Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pd-catalyzed access to mono- and di-fluoroallylic amines from primary anilines†
Xingben
Wang
and
Frederic W.
Patureau
 *
*
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, Aachen 52074, Germany. E-mail: Frederic.patureau@rwth-aachen.de; Web: https://www.patureau-oc-rwth-aachen.de
First published on 16th December 2022
Abstract
The Pd-catalyzed highly selective synthesis of mono- and di-2-fluoroallylic amines from gem-difluorocyclopropanes and ubiquitous unprotected primary anilines is herein described. Initial kinetic investigations suggest a first order in the gem-difluorocyclopropane substrate, as well as a circa zeroth order in the aniline coupling partner. The newly produced fluoroallylic motifs should find important applications in synthetic as well as medicinal chemistry and stimulate the further development of coupling methods based on strained cyclic building blocks.
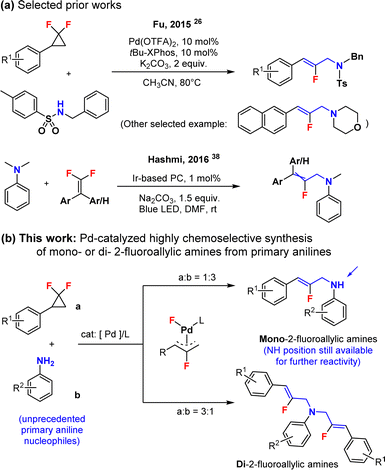
Allylamines are important structures found in both natural products and biologically active molecules.1–3 This is the case for example in naftifine,4,5 an antifungal drug, or flunarizine,6,7 a calcium blocker. Meanwhile, research and development of fluorine-containing drugs8–12 have considerably expanded in the last 20 years. This is because fluorine incorporation can impose remarkable electronic, physical and biological properties on organic compounds. Thus, we envisioned that developing simple synthetic methods granting access to fluorinated allylamines would represent an important objective in order to develop new potent drugs, or fluorinated variants of the existing ones. It is noteworthy that the synthesis of allylamine structures with transition metal catalysts13–22 is a frequently utilized and efficient approach. However, the synthesis of their fluorinated equivalents is starkly underdeveloped, and constitutes a major challenge. In this context, gem-difluorocyclopropanes23–25 are composed of a fluorine-containing strained three-membered ring structure, and are easily accessible (see the ESI†). Moreover, these motifs can be readily activated at the C–C and C–F bonds under transition metal catalysis, such as with Pd,26–36 Rh,37 or other metals, usually leading to the corresponding fluorinated allylic metal complexes. These organometallic intermediates can then be utilized to make fluorine-containing allylamide and allylamine compounds, when combined with N-derived nucleophiles. This was elegantly demonstrated by the seminal work of Fu and coauthors in 2015 (Scheme 1a).26 We propose herein such a C–N bond forming method, however with unprecedented primary aniline N-nucleophiles, which unfortunately perform very poorly under the method described by Fu,26 either towards the mono-, or alternatively towards the di-fluoroallylic amines, selectively (Scheme 1b). This strategy moreover represents a different retrosynthetic disconnection compared to the Ir-catalyzed photochemical C–C coupling method of Hashmi and collaborators (Scheme 1a),38 and allows for the direct synthesis of elusive NH-unprotected fluoroallylic anilines.
Therefore, 4-methyl gem-difluorocyclopropane a1 (1-(2,2-difluorocyclopropyl)-4-methylbenzene) and simple aniline (b1) were originally selected as model substrates. Setting the a1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b1 ratio to 1
b1 ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, under Pd(dba)2 catalysis (5 mol%), with XPhos (12.5 mol%) and K3PO4 (2 equiv.) in p-xylene at 110 °C for 12 h afforded the mono-functionalized product c1 in 29% yield. Under these conditions, the di-functionalized product d1 was obtained in 4% yield (entry 1, Table S1, see the ESI†). Increasing the amount of aniline b1 afforded the mono-functionalized 2-fluoroallylic amine product c1 in an impressive 89% isolated yield, with a selectivity as high as 20
1, under Pd(dba)2 catalysis (5 mol%), with XPhos (12.5 mol%) and K3PO4 (2 equiv.) in p-xylene at 110 °C for 12 h afforded the mono-functionalized product c1 in 29% yield. Under these conditions, the di-functionalized product d1 was obtained in 4% yield (entry 1, Table S1, see the ESI†). Increasing the amount of aniline b1 afforded the mono-functionalized 2-fluoroallylic amine product c1 in an impressive 89% isolated yield, with a selectivity as high as 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (a1
1 (a1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b1 ratio of 1
b1 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, entries 2 and 3). The higher XPhos/Pd catalytic ratio was moreover found necessary, as reducing the XPhos loading to only 5 mol% almost shuts down the reaction (entry 4). In contrast to entry 3, an a1
3, entries 2 and 3). The higher XPhos/Pd catalytic ratio was moreover found necessary, as reducing the XPhos loading to only 5 mol% almost shuts down the reaction (entry 4). In contrast to entry 3, an a1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b1 ratio of 3
b1 ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 afforded the new di-2-fluoroallylic amine substance d1 in an impressive 90% isolated yield and also high chemical selectivity (20
1 afforded the new di-2-fluoroallylic amine substance d1 in an impressive 90% isolated yield and also high chemical selectivity (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, entries 5 and 6). Finally, we verified that the original reaction conditions reported by Fu26 (Scheme 1b) do not provide product c1 or d1 in any significant yield regardless of the substrate ratio (entries 7 and 8), thus validating the superiority of our method for the challenging primary anilines.
1, entries 5 and 6). Finally, we verified that the original reaction conditions reported by Fu26 (Scheme 1b) do not provide product c1 or d1 in any significant yield regardless of the substrate ratio (entries 7 and 8), thus validating the superiority of our method for the challenging primary anilines.
In terms of scope, many functional groups were found well tolerated in very diverse positions (c1–c28), with typically excellent yields and mono-selectivity (usually m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d > 20
d > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Scheme 2). Moreover, a broad series of bioactive moieties (c29–c33) were well accommodated, with high isolated yields (26–99%) and good to excellent mono-selectivity (m
1, Scheme 2). Moreover, a broad series of bioactive moieties (c29–c33) were well accommodated, with high isolated yields (26–99%) and good to excellent mono-selectivity (m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d 7
d 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 20
1 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in some cases. In the low yielding case of c30, the substrate ratio was adjusted to 1
1) in some cases. In the low yielding case of c30, the substrate ratio was adjusted to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in order to minimize di-functionalization as well as other undefined side products. These results arguably demonstrate the high relevance of the herein described synthetic method, in spite of some other substrate limitations such as amide or aliphatic amine N-nucleophiles (c34–c38). This includes Fu's typical N-nucleophile,26 which did not efficiently furnish product c39. In such cases, some unreacted gem-difluorocyclopropane is typically observed at the end of the reaction. This indicates the high specificity of our method for anilines.
1 in order to minimize di-functionalization as well as other undefined side products. These results arguably demonstrate the high relevance of the herein described synthetic method, in spite of some other substrate limitations such as amide or aliphatic amine N-nucleophiles (c34–c38). This includes Fu's typical N-nucleophile,26 which did not efficiently furnish product c39. In such cases, some unreacted gem-difluorocyclopropane is typically observed at the end of the reaction. This indicates the high specificity of our method for anilines.
We then turned our attention to the new di-2-fluoroallylic amines and their synthesis (Scheme 3, d1–d17), affording generally good yields and selectivities (d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m > 20
m > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Interestingly, even sterically hindered 2-methyl aniline converted to the di-functionalized product (d18), although with a reaction time extended to 24 h, affording 82% yield and an encouraging di-selectivity (d
1). Interestingly, even sterically hindered 2-methyl aniline converted to the di-functionalized product (d18), although with a reaction time extended to 24 h, affording 82% yield and an encouraging di-selectivity (d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m = 9
m = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In addition, multifunctional and heterocyclic anilines were also found to be competent (d19–d24), in addition to a broad range of bioactive and natural fragments (d25–d29). In order to further explore the synthetic utility of the method, a gram scale reaction was conducted for mono-functionalized product c1 (Scheme 4). This target was thus obtained in a remarkably preserved 80% isolated yield (0.96 g), with high mono-selectivity (m
1). In addition, multifunctional and heterocyclic anilines were also found to be competent (d19–d24), in addition to a broad range of bioactive and natural fragments (d25–d29). In order to further explore the synthetic utility of the method, a gram scale reaction was conducted for mono-functionalized product c1 (Scheme 4). This target was thus obtained in a remarkably preserved 80% isolated yield (0.96 g), with high mono-selectivity (m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d > 20
d > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Furthermore, we verified that diphenylamine reacts in a similar fashion to the primary anilines described in this study, with 1a, affording indeed 95% isolated yield of the corresponding product (e1). Moreover, scaffold e1 could also be obtained from c1 in likewise excellent 95% yield with a classical Buchwald–Hartwig coupling reaction.39 Finally, unsymmetrical di-2-fluoroallylic product d30, with two different allylic arms, could be accessed from c1 reacting with a different gem-difluorocyclopropane, under otherwise similar reaction conditions (64%, Scheme 4). This demonstrates the feasibility of attaching two different fluoroallyl functional groups on primary anilines in a sequential fashion.
1). Furthermore, we verified that diphenylamine reacts in a similar fashion to the primary anilines described in this study, with 1a, affording indeed 95% isolated yield of the corresponding product (e1). Moreover, scaffold e1 could also be obtained from c1 in likewise excellent 95% yield with a classical Buchwald–Hartwig coupling reaction.39 Finally, unsymmetrical di-2-fluoroallylic product d30, with two different allylic arms, could be accessed from c1 reacting with a different gem-difluorocyclopropane, under otherwise similar reaction conditions (64%, Scheme 4). This demonstrates the feasibility of attaching two different fluoroallyl functional groups on primary anilines in a sequential fashion.
Based on previous literature,26 we envisioned a possible mechanism as outlined in Scheme 5. First Pd(0) would activate the strained C–C bond of the gem-difluorocyclopropane to form intermediate I, followed by β-F elimination40 to give π-allylpalladium species II. Intermediate II would then be attacked by the N-nucleophile to give species III. Finally, the product would be obtained by C–N bond reductive elimination, thus regenerating the Pd(0) active catalyst. Experimentally, we observed first order kinetics with respect to the gem-difluorocyclopropane building block a1 in the 0.05 to 0.40 M concentration range. In contrast, the reaction has an approximatively zeroth order in aniline substrate b1 (see the ESI† for details). These results suggest an early rate limiting step, such as the strained C–C bond activation, or the subsequent β-F elimination step towards intermediate II.
In summary, we have developed a Pd-catalyzed highly selective synthesis of mono- and di- 2-fluoroallylic amines from primary anilines and gem-difluorocyclopropanes. In addition to the newly opened chemical space in terms of potentially interesting fluorinated drug candidates, these results should encourage the further development of cross coupling methods based on the very versatile gem-difluorocyclopropane41–43 and related strained building blocks.
The ERC project 716136:2O2ACTIVATION (FWP) and the Chinese Scholarship Council (XW, No. 202008330337) are gratefully acknowledged for financial support.
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. D. Roughley and A. M. Jordan, J. Med. Chem., 2011, 54, 3451 CrossRef CAS PubMed.
- A. Trowbridge, S. M. Walton and M. J. Gaunt, Chem. Rev., 2020, 120, 2613 CrossRef CAS PubMed.
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257 CrossRef CAS PubMed.
- G. Petranyi, N. S. Ryder and A. Stütz, Science, 1984, 224, 1239 CrossRef CAS PubMed.
- H. Kanno and R. J. K. Taylor, Tetrahedron Lett., 2002, 43, 7337 CrossRef CAS.
- J. Olesen, J. Neurol., 1991, 238, S23 CrossRef PubMed.
- M. T. Marın, M. V. Margarit and G. E. Salcedo, Il Farmaco, 2002, 57, 723 CrossRef PubMed.
- H. Mei, J. Han, S. Fustero, M. Medio-Simon, D. M. Sedgwick, C. Santi, R. Ruzziconi and V. A. Soloshonok, Chem. – Eur. J., 2019, 25, 11797 CrossRef CAS PubMed.
- H. Chen, S. Viel, F. Ziarelli and L. Peng, Chem. Soc. Rev., 2013, 42, 7971 RSC.
- N. C. Yoder and K. Kumar, Chem. Soc. Rev., 2002, 31, 335 RSC.
- M. Salwiczek, E. K. Nyakatura, U. I. M. Gerling, S. Ye and B. Koksch, Chem. Soc. Rev., 2012, 41, 2135 RSC.
- E. N. G. Marsh, Acc. Chem. Res., 2014, 47, 2878 CrossRef CAS PubMed.
- T. Ohshima, Y. Miyamoto, J. Ipposhi, Y. Nakahara, M. Utsunomiya and K. Mashima, J. Am. Chem. Soc., 2009, 131, 14317 CrossRef CAS PubMed.
- K. Das, R. Shibuya, Y. Nakahara, N. Germain, T. Ohshima and K. Mashima, Angew. Chem., Int. Ed., 2012, 51, 150 CrossRef CAS PubMed.
- N. D. Knöfel, H. Rothfuss, J. Willenbacher, C. Barner-Kowollik and P. W. Roesky, Angew. Chem., Int. Ed., 2017, 56, 4950 CrossRef PubMed.
- I. Šolić, D. Reich, J. Lim and R. W. Bates, Asian J. Org. Chem., 2017, 6, 658 CrossRef.
- F. Ozawa, H. Okamoto, S. Kawagishi, S. Yamamoto, T. Minami and M. Yoshifuji, J. Am. Chem. Soc., 2002, 124, 10968 CrossRef CAS PubMed.
- S. Sawadjoon and J. S. M. Samec, Org. Biomol. Chem., 2011, 9, 2548 RSC.
- E. N. Bahena, S. E. Griffin and L. L. Schafer, J. Am. Chem. Soc., 2020, 142, 20566 CrossRef CAS PubMed.
- A. Cai, W. Guo, L. Martínez-Rodríguez and A. W. Kleij, J. Am. Chem. Soc., 2016, 138, 14194 CrossRef CAS PubMed.
- E. Skucas, M. Y. Ngai, V. Komanduri and M. J. Krische, Acc. Chem. Res., 2007, 40, 1394 CrossRef CAS PubMed.
- S. Z. Ali, B. G. Budaitis, D. F. A. Fontaine, A. L. Pace, J. A. Garwin and M. C. White, Science, 2022, 376, 276 CrossRef CAS PubMed.
- D. Seyferth, H. Dertouzos, R. Suzuki and J. Y. P. Mui, J. Org. Chem., 1967, 32, 2980 CrossRef CAS.
- A. P. Thankachan, K. S. Sindhu, K. K. Krishnan and G. Anilkumar, Org. Biomol. Chem., 2015, 13, 8780 RSC.
- D. M. Volochnyuk and O. O. Grygorenko, Emerging Fluorinated Motifs: Synthesis, Properties, and Applications, ed. D. Cahard and J.-A. Ma, Wiley, 2020, vol. 1, p. 135 Search PubMed.
- J. Xu, E. A. Ahmed, B. Xiao, Q. Q. Lu, Y. L. Wang, C. G. Yu and Y. Fu, Angew. Chem., Int. Ed., 2015, 54, 8231 CrossRef CAS PubMed.
- L. Lv, H. Qian, Y. Ma, S. Huang, X. Yan and Z. Li, Chem. Sci., 2021, 12, 15511 RSC.
- J. Wenz, C. A. Rettenmeier, H. Wadepohl and L. H. Gade, Chem. Commun., 2016, 52, 202 RSC.
- E. A. M. A. Ahmed, A. M. Y. Suliman, T. J. Gong and Y. Fu, Org. Lett., 2019, 21, 5645 CrossRef CAS PubMed.
- E. A. M. A. Ahmed, A. M. Y. Suliman, T. J. Gong and Y. Fu, Org. Lett., 2020, 22, 1414 CrossRef CAS PubMed.
- A. M. Y. Suliman, E. A. M. A. Ahmed, T. J. Gong and Y. Fu, Org. Lett., 2021, 23, 3259 CrossRef CAS PubMed.
- P. X. Zhou, X. Yang, J. Wang, C. Ge, W. Feng, Y. M. Liang and Y. Zhang, Org. Lett., 2021, 23, 4920 CrossRef CAS PubMed.
- A. M. Y. Suliman, E. A. M. A. Ahmed, T. J. Gong and Y. Fu, Chem. Commun., 2021, 57, 6400 RSC.
- L. Lv and C. J. Li, Angew. Chem., Int. Ed., 2021, 60, 13098 CrossRef CAS PubMed.
- Z. Fu, J. Zhu, S. Guo and A. Lin, Chem. Commun., 2021, 57, 1262 RSC.
- B. Xiong, X. Chen, J. Liu, X. Zhang, Y. Xia and Z. Lian, ACS Catal., 2021, 11, 11960 CrossRef CAS.
- Z. T. Jiang, J. Huang, Y. Zeng, F. Hu and Y. Xia, Angew. Chem., Int. Ed., 2021, 60, 10626 CrossRef CAS PubMed.
- J. Xie, J. Yu, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2016, 55, 9416 CrossRef CAS PubMed.
- M. A. Topchiy, A. F. Asachenko and M. S. Nechaev, Eur. J. Org. Chem., 2014, 3319 CrossRef CAS.
- T. Fujita, K. Fuchibe and J. Ichikawa, Angew. Chem., Int. Ed., 2019, 58, 390 CrossRef CAS PubMed.
- For a recent review: Y. Zhu, Y. Zeng, Z.-T. Jiang and Y. Xia, Synlett, 2022 DOI:10.1055/a-1912-3059.
- L. Lv, H. Qian, A. B. Crowell, S. Chen and Z. Li, ACS Catal., 2022, 12, 6495 CrossRef CAS.
- W. Yuan, X. Li, Z. Qi and X. Li, Org. Lett., 2022, 24, 2093 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Optimization, methods, characterization and spectra. See DOI: https://doi.org/10.1039/d2cc05844h |
| This journal is © The Royal Society of Chemistry 2023 |