DCM. Highlighting the decisive role of the calix[4]arene scaffold, the spectroscopic characteristics of the bis(triphenylphosphine) analogue trans-[Rh(biph)(PPh3)2(κ1-ClCH2Cl)][Al(ORF)4] (2-DCM) are unchanged under an atmosphere of dinitrogen. Encouraged by these findings, the reaction between 1-OH2 and [ZrCp2Me2] was repeated under an atmosphere of dinitrogen in the more weakly coordinating solvent fluorobenzene.10 Consistent with our interpretation so far, 1-N2 (δ31P 16.0; 1JRhP = 116 Hz) was the only dehydration product observed by NMR spectroscopy.11 Subsequent analysis of 1-N2 by solution-phase IR spectroscopy provided direct evidence for coordination of dinitrogen. A very low intensity signal was observed at 2290 cm−1 and is tentatively assigned to the ν(N
5 dynamic equilibrium mixture of new rhodium(III) CxP2 complexes we assign as 1-N2 (δ31P 16.1, 1JRhP = 117 Hz) and 1-DCM (δ31P 4.4, 1JRhP = 117 Hz) within 24 h at room temperature. These assignments were substantiated by freeze-pump-thaw degassing the solution to remove dinitrogen and a control reaction carried out under an atmosphere of argon, both of which resulted in exclusive formation of 1-DCM. Highlighting the decisive role of the calix[4]arene scaffold, the spectroscopic characteristics of the bis(triphenylphosphine) analogue trans-[Rh(biph)(PPh3)2(κ1-ClCH2Cl)][Al(ORF)4] (2-DCM) are unchanged under an atmosphere of dinitrogen. Encouraged by these findings, the reaction between 1-OH2 and [ZrCp2Me2] was repeated under an atmosphere of dinitrogen in the more weakly coordinating solvent fluorobenzene.10 Consistent with our interpretation so far, 1-N2 (δ31P 16.0; 1JRhP = 116 Hz) was the only dehydration product observed by NMR spectroscopy.11 Subsequent analysis of 1-N2 by solution-phase IR spectroscopy provided direct evidence for coordination of dinitrogen. A very low intensity signal was observed at 2290 cm−1 and is tentatively assigned to the ν(N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) band. This band is red-shifted relative to free N2 (2330 cm−1), but considerably higher frequency than previously reported for terminal group 9 examples (1910–2236 cm−1).1,3 The assignment is supported by exposure of the sample to air, which resulted in disappearance of the ν(N
N) band. This band is red-shifted relative to free N2 (2330 cm−1), but considerably higher frequency than previously reported for terminal group 9 examples (1910–2236 cm−1).1,3 The assignment is supported by exposure of the sample to air, which resulted in disappearance of the ν(N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) band and formation of 1-OH2 within 5 seconds, slow evaporation of the solvent and analysis of the residue by ATR-IR spectroscopy within a dinitrogen filled glovebox, and computational analysis.
N) band and formation of 1-OH2 within 5 seconds, slow evaporation of the solvent and analysis of the residue by ATR-IR spectroscopy within a dinitrogen filled glovebox, and computational analysis.
Despite numerous attempts, our efforts to isolate analytically pure samples of 1-N2 from solution were frustrated by the extremely strong affinity of 1 for water, resulting in contamination of samples with 1-OH2 by reaction with adventurous water.12 In one instance we were, however, able to obtain a single crystal of 1-N2 suitable for analysis by X-ray diffraction, by slow diffusion of hexane into a CH2Cl2 solution of 1-N2 generated in situ using [ZrCp2Me2] (Fig. 1B). The dinitrogen ligand was readily located from the Fourier difference map, was freely refined with 100% crystallographic occupancy, and there is no evidence for significant disorder (Fourier peaks <0.5 e Å−3). Whilst this crystal was not representative of the bulk of the sample, it is the first structurally characterised example of a rhodium(III) dinitrogen complex. The solid-state structure of 1-N2 is isomorphous to 1-OH2 and the bulk geometric features of the {Rh(biph)(CxP2)}+ fragment are consequently similar. There are, however, statistically significant perturbations to the metal-centred metrics. For instance, the Rh1–P20 (2.3732(6) vs. 2.3505(5) Å) and Rh1–P40 (2.3630(6) vs. 2.3403(5) Å) bonds are elongated in 1-N2, whilst the P20–Rh1–P40 bond angle is contracted (169.79(2) vs. 171.90(2)°) relative to 1-OH2. Coordination of dinitrogen is also associated with a straighter C15–Rh1–N1 angle (179.43(10)°) than the corresponding metric in 1-OH2 (172.17(7)°), presumably to accommodate the linear diatomic within the calix[4]arene cavity. Both terminal and bridging end-on rhodium(I) dinitrogen complexes have been structurally characterised in the solid-state by X-ray diffraction, with the corresponding Rh–N bond lengths ranging from 1.85 to 2.08 Å (CSD version 5.43).13 Consistent with weak binding to the higher metal oxidation state, the Rh1–N1 bond length observed in 1-N2 is substantially longer than all these examples (2.160(2) Å). As for the aqua derivative, 1-N2 is fluxional in solution on the NMR time scale, adopting time-averaged C2v symmetry in solution at 298 K. We attempted to probe coordination of dinitrogen by 15N NMR spectroscopy using an isotopically enriched sample in fluorobenzene, but only free dinitrogen was observed. Presumably ligand exchange is fast on the timescale of the NMR experiment at 298 K.
To help delineate the role of the calix[4]arene scaffold, a DFT-based energy decomposition analysis was performed in combination with natural orbitals for chemical valence (EDA-NOCV; PBE-D3(BJ)/TZ2P-ZORA level of theory) using minimum energy structures of 1-L and 2-L (L = H2O, N2, DCM; optimised at the PBE-D3(BJ)/def2-SVP level of theory).14 Consistent with our hypothesis that CxP2 destabilises solvent over dinitrogen coordination, the calculated bond dissociation energies (De/kJ mol−1) decrease in the order H2O (89.6) > DCM (71.3) > N2 (68.6) for the bis(triphenylphosphine) complexes 2-L, but H2O (118.9) > N2 (81.9) ≫ DCM (33.9) for 1-L. Dichloromethane is not only too large to be accommodated within the calix[4]arene scaffold in 1, but requires a destabilising conformational change to permit metal coordination adjacent to the upper rim of the macrocycle (ΔEprep = +50.7, cf. +16.4 kJ mol−1 for 2). The interaction between 1 and dinitrogen is characterised by a greater extent of σ-donation (ΔEL→M = 48.9%) than π-back bonding (ΔEM→L = 44.6%) and no meaningful covalent interactions with the cavity were identified from the NOCV analysis (Fig. S38, ESI†). This net charge transfer to the metal is unusual for dinitrogen complexes observed in the condensed phase, but in line with the high N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching frequency measured.15
N stretching frequency measured.15
In summary, structural and spectroscopic characterisation of a well-defined rhodium(III) dinitrogen complex is reported. This complex is notable for a remarkably long rhodium–nitrogen bond (2.160(2) Å), a high N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N vibrational band (2290 cm−1), and showcases the utility of donor-functionalised cavitands for interrogating small molecular activation reactions mediated by transition metals.
N vibrational band (2290 cm−1), and showcases the utility of donor-functionalised cavitands for interrogating small molecular activation reactions mediated by transition metals.
We thank Andy Ashley (Imperial College London) for supply of 15N2. We gratefully acknowledge the EPSRC (DTA studentship to J. E.-K.), European Research Council (ERC, grant agreement 637313; M. R. G.), and Royal Society (UF100592, UF150675, A. B. C.) for financial support. Crystallographic data were collected using an instrument that received funding from the ERC under the European Union's Horizon 2020 research and innovation programme (grant agreement no. 637313). Computational resources were provided by ZIH Dresden and GOETHE-CSC Frankfurt.
Conflicts of interest
The authors declare no conflicts of interest.Notes and references
- Transition Metal-Dinitrogen Complexes – Preparation and Reactivity, ed. Y. Nishibayashi, Wiley-VCH, 2019 Search PubMed.
- (a) L. S. Yamout, M. Ataya, F. Hasanayn, P. L. Holland, A. J. M. Miller and A. S. Goldman, J. Am. Chem. Soc., 2021, 143, 9744–9757 CrossRef CAS PubMed; (b) P. L. Holland, Chem. Rev., 2020, 120, 4919–4920 CrossRef CAS PubMed; (c) J. G. Chen, R. M. Crooks, L. C. Seefeldt, K. L. Bren, R. M. Bullock, M. Y. Darensbourg, P. L. Holland, B. Hoffman, M. J. Janik, A. K. Jones, M. G. Kanatzidis, P. King, K. M. Lancaster, S. V. Lymar, P. Pfromm, W. F. Schneider and R. R. Schrock, Science, 2018, 360, eaar6611 CrossRef PubMed; (d) J. W. Erisman, M. A. Sutton, J. Galloway, Z. Klimont and W. Winiwarter, Nat. Geosci., 2008, 1, 636–639 CrossRef CAS.
- Crystallographically charactered terminal rhodium dinitrogen complexes: (a) J. T. Moore and C. C. Lu, J. Am. Chem. Soc., 2020, 142, 11641–11646 CrossRef CAS PubMed; (b) I. Fujii, K. Semba, Q.-Z. Li, S. Sakaki and Y. Nakao, J. Am. Chem. Soc., 2020, 142, 11647–11652 CrossRef CAS PubMed; (c) R. Kawakami, S. Kuriyama, H. Tanaka, K. Arashiba, A. Konomi, K. Nakajima, K. Yoshizawa and Y. Nishibayashi, Chem. Commun., 2019, 55, 14886–14889 RSC; (d) C. Rebreyend, Y. Gloaguen, M. Lutz, J. I. van der Vlugt, I. Siewert, S. Schneider and B. de Bruin, Chem. – Eur. J., 2017, 23, 17438–17443 CrossRef CAS PubMed; (e) N. Zhang, R. S. Sherbo, G. S. Bindra, D. Zhu and P. H. M. Budzelaar, Organometallics, 2017, 36, 4123–4135 CrossRef CAS; (f) G. M. Adams, F. M. Chadwick, S. D. Pike and A. S. Weller, Dalton Trans., 2015, 44, 6340–6342 RSC; (g) M. G. Scheibel, Y. Wu, A. C. Stückl, L. Krause, E. Carl, D. Stalke, B. de Bruin and S. Schneider, J. Am. Chem. Soc., 2013, 135, 17719–17722 CrossRef CAS PubMed; (h) O. V. Zenkina, E. C. Keske, R. Wang and C. M. Crudden, Angew. Chem., Int. Ed., 2011, 50, 8100–8104 CrossRef CAS PubMed; (i) J. Schöffel, N. Šušnjar, S. Nückel, D. Sieh and P. Burger, Eur. J. Inorg. Chem., 2010, 4911–4915 CrossRef; (j) S. K. Hanson, D. M. Heinekey and K. I. Goldberg, Organometallics, 2008, 27, 1454–1463 CrossRef CAS; (k) J. M. Praetorius, R. Wang and C. M. Crudden, Eur. J. Inorg. Chem., 2009, 1746–1751 CrossRef CAS; (l) A. Y. Verat, M. Pink, H. Fan, J. Tomaszewski and K. G. Caulton, Organometallics, 2008, 27, 166–168 CrossRef CAS; (m) J. D. Masuda and D. W. Stephan, Can. J. Chem., 2005, 83, 324–327 CrossRef CAS; (n) A. Vigalok, H. Kraatz, L. Konstantinovsky and D. Milstein, Chem. – Eur. J., 1997, 3, 253–260 CrossRef CAS PubMed; (o) P. R. Hoffman, T. Yoshida, T. Okano, S. Otsuka and J. A. Ibers, Inorg. Chem., 1976, 15, 2462–2466 CrossRef CAS.
- (a) G. R. F. Orton, B. S. Pilgrim and N. R. Champness, Chem. Soc. Rev., 2021, 50, 4411–4431 RSC; (b) D. Matt and J. Harrowfield, ChemCatChem, 2021, 13, 153–168 CrossRef CAS; (c) S. H. A. M. Leenders, R. Gramage-Doria, B. de Bruin and J. N. H. Reek, Chem. Soc. Rev., 2015, 44, 433–448 RSC; (d) C. Gibson and J. Rebek, Org. Lett., 2002, 4, 1887–1890 CrossRef CAS PubMed; (e) C. Wieser-Jeunesse, D. Matt and A. D. Cian, Angew. Chem., Int. Ed., 1998, 37, 2861–2864 CrossRef CAS.
- (a) T. M. Hood and A. B. Chaplin, Dalton Trans., 2021, 50, 2472–2482 RSC; (b) M. R. Gyton, A. E. Kynman, B. Leforestier, A. Gallo, J. R. Lewandowski and A. B. Chaplin, Dalton Trans., 2020, 49, 5791–5793 RSC; (c) T. M. Hood, M. R. Gyton and A. B. Chaplin, Dalton Trans., 2020, 49, 2077–2086 RSC; (d) T. M. Hood, B. Leforestier, M. R. Gyton and A. B. Chaplin, Inorg. Chem., 2019, 58, 7593–7601 CrossRef CAS PubMed; (e) J. Emerson-King, I. Prokes and A. B. Chaplin, Chem. – Eur. J., 2019, 25, 6317–6319 CrossRef CAS PubMed; (f) M. R. Gyton, B. Leforestier and A. B. Chaplin, Organometallics, 2018, 37, 3963–3971 CrossRef CAS PubMed; (g) R. C. Knighton, J. Emerson-King, J. P. Rourke, C. A. Ohlin and A. B. Chaplin, Chem. – Eur. J., 2018, 24, 4927–4938 CrossRef CAS PubMed.
- J. Emerson-King, PhD thesis, University of Warwick, 2019.
- X. Fang, B. L. Scott, J. G. Watkin, C. A. G. Carter and G. J. Kubas, Inorg. Chim. Acta, 2001, 317, 276–281 CrossRef CAS.
- M. Brookhart, M. L. H. Green and G. Parkin, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 6908–6914 CrossRef CAS PubMed.
- (a) N. A. Yakelis and R. G. Bergman, Organometallics, 2005, 24, 3579–3581 CrossRef CAS PubMed; (b) G. Proulx and R. G. Bergman, Organometallics, 1996, 15, 684–692 CrossRef CAS.
- S. D. Pike, M. R. Crimmin and A. B. Chaplin, Chem. Commun., 2017, 53, 3615–3633 RSC.
- Complete decomposition to an intractable precipitate via a transient intermediate assumed to be ‘naked’ 1 (δ31P 17.1, 1JRhP = 122 Hz) was observed on a similar timescale when the reaction was repeated under an atmosphere of argon.
- 1-N2 extracts water from vacuum dried 3 Å molecular sieves in solution.
- The longest Rh–N bond length reported in the literature is 2.076(2) Å for a neutral rhodium(I) dimer of the form [(PBP)Rh–N
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–Rh(PBP)] M. Hasegawa, Y. Segawa, M. Yamashita and K. Nozaki, Angew. Chem., Int. Ed., 2012, 51, 6956–6960 CrossRef CAS PubMed.
N–Rh(PBP)] M. Hasegawa, Y. Segawa, M. Yamashita and K. Nozaki, Angew. Chem., Int. Ed., 2012, 51, 6956–6960 CrossRef CAS PubMed. - (a) F. M. Bickelhaupt and E. J. Baerends, in Reviews in Computational Chemistry, ed. K. B. Lipkowitz and D. B. Boyd, Wiley-VCH, 2000, ch. 1, vol. 15, pp. 1–86 Search PubMed; (b) L. Zhao, M. Hermann, W. H. E. Schwarz and G. Frenking, Nat. Rev. Chem., 2019, 3, 48–63 CrossRef CAS; (c) M. P. Mitoraj, A. Michalak and T. Ziegler, J. Chem. Theor. Comput., 2009, 5, 962–975 CrossRef CAS PubMed.
- M. Schmitt and I. Krossing, J. Comput. Chem., 2023, 44, 149–158 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and computational details (PDF) and optimised geometries (XYZ). CCDC 2225292 (1-OH2), 2225293 (1-N2). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc06837k |
| This journal is © The Royal Society of Chemistry 2023 |