Integrating a zwitterionic peptide with a two-photoelectrode system for an advanced photoelectrochemical immunosensing platform†
Yaqun
Xu
a,
Huimin
Chen
a,
Zhi-Ling
Song
 a,
Gao-Chao
Fan
a,
Gao-Chao
Fan
 *ab and
Xiliang
Luo
*ab and
Xiliang
Luo
 *a
*a
aShandong Key Laboratory of Biochemical Analysis, College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao 266042, China. E-mail: gcfan@qust.edu.cn; xiliangluo@qust.edu.cn
bState Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan 410082, China
First published on 23rd November 2022
Abstract
An ingenious strategy with the integration of a zwitterionic peptide into a two-photoelectrode system was reported to construct an advanced photoelectrochemical immunosensing platform. The strategy has endowed the platform with both excellent photoelectric properties and an antifouling ability, and was capable of accurate and sensitive detection of target biomarkers in biological specimens.
Accurate probing of important biomarkers contributes greatly to early prediction, pathological surveillance, and drug therapy. Among biosensing communities, photoelectrochemical (PEC) immunosensing has shown its salient features for the monitoring of target biomarkers, deriving from its characteristics of needing simple equipment, rapid testing, low background interference and high sensibility.1–3 Photoelectrodes as signal transducers of PEC immunosensors harvest light energy and convert it into current signals. The photoelectrodes can be classified into photoanodes and photocathodes.4 Photoanodes that use electrons as the main charge carriers have proven to exhibit evident current signals but poor anti-interference towards reductive species.5,6 While, the photocathodes that use holes as the main charge carriers have been demonstrated to show excellent anti-interference towards reductive species but with relatively poor current signals.7,8 Against detection in real biofluids, evident current signals and good anti-interference abilities are highly sought after. From the above situation, solitary photoanodes or photocathodes cannot meet this demanding criterion. Two-photoelectrode systems that refer to a series connection of a photocathode and photoanode can solve the puzzle:9,10 (i) capture probes are anchored on the photocathode, and the photoanode need not be incubated in the biofluid, which absolutely avoids the interference of the photoanode by reductive species; (ii) the useful series structure can motivate the promotion mechanism of the photocathode, and spark a more conspicuous current signal than the individual photoanode. In spite of this distinct superiority of the two-photoelectrode system, however, related PEC immunosensors have rarely been reported.
In fact, biofouling that is mainly generated by nonspecific adsorption of interfering proteins is indeed a challenge for practical immunosensors, because many potential proteins coexist within biological fluids (such as serum, plasma or blood) and are liable to adhere on the sensor surface.11,12 Since the biofouling inevitably brings false signals, impaired specificity and reduced accuracy for the majority of practical immunosensors, antifouling strategies are urgently needed for clinical applications. Currently, engineering antifouling biointerfaces via hydrophilic coatings are the most popular antifouling strategies to minimize surface biofouling.13 Among the hydrophilic coating communities, zwitterionic peptides as building blocks have aroused great interest owing to their characteristics of nontoxicity, easy synthesis, biocompatibility and structural stability.14,15 It is reported that zwitterionic peptides with repeating EK units of positively charged lysine residues (K) and negatively charged glutamic residues (E) can produce a strong hydrate layer to reduce protein adsorption.16 However, the direct coupling of E and K would cause interchain electrostatic interactions and limits the conformation of the zwitterionic peptides, preventing further strengthened antifouling performance. Studies have proved that inserting neutral spacers of hydrophilic amino acids such as serine (S) or asparagine (A) can address this issue, because they can break electrostatic interactions between the charged amino acid residues and prevent the formation of stable or metastable secondary structures.17 In addition, the PPPPC segment is usually used as a linker for surface anchoring owing to its rigid and regular structure, which can increase the peptide density in antifouling coatings.16 Taking these aspects into account, a zwitterionic peptide SKSESKSEPPPPC was designed, and the molecular structure is shown in Fig. S1 (ESI†).
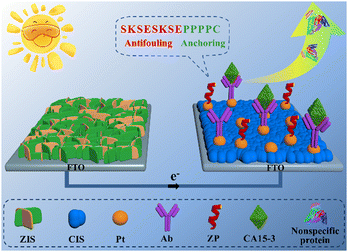
Inspired by the aforementioned background, a smart and feasible PEC immunosensing platform with the integration of a zwitterionic peptide and a two-photoelectrode system was reported to achieve accurate and sensitive probing of the target biomarker carbohydrate antigen 15-3 (CA15-3), as illustrated in Scheme 1. The two-photoelectrode system was the series connection of a photocathode and a photoanode. The ZIS/FTO photoanode was a coating of a ZnIn2S4 (ZIS) nanofilm on a fluorine-doped tin oxide (FTO) electrode; the Pt/CIS/FTO photocathode was a modification of CuInS2 (CIS) and Pt nanoparticles on a FTO electrode. The photoanode was used solely for the production of an evident current signal, whereas the photocathode was utilized to anchor the CA15-3 capture antibody (Ab) for the specific recognition of target CA15-3. After the designed zwitterionic peptide SKSESKSEPPPPC was then applied to the modified photocathode for generating a robust antifouling biointerface, the sensing electrode was complete. With this elaborated PEC immunosensing platform, the target CA15-3 was sensitively and accurately detected in human serum via a marked decrease of the current signal.
 | ||
| Scheme 1 Depicted PEC immunosensing platform integrating a zwitterionic peptide with a two-photoelectrode system. | ||
Scheme 2 illustrates the charge-transfer mechanism of the PEC immunosensing platform. The two-photoelectrode system as a signal transducer of the platform converted light energy into current signal. For the ZIS/FTO photoanode, ZIS as an n-type semiconductor has a proper band gap of 2.4 eV, which makes its absorption correspond well with the solar spectrum.18 Under light irradiation, electrons were excited from the valence band (VB) to the conduction band (CB) of the ZIS/FTO photoanode and flowed into the external circuit. With the assistance of the electron donor of ascorbic acid (AA), the photo-generated holes were neutralized, and hereby an evident current response appeared. For the Pt/CIS/FTO photocathode, CIS as a p-type semiconductor has a narrow band gap of 1.5 eV, endowing it with efficient visible-light adsorption.19 In addition, Pt nanoparticles have not only a good electrical conductivity but also an efficient catalytic ability to the O2 reduction reaction.20 As a result, the Pt/CIS/FTO photocathode produced abundant hole carriers to strengthen the attraction against electron carriers from the ZIS/FTO photoanode, and the electron carriers were easily captured by an electron acceptor of the dissolved O2 with the catalysis action of the Pt nanoparticles. With the synergistic action of the photoanode and the photocathode, the two-photoelectrode system sparked a more conspicuous current response.
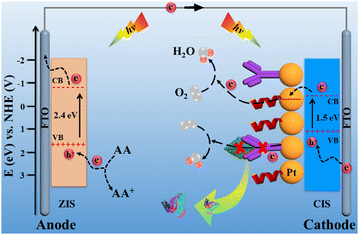 | ||
| Scheme 2 Charge-transfer mechanism of the elaborated PEC immunosensing platform. Band positions of the materials refer to the literature.18,19 | ||
Towards biological recognition, the capture Ab of CA15-3 was modified just on the photocathode, which granted the sensing platform with an anti-interference ability to the potential reductive species, since the photoanode definitely did not need sample incubation. Aiming at possible nonspecific adsorption from the interfering proteins, an antifouling element of the zwitterionic peptide SKSESKSEPPPPC was anchored on the photocathode. It has been proven that zwitterionic peptides with electric neutrality and strong hydrophilicity exhibit competent antifouling ability, because the characteristics can resist allowing biofouling molecules to access the electrode surface via charge attraction and hydrophobic interactions.21 In addition, compared to traditional large-sized protein blocking reagents, zwitterionic peptides have a small steric hindrance and can occupy more nonspecific binding sites, forming a dense antifouling layer and further promoting the antifouling capabilities of the biointerface.22 Based on the superior photoelectric property of the two-photoelectrode system and the antifouling ability of the zwitterionic peptide, accurate and sensitive probing of the target was realized.
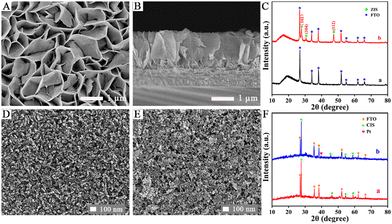
The surface topography of the ZIS/FTO photoanode was first monitored by scanning electron microscopy (SEM). As displayed in Fig. 1A, ZIS nanosheets about 40 nm in thickness grew uniformly on the FTO substrate, and they were interconnected to appear as a dense 2D layered structure with a large specific surface area. As presented in Fig. 1B in the cross-sectional view, a distinct growth layer could be distinguished and the nanosheets grew perpendicularly to the FTO substrate, forming a compact mesoporous film which was about 1.5 μm in height. The crystalline structure of the ZIS/FTO photoanode was studied by X-ray diffraction (XRD) in Fig. 1C. Curve a reveals the XRD pattern of a bare FTO substrate, in which all the diffraction peaks were indexed to the crystal panels of the tetragonal rutile phase of SnO2 (PDF no. 41-1445). Whereas with ZIS deposition, the new diffraction peaks in curve b appeared at 2θ = 27.9°, 30.4° and 47.7° could be assigned to the (102), (104) and (112) crystal planes of the hexagonal phase of ZIS (PDF no. 72-0773). Except for the FTO substrate, no diffraction peaks of other impurity phases were found, indicating a pure structure of the ZIS/FTO photoanode.
The Pt/CIS/FTO photocathode was also monitored first by SEM observations. As shown in Fig. 1D the surface morphology of the CIS/FTO electrode, CIS film was produced by the aggregates of spherical particles that were about 25 nm in size, and these particles were connected to each other, forming a relatively smooth and dense surface. After decoration of the Pt nanoparticles, as depicted in Fig. 1E, many small particles that were about 4–6 nm in average size gathered on the CIS film and the surface roughness increased. The XRD shown in Fig. 1F was then used as evidence to support modification of the Pt/CIS/FTO photocathode. For the CIS/FTO electrode in curve a, excluding the diffraction peaks from the FTO substrate, other peaks at 2θ = 27.8°, 46.2°, 54.7° and 57.4° corresponded to (112), (204)/(220), (116)/(312) and (224) crystal planes of chalcopyrite CIS (PDF no. 27-0159). With further deposition of Pt, as shown in curve b, the newly appeared diffraction peak at 2θ = 39.7° was indexed to the (111) crystal plane of Pt (PDF no. 04-0802), and no other diffraction peaks for impurity phases were detected. Incidentally, the TEM image (Fig. S3) and concentration estimation of the Pt nanoparticles are presented in the ESI.†
Subsequently, the photoelectric behaviour of the ZIS/FTO photoanode was inspected (Fig. S4), and further the stepwise fabrication of the antifouling biocathode was confirmed by both PEC monitoring and the EIS patterns (Fig. S5 and S6), and the details are exhibited in the ESI.†
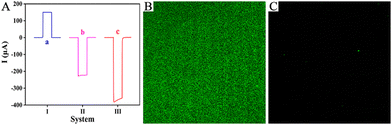
To highlight the superior photoelectric properties of the two-photoelectrode system, a control experiment was conducted against current outputs of different PEC systems, and the results are shown in Fig. 2A. Curve a shows current output of the ZIS/FTO photoanode system with Pt wire as the counter electrode, and the response value was evident (curve a, I = 150.04 μA). Curve b presents the current output of the immature two-photoelectrode system made up of a CIS/FTO photocathode and a ZIS/FTO photoanode, and the response value had an obvious elevation (curve b, I = −227.65 μA), increasing by 52% more than the photoanode system. Curve c reveals the current output of the just stated two-photoelectrode system consisting of the Pt/CIS/FTO photocathode and the ZIS/FTO photoanode, and the response value enhanced further (curve c, I = −372.28 μA), increasing by 148% more than the photoanode system. The results thus confirm that the described two-photoelectrode system has outstanding photoelectric properties, showing bright prospects.
The rational design of a zwitterionic peptide directly affects the antifouling ability of the immunosensing platform. Based on this, the characteristics of the zwitterionic peptide SKSESKSEPPPPC were first studied (Fig. S7 and S8), as presented in the ESI.† To further visually inspect the antifouling ability of the sensing platform, laser confocal microscopy was used to conduct optical imaging of different electrodes after incubation with fluorescein-labelled interfering protein FITC-BSA. As shown, the Ab-bound photocathode appeared with an obvious green fluorescence (Fig. 2B), implying evident nonspecific absorption of the electrode. Whereas with zwitterionic peptide anchoring, the electrode had almost no green fluorescence (Fig. 2C), indicating that the biointerface was not contaminated or affected by interfering proteins. Optical imaging further confirmed the excellent antifouling ability of the sensing platform.
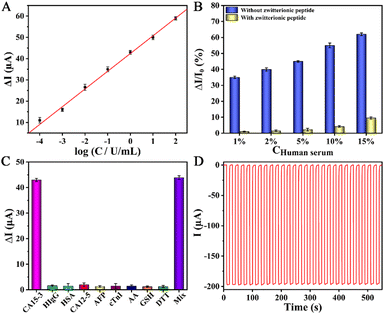
The detection signal of the PEC immunosensing platform depended on the quantity of the captured target CA15-3 on the antifouling biocathode. Prior to analysis, the optimization of the immunosensing platform was explored (Fig. S9, ESI†). Fig. 3A depicts the calibration curve of the sensing platform against target CA15-3 detection. In the concentration scope of 0.1 mU mL−1 to 100 U mL−1, a good linear relationship was observed, and the regression equation was acquired as ΔI = 42.40 + 8.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C (U mL−1) with a correlation coefficient of 0.9983. The limit of detection (LOD) was then calculated to be 0.027 mU mL−1 (S/N = 3), illustrating its high sensitivity.
C (U mL−1) with a correlation coefficient of 0.9983. The limit of detection (LOD) was then calculated to be 0.027 mU mL−1 (S/N = 3), illustrating its high sensitivity.
To inspect the antifouling performance of the sensing platform, as revealed in Fig. 3B, the signal changes (ΔI/I0) of the platforms that implanted without and with the zwitterionic peptide were compared after incubation in the biological specimen of human serum. As observed, the robust sensing platform implanted with the zwitterionic peptide exhibited a very tiny signal change, and meanwhile, projected a nice antifouling capability even against 10% diluted human serum. Furthermore, its antifouling durability with an extension of the incubation time was inspected, as shown in Fig. S10 (ESI†). The results hereby illustrate the potential application of the platform to real biofluids.
Excluding the target biomarker, the biological fluids often co-exist with some typical species of reducing agents and protein molecules. Accordingly, a few familiar substances such as human immunoglobin G (HIgG), human serum albumin (HSA), carbohydrate antigen 12-5 (CA12-5), α-fetoprotein (AFP), cardiac troponin I (cTnI), ascorbic acid (AA), glutathione (GSH) and dithiothreitol (DTT) were singled out to be incorporated into the 2% dilute human serum for an interference test. As perceived in Fig. 3C, the detection signals of the platform against the interfering proteins and reducing agents were tiny and negligible with respect to target CA15-3, verifying high specificity.
The stability of the PEC sensing platform was monitored by a time-based current response. As shown in Fig. 3D, the current intensity had almost no decay whereas the platform continuously suffered from dozens of irradiation cycles, confirming its good stability. In addition, the reproducibility, storage stability, and feasibility of the sensing platform were also evaluated (see the ESI†), and good results were acquired.
In summary, an efficient and feasible PEC immunosensing platform with the integration of a zwitterionic peptide and a two-photoelectrode system was built. The two-photoelectrode system that referred to a series connection of the Pt/CIS/FTO photocathode and the ZIS/FTO photoanode sparked a conspicuous current signal. The zwitterionic peptide engineered antifouling biointerface exhibited a good capability to resist nonspecific protein adsorption. Combing the prominent photoelectric property of the two-photoelectrode system with the antifouling characteristics of the zwitterionic peptide, the platform demonstrated high sensitivity and specificity for target biomarker detection, and was competent for assaying in human serum.
The present work is supported by the National Natural Science Foundation of China (22074073, 21275087), and the Natural Science Foundation of Shandong Province of China (ZR2021YQ11).
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. C. Fan, H. Zhao, L. Ma, Y. Lu and X. Luo, Chem. Commun., 2020, 56, 1345–1348 RSC.
- Y. X. Lin, Q. Zhou, D. P. Tang, R. Niessner and D. Knopp, Anal. Chem., 2017, 89, 5637–5645 CrossRef CAS PubMed.
- G. C. Fan, H. Zhu, Q. Shen, L. Han, M. Zhao, J. R. Zhang and J. J. Zhu, Chem. Commun., 2015, 51, 7023–7026 RSC.
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43, 7520–7535 RSC.
- G. L. Wang, K. L. Liu, J. X. Shu, T. T. Gu, X. M. Wu, Y. M. Dong and Z. J. Li, Biosens. Bioelectron., 2015, 69, 106–112 CrossRef CAS PubMed.
- W. X. Dai, L. Zhang, W. W. Zhao, X. D. Yu, J. J. Xu and H. Y. Chen, Anal. Chem., 2017, 89, 8070–8078 CrossRef CAS.
- G. C. Fan, L. Ma, S. Jayachandran, Z. Li and X. Luo, Chem. Commun., 2018, 54, 7062–7065 RSC.
- S. Z. Lv, K. Y. Zhang, Z. Z. Lin and D. P. Tang, Biosens. Bioelectron., 2017, 96, 317–323 CrossRef CAS PubMed.
- Q. Zeng, J. Bai, J. Li, Y. Li, X. Li and B. Zhou, Nano Energy, 2014, 9, 152–160 CrossRef CAS.
- X. Liang, P. Wang, F. Tong, X. Liu and C. Wang, Adv. Funct. Mater., 2021, 31, 2008656 CrossRef CAS.
- H. Qi, W. Zheng, X. Zhou, C. Zhang and L. Zhang, Chem. Commun., 2018, 54, 11328–11331 RSC.
- J. S. del Río, O. Y. F. Henry, P. Jolly and D. E. Ingber, Nat. Nanotechnol., 2019, 14, 1143–1149 CrossRef.
- C. Jiang, G. Wang, R. Hein, N. Liu, X. Luo and J. Davis, Chem. Rev., 2020, 120, 3852–3889 CrossRef CAS.
- B. E. I. Ramakers, J. C. M. van Hest and D. W. P. M. Löwik, Chem. Soc. Rev., 2014, 43, 2743–2756 RSC.
- G. Wang, X. Su, Q. Xu, G. Xu, J. Lin and X. Luo, Biosens. Bioelectron., 2018, 101, 129–134 CrossRef CAS.
- A. K. Nowinski, F. Sun, A. D. White, A. J. Keefe and S. Jiang, J. Am. Chem. Soc., 2012, 134, 6000–6005 CrossRef CAS PubMed.
- C. Li, M. Li, W. Qi, R. Su and J. Yu, Langmuir, 2021, 37, 8455–8462 CrossRef CAS PubMed.
- Q. Liu, H. Lu, Z. W. Shi, F. L. Wu, J. Guo, K. M. Deng and L. Li, ACS Appl. Mater. Interfaces, 2014, 6, 17200–17207 CrossRef CAS PubMed.
- A. Enesca, M. Baneto, D. Perniu, L. Isac, C. Bogatu and A. Duta, Appl. Catal., B, 2016, 186, 69–76 CrossRef CAS.
- Y. Nie, L. Li and Z. D. Wei, Chem. Soc. Rev., 2015, 44, 2168–2201 RSC.
- B. Liu, X. Liu, S. Shi, R. Huang, R. Su, W. Qi and Z. He, Acta Biomater., 2016, 40, 100–118 CrossRef CAS PubMed.
- S. Gu, X. M. Shi, D. Zhang, G. C. Fan and X. Luo, Anal. Chem., 2021, 93, 2706–2712 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details; characterization of Pt; PEC behaviour of photoanode; monitoring of biocathode; characteristics of peptide; optimization, antifouling durability, reproducibility, storage stability, and feasibility. See DOI: https://doi.org/10.1039/d2cc05721b |
| This journal is © The Royal Society of Chemistry 2023 |